Abstract
Tissue engineering may provide a technique to generate cartilage grafts for laryngotracheal reconstruction in children. The present study used a rabbit model to characterize cartilage generated by a candidate tissue engineering approach to determine, under baseline conditions, which chondrocytes in the rabbit produce tissue-engineered cartilage suitable for in vivo testing in laryngotracheal reconstruction. We characterized tissue-engineered cartilage generated in perfused bioreactor chambers from three sources of rabbit chondrocytes: articular, auricular, and nasal cartilage. Biomechanical testing and histological, immunohistochemical, and biochemical assays were performed to determine equilibrium unconfined compression (Young's) modulus, and biochemical composition and structure. We found that cartilage samples generated from articular or nasal chondrocytes lacked the mechanical integrity and stiffness necessary for completion of the biomechanical testing, but five of six auricular samples completed the biomechanical testing (moduli of 210 ± 93 kPa in two samples at 3 weeks and 100 ± 65 kPa in three samples at 6 weeks). Auricular samples showed more consistent staining for proteoglycans and collagen II and had significantly higher glycosaminoglycan (GAG) content and concentration and higher collagen content than articular or nasal samples. In addition, the delayed gadolinium enhanced MRI of cartilage (dGEMRIC) method revealed variations in GAG spatial distribution in auricular samples that were not present in articular or nasal samples. The results indicate that, for the candidate tissue engineering approach under baseline conditions, only rabbit auricular chondrocytes produce tissue-engineered cartilage suitable for in vivotesting in laryngotracheal reconstruction. The results also suggest that this and similar tissue engineering approaches must be optimized for each potential source of chondrocytes.
INTRODUCTION
SUBGLOTTIC STENOSIS is a narrowing of the subglottic airway, which can cause difficulty in breathing and is a significant cause of morbidity and mortality in the pediatric population.1Subglottic stenosis can be congenital or acquired. Fibrous subglottic stenosis caused by trauma due to endotracheal intubation is the most common form that requires surgery.2The pathophysiology of this form of stenosis stems from mechanical irritation caused by the endotracheal tube, which leads to damage of the subglottic mucosa followed by a bacterial invasion, resulting in perichondritis, chondritis, and ultimately a hard, fibrotic stenosis of the cricoid cartilage and the adjacent tracheal rings.3,4Approximately 90% of acquired stenoses are the result of endotracheal intubation, and 1% to 8% of previously intubated neonatal intensive care unit patients acquire subglottic stenosis.5Conservative treatment modalities such as corticosteroid injection and balloon dilation have been shown to be unsuccessful in most cases, and open surgery is considered the standard of care for fibrous subglottic stenosis.3,4
The surgical approach used depends on the severity of the stenosis. For severe stenosis requiring surgery, the preferred method of treatment is cricotracheal resection, in which the stenosed segment is resected and anastamosis of the patent airway is performed. For mild to moderate stenosis requiring surgery, the preferred method of treatment is laryngotracheal reconstruction, a less extensive procedure in which the cricoid cartilage is split and expanded with a graft.2
There are currently a number of significant challenges to performing laryngotracheal reconstruction in children. Autologous costal cartilage grafts are the current gold standard,6but their harvest carries the risk of pneumothorax, pneumonia, or wound infection associated with a chest wall procedure.7Because subglottic stenosis is commonly diagnosed in the neonatal period, there is a need for a temporizing measure to stabilize the airway and allow time for adequate growth and development. The common practice for neonates and infants is to perform a tracheotomy, thereby bypassing the stenotic area and providing an adequate airway. The tracheotomy tube is generally left in place until the child's laryngotracheal segment is large enough for laryngotracheal reconstruction, normally at 18 to 36 months of age, at which time most patients have a body weight of at least 10 kg.5This long-term use of a tracheotomy tube carries the risk of high morbidity and mortality rates (mortality rates of up to 4.6%), as well as impairment of speech and language development.8
Tissue engineering methodologies have the potential to facilitate early and definitive laryngotracheal reconstruction in the pediatric population. Specifically, cartilage tissue engineering may provide a technique to generate autologous cartilage grafts for use in children. By eliminating the need for a chest wall procedure, the use of tissue-engineered grafts could reduce morbidity rates and shorten operative time.
The success of tissue engineering for pediatric laryngotracheal reconstruction is likely to depend not only on the tissue engineering techniques employed but also on the choice of the anatomic location from which the chondrocytes are harvested. Cartilage from different anatomic locations9and from different zones of articular cartilage10–13possess unique matrix compositions and structures. Similarly, functional qualities of cartilage engineered using autologous chondrocytes depend on the anatomic location from which the chondrocytes are harvested.14–16 These relatively recent findings have two important implications for cartilage tissue engineering. First, tissue engineering approaches should be optimized with respect to anatomic location of chondrocyte harvest. Consequently, animal models used for development of tissue engineering approaches should be likewise optimized. Second, a fundamental, as yet unanswered, question in cartilage tissue engineering is whether chondrocytes harvested from one anatomic location are capable of producing functional tissue-engineered cartilage for use in another anatomic location. Previous investigations of autologous tissue-engineered cartilage grafts for use in laryngotracheal reconstruction have had mixed success when the cartilage grafts were implanted in vivo.17,18 We hypothesize that the success or failure of the approach may be due in large part to the anatomic location from which the chondrocytes are harvested.
We developed a rabbit model with the long-term goal of tissue engineering autologous cartilage grafts for pediatric laryngotracheal reconstruction. In choosing the rabbit, a primary consideration was that the size of the laryngotracheal segment in skeletally mature rabbits should approximate that of the target pediatric population. Based on the issues discussed above, an important step in establishing the rabbit model was optimization, with respect to anatomic location of chondrocyte harvest, of the tissue engineering approach being developed.
The primary goal of the present study was to determine which chondrocytes (if any) in the rabbit produced tissue-engineered cartilage suitable for in vivo testing in laryngotracheal reconstruction, by applying a candidate tissue engineering approach under baseline conditions. The main criteria for suitability were biomechanical stability and stiffness. A secondary goal was to investigate whether chondrocytes harvested from one anatomic location were capable of producing functional tissue-engineered cartilage for use in another anatomic location. To achieve these goals, we used a hyaluronan-based scaffold and a bioreactor culture system19 to generate tissue-engineered cartilage from three sources of rabbit chondrocytes. The tissue-engineered cartilage was then characterized to determine which chondrocytes produced cartilage suitable for in vivo testing in laryngotracheal reconstruction in rabbits.
MATERIALS AND METHODS
Cartilage tissue engineering
Chondrocyte harvest, isolation, and expansion
Native cartilage was harvested during nonsurvival surgeries from three skeletally mature male New Zealand white rabbits (ages 9, 15, and 15 months) in accordance with the guidelines of the Animal Care and Use Committee of Case Western Reserve University. Articular cartilage was obtained from the proximal humerus. Auricular and nasal cartilage samples were obtained, as previously described,9 from the central region of the ear cartilage and from the nasal septum, respectively (Fig. 1). Cartilage samples were manually cleaned of noncartilaginous soft tissue and diced to approximately 1 mm3 pieces. Diced cartilage was digested sequentially in testicular hyaluronidase (261U/mL, 15 minutes; H-3506, Sigma Chemical Co, St. Louis, MO), trypsin-EDTA (0.25%, 30minutes; Invitrogen, Carlsbad, CA), and collagenase Type II (422 U/mL, 24 hours; CLS 2, Worthington, Lakewood, NJ), with all digestions performed on a rocker incubated at 37°C. Cells isolated from each cartilage sample were counted in a hemocytometer, plated on 100 mm cell culture dishes at densities not greater than 5700 cells/cm2, and expanded in expansion medium (Dulbecco's modified Eagle's medium [DMEM] with 1 g/L glucose supplemented with 10% fetal bovine serum [FBS, Invitrogen, lot no. 1256415]). Medium was changed every 3 or 4 days. When cell cultures reached confluence (approximately 3.0×104 cells/ cm2), cells were trypsinized and three chondrogenic pellets were generated from each group of cells (to provide quality control of chondrogenic capacity)and cultured as previously described.20 The remaining cells, for use in generating tissue-engineered cartilage, were frozen in expansion medium containing 10% dimethyl sulfoxide (Sigma) and stored in liquid nitrogen to allow their subsequent use in bioreactor culture. Prior to generation of tissue-engineered cartilage, the nine groups of cells were thawed and expanded (as above) with one additional passage at confluence (approximately 5.3×104 cells/cm2). Second-passage cells were trypsinized at confluence (approximately 3.8×104 cells/cm2), one to three chondrogenic pellets (depending on cell availability) were again generated and cultured as above, and the remaining cells were used for bioreactor culture. After 3 weeks of culture, pellets were stained with toluidine blue, as described below.
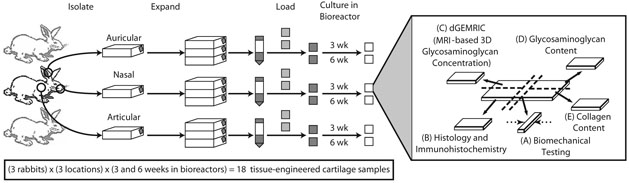
FIG. 1.
Study design. Chondrocytes were isolated from articular, auricular, and nasal cartilage from each of the three rabbits. Isolated cells were culture-expanded, loaded onto scaffolds, and cultured in bioreactors for 3 or 6 weeks. Once harvested, each sample of engineered cartilage was cut into five pieces for characterization by (A) biomechanical testing, (B) histological and immunohistochemical staining, (C) dGEMRIC, an MRI-based measure of GAG concentration and spatial distribution, (D) GAG content assay, and (E) collagen content assay. Dashed arrows indicate the loading direction during biomechanical testing.
Bioreactor culture
For each of the nine groups of cells, 4 million cells (in 150 μL of bioreactor medium) were loaded onto each of two 1 cm×1 cm pieces of Hyalograft-C scaffold (Fidia Advanced Biopolymers, Terme, Italy) and allowed to attach at 37°C and 5% carbon dioxide (CO2) for 2 hours, with the scaffolds flipped at 45 minutes and 90 minutes. Bioreactor medium was essentially as described by Penick and colleagues21: DMEM with 4.0g/L glucose supplemented with 1% ITS+Premix™ (BD Biosciences, San Jose, CA), 37.5 μg/mL ascorbate-2-phosphate (Wako Chemicals, Richmond, VA), and 10−7M dexamethasone (Sigma). Additional supplements (200mM L-glutamine, antibiotic-antimycotic [10,000U/mL penicillin G sodium, 10°g/mL streptomycin sulfate, and 25 μg/mL amphotericin B in 0.85% saline], nonessential amino acids, and sodium pyruvate [all from Invitrogen]) were also added to the medium at 1%, but the bioreactor medium in the present study was not supplemented with transforming growth factor β1. The resulting 18 samples were then placed into separate bioreactor chambers and cultured, as previously described,19 for 3 or 6 weeks. Briefly said, each bioreactor chamber was perfused with bioreactor medium at 0.25mL per hour and mounted on a rocker (30° arc at 0.5Hz) to improve mixing, with the entire system located inside an incubator at 37°C and 7.5% CO2. At the scheduled time (3 or 6 weeks), the samples were removed from the bioreactor chambers and cut into five pieces each (Fig. 1), and wet weight of each piece was taken after blotting on filter paper. One piece of each sample was fixed in formalin, dehydrated, and embedded for histology, and the remaining four pieces were snap-frozen using dry ice and 95% ethanol and stored at −70°C for subsequent biomechanical and biochemical analysis.
Biomechanical and biochemical analysis
Proliferation rates during monolayer expansion
Proliferation rates were determined to allow comparison of expansion potential of chondrocytes from different anatomic locations. Proliferation rate was determined as number of cell doublings per day during the combined pre- and postthaw expansion and was calculated as the logarithm (in base 2) of the fold increase in the number of cells during expansion divided by the number of days in the expansion.
Biomechanical testing
Unconfined compression creep testing was performed on all cartilage samples to determine their equilibrium unconfined compression (Young's) modulus. Prior to testing, each sample was thawed for 20 minutes at room temperature in phosphate buffered saline solution (0.15M NaCl, pH 7.35) supplemented with enzyme inhibitors (10mMEDTA, 5 mM benzamidine hydrochloride, 0.1 M 6-aminocaproic acid, and 0.1 mM phenylmethylsulfonyl fluoride [all from Sigma]). Throughout imaging and testing, the samples were kept submerged in the saline/inhibitor solution. Digital images of the two opposing sides of each sample that represented the cross-sectional area to be tested were taken at low (1.25× or 2.5×) magnification. The areas of these sides were calculated using NIH ImageJ (NIH, Bethesda, MD) and averaged to determine the cross-sectional area of the sample. All testing was performed on a custom-designed testing apparatus with rigid, smooth, impermeable Delrin (DuPont, Wilmington, DE) loading platens. Initial undeformed height of the samples was measured after application and equilibration of a 9.8 mN (1 gram force, gf) tare load. Unconfined compression creep testing was performed at loads of 68.7, 147.2, and 215.8 mN (7, 15, and 22 gf). Specimens were not allowed to recover between load levels. If a sample was damaged by the minimal handling required for imaging, or if the range (equivalent to uniaxial sample strain of approximately 50%) of the linear variable displacement transducer used to measure displacement was reached prior to completion of creep testing, the test was terminated. The equilibrium unconfined compression modulus of each tissue-engineered cartilage sample was calculated using a linear regression of all the stress-strain data points through the origin and was converted to kilopascals (kPa). The maximum applied load of 215.8 mN was chosen to simulate the maximum applied load the engineered cartilage is expected to experience when implanted into the rabbit trachea, and was determined by measuring the maximum compressive force produced by expansion of an incision in the trachea. For this, three rabbit tracheas were harvested and a 1 cm incision was made at the site where in vivo reconstruction would be performed. The edges of each incision were retracted to produce a 3 mm opening between the edges, and the compressive force produced by the retraction was measured immediately (1–2 s) following retraction. In addition, the equilibrium unconfined compression modulus of dry, empty scaffold was determined from four pieces of Hyalograft-C that were cut to the same dimensions as the tissue-engineered samples (the scaffold was not tested wet because hydrated empty scaffold was too compliant to be positioned upright between the testing platens).
Histology and immunohistochemistry
Histology and immunohistochemistry were performed to determine sample structure, composition, and size. Samples were dehydrated, embedded in paraffin, and 5 μm sections were cut. Forhistochemical staining of glycosamininoglycans(GAGs), representative sections were stained with 0.2% toluidine blue (T-3260; Sigma) for 5 minutes. For immunohistochemical analysis, sections were rehydrated and stained with antibodies to Type I and II collagen and elastin, with sources, dilutions for the primary antibodies, and the staining protocol as previously described.9 There were two modifications to the protocol: a 2-minute, rather than 15-minute, pronase digestion was used and the dilution of the elastin primary antibody was 1:200 rather than 1:300. In addition, sections were stained for the proteoglycans chondroitin 6-sulfate and chondroitin 4-sulfate/dermatan sulfate using the antibodies 3B3 and 2B6 (Seikagaku, Tokyo, Japan), respectively, using the same protocol as for collagen and elastin but with the following modifications: chondroitinase ABC was applied for 30 minutes at 37°C to digest the samples, rather than the 2-minute pronase digestion, and the 3B3 and 2B6 antibodies were diluted 1:500.
Biochemical composition and structure
Glycosaminoglycan and DNA analyses: GAG and DNA analyses were performed to determine the total GAG content per wet weight and per DNA weight of each sample. Samples were enzymatically digested, and the total GAG content was assayed colorimetrically by a Safranin O assay as previously described. 9,22 DNA content was determined on the same sample using Hoechst dye 33258 (General Electric [GE], Piscataway, NJ) at 0.67 μg/mL and measured on a Genios Pro multiwell plate reader (Tecan, Durham, NC). DNA signal was assessed with multiple readings at 485 nm, and calf thymus DNA (GE) was used as a standard. The final results were expressed as micrograms of GAG per milligram of sample wet weight and as micrograms of GAG per microgram DNA weight.
dGEMRIC analysis
The delayed gadolinium enhanced MRI of cartilage (dGEMRIC) method was performed to quantify GAG concentration and spatial distribution.23,24 Briefly said, constructs (n = 18) and empty scaffolds (n = 3) were equilibrated in 1 mM (GdDTPA)−2 (Magnevist, Berlex Imaging, Wayne, NJ), a charged MRI contrast agent diluted in Hanks' balanced salt solution (HBSS, Invitrogen). GAG maps were computed from an MRI measurement of T1Gd (T1 in the presence of GdDTPA2−). Since this calculation requires the measurement of T1o (T1 in saline, without contrast agent), a subset of the constructs (n = 3) was also equilibrated in HBSS, and T1o was measured. MRI images were acquired on an 8.45 T magnetic resonance microimaging system (Bruker Instruments, Billerica, MA) with a 10 mm coil. A saturation recovery sequence was used to measure the MRI relaxation parameter T1. For T1Gd, the TRs (time-to-repeats) used were 100, 125, 175, 275, 375, 475, 600, 900, 1800, and 2700 ms; and for T1o, the TRs used were 100, 150, 300, 400, 600, 900, 1400, 2000, 3200, and 5000 ms. Each image “slice” was 400 μm thick, and had an in-plane resolution of 100 μm (i.e., each image voxel represented a volume of tissue that was 100×100×400 μm). Multiple adjacent image slices were taken such that each sample was represented by a set of three to four image slices. T1 maps were generated by curve fitting each T1-weighted image series with custom software written in Matlab (The Mathworks, Natick, MA). GAG was computed from the T1Gd maps using a previously validated modified Donnan Theory, using the average measured T1o of 2.15 s and assuming relaxivity, R = 4.6 (mM/s)−1.23–25
Collagen content
Total collagen content was assayed to quantify collagen content of the extracellular matrix present in each sample. Samples of native articular, auricular, and nasal cartilage (harvested from three rabbits of equal age sacrificed for a separate study) were also assayed to provide a baseline with which to compare the collagen content of the tissue-engineered cartilage. The individual cartilage tissues were dried in a Savant concentrator and then weighed. The weighed tissues were hydrolyzed in 6 M HCl at 108°C for 18 hours. The hydrolysates were neutralized by dilution in water, dried on a speed Vac concentrator (Savant Instruments, Farmingdale, NY), and the samples were then reconstituted in 1% n-heptafluorobutyric acid. Aliquots of the samples were taken for hydroxyproline determination to quantify the collagen content. The hydroxyproline and collagen content determination were carried out using the methods of Woessner.26
Statistical analysis
All quantitative data are presented as means ± standard deviations. Differences between chondrocytes from different anatomic locations were assessed by repeated measure analysis of variance (ANOVA) and post hoc Holm t tests. Differences between 3-week and 6-week samples of chondrocytes from the same anatomic location were assessed by paired t tests, unless otherwise indicated. Values of p < 0.05 were considered to indicate statistically significant differences.
RESULTS
Proliferation rates during monolayer expansion
Over the course of the pre-freeze and post-thaw expansions, the auricular chondrocytes expanded most quickly (0.56 ± 0.12 doublings/day in three groups), followed by the nasal (0.54 ± 0.09 doublings/day) and then articular (0.39 ± 0.06 doublings/day) chondrocytes, although the expansion rates were not statistically different (p = 0.10).
Biomechanical testing
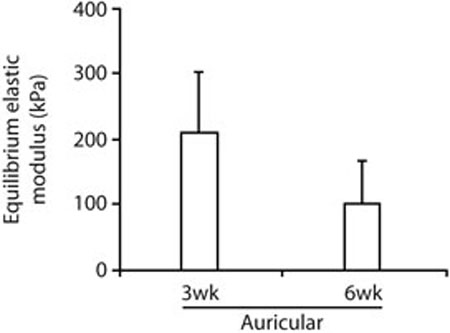
Biomechanical testing indicated that under the current baseline conditions only the auricular chondrocytes produced tissue-engineered cartilage that was mechanically suitable for in vivo testing in laryngotracheal reconstruction in rabbits. Five of six auricular samples successfully completed biomechanical testing and had measured equilibrium unconfined compression moduli of 210 ± 93 kPa in two samples at 3weeks and 100 ± 65 kPa in three samples at 6 weeks (p = 0.21, by unpaired t test, Fig. 2). The one auricular sample test that was not completed was terminated when the displacement of the sample exceeded the range of the linear variable displacement transducer during the 215.8 mN load level. Due to the large deformation, buckling of this sample could not be ruled out and the modulus of the sample was not calculated or included in subsequent analysis. All tissue-engineered cartilage samples generated from articular or nasal chondrocytes lacked the mechanical integrity and stiffness necessary for completion of the biomechanical testing. Four samples (one artiular and three nasal) were damaged by the minimal handling required for imaging. Eight samples (five articular and three nasal) deformed to such an extent that the range of the linear variable displacement transducer used to measure displacement was reached during the first experimental load level of 68.7 mN. The higher modulus of auricular samples at 3 weeks than at 6 weeks was not statistically significant, and when the results of preliminary testing (one 3-week sample, one 3-week and 2-day sample, and one 6-week sample, data not shown) were added to the current results, the difference between 3 weeks and 6 weeks increased slightly but remained nonsignificant (260 ± 180 kPa in four samples at 3 weeks and 110 ± 54 kPa in four samples at 6 weeks, p = 0.16, by unpaired t test). This finding suggests that extended periods of bioreactor culture using the current approach may lead to degeneration of the scaffold or of the tissue-engineered cartilage. Dry, empty scaffold had a measured equilibrium unconfined compression modulus of 12 ± 2.8 kPa, indicating that the scaffold's contribution to a decrease in sample modulus, if present, would be relatively small. It should be noted, however, that the test of the 3-week auricular sample that was terminated during the 215.8 mN load level and, therefore, was not analyzed may have resulted from a low modulus. Further investigation is necessary to characterize the effects, if any, of extended bioreactor culture.
FIG. 2.
Quantitative results from biomechanical testing. Five of six auricular samples successfully completed biomechanical testing. All tissue-engineered cartilage samples generated from articular or nasal chondrocytes lacked the mechanical integrity and stiffness necessary for completion of the test. The higher equilibrium elastic modulus of auricular samples at 3 weeks than at 6 weeks was not statistically significant (p = 0.21, by unpaired t test).
Histology and immunohistochemistry
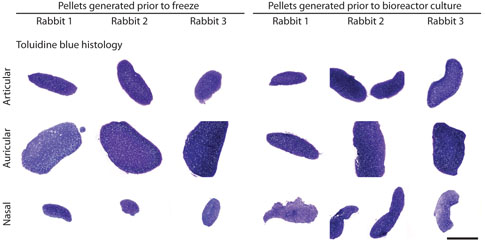
The chondrogenic pellet assay confirmed that all groups of cells were capable of generating cartilaginous tissue both prior to freeze and prior to use in bioreactor culture. Although primarily performed to provide quality control, the results of the pellet assay also illustrated that the nine groups of isolated chondrocytes possessed differences in chondrogenic capacity early in the tissue engineering process. For example, auricular chondrocytes formed much larger aggregates than articular or nasal chondrocytes, both prior to freeze and prior to use in bioreactor culture (Fig. 3).
FIG. 3.
Toluidine blue stained sections from pellets generated prior to freezing the chondrocytes (primary cells) and prior to use of the chondrocytes in bioreactor culture (second-passage cells). Pellets generated from the auricular chondrocytes were consistently larger than pellets generated from the articular or nasal chondrocytes. Pellets generated prior to freeze showed the most substantial variation in size, with pellet size decreasing from auricular to articular to nasal pellets. Bar = 1 mm. Color images available online at www.liebertpub.com/ten.
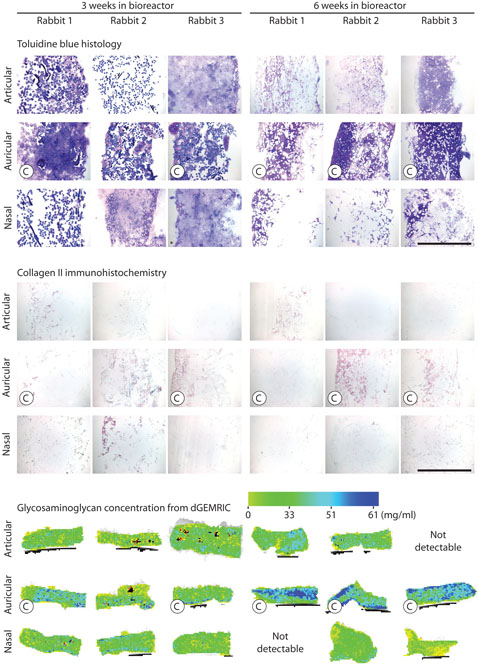
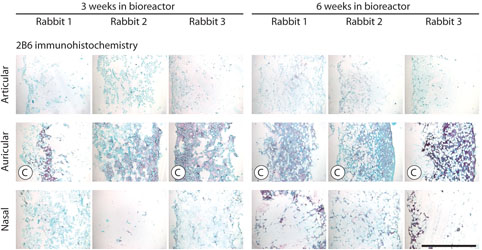
The variations in gross structure of the 18 tissue-engineered cartilage samples, as visualized using toluidine blue staining (Fig. 4), were consistent with the results of biomechanical testing. Toluidine blue staining showed the most metachromasia in the auricular samples. In these samples, cell-produced extracellular matrix was apparent and the Hyalograft-C scaffold material was relatively unchanged over time. In contrast, in the articular and nasal samples, cell-produced extracellular matrix was sparse and the Hyalograft-C scaffold material appeared to be swelling and degrading over time. These findings suggest that the more abundant extracellular matrix in auricular samples contributes directly to biomechanical stiffness, as would be expected, but the matrix may also indirectly contribute to biomechanical stiffness by stabilizing the Hyalograft-C scaffold and slowing down scaffold degradation. Immunostaining for collagen II, the primary collagen of cartilage, was most extensive in the auricular samples (Fig. 4). Immunostaining for collagen I, a collagen not present in native cartilage, and elastin showed these were absent in all samples (data not shown). Immunostaining for chondroitin 4-sulfate and dermatan sulfate by antibody 2B6 was most extensive in the auricular samples (Fig. 5). Immunostaining for chondroitin 6-sulfate by antibody 3B3 was absent in all samples (data not shown).
FIG. 4.
Structure and composition of tissue-engineered cartilage samples as analyzed by histology, immunohistochemistry, and dGEMRIC. Top: Images of sections stained by toluidine blue. Bar = 1 mm. Middle: Images of sections immunostained for collagen II. Bar = 1 mm. Bottom: Pseudo color representation of GAG concentration (mg GAG per mL tissue water) as quantified by dGEMRIC. Each dGEMRIC slice transects an entire sample. “C” indicates a sample that completed biomechanical testing.
FIG. 5.
Histological appearance of tissue-engineered cartilage samples immunostained with 2B6. Bar = 1 mm. “C” indicates a sample that completed biomechanical testing. Color images available online at www.liebertpub.com/ten.
Biochemical composition and structure
Glycosaminoglycan and DNA analyses
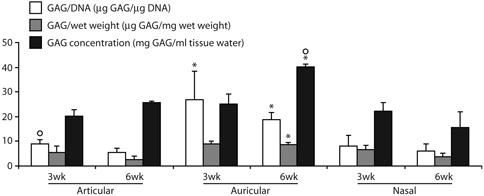
Colorimetric GAG and DNA assays showed that at 3 weeks GAG content per DNA content of the auricular samples was 210% higher than the articular and 230% higher than the nasal samples (p < 0.05, Fig. 6). This difference persisted at 6 weeks, when GAG content per DNA content of the auricular samples was 260% higher than the articular and 210% higher than the nasal samples (p < 0.05). Similarly, at 6 weeks the GAG content per sample wet weight of the auricular samples was 240% higher than the articular and 130% higher than the nasal samples (p < 0.05, Fig. 6).
FIG. 6.
Quantitative results from biochemical analysis: GAG content per DNA content, GAG content per sample wet weight, and GAG concentration. Units for the y-axis are result specific, as indicated. *Significantly greater than articular or nasal samples at the same time point (3 weeks or 6 weeks). ºSignificantly greater than paired samples from the same anatomic location.
dGEMRIC analysis
dGEMRIC imaging of empty scaffolds showed a T1Gd similar to that of the surrounding medium, indicating that the empty scaffold has negligible net charge, so that any dGEMRIC measurements (which are, in reality, measurements of charge), made for tissue-engineered constructs will reflect charge accumulated during culture through the synthesis and retention of GAG. Consistent with the results of the colorimetric GAG assay, dGEMRIC showed that at 6 weeks GAG concentration of the auricular samples was 54% higher than the articular and 150% higher than the nasal samples (p < 0.05, Figs. 4 and 6). dGEMRIC imaging of one 6-week articular sample and one 6-week nasal sample showed negligible contrast between the bathing solution and the sample, indicating negligible GAG concentrations, and were not included in the analyses. In contrast to biochemical measurement of GAG, which showed no significant difference between the 3-week and 6-week auricular samples, dGEMRIC showed that the GAG concentration of the auricular samples was 60% higher at 6 weeks than at 3 weeks (p = 0.01). In addition, whereas the articular and nasal samples had relatively uniform distributions of low GAG concentration, the distribution of GAG concentration in some auricular samples revealed a nonuniform layered structure, which may be indicative of reduced matrix synthesis at the center of samples, as increased GAG concentration limits nutrient and waste movement through the sample (Fig. 4).
Collagen content
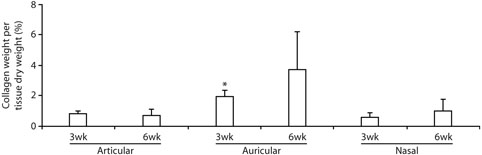
Collagen content analysis showed that at 3 weeks collagen content per tissue dry weight of the auricular samples was 130% higher than the articular and 230% higher than the nasal samples (p < 0.05, Fig. 7). There was a similar trend at 6 weeks, when collagen content per tissue dry weight of the auricular samples was 440% higher than the articular and 270% higher than the nasal samples, although the differences were not statistically significant (p = 0.14). The collagen content of all of engineered samples, including auricular samples, was low when compared to the collagen content in native rabbit cartilages: collagen contents of 0.81 ± 0.17% and 0.69 ± 0.43% for 3-week and 6-week engineered articular cartilage vs. 56 ± 5.8% for native articular cartilage; 1.9 ± 0.46% and 3.7 ± 2.5% vs. 37 ± 4.7% for auricular cartilages; and 0.57 ± 0.33% and 1.0 ± 0.69% vs. 30 ± 6.5% for nasal cartilages.
FIG. 7.
Quantitative results from collagen content analysis: collagen content per tissue dry weight. *Significantly greater than articular or nasal samples at the same time point (3 weeks).
DISCUSSION
This study shows that, using the current tissue engineering approach under baseline conditions, only rabbit auricular chondrocytes produce tissue-engineered cartilage that is suitable for in vivo testing in laryngotracheal reconstruction in rabbits. In vitro biomechanical testing designed around the functional requirements of the cartilage graft indicated that tissue-engineered cartilage generated from auricular chondrocytes, but not articular or nasal chondrocytes, had the mechanical properties necessary for in vivo testing as a graft. Consistent with the biomechanical results, biochemical characterization showed that, compared to the articular and nasal samples, auricular samples generally had more cell-produced extracellular matrix, more extensive immunostaining for collagen II, significantly higher GAG content and concentration, and significantly higher collagen content. The dGEMRIC method, a recently developed MRI method to assess cartilage GAG concentration and spatial distribution, revealed variations in GAG spatial distribution in auricular samples that were not present in articular or nasal samples.
Biomechanical testing was performed to determine the equilibrium unconfined compression modulus of the tissue-engineered cartilage samples. The in vitro conditions were chosen to simulate the in vivo loading conditions and the maximum applied load the engineered cartilage is expected to experience when implanted into the rabbit trachea, as determined by in situ measurements. For this reason, in vitro biomechanical tests were performed under load control, rather than displacement control, to ensure that samples were tested up to the maximum expected in vivo load.
Biomechanical testing revealed a profound difference in mechanical integrity and stiffness of the auricular samples when compared to the articular and nasal samples. Five of six auricular samples successfully completed biomechanical testing and had measured equilibrium unconfined compression moduli approaching those of native hyaline cartilages tested under similar conditions of unconfined compression (210 ± 93 kPa at 3 weeks and 100 ± 65 kPa at 6 weeks in the present study, compared to 270 kPa for trochlear groove cartilage of 6-month-old cows,27 677 ± 223 kPa for humeral head cartilage of 1- to 2-year-old cows,28 and 310 ± 180, 570 ± 170, and 800 ± 330 kPa for femoral, patellar, and humeral cartilage from cows of unreported age29). The one auricular sample that did not complete testing exceeded the range of the linear variable displacement transducer during the highest load level (215.8 mN). In contrast, all tissue-engineered cartilage samples generated from articular or nasal chondrocytes lacked the mechanical integrity and stiffness for completion of the biomechanical testing. These samples were either damaged during handling or exceeded the range of the linear variable displacement transducer during the first experimental load level (68.7 mN), preventing estimation of equilibrium unconfined compression modulus. Based on these findings, it is clear that the articular and nasal samples were unsuitable for in vivo testing in laryngotracheal reconstruction in rabbits, because handling less demanding than that present during implantation damaged some samples, and small loads (less than one-third of that expected to exist in vivo) compressed the remaining samples by 50% or more.
Determining whether the tissue-engineered cartilage generated by auricular chondrocytes in the present study is truly suitable for laryngotracheal reconstruction in rabbits will ultimately require in vivo testing, particularly since relatively little is known about the biomechanics of laryngotracheal reconstruction. The equilibrium unconfined compression modulus of human costal cartilage, the current gold standard for laryngotracheal reconstruction in children, has not, to our knowledge, been reported. In the present study, it proved infeasible to harvest a sample of rabbit costal cartilage of the appropriate size and shape for biomechanical testing. In addition, the equilibrium unconfined compression modulus of cricoid cartilage or tracheal rings have also not been reported, and would not represent a standard with which to compare the properties of engineered cartilage because of the composite nature of the trachea.
The differences in quality of cartilage generated in the present study likely indicate that the baseline conditions used in this study are more favorable to auricular chondrocytes than to articular or nasal chondrocytes, not that articular and nasal chondrocytes are fundamentally unsuitable for use in this tissue engineering application. It has previously been shown that a number of factors can affect the behavior of chondrocytes during tissue engineering, and these factors can affect chondrocytes from different anatomic locations in different ways. Exposure to growth factors during expansion 16,30–32 or redifferentiation30,33,34 can improve the chondrogenic capacity of chondrocytes, and chondrocytes harvested from different anatomic locations have been found to respond to the same growth factors in different ways or to different extents.16 Similarly, scaffold composition and architecture can have a significant effect on chondrocyte behavior 35 (and, in purely mechanical terms, use of a stiffer scaffold in the present application might allow more samples to withstand the loads the engineered cartilage is expected to experience when implanted). In addition, dynamic mechanical loading of agarose cartilage constructs has been shown to stimulate accumulation of GAGs and increase stiffness.36,37 Although the responses to mechanical loading of chondrocytes from different anatomic locations have not been compared, it is reasonable to expect that chondrocytes conditioned by different mechanical environments in vivo, such as the mechanical environment of the shoulder versus the mechanical environment of the ear, would respond differently to mechanical signals applied during culture in vitro. Experiments to optimize the present tissue engineering approach, with respect to growth factors, scaffold composition and architecture, and mechanical loading, are ongoing. Such optimization may result in chondrocytes from articular, nasal, or other cartilages being viable, if not preferred, alternatives to the use of auricular chondrocytes.
It is potentially very useful to use chondrocytes from different anatomic locations to produce engineered cartilage. The present results suggest that chondrocytes harvested from one anatomic location, the ear, are capable of producing functional tissue-engineered cartilage for use in another anatomic location, the laryngotracheal segment. The results also suggest that chondrocytes from different anatomic locations will require different isolation and culture conditions to permit their use in this specific tissue engineering application for laryngotracheal reconstruction. Other cartilage tissue engineering applications that employ comparable approaches can be expected to require similar optimization with respect to anatomic location of chondrocyte harvest.
Viewing these findings in the context of other tissue engineering studies,13–16,38,39 we suggest that optimization for specific sources of chondrocytes, whether anatomic location or even articular cartilage zone, is emerging as an important consideration when developing cartilage tissue engineering approaches that use autologous chondrocytes. The results demonstrate that the rabbit is a valuable model for continued development of the present cartilage tissue engineering approach for pediatric laryngotracheal reconstruction. The rabbit also provides a useful model for investigating the different ways in which chondrocytes from different anatomic locations behave when applied in tissue engineering approaches. Continued development of the approach presented here will allow further investigation of differences in chondrocytes from different anatomic locations while pursuing the long-term goal of tissue engineering autologous cartilage grafts for pediatric laryngotracheal reconstruction.
ACKNOWLEDGMENTS
The authors would like to thank David Carrino for assistance with the GAG assays, Kevin Davis for biomechanical testing, Michelle Farley and Martha Gray for dGEMRIC analysis, Mark Weidenbecher for helpful discussion of the manuscript, and Amad Awadallah, Nell Ginley, Kitsie Penick, and Lisa Walsh for technical support. Fidia Advanced Biopolymers supplied the Hyalograft-C scaffold material. This work was supported by NIH/NIDCR R01 DE015322-01 (JED), NIH/NIAMS Training Program in Musculoskeletal Diseases AR07505 (JHH), and an Arthritis Foundation Post-doctoral Fellowship (JHH). Development of the bioreactor system was supported by the Arthritis Foundation and NIH R01 AR050802 (JFW).
Footnotes
Presented at the 6th Symposium of the International Cartilage Repair Society, January 2006.
REFERENCES
- 1.Silva AB, Lusk RP, Muntz HR. Update on the use of auricular cartilage in laryngotracheal reconstruction. Ann Otol Rhinol Laryngol. 2000;109:343. doi: 10.1177/000348940010900401. [DOI] [PubMed] [Google Scholar]
- 2.Hartley EJ, Cotton RT. Paediatric airway stenosis: laryngotracheal reconstruction or cricotracheal resection? Clin Otolaryngol. 2000;25:342. doi: 10.1046/j.1365-2273.2000.00399.x. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin B. Prolonged intubation injuries of the larynx: endoscopic diagnosis, classification, and treatment. Ann Otol Rhinol Laryngol. 1993;1(Suppl 160) doi: 10.1177/00034894931020s401. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell SA. The mechanism of tracheal stenosis. Ear Nose Throat J. 1979;58:28. [PubMed] [Google Scholar]
- 5.Holinger LD, Lusk RP, Green CG. Pediatric Laryngology and Bronchoesophagology. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 6.Gerek M. Laryngotracheal reconstruction update. Curr Opin Otolaryngol Head Neck Surg. 2001;9:209. [Google Scholar]
- 7.Lusk RP, Kang DR, Muntz HR. Auricular cartilage grafts in laryngotracheal reconstruction. Ann Otol Rhinol Laryngol. 1993;102:247. doi: 10.1177/000348949310200402. [DOI] [PubMed] [Google Scholar]
- 8.Zalzal GH, Choi SS, Patel KM. Ideal timing of pediatric laryngotracheal reconstruction. Arch Otolaryngol Head Neck Surg. 1997;123:206. doi: 10.1001/archotol.1997.01900020094014. [DOI] [PubMed] [Google Scholar]
- 9.Naumann A, Dennis JE, Awadallah A, Carrino DA, Mansour JM, Kastenbauer E, Caplan AI. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J Histochem Cytochem. 2002;50:1049. doi: 10.1177/002215540205000807. [DOI] [PubMed] [Google Scholar]
- 10.Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tissue Res. 1988;18:223. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- 11.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 1999;254:535. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzo P, Bayliss MT, Heinegard D. A novel cartilage protein (CILP) present in the mid-zone of human articular cartilage increases with age. J Biol Chem. 1998;273:23463. doi: 10.1074/jbc.273.36.23463. [DOI] [PubMed] [Google Scholar]
- 13.Waldman SD, Grynpas MD, Pilliar RM, Kandel RA. The use of specific chondrocyte populations to modulate the properties of tissue-engineered cartilage. J Orthop Res. 2003;21:132. doi: 10.1016/S0736-0266(02)00105-5. [DOI] [PubMed] [Google Scholar]
- 14.Isogai N, Kusuhara H, Ikada Y, Ohtani H, Jacquet R, Hillyer J, Lowder E, Landis WJ. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12:691. doi: 10.1089/ten.2006.12.691. [DOI] [PubMed] [Google Scholar]
- 15.Kafienah W, Jakob M, Demarteau O, Frazer A, Barker MD, Martin I, Hollander AP. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8:817. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 16.Tay AG, Farhadi J, Suetterlin R, Pierer G, Heberer M, Martin I. Cell yield, proliferation, and postexpansion differentiation capacity of human ear, nasal, and rib chondrocytes. Tissue Eng. 2004;10:762. doi: 10.1089/1076327041348572. [DOI] [PubMed] [Google Scholar]
- 17.Grimmer JF, Gunnlaugsson CB, Alsberg E, Murphy HS, Kong HJ, Mooney DJ, Weatherly RA. Tracheal reconstruction using tissue-engineered cartilage. Arch Otolaryngol Head Neck Surg. 2004;130:1191. doi: 10.1001/archotol.130.10.1191. [DOI] [PubMed] [Google Scholar]
- 18.Kamil SH, Eavey RD, Vacanti MP, Vacanti CA, Hartnick CJ. Tissue-engineered cartilage as a graft source for laryngotracheal reconstruction: a pig model. Arch Otolaryngol Head Neck Surg. 2004;130:1048. doi: 10.1001/archotol.130.9.1048. [DOI] [PubMed] [Google Scholar]
- 19.Penick KJ, Solchaga LA, Berilla JA, Welter JF. Performance of polyoxymethylene plastic (POM) as a component of a tissue engineering bioreactor. J Biomed Mater Res A. 2005;75:168. doi: 10.1002/jbm.a.30351. [DOI] [PubMed] [Google Scholar]
- 20.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 21.Penick KJ, Solchaga LA, Welter JF. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques. 2005;39:687. doi: 10.2144/000112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrino DA, Arias JL, Caplan AI. A spectrophotometric modification of a sensitive densitometric Safranin O assay for glycosaminoglycans. Biochem Int. 1991;24:485. [PubMed] [Google Scholar]
- 23.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med. 1996;36:665. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 24.Gillis A, Gray M, Burstein D. Relaxivity and diffusion of gadolinium agents in cartilage. Magn Reson Med. 2002;48:1068. doi: 10.1002/mrm.10327. [DOI] [PubMed] [Google Scholar]
- 25.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magn Reson Med. 1999;41:857. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 27.Woodfield TB, Malda J, de Wijn J, Peters F, Riesle J, van Blitterswijk Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials. 2004;25:4149. doi: 10.1016/j.biomaterials.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 28.Jurvelin JS, Buschmann MD, Hunziker EB. Optical and mechanical determination of Poisson's ratio of adult bovine humeral articular cartilage. J Biomech. 1997;30:235. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen RK, Laasanen MS, Toyras J, Rieppo J, Hirvonen J, Helminen HJ, Jurvelin JS. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 30.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J Cell Biochem. 2001;81:368. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Mandl EW, Jahr H, Koevoet JL, van Leeuwen JP, Weinans H, Verhaar JA, van Osch GJ. Fibroblast growth factor-2 in serum-free medium is a potent mitogen and reduces dedifferentiation of human ear chondrocytes in monolayer culture. Matrix Biol. 2004;23:231. doi: 10.1016/j.matbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Serum-free medium supplemented with high-concentration FGF2 for cell expansion culture of human ear chondrocytes promotes redifferentiation capacity. Tissue Eng. 2002;8:573. doi: 10.1089/107632702760240490. [DOI] [PubMed] [Google Scholar]
- 33.Chaipinyo K, Oakes BW, van Damme MP. Effects of growth factors on cell proliferation and matrix synthesis of low-density, primary bovine chondrocytes cultured in collagen I gels. J Orthop Res. 2002;20:1070. doi: 10.1016/S0736-0266(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 34.Jakob M, Demarteau O, Suetterlin R, Heberer M, Martin I. Chondrogenesis of expanded adult human articular chondrocytes is enhanced by specific prostaglandins. Rheumatology (Oxford) 2004;43:852. doi: 10.1093/rheumatology/keh197. [DOI] [PubMed] [Google Scholar]
- 35.Miot S, Woodfield T, Daniels AU, Suetterlin R, Peterschmitt I, Heberer M, van Blitterswijk CA, Riesle J, Martin I. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials. 2005;26:2479. doi: 10.1016/j.biomaterials.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 36.Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 37.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 38.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Kojima K, Bonassar LJ, Ignotz RA, Syed K, Cortiella J, Vacanti CA. Comparison of tracheal and nasal chondrocytes for tissue engineering of the trachea. Ann Thorac Surg. 2003;76:1884. doi: 10.1016/s0003-4975(03)01193-7. [DOI] [PubMed] [Google Scholar]