Abstract
During functional electrical stimulation (FES), both the frequency and intensity can be increased to increase muscle force output and counteract the effects of muscle fatigue. Most current FES systems, however, deliver a constant frequency and only vary the stimulation intensity to control muscle force. This study compared muscle performance and fatigue produced during repetitive electrical stimulation using three different strategies: (1) constant pulse-duration and stepwise increases in frequency (frequency-modulation); (2) constant frequency and stepwise increases in pulse-duration (pulse-duration-modulation); and (3) constant frequency and pulse-duration (no-modulation). Surface electrical stimulation was delivered to the quadriceps femoris muscles of 12 healthy individuals and isometric forces were recorded. Muscle performance was assessed by measuring the percent changes in the peak forces and force–time integrals between the first and the last fatiguing trains. Muscle fatigue was assessed by measuring percent declines in peak force between the 60 Hz pre- and post-fatigue testing trains. The results showed that frequency-modulation showed better performance for both peak forces and force–time integrals in response to the fatiguing trains than pulse-duration-modulation, while producing similar levels of muscle fatigue. Although frequency-modulation is not commonly used during FES, clinicians should consider this strategy to improve muscle performance.
Keywords: Functional electrical stimulation (FES), Pulse-duration, Low-frequency fatigue, Muscle performance
1. Introduction
The central nervous system achieves a precise and task-specific control of skeletal muscle forces by controlling the number of activated motor units (recruitment) and the firing frequency of the activated motor units (rate-coding) (Kamen and Du, 1999; Kanosue et al., 1979). In individuals with paralysis due to upper motor neuron dysfunction, electrical stimulation is used to substitute for the loss of voluntary motor control to enable patients to stand, walk, and grasp objects (Kralj et al., 1988; Liberson et al., 1961). This application of electrical stimulation is called functional electrical stimulation (FES). Analogous to the two mechanisms of recruitment and rate-coding, stimulation intensity and frequency can be modulated to control muscle force output during FES. Although FES has immense potential application, it has not gained widespread popularity due to the limitations in current FES systems (Brissot et al., 2000; Popovic et al., 2001; Riener, 1999). An important limitation that discourages FES users is the rapid onset of muscle fatigue (Andrews et al., 1988; Brindley et al., 1979; Marsolais and Edwards, 1988; Rabischong and Chavet, 1997; Riener, 1999). Muscle fatigue may impede efficient task performance during FES by limiting the number of steps that FES users can perform or the number of minutes that FES users can stand or grasp an object (Andrews et al., 1988; Brindley et al., 1979; Marsolais and Edwards, 1988; Rabischong and Chavet, 1997; Riener, 1999).
Stimulation trains of different combinations of frequency and intensity can be used to generate the muscle force required to perform a functional task during FES. However, with repetitive activation, the muscle will fatigue and an increase in either the frequency or the intensity of stimulation will be required to enable the targeted muscle force to be maintained. Interestingly, although both the stimulation intensity and frequency can be modulated, most current FES systems deliver a constant frequency and only increase the stimulation intensity to increase force output as the muscle fatigues (Donaldson et al., 2000; Petrofsky and Stacy, 1992; Raymond et al., 1999; Taylor et al., 1999). Previous studies on animal muscles show that compared to modulating either the pulse-duration or frequency, a simultaneous modulation of both stimulation pulse-duration and frequency produces improved control of isometric torque during FES (Chizeck et al., 1991). However, no previous studies on human muscles have systematically compared the effects of increasing stimulation frequency versus stimulation intensity on muscle fatigue and performance during repetitive electrical stimulation. The purpose of this study, therefore, was to compare the muscle fatigue and performance during repetitive stimulation using trains consisting of: (1) constant pulse-duration and stepwise increases in frequency (frequency-modulation); (2) constant-frequency and stepwise increases in pulse-duration (pulse-duration-modulation); and (3) constant-frequency and constant-pulse-duration (no-modulation).
During FES, single pulses are grouped together to form stimulation trains. The stimulation frequency within each train can be controlled by varying the inter-pulse interval. The stimulation intensity can be controlled by varying either the amplitude or the duration of each pulse within a train. In this study, we kept the stimulation amplitude constant during each testing session and varied pulse-duration to modulate the stimulation intensity. Previous studies have shown that modulating the pulse-duration to control intensity requires a smaller charge per stimulus to produce a particular force and allows a greater selectivity of recruitment thresholds compared to modulating the pulse-amplitude (Crago et al., 1980).
2. Materials and methods
2.1. Subjects
Twelve healthy individuals (six males + six females) aged 22–30 years with no history of lower extremity orthopedic, neurological, or vascular problems participated in the study. The subjects were requested to refrain from strenuous exercise for at least 48 h before testing. The subjects were informed about the testing procedures and signed informed consent forms approved by the Human Subjects Review Board of the University of Delaware.
2.2. Apparatus and setup
The subject was seated on an electromechanical force dynamometer (KinCom III 500-11, Chattecx Corp., Chattanooga, TN), with the back supported, hips flexed to approximately 75°, and knees flexed at 90°. Velcro straps were used to stabilize the subject's upper trunk, waist, and thigh. The subject's ankle was stabilized with a strap placed approximately 5 cm proximal to the lateral malleolus. The isometric force output of the quadriceps femoris muscle was recorded via a force transducer placed against the anterior aspect of the leg, about 5 cm proximal to the lateral malleolus. The subject could see a representation of the force recorded by the KinCom force transducer on a display screen.
Electrical stimulation was delivered via two self-adhesive surface electrodes (Versa-Stim, CONMED Corp., New York, USA, 76 mm × 127 mm). The proximal electrode was placed over the upper thigh, covering the proximal portion of the rectus femoris and vastus lateralis muscles. The distal electrode was placed over the lower aspect of the thigh, covering the vastus medialis and distal portion of the rectus femoris. A Grass S8800 (Grass Instrument Company, Quincy, MA) stimulator with a SIU8T stimulus isolation unit was used to deliver the electrical stimulation. A personal computer equipped with a PCI-6024E data acquisition board and a PCI-6602 counter-timer board was used for data-acquisition. A custom-made switch was connected in series with the stimulator to modulate the duration and timing of the pulses. A custom-written LabVIEW program was used for data-acquisition.
2.3. Experimental procedures
Each subject participated in four sessions with a minimum of 48 h separating consecutive sessions. During the 1st session, subjects were familiarized with the testing procedures and equipment, their maximal voluntary isometric contraction (MVIC) forces were determined, and data were collected for plotting their force versus frequency and force versus pulse-duration curves. During the 2nd– 4th sessions, one of the three fatigue protocols was tested each day in random order. Except during the MVIC testing, the subjects were requested to relax during the testing. A series of trains were delivered to the subjects' quadriceps muscles to train the subjects to relax their muscles while the stimulation was being delivered. The following procedures were performed during the four sessions (Fig. 1):
Fig. 1.
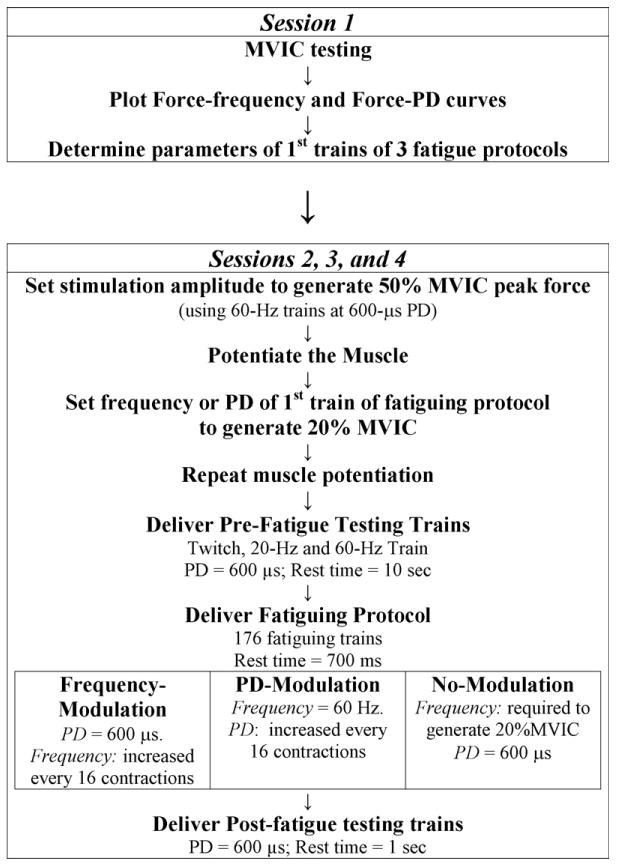
Flowchart showing the experimental protocol for the four testing sessions. (PD: pulse duration). See text for details.
2.3.1. Initial data-collection (1st session)
At the start of the 1st session, subjects were informed about the testing procedures, requested to sign the informed consent forms, and trained to perform the MVIC test.
MVIC Testing
The burst superimposition technique was used to determine each subject's MVIC force (Snyder-Mackler et al., 1994). During the MVIC testing, the subjects were asked to produce as much knee extension force as possible. While the subjects were generating knee extension force, a supra-maximal electrical stimulation train (amplitude, 130 V; train duration, 100 ms; frequency, 100 Hz; pulse-duration, 600 μs) was delivered to the quadriceps femoris muscle. If the electrical stimulation train increased the muscle force by <10%, the subject's MVIC was recorded. If the electrical stimulation train increased the force output ≥10%, the MVIC testing was repeated after a 10-min rest. If the subjects failed to complete the MVIC testing within three repetitions, they were not tested on that day.
Data-collection for force–frequency and force–pulse-duration curves
The MVIC test was followed by a 10-min rest. Next, the stimulation amplitude was set to elicit 50% of the subject's MVIC. The train with the highest frequency and pulse duration delivered during our study, i.e., a 300-ms long 100 Hz train with a 600 μs pulse-duration, was used to set the stimulation amplitude. The subjects were asked to refrain from testing if they considered the stimulation amplitude uncomfortable or intolerable. All subjects were able to tolerate the amplitude with no complaints of discomfort. After setting the stimulation amplitude, a series of trains were delivered to collect data for plotting the subject's force versus frequency and force versus pulse-duration curves. After the stimulation amplitude was set, eleven 770-ms long 14 Hz trains with 600 μs pulse-duration were delivered to potentiate the muscle (Binder-Macleod et al., 2002). Next, twenty-two 300-ms long trains with 600 μs pulse-duration and frequencies ranging from 10 to 100 Hz were delivered in random order. Finally, twenty-two 300-ms long trains with 60 Hz frequency and pulse-durations varying from 100 to 600 μs were delivered in random order. There was a 5-s rest time between consecutive trains.
Determination of parameters of 1st trains of the fatigue protocols
The stimulation frequency that generated 20% MVIC peak force in response to a 300-ms long train at 600 μs pulse-duration was recorded, and used for all the fatiguing trains of the no-modulation protocol and the first 16 trains of the frequency-modulation protocol. The stimulus pulse-duration that generated 20% MVIC peak force in response to a 300-ms long 60 Hz train was recorded and used for the first 16 trains of the pulse-duration-modulation protocol. The 600 μs pulse-duration was selected for the no-modulation and the frequency-modulation protocols and as the longest pulse-duration used during the pulse-duration-modulation protocol because it was the shortest pulse-duration found to be at the commencement of the plateau region of the force versus pulse-duration curve (Fig. 2). Similarly, 60 Hz frequency was selected for the pulse-duration-modulation protocol and as the highest frequency used during the frequency-modulation protocol because it was the lowest frequency able to produce near-maximal force (Fig. 2) while avoiding the occurrence of high-frequency fatigue (Jones, 1996) and was within the range of frequencies used clinically during FES (Bajd et al., 1999; Malezic and Hesse, 1995). Please also note that the frequencies and pulse-durations for the three protocols were selected such that they generated the same initial peak forces (∼20% MVIC) to prevent the effects of the differences in initial peak forces generated in response to the electrical stimulation from influencing the amount of muscle fatigue produced by the fatigue protocols (Russ et al., 2002).
Fig. 2.
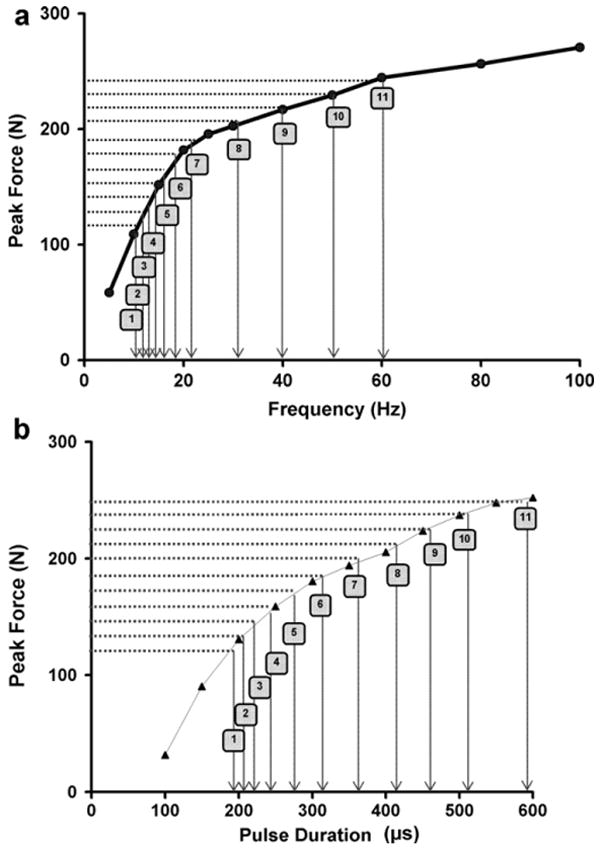
A schematic showing the peak force versus frequency (a) and peak force versus pulse duration (PD) (b) curves for a representative subject and the method used to determine steps for frequency-modulation (a) and PD-modulation (b). The horizontal shaded lines point to the 11 equal force-steps. The vertical arrows point to the corresponding frequency (a) and PD (b) steps used for modulation. Note that the frequency- and PD-steps were spaced on the curves such that each stepwise increase in frequency or PD caused an approximately equal increase in force output.
2.3.2. Determination of steps for the modulation-protocols
Steps for the frequency and pulse-duration-modulation protocols were custom-designed for each subject based on their force–frequency and force–pulse-duration curves, respectively. First, the force–frequency and force–intensity curves were plotted. The frequency of the first 16 trains was set at the frequency of the 300-ms long train with pulses of 600 μs duration that generated 20% of the subject's MVIC, as explained above, to determine the steps for the frequency-modulation protocol. Following this, the portion of the y-axis (peak force) of the force–frequency curve between the 20% MVIC peak force and the peak force generated at 60 Hz was divided into 10 equal parts to obtain 11 equal ‘force-steps’ (See Fig. 2). Eleven stepwise increases in frequency and pulse-duration were used for the modulation protocols because preliminary data showed better performance with 11 force steps compared to using fewer steps and only a marginal improvement with a greater number of steps. The frequencies corresponding to the peak forces at each of the force-steps were then recorded from the x-axis of the force–frequency curve. During frequency-modulation, the stimulus pulse-duration was fixed at 600 μs and the frequency was increased every 16 stimulation trains according to the 11 steps obtained as explained above. Similarly, 11 equal force steps for the pulse-duration-modulation protocol were determined using the force versus pulse-duration curve for each subject (see Fig. 2). During the pulse-duration-modulation protocol, the stimulation frequency was fixed at 60 Hz and the pulse-duration was increased every 16 contractions.
2.3.3. Fatigue protocols (2nd–4th sessions)
One of the three fatigue protocols was tested in random order during each of the remaining three sessions. During each session, after electrode placement, the stimulation amplitude was set to produce 50% of the subject's MVIC force using 300-ms long, 60 Hz trains with 600 μs pulse-duration.
The electrical stimulation protocols for the 2nd–4th sessions consisted of potentiation trains, pre-fatigue testing trains, fatiguing trains, and post-fatigue testing trains as explained below (See Fig. 1):
Potentiation trains
Eleven 770-ms long 14 Hz trains with 600 μs pulse-duration were delivered with a 5-s rest time between trains to potentiate the muscle (Binder-Macleod et al., 2002).
Pre- and post-fatigue testing trains
A twitch and a pair of 60- and 20 Hz testing trains at 600 μs pulse-duration were delivered before and after the fatiguing trains to measure the force generating ability of the muscle. The rest time between pre-fatigue testing trains was 10 s. The rest time between post-fatigue testing trains was 1 s.
Fatiguing trains
A total of 176 trains were delivered at the rate of 1 train every second to fatigue the muscle. All stimulation trains were 300-ms long. The activation pattern used to fatigue the muscles (300-ms long trains with 700-ms rest time) is similar to the activation patterns recorded in the quadriceps muscle during normal walking (Pierrynowski and Morrison, 1985). The stimulation parameters of the fatiguing trains during the three fatigue protocols were as follows:
Frequency-modulation protocol. The pulse-duration was fixed at 600 μs. The frequency of the 1st train was set to the frequency required to generate 20% MVIC peak force and the frequency of the 176th train was 60 Hz. Stimulation frequency was increased stepwise every 16 contractions in 11 equal force steps.
Pulse-duration-modulation protocol. The frequency was fixed at 60 Hz. The pulse-duration of the 1st train was set to the pulse-duration required to generate 20% MVIC peak force and the pulse-duration of the 176th train was 600 μs. Stimulus pulse-duration was increased stepwise every 16 contractions in 11 equal force-steps.
No-modulation protocol. The pulse-duration was fixed at 600 μs. The frequency of the 1st train was set to the frequency required to generate 20% MVIC peak force. Both the pulse-duration and the frequency were kept constant for all the trains in the protocol. This protocol was compared to the two modulation protocols because out of three combinations of frequency and pulse-duration tested in a concurrent study, repetitive stimulation with the combination of a low-frequency and 600 μs pulse-duration produced better performance in response to fatiguing trains compared to either a medium-pulse-duration and medium-frequency, or a high-frequency (60 Hz) and short-pulse-duration (unpublished observations).
3. Data analyses
The peak forces and force–time integrals in response to each stimulation train were calculated for the three fatigue protocols. The percent change in peak force and force–time integral from the first to the last fatiguing train were used as a measures of the muscle's ‘performance’ or its ability to generate force in response to the fatiguing trains. A positive percentage change indicated that the last fatiguing train generated greater force than the first fatiguing train and a negative percentage change indicated that the last fatiguing train generated less force than the first fatiguing train. In addition, the average peak forces and force–time integrals produced in response to the 1st–176th, 1st–60th, 61st–120th, and 121st–176th fatiguing trains were used as additional measures of muscle performance. The percentage declines in peak force between the pre- and post-fatigue 60 Hz testing trains were used as a measure of muscle fatigue. The ratio of peak forces produced in response to 20 Hz versus 60 Hz pre- and post-fatigue testing trains (20 Hz:60 Hz peak force ratio) were used as measures of the degree of low-frequency fatigue in the muscle (Russ and Binder-Macleod, 1999; Vollestad, 1997).
3.1. Statistical analyses
Each of the dependant variables, except the 20 Hz:60 Hz peak force ratios, were compared using one-way repeated measures ANOVAs. Pair-wise post hoc comparisons were performed if significant differences were present. The 20 Hz:60 Hz peak force ratios were compared using 2-way (protocol × fatigue) repeated measures ANOVAs. The significance level was set at p ≤ 0.05.
4. Results
The average ages of the 12 subjects tested were 24.8 ± 2.4 years and their MVIC forces were 949.4 ± 246.2 N. Table 1 shows the frequencies and pulse-durations of the first and last trains for the three fatigue protocols. The peak forces produced in response to each of the 176 fatiguing trains (Fig. 3c), and the forces generated in response to the first (Fig. 3a) and last (Fig. 3b) fatiguing trains for a representative subject are shown in Fig. 3. Similar to the force data shown in Fig. 3, for nine out of the 12 subjects tested, frequency-modulation produced larger peak forces in response to the last compared to the first fatiguing trains (Fig. 3).
Table 1.
The stimulation frequency and pulse duration (PD) of the first (average ± SD) and last trains for each of the three fatigue protocols
| Frequency (Hz) | PD (μs) | |
|---|---|---|
| Frequency-modulation | ||
| First train | 11.6 ± 1.5 | 600 |
| Last train | 60.0 | 600 |
| PD-modulation | ||
| First train | 60.0 | 131 ± 23 |
| Last train | 60.0 | 600 |
| No-modulation | ||
| First train | 11.3 ± 1.6 | 600 |
| Last train | 11.3 ± 1.6 | 600 |
Fig. 3.
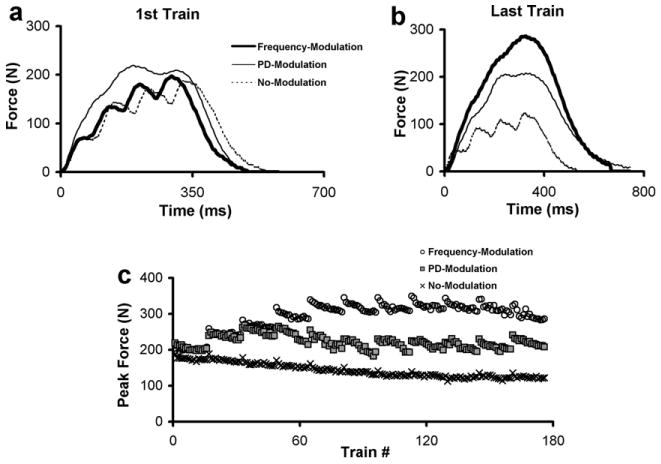
The peak forces produced in response to each fatiguing train during the three fatigue protocols (c) for a representative subject. Raw force responses of the first (a) and last (b) fatiguing trains for each of the three fatigue protocols. Note that for the modulation protocols, the force in response to the last train is either equal to or greater than the force in response to the first train. In contrast, the no-modulation protocol causes a decline in force from the first to the last train.
4.1. Fatiguing trains (measures of muscle performance)
Analyses of the percentage change in peak force between the first and last fatiguing trains showed that the frequency-modulation protocol produced an increase in peak force (percent change = 15.5 ± 28.7%), the pulse-duration-modulation produced a small decrease in peak force (percent change = −6.2 ± 20.3%), and the no-modulation protocol produced a large decline in peak force (percent change = −31.2 ± 9.4%) (p < 0.01) (Fig. 4a). The percent changes in force–time integrals of the fatiguing trains showed a similar trend; however, both frequency- and pulse-duration-modulation protocols showed increases in the force–time integrals from the first to the last fatiguing train (Fig. 4b).
Fig. 4.
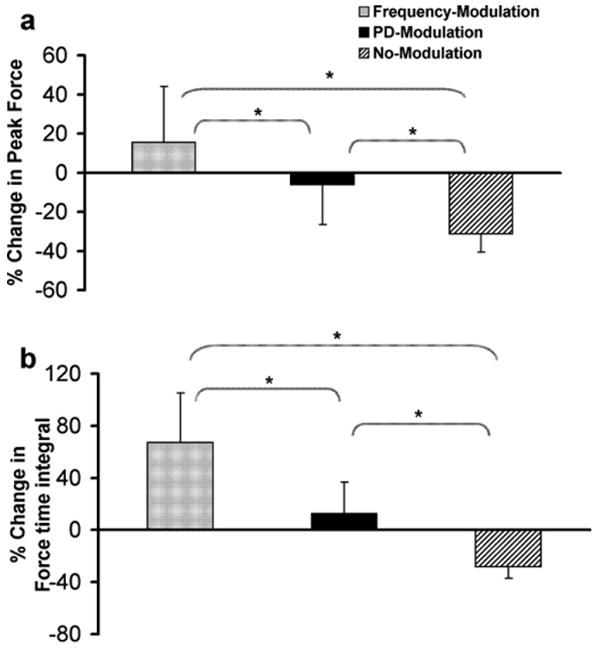
The percent change in peak force (a) and force time integrals (b) between the first and the last fatiguing trains during the three fatigue protocols. Note the positive percentage change in peak force for the frequency-modulation protocol (a) and the positive percentage change in force time integral for frequency-modulation and PD-modulation protocols (b). *Significant differences between protocols (p ≤ 0.01).
The frequency-modulation protocol produced the largest and the no-modulation protocol produced the smallest average peak forces in response to the 1st–176th, 1st–60th, 61st–120th, and 121st–176th fatiguing trains (p < 0.01) (Fig. 5a). Similarly, frequency-modulation protocol produced the largest and no-modulation the smallest average force–time integrals in response to the 1st–60th and 121st–176th fatiguing trains (p < 0.01) (Fig. 5b). In response to 61st–120th and 1st–176th fatiguing trains, the no-modulation protocol produced the smallest sum of the force–time integrals, but there were no differences in the sum of the force–time integrals between frequency- and pulse-duration-modulation (Fig. 5b).
Fig. 5.
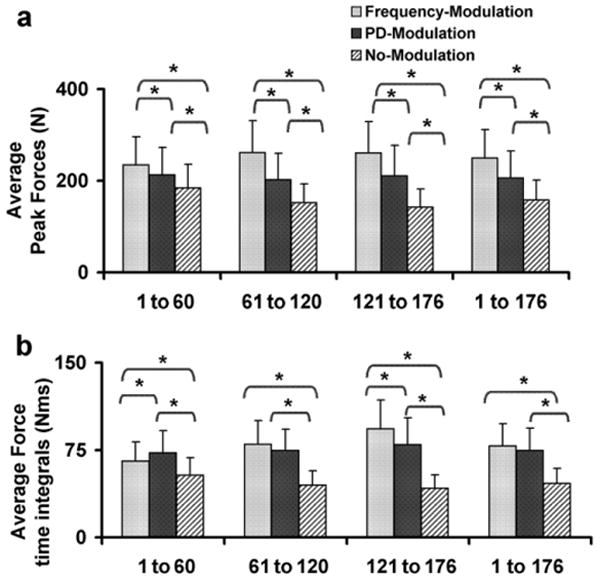
Average peak forces (a) and force time integrals (b) generated in response to the 1st–60th, 61st–120th, 121st–176th, and 1st–176th fatiguing trains during the three fatigue protocols. *Significant difference between fatigue protocols (p ≤ 0.01).
4.2. Testing trains (measures of muscle fatigue)
The no-modulation protocol produced a significantly smaller decline in peak force of the 60 Hz testing trains (23.6 ± 8.4%) compared to both the frequency-modulation and the pulse-duration-modulation protocols (p < 0.01) (Fig. 6a). There was no significant difference in percentage decline in peak force of the 60 Hz testing trains between the frequency-modulation (46.6 ± 9.4%) and the pulse-duration-modulation (48.0 ± 10.6%) protocols (Fig. 6a).
Fig. 6.

The percent decline in peak force between pre-fatigue and post-fatigue 60 Hz testing trains (a) and the pre- and post-fatigue 20 Hz:60 Hz peak force ratios (b) for the three fatigue protocols tested. There were significant differences between the pre- and post-fatigue 20 Hz:60 Hz peak force ratios for each of the three fatigue protocols (p < 0.01). *Significant differences between protocols (p ≤ 0.05).
The 2-way ANOVA for the 20 Hz:60 Hz peak force ratios showed significant effects of protocol (F = 22.8, p < 0.01), fatigue (F = 312.8, p < 0.01), and a significant interaction (F = 50.7, p < 0.01) (Fig. 6b). There were no differences in the pre-fatigue 20 Hz:60 Hz peak force ratios across protocols (F = 0.37, p = 0.70). The no-modulation protocol showed the smallest post-fatigue 20 Hz:60 Hz peak force ratios (p < 0.01). There were no differences between frequency- and pulse-duration-modulation protocols in the post-fatigue 20 Hz:60 Hz peak force ratios (p = 0.77) (Fig. 6b). For all three protocols, post-fatigue ratios were smaller than pre-fatigue 20 Hz:60 Hz ratios (all p < 0.01) (Fig. 6b).
5. Discussion
An interesting finding of the present study was that although the frequency- and pulse-duration-modulation protocols showed no differences in the amount of muscle fatigue or low-frequency fatigue (Fig. 6), the frequency-modulation protocol produced better isometric muscle performance in response to the fatiguing trains than the pulse-duration-modulation protocol (Figs. 4 and 5). The better isometric performance demonstrated during frequency-compared to pulse-duration-modulation for similar levels of muscle fatigue may have important implications for clinical applications of FES because most current FES systems deliver a constant frequency and only increase the stimulation intensity to maintain muscle force as the muscle fatigues (Petrofsky and Stacy, 1992; Raymond et al., 1999). Thus, these results suggest that there is a need to further investigate strategies of control of force during FES in patient populations.
We compared the peak forces generated in response to the last fatiguing trains and the post-fatigue 60 Hz testing trains during the frequency- and pulse-duration-modulation protocols to further analyze differences between peak force responses of the two modulation protocols (Fig. 7). We are not sure how to explain the finding that an additional 1 s of recovery time between the last fatiguing trains and the post-fatigue testing trains (see Section 2.3 and Fig. 1 for details) allowed for greater recovery of peak forces for the pulse-duration-modulation than the frequency-modulation protocol (see Fig. 7). After additional recovery of peak forces in response to the 60 Hz testing trains for the pulse-duration-modulation protocol, the differences in the amount of muscle fatigue between the frequency- and pulse-duration-modulation protocols were not significant. These results could be due to differences in the level of metabolic energy expenditure, the extent of activation failure, or differences in recovery of force-generating ability during the frequency- and pulse-duration-modulation protocols. However, we did not have adequate data to further investigate the mechanisms underlying muscle fatigue and performance during the two modulation protocols. Future studies that measure the metabolic energy expenditure and/or electrophysiological activity of motor units during different modulation strategies may help to gain a better understanding of the mechanisms underlying muscle performance and fatigue during repetitive stimulation.
Fig. 7.
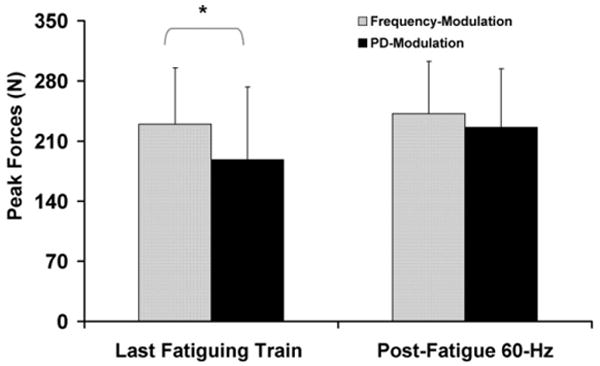
Average peak forces generated in response to the last fatiguing train (left) and the post-fatigue 60 Hz testing train (right) during the frequency-modulation and the pulse-duration (PD)-modulation protocol. Both the last fatiguing train and the post-fatigue testing trains were 60 Hz trains with 600 μs PD and were separated by a 1 s rest time and a twitch. Note the larger increase in peak force from the last fatiguing train to the post-fatigue 60 Hz testing train during the PD-Modulation than the frequency-modulation protocol. *p ≤ 0.05.
Although the no-modulation protocol produced the least muscle fatigue (Fig. 6a) and low-frequency fatigue (Fig. 6b), both modulation protocols showed better performance in response to the fatiguing trains than the no-modulation protocol (Figs. 4 and 5). The no-modulation protocol maintained a lower stimulation frequency (11.3 ± 1.6 Hz) than either the pulse-duration- (60 Hz throughout) or the frequency-modulation protocol (stepwise increase from 11.6 ± 1.5 to 60 Hz), which may have contributed to the lower muscle fatigue (Binder-Macleod et al., 1995; Frank et al., 1998; Marsden et al., 1983) and low-frequency fatigue (Chin and Allen, 1996; Chin et al., 1997; Westerblad et al., 1993) (Fig. 6). Low-frequency fatigue is characterized by the selective loss of force at low- versus high-frequencies during fatigue or recovery from fatigue (Edwards et al., 1977; Stokes et al., 1989). Low-frequency fatigue causes a rightward shift in the force–frequency curve, which requires higher frequencies to produce comparable forces to the pre-fatigued state (Binder-Macleod and McDermond, 1992; Binder-Macleod et al., 1998; Fuglevand et al., 1999; Thomas et al., 1991). Thus, low-frequency fatigue, as well as overall muscle fatigue, would cause an attenuation of performance in response to the low-frequency trains (11.3 ± 1.6 Hz) used during the no-modulation protocol. However, during the latter half of the frequency-modulation protocol, and throughout the pulse-duration-modulation-protocol, the muscle was stimulated with frequencies in the high-frequency range of the force–frequency curve (Fig. 2). This high-frequency stimulation would overcome the effects of low-frequency fatigue (Edwards et al., 1977; Russ and Binder-Macleod, 1999; Stokes et al., 1989) and perhaps contribute to the better performance of the two modulation protocols compared to the no-modulation protocol.
Previously, Graupe and colleagues showed that the stochastic modulation of the inter-pulse intervals within stimulation trains decreased the rate of muscle fatigue of the quadriceps femoris muscle compared to stimulation at a constant frequency on a single subject (Graupe et al., 2000). In contrast, Thrasher and colleagues recently showed that random modulation of frequency (mean: 40 Hz), amplitude (mean: 75% maximal tetanic force), and pulse-duration (mean: 250 μs) by ±15% of their mean values every 100-ms did not effect the rate of fatigue during isometric contractions of the tibialis anterior and quadriceps femoris muscles of seven spinal cord injured subjects (Thrasher et al., 2005). Our present study is the first to show improvement in isometric muscle performance using stepwise increases in stimulation frequency during repetitive stimulation. In addition, Kebaetse and Binder-Macleod recently showed that for healthy subjects and subjects with spinal cord injury, starting at a low- and later switching to a high-frequency produced better performance during repetitive non-isometric contractions than stimulation using either a low- or high-frequency alone (Kebaetse and Binder-Macleod, 2004; Kebaetse et al., 2005). However, unlike the present study, Kebaetse and colleagues only increased frequency once in their study (i.e., used one frequency step) and did not modulate the stimulation intensity (Kebaetse et al., 2005). The present study was the first to compare muscle fatigue and performance during systematic stepwise increases of frequency or pulse-duration during repetitive stimulation.
During our study, surface electrical stimulation was used to test isometric muscle fatigue and performance. Surface electrodes are commonly used for FES (Popovic et al., 2001; Prochazka et al., 1997; Snoek et al., 2000) and for the rehabilitation training of individuals with acute paralysis (Chae et al., 1998) because they are easily placed by the FES-users and are non-invasive. However, implanted nerve cuff electrodes are also used during FES, especially for individuals with chronic paralysis following spinal cord injury or stroke (Chae et al., 2001; Daly and Ruff, 2000; Peckham and Knutson, 2005; Peckham et al., 2002). Further studies are therefore needed to assess the validity of our current findings during stimulation using implanted electrodes.
During FES, muscle force must repetitively reach a targeted level to enable efficient task performance. The stepwise increases in frequency and pulse-duration in our study caused the peak force to rise above the 20% MVIC targeted force level (Fig. 3c). This overshoot of force may have caused greater metabolic energy expenditure (Boska, 1994; Potma et al., 1994; Stienen et al., 1995), and produced greater muscle fatigue (Cooke et al., 1988; Sahlin et al., 1998; Westerblad et al., 1998) than would have been produced if the targeted force was not exceeded. A better strategy would have been to only increase the stimulation frequency or pulse-duration to the level needed to produce the targeted force with minimal overshoot. The combined modulation of frequency and pulse-duration may be a better strategy to improve muscle performance during FES. It is important to note that changes in skeletal muscle fiber-type composition and atrophy following paralysis (Gerrits et al., 2003) may cause differences in the responses of paralyzed muscles versus muscles of able-bodied individuals. Future studies will use predictive mathematical models (Ding et al., 1998, 2000) that account for changes in the force–frequency curve due to fatigue to determine the appropriate frequency and pulse-duration steps required to generate the targeted force (Chou et al., 2005; Ding et al., 1998, 2000) in muscles of paralyzed individuals.
6. Conclusion
During repetitive electrical stimulation, for similar levels of muscle fatigue, the strategy of frequency-modulation produced better performance in response to the fatiguing trains compared to the strategy of pulse-duration-modulation. Although frequency-modulation is not a strategy currently used in FES, clinicians and researchers in the field of FES should consider incorporating frequency-modulation as a strategy for skeletal muscle force control in FES systems. Future work is needed to develop stimulation strategies that can minimize fatigue and improve skeletal muscle performance during FES applications. These strategies may involve combining frequency- and pulse-duration-modulation to maximize muscle performance.
Acknowledgments
Funding source: NIH Grant Nos. HD36797 and HD38582. The authors thank Dr. R. Perumal and Mr. R. Maladen for their helpful comments on an early draft of this manuscript and for the development of the software and hardware for data-acquisition.
Biography
 Trisha Kesar completed her Bachelors in Physical Therapy (2002) from Post Graduate Institute of Medical Education and Research, Chandigarh, India. She completed her MS (2005) in Biomechanics and Movement Science from University of Delaware, Newark, Delaware. She has been a Research Assistant at the Muscle Performance Laboratory, Department of Physical Therapy, University of Delaware since 2003. She is currently pursuing her PhD in the Inter-disciplinary Graduate Program in Biomechanics and Movement Sciences, University of Delaware. Her research interests include the use of electrical stimulation in rehabilitation and development of novel interventions for neuro-rehabilitation.
Trisha Kesar completed her Bachelors in Physical Therapy (2002) from Post Graduate Institute of Medical Education and Research, Chandigarh, India. She completed her MS (2005) in Biomechanics and Movement Science from University of Delaware, Newark, Delaware. She has been a Research Assistant at the Muscle Performance Laboratory, Department of Physical Therapy, University of Delaware since 2003. She is currently pursuing her PhD in the Inter-disciplinary Graduate Program in Biomechanics and Movement Sciences, University of Delaware. Her research interests include the use of electrical stimulation in rehabilitation and development of novel interventions for neuro-rehabilitation.
 Li-Wei Chou received a BS (1997) degree in Physical Therapy from National Yang Ming University, Taiwan, and MS (1999) degree in Sports Science from National College of Physical Education and Sports, Taiwan. He recently earned his PhD (2006) in Biomechanics and Movement Science from University of Delaware, Newark, Delaware. His main research interests include motor unit activities during electrically elicited contractions and development of better stimulation strategies for FES.
Li-Wei Chou received a BS (1997) degree in Physical Therapy from National Yang Ming University, Taiwan, and MS (1999) degree in Sports Science from National College of Physical Education and Sports, Taiwan. He recently earned his PhD (2006) in Biomechanics and Movement Science from University of Delaware, Newark, Delaware. His main research interests include motor unit activities during electrically elicited contractions and development of better stimulation strategies for FES.
 Stuart A. Binder-Macleod received a BS (1974) in Physical Therapy from the State University of New York at Buffalo. He received his Master of Medical Science (1979) in Physical Therapy from Emory University and his Ph.D. degree (1987) in Physiology from the Medical College of Virginia. He is a Fellow of the American Physical Therapy Association. He received the American Physical Therapy Association's Eugene Michels New Investigator Award in 1993, Marion Williams Award for Research in Physical Therapy in 1999, and the Golden Pen Award in recognition of significant contributions to the advance of Physical Therapy in 2004. Presently, he is the Edward L. Ratledge Professor of Physical Therapy and the Chair of the Department of Physical Therapy at the University of Delaware. He is also a Professor in the Interdisciplinary Graduate Program in Biomechanics and Movement Sciences and the Director of the Muscle Performance Laboratory at the University of Delaware. His main areas of research are optimization of human muscle function, mathematical modeling of muscle performance, and the development of rehabilitation interventions that utilize novel functional electrical stimulation strategies.
Stuart A. Binder-Macleod received a BS (1974) in Physical Therapy from the State University of New York at Buffalo. He received his Master of Medical Science (1979) in Physical Therapy from Emory University and his Ph.D. degree (1987) in Physiology from the Medical College of Virginia. He is a Fellow of the American Physical Therapy Association. He received the American Physical Therapy Association's Eugene Michels New Investigator Award in 1993, Marion Williams Award for Research in Physical Therapy in 1999, and the Golden Pen Award in recognition of significant contributions to the advance of Physical Therapy in 2004. Presently, he is the Edward L. Ratledge Professor of Physical Therapy and the Chair of the Department of Physical Therapy at the University of Delaware. He is also a Professor in the Interdisciplinary Graduate Program in Biomechanics and Movement Sciences and the Director of the Muscle Performance Laboratory at the University of Delaware. His main areas of research are optimization of human muscle function, mathematical modeling of muscle performance, and the development of rehabilitation interventions that utilize novel functional electrical stimulation strategies.
References
- Andrews BJ, Baxendale RH, Barnett R, Phillips GF, Yamazaki T, Paul JP, et al. Hybrid FES orthosis incorporating closed loop control and sensory feedback. J Biomed Eng. 1988;10:189–95. doi: 10.1016/0141-5425(88)90099-4. [DOI] [PubMed] [Google Scholar]
- Bajd T, Kralj A, Stefancic M, Lavrac N. Use of functional electrical stimulation in the lower extremities of incomplete spinal cord injured patients. Artif Organs. 1999;23:403–9. doi: 10.1046/j.1525-1594.1999.06360.x. [DOI] [PubMed] [Google Scholar]
- Binder-Macleod SA, McDermond LR. Changes in the force–frequency relationship of the human quadriceps femoris muscle following electrically and voluntarily induced fatigue. Phys Ther. 1992;72:95–104. doi: 10.1093/ptj/72.2.95. [DOI] [PubMed] [Google Scholar]
- Binder-Macleod SA, Halden EE, Jungles KA. Effects of stimulation intensity on the physiological responses of human motor units. Med Sci Sports Exerc. 1995;27:556–65. [PubMed] [Google Scholar]
- Binder-Macleod SA, Lee SC, Fritz AD, Kucharski LJ. New look at force–frequency relationship of human skeletal muscle: effects of fatigue. J Neurophysiol. 1998;79:1858–68. doi: 10.1152/jn.1998.79.4.1858. [DOI] [PubMed] [Google Scholar]
- Binder-Macleod SA, Dean JC, Ding J. Electrical stimulation factors in potentiation of human quadriceps femoris. Muscle Nerve. 2002;25:271–9. doi: 10.1002/mus.10027. [DOI] [PubMed] [Google Scholar]
- Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med. 1994;32:1–10. doi: 10.1002/mrm.1910320102. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Polkey CE, Rushton DN. Electrical splinting of the knee in paraplegia. Paraplegia. 1979;16:428–37. doi: 10.1038/sc.1978.78. [DOI] [PubMed] [Google Scholar]
- Brissot R, Gallien P, Le Bot MP, Beaubras A, Laisne D, Beillot J, et al. Clinical experience with functional electrical stimulation-assisted gait with Parastep in spinal cord-injured patients. Spine. 2000;25:501–8. doi: 10.1097/00007632-200002150-00018. [DOI] [PubMed] [Google Scholar]
- Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29:975–9. doi: 10.1161/01.str.29.5.975. [DOI] [PubMed] [Google Scholar]
- Chae J, Fang ZP, Walker M, Pourmehdi S. Intramuscular electromyographically controlled neuromuscular electrical stimulation for upper limb recovery in chronic hemiplegia. Am J Phys Med Rehabil. 2001;80:935–41. doi: 10.1097/00002060-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Chin ER, Allen DG. The role of elevations in intracellular [Ca2+] in the development of low frequency fatigue in mouse single muscle fibres. J Physiol. 1996;491(Pt 3):813–24. doi: 10.1113/jphysiol.1996.sp021259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Balnave CD, Allen DG. Role of intracellular calcium and metabolites in low-frequency fatigue of mouse skeletal muscle. Am J Physiol. 1997;272:C550–9. doi: 10.1152/ajpcell.1997.272.2.C550. [DOI] [PubMed] [Google Scholar]
- Chizeck HJ, Lan N, Palmieri LS, Crago PE. Feedback control of electrically stimulated muscle using simultaneous pulse width and stimulus period modulation. IEEE Trans Biomed Eng. 1991;38:1224–34. doi: 10.1109/10.137288. [DOI] [PubMed] [Google Scholar]
- Chou LW, Ding J, Wexler AS, Binder-Macleod SA. Predicting optimal electrical stimulation for repetitive human muscle activation. J Electromyogr Kinesiol. 2005;15:300–9. doi: 10.1016/j.jelekin.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Cooke R, Franks K, Luciani GB, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. J Physiol. 1988;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Peckham PH, Thrope GB. Modulation of muscle force by recruitment during intramuscular stimulation. IEEE Trans Biomed Eng. 1980;27:679–84. doi: 10.1109/TBME.1980.326592. [DOI] [PubMed] [Google Scholar]
- Daly JJ, Ruff RL. Electrically induced recovery of gait components for older patients with chronic stroke. Am J Phys Med Rehabil. 2000;79:349–60. doi: 10.1097/00002060-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Ding J, Binder-Macleod SA, Wexler AS. Two-step, predictive, isometric force model tested on data from human and rat muscles. J Appl Physiol. 1998;85:2176–89. doi: 10.1152/jappl.1998.85.6.2176. [DOI] [PubMed] [Google Scholar]
- Ding J, Wexler AS, Binder-Macleod SA. A predictive model of fatigue in human skeletal muscles. J Appl Physiol. 2000;89:1322–32. doi: 10.1152/jappl.2000.89.4.1322. [DOI] [PubMed] [Google Scholar]
- Donaldson N, Perkins TA, Fitzwater R, Wood DE, Middleton F. FES cycling may promote recovery of leg function after incomplete spinal cord injury. Spinal Cord. 2000;38:680–2. doi: 10.1038/sj.sc.3101072. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–78. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K, Bolck B, Bavendiek U, Schwinger RH. Frequency dependent force generation correlates with sarcoplasmic calcium ATPase activity in human myocardium. Basic Res Cardiol. 1998;93:405–11. doi: 10.1007/s003950050109. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Macefield VG, Bigland-Ritchie B. Force–frequency and fatigue properties of motor units in muscles that control digits of the human hand. J Neurophysiol. 1999;81:1718–29. doi: 10.1152/jn.1999.81.4.1718. [DOI] [PubMed] [Google Scholar]
- Gerrits HL, Hopman MT, Offringa C, Engelen BG, Sargeant AJ, Jones DA, et al. Variability in fibre properties in paralysed human quadriceps muscles and effects of training. Pflugers Arch. 2003;445:734–40. doi: 10.1007/s00424-002-0997-4. [DOI] [PubMed] [Google Scholar]
- Graupe D, Suliga P, Prudian C, Kohn KH. Stochastically-modulated stimulation to slow down muscle fatigue at stimulated sites in paraplegics using functional electrical stimulation for leg extension. Neurol Res. 2000;22:703–4. doi: 10.1080/01616412.2000.11740743. [DOI] [PubMed] [Google Scholar]
- Jones DA. High-and low-frequency fatigue revisited. Acta Physiol Scand. 1996;156:265–70. doi: 10.1046/j.1365-201X.1996.192000.x. [DOI] [PubMed] [Google Scholar]
- Kamen G, Du DC. Independence of motor unit recruitment and rate modulation during precision force control. Neuroscience. 1999;88:643–53. doi: 10.1016/s0306-4522(98)00248-6. [DOI] [PubMed] [Google Scholar]
- Kanosue K, Yoshida M, Akazawa K, Fujii K. The number of active motor units and their firing rates in voluntary contraction of human brachialis muscle. Jpn J Physiol. 1979;29:427–43. doi: 10.2170/jjphysiol.29.427. [DOI] [PubMed] [Google Scholar]
- Kebaetse MB, Binder-Macleod SA. Strategies that improve human skeletal muscle performance during repetitive, non-isometric contractions. Pflugers Arch. 2004;448:525–32. doi: 10.1007/s00424-004-1279-0. [DOI] [PubMed] [Google Scholar]
- Kebaetse MB, Lee SC, Johnston TE, Binder-Macleod SA. Strategies that improve paralyzed human quadriceps femoris muscle performance during repetitive, nonisometric contractions. Arch Phys Med Rehabil. 2005;86:2157–64. doi: 10.1016/j.apmr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Kralj A, Bajd T, Turk R. Enhancement of gait restoration in spinal injured patients by functional electrical stimulation. Clin Orthop. 1988:34–43. [PubMed] [Google Scholar]
- Liberson WT, Holmquest HJ, Scot D, Dow M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Arch Phys Med Rehabil. 1961;42:101–5. [PubMed] [Google Scholar]
- Malezic M, Hesse S. Restoration of gait by functional electrical stimulation in paraplegic patients: a modified programme of treatment. Paraplegia. 1995;33:126–31. doi: 10.1038/sc.1995.28. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC, Merton PA. “Muscular wisdom” that minimizes fatigue during prolonged effort in man: peak rates of motoneuron discharge and slowing of discharge during fatigue. Adv Neurol. 1983;39:169–211. [PubMed] [Google Scholar]
- Marsolais EB, Edwards BG. Energy costs of walking and standing with functional neuromuscular stimulation and long leg braces. Arch Phys Med Rehabil. 1988;69:243–9. [PubMed] [Google Scholar]
- Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–60. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- Peckham PH, Kilgore KL, Keith MW, Bryden AM, Bhadra N, Montague FW. An advanced neuroprosthesis for restoration of hand and upper arm control using an implantable controller. J Hand Surg [Am] 2002;27:265–76. doi: 10.1053/jhsu.2002.30919. [DOI] [PubMed] [Google Scholar]
- Petrofsky JS, Stacy R. The effect of training on endurance and the cardiovascular responses of individuals with paraplegia during dynamic exercise induced by functional electrical stimulation. Eur J Appl Physiol Occup Physiol. 1992;64:487–92. doi: 10.1007/BF00843755. [DOI] [PubMed] [Google Scholar]
- Pierrynowski MR, Morrison JB. Estimating the muscle forces generated in the human lower extremity when walking: a physiological solution. Math Biosci. 1985;75:43–68. [Google Scholar]
- Popovic MR, Curt A, Keller T, Dietz V. Functional electrical stimulation for grasping and walking: indications and limitations. Spinal Cord. 2001;39:403–12. doi: 10.1038/sj.sc.3101191. [DOI] [PubMed] [Google Scholar]
- Popovic MR, Keller T, Pappas IP, Dietz V, Morari M. Surface-stimulation technology for grasping and walking neuroprosthesis. IEEE Eng Med Biol Mag. 2001;20:82–93. doi: 10.1109/51.897831. [DOI] [PubMed] [Google Scholar]
- Potma EJ, Stienen GJ, Barends JP, Elzinga G. Myofibrillar ATPase activity and mechanical performance of skinned fibres from rabbit psoas muscle. J Physiol. 1994;474:303–17. doi: 10.1113/jphysiol.1994.sp020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil. 1997;78:608–14. doi: 10.1016/s0003-9993(97)90426-3. [DOI] [PubMed] [Google Scholar]
- Rabischong E, Chavet P. Regression-based indices of fatigue in paraplegics' electrically stimulated quadriceps. Med Eng Phys. 1997;19:749–54. doi: 10.1016/s1350-4533(97)00038-6. [DOI] [PubMed] [Google Scholar]
- Raymond J, Davis GM, Climstein M, Sutton JR. Cardiorespiratory responses to arm cranking and electrical stimulation leg cycling in people with paraplegia. Med Sci Sports Exerc. 1999;31:822–8. doi: 10.1097/00005768-199906000-00010. [DOI] [PubMed] [Google Scholar]
- Riener R. Model-based development of neuroprosthesis for paraplegic patients. Philos Trans R Soc Lond B Biol Sci. 1999;354:877–94. doi: 10.1098/rstb.1999.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ DW, Binder-Macleod SA. Variable-frequency trains offset low-frequency fatigue in human skeletal muscle. Muscle Nerve. 1999;22:874–82. doi: 10.1002/(sici)1097-4598(199907)22:7<874::aid-mus10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Russ DW, Vandenborne K, Binder-Macleod SA. Factors in fatigue during intermittent electrical stimulation of human skeletal muscle. J Appl Physiol. 2002;93:469–78. doi: 10.1152/japplphysiol.01010.2001. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Tonkonogi M, Soderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998;162:261–6. doi: 10.1046/j.1365-201X.1998.0298f.x. [DOI] [PubMed] [Google Scholar]
- Snoek GJ, MJ IJ, in 't Groen FA, Stoffers TS, Zilvold G. Use of the NESS handmaster to restore handfunction in tetraplegia: clinical experiences in ten patients. Spinal Cord. 2000;38:244–9. doi: 10.1038/sj.sc.3100980. [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR., 3rd Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:555–60. doi: 10.2106/00004623-199404000-00010. [DOI] [PubMed] [Google Scholar]
- Stienen GJ, Zaremba R, Elzinga G. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J Physiol. 1995;482(Pt 1):109–22. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MJ, Edwards RH, Cooper RG. Effect of low frequency fatigue on human muscle strength and fatigability during subsequent stimulated activity. Eur J Appl Physiol Occup Physiol. 1989;59:278–83. doi: 10.1007/BF02388329. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80:1577–83. doi: 10.1016/s0003-9993(99)90333-7. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Bigland-Richie B, Johansson RS. Force–frequency relationships of human thenar motor units. J Neurophysiol. 1991;65:1509–16. doi: 10.1152/jn.1991.65.6.1509. [DOI] [PubMed] [Google Scholar]
- Thrasher A, Graham GM, Popovic MR. Reducing muscle fatigue due to functional electrical stimulation using random modulation of stimulation parameters. Artif Organs. 2005;29:453–8. doi: 10.1111/j.1525-1594.2005.29076.x. [DOI] [PubMed] [Google Scholar]
- Vollestad NK. Measurement of human muscle fatigue. J Neurosci Meth. 1997;74:219–27. doi: 10.1016/s0165-0270(97)02251-6. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol. 1993;75:382–8. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Bruton JD, Andrade FH, Lannergren J. Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue. Acta Physiol Scand. 1998;162:253–60. doi: 10.1046/j.1365-201X.1998.0301f.x. [DOI] [PubMed] [Google Scholar]


