Abstract
Mouse hepatitis virus induces a biphasic disease in BALB/c mice that consists of an acute retinitis followed by progression to a chronic retinal degeneration with autoimmune reactivity. Retinal degeneration resistant CD-1 mice do not develop the late phase. What host factors contribute to the distinct responses to the virus are unknown. Herein, we show that IFN-α, IFN-β and IFN-γ act in concert as part of the innate immune response to the retinal infection. At day 2, high serum levels of IFN-γ, CXCL9 and CXCL10, were detected in BALB/c mice. Moreover, elevated levels of CXCL9 and CXCL10 gene expression were detected in retinal tissue. Although IFN-γ and the chemokines were detected in CD-1 mice, they were at significantly lower levels compared to BALB/c mice. These augmented innate responses observed correlated with the development of autoimmune reactivity and retinal degeneration and thus may contribute to the pathogenic processes.
Keywords: Coronavirus, Retinal degeneration, Chemokines, Interferon, Autoimmunity
1. Introduction
Degenerative and inflammatory diseases of the retina are a leading cause of visual impairment and blindness. In fact, approximately one million Americans suffer severe visual impairment from retinal and choroidal diseases. Retinal degenerative disorders consist of a diverse group of diseases frequently associated with a genetic predisposition; however, many cases are of unknown cause. Although considerable effort has been made to expand our understanding of these degenerative diseases, the exact mechanisms remain elusive. Over the years, animal models have provided us with unique opportunities to learn more about the pathogenesis and treatment of a variety of human diseases. One such example is coronavirus infections that serve as an excellent prototype for CNS and ocular diseases (Bergmann et al., 2006, Holmes, 2001, Hooks et al., 2001). Murine coronavirus infection of BALB/c mice results in a progressive retinal degeneration associated with autoimmune reactivity (Hooks et al., 1993, Robbins et al., 1990). This animal model, referred to as experimental coronavirus retinopathy (ECOR), was established to investigate pathologic processes within the retina. Intravitreal injection of mouse hepatitis virus (MHV), JHM strain, into BALB/c mice results in a biphasic disease. Acute virus induced retinitis develops in susceptible BALB/c mice from day 3 to 7 post-inoculation (PI) and progresses to chronic retinal degeneration from day 20 to 100. Injection of MHV into resistant CD-1 mice also results in acute infection without the late retinal degenerative phase of the disease (Wang et al., 1996).
In both mouse strains, the acute phase is characterized by the detection of infectious virus within the retina and the retinal pigment epithelium (RPE) (Wang et al., 1993). Viral infectivity titers peak on day 6 and after day 8 infectious virus is not detected. Nevertheless, viral RNA persists within the retina throughout the life of the animal (Komurasaki et al., 1996). Beginning at day 7, anti-viral neutralizing antibody is detectable in the sera and continues to rise during the course of the disease. On day 3, immune cells infiltrate the retina and this infiltration peaks at day 6. The most prominent infiltrating cell is the macrophage. In addition, CD4 T cells are seen at day 3 and 6, whereas, CD8 T cells are not detected until days 6 and 8 PI (Hooks et al., 2003, Vinores et al., 2001).
The chronic degenerative disease is seen only in BALB/c mice and is associated with a progressive loss of photoreceptors, ganglion cells and RPE cells. In the degeneration resistant CD-1 mice, only the early inflammatory phase is observed without retinal degeneration. Since viral replication and clearance of infectious virus are nearly identical in the two mouse strains, it is plausible that the development of the degenerative phase is associated with host responses to the infection. We have previously identified an autoimmune component in BALB/c mice that is absent in CD-1 mice (Hooks et al., 1993). Autoantibodies from BALB/c sera were shown to react with retinal tissue and the RPE cell. Furthermore, BALB/c mouse CD4 T cells proliferate in response to retinal antigens. What factors contribute to these distinct host responses are unknown. However, it is well established that the innate immune system is essential for the development of protective adaptive immune responses. We therefore hypothesize that the intensity of the cytokine and chemokine responses very early in the course of the infection may contribute to these diverse host responses. In order to test this hypothesis, we focused on the interferons (IFN) and the IFN inducible chemokines, CXCL9 and CXCL10.
The IFNs were discovered as antiviral molecules that inhibit a variety of viruses. Today, they are also recognized as key components of innate immunity (Pestka et al., 2004). The type 1 IFNs include IFN-α, IFN-β and IFN-ω, that share a cellular receptor. The type II IFN is IFN-γ, which is encoded by a single gene, has a separate cellular receptor and is produced by T cells and NK cells. All the IFNs have overlapping pleiotrophic effects on a variety of cellular functions. Studies using IFN-α/β and IFN-γ receptor knockout mice have demonstrated that both IFN systems are essential for antiviral defense and are functionally non-redundant (Muller et al., 1994). Numerous studies have demonstrated that IFN-γ is important in controlling virus infections in the central nervous system (CNS) and this is a critical component in ocular inflammation (Hooks et al., 1988, Parra et al., 1999). Using our ECOR model system, we have demonstrated that the generation of IFN-γ by cells infiltrating the retina is an essential immune component responsible for non-cytolytic clearance of infectious virus from the retina (Hooks et al., 2003).
Chemokines are a superfamily of small proteins with a crucial role in immune and inflammatory reactions, specifically, the induction of leukocyte migration (Coelho et al., 2005, Medoff and Luster, 2006). CXCL9 (MIG) and CXCL10 (IP-10) are IFN inducible chemokines that attract T cells and NK cells to the site of inflammation. These cells contain the receptor CXCR3 on their surface. Several studies have identified these molecules as key components of host responses in virus infections and in autoimmune diseases (Christen et al., 2003, Khan et al., 2000, Salomon et al., 2002, Wallace et al., 2004). For example, MHV infections of the CNS have identified a critical role for CXCL10 and CXCR3 in attracting T cells into the CNS and thus provide protection to the host (Trifilo et al., 2004). An example of CXCL9 involvement in autoimmunity was clearly demonstrated by Lang et al. (2006). In a murine model system they showed that the localized expression of CXCL9, incited an enhanced infiltration of liver specific CD8 T cells and subsequent autoimmune liver damage. These studies clearly showed that autoimmune liver disease could be induced only after CXCL9 was expressed within the liver.
These examples provide evidence that the chemokines, CXCL9 and CXCL10, attract T cells to the site of infection and may provide in some cases, optimal defense against microbial pathogens. Alternatively, these processes may also provide the appropriate environment for autoimmune reactivity. ECOR is characterized by having both an infectious component and an autoimmune component and it is therefore possible that these chemokines may contribute to the pathogenic processes in ECOR.
In an effort to better define the pathologic events in ECOR, we have evaluated host responses to this infection by studying cytokine profiles during the acute phase (day 3–6) and the late phase (day 20) (Hooks et al., 2003, Hooper et al., 2005). Our initial studies did not evaluate the day 1 and day 2 time points in which the innate immune responses to the retinal virus infection could be measured. Therefore, the purpose of this study was to evaluate very early cytokine and chemokine profiles and compare the intensity of immune reactivity in coronavirus infected mice with a retinal degeneration susceptible and a retinal degeneration resistant background. We noted that immune reactivity is more robust at early time points in the retinal degeneration susceptible BALB/c mice compared to the degeneration resistant CD-1 mice. The key cytokines and chemokines identified are IFNs, CXCL9 and CXCL10. Moreover, in the susceptible BALB/c mice we detected elevated levels of CXCL9 and CXCL10 gene expression in retinal tissue which correlates with the robust chemokine levels detected in the sera.
2. Materials and methods
2.1. Animals
Male BALB/c (Harlan Sprague Dawley, Indianapolis, IN) and CD-1 (Charles River, Raleigh, NC) mice (8–13 weeks old, 25–30 g) were used. All experimental procedures conformed to the Association for Research in Vision and Ophthalmology resolution for the use of animals in ophthalmic and vision research.
2.2. Virus preparation
Mouse hepatitis virus (MHV), strain JHM, was obtained from the American Type Tissue Collection (Manassas, VA) and was propagated in mouse 17CL1, 3T3 or mouse L2 cells. Briefly, infected cultures were frozen and thawed, centrifuged at 2000 rpm for 20 min to remove cellular debris and the supernatant was centrifuged at 15,000 rpm for 2 h to pellet the virus. The viral pellet was resuspended in DMEM with 2% heat-inactivated fetal bovine sera (HI FBS), divided into small aliquots and stored at − 70 °C. Viral infectivity titers were determined by plaque assay on mouse L2 cells.
2.3. Mouse inoculations
As previously described, eyes were injected intravitreally with 5 μl of either 1.35 × 106 PFU/ml of MHV (virus-infected) or with MEM containing 2% HI FBS (mock-infected) (Wang et al., 1996). Blood was collected in Microtainers (Becton Dickinson, Franklin Lakes, NJ) from un-injected, mock-injected and virus-injected mice at 2, 4, 8, and 10 days after inoculation for the first set of experiments and at 1, 2, 3, and 4 days after inoculation for the second set of experiments. Sera was separated from the cells and stored at − 70 °C until analyzed. The mice were euthanized by cervical dislocation, and eyes were removed and fixed in 10% buffered formalin for hematoxylin and eosin staining.
2.4. Cytokine analysis by EIA
The serum levels of IFN-γ, CXCL9, CXCL10, IL-10 and IL-12 were determined using commercially available enzyme immunoassay (EIA) kits (R&D systems, Minneapolis, MN). EIA kits were also used to identify IFN-α and IFN-β (PBL Biomedical, Piscataway, NJ, through R&D Systems). All serum samples were tested in duplicate according to the manufacturer's instructions. The optical density of each sample was determined using the VERSAmax tunable microplate reader (Molecular Devices, Sunnyvale, CA). Results were calculated from a standard curve and reported accordingly in picograms per milliliter. The minimal detectable dose for each cytokine/chemokine measured is as follows: IFN-γ is < 2.5 pg/ml, CXCL9 is 3 pg/ml, CXCL10 is 2.2 pg/ml, IL-10 is < 4 pg/ml, IL-12 is < 2.5 pg/ml. The value for the lowest standard in the IFN-α assay and IFN-β assay was: 12.5 pg/ml for IFN-α and 15.6 pg/ml for IFN-β. All results are expressed as mean values +/− SD. The unpaired student t-test was used to analyze the serum samples.
2.5. Real time reverse-transcriptase PCR
Retinas were dissected from enucleated eyes on the days indicated and six to twelve retinas were pooled. Total RNA was isolated from each pool using RNA Stat (Friendswood, TX). Total RNA (0.2 µg per point) was reverse transcribed to cDNA and amplified using mouse specific Taqman primers for GAPDH, CXCL9 and CXCL10 (Applied Biosystems, Foster City, CA) and RT-PCR and qPCR kits from Eurogentec (San Diego, CA) according to the manufacturer's instructions. All samples were run in duplicate and relative amounts of each message were determined by comparison with serially diluted standards followed by normalization to GAPDH levels and represented as a ratio.
2.6. Immunohistochemistry
Mice eyes were enucleated on days 2, 3, 4, 6, 8 and 10, snap frozen, and sectioned via papillary-optic nerve plane. Ocular sections were stained using the avidin-biotin-immunoperoxidase technique. The following anti-mouse mAbs were used: for CD4 T cell identification, rat IgG2b reacting with L3/T4 (sera-Laboratory, Belton, UK); for CD8 T cell identification, rat IgG2b reacting with Lyt-2 (Sera-Laboratory); for macrophage identification, rat IgG2b reacting with Mac-1 (CD11b/CDd18).
3. Results
3.1. Evaluation of infiltrating cells in ECOR
Inoculation of BALB/c and CD-1 mice with JHM virus by the intravitreal route (5 µl) results in an inflammatory disease beginning on day 3 and lasting until day 8. Day 3 PI is the first day when infiltrating cells are seen (Fig. 1 ). Immunohistochemistry analysis at day 3 revealed that the primary infiltrating cell was the macrophage in both mouse strains. At this time, scattered CD4 T cells were seen in 2/4 eyes from BALB/c mice and in 1/4 eyes from CD-1 mice. These scattered CD4 T cells were seen in the ciliary body and iris. CD8 T cells were not observed until day 6 PI (Hooks et al., 2003).
Fig. 1.
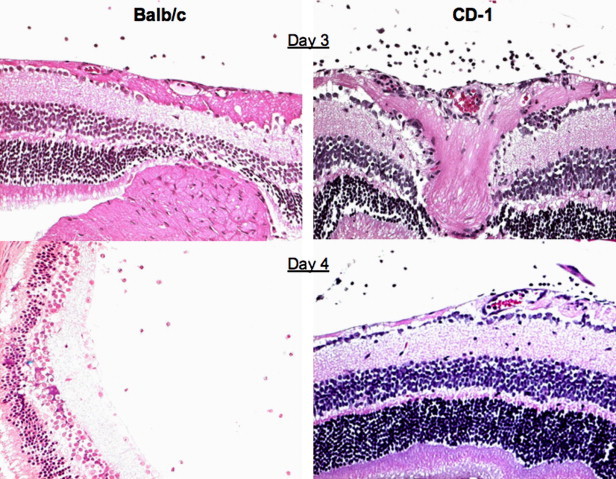
Retinal tissue from MHV infected BALB/c and CD-1 mice, day 3 and 4 PI.
The infection is resolved in CD-1 mice and at day 20 PI and the retinas from these animals have a normal architecture. In contrast, in BALB/c mice, the retinas that do not recover begin to degenerate. Associated with the retinal degenerative process is the development of anti-retinal autoantibodies in BALB/c mice. These autoantibodies are not observed in sera from CD-1 mice.
3.2. Comparison of serum cytokine and chemokine responses to JHM virus infection in BALB/c and CD-1 mice
Our earlier studies on the evaluation of cytokine profiles during the acute phase of the disease (day 3 to 8) did not reveal differences between the two mouse strains. Since the very early or innate immune responses (day 1 and day 2) may contribute to the intensity of the immune response to the infection, we initiated studies to evaluate these time points. BALB/c and CD-1 mice were inoculated with JHM virus, media (mock) or they remained untreated. At day 2, 4, 8 and 10 sera were collected and analyzed for IFN-γ, CXCL9, CXCL10, IL-10 and IL-12. IFN-γ, CXCL9 and CXCL10 were not detected in the sera from untreated mice or from mock injected mice. As is seen in Fig. 2 , IFN-γ, CXCL9 and CXCL10 were detected in the sera from both infected mouse strains. However, significantly different levels were observed in the BALB/c and CD-1 mice. Overall, the levels of IFN-γ, CXCL9 and CXCL10 were present earlier and at higher levels in BALB/c mice compared to CD-1 mice. As is noted in Fig. 2, at day 2, IFN-γ (233 pg/ml) was detected in sera from BALB/c mice, whereas, at this time point, IFN-γ was not detected in sera from CD-1 mice. As we have published previously, similar low levels of IFN-γ were detected in both mouse strains on day 4 and this cytokine was not detected at days 8 and 10. Fig. 2 illustrates that sera levels of CXCL10 were higher on day 2 (1021 pg/ml) and day 4 (547 pg/ml) in BALB/c mice in comparison to CD-1 mice at day 2 (120 pg/ml) at day 4 (289 pg/ml). Likewise, sera levels of CXCL9 were higher at day 2 (547 pg/ml) and at day 4 (789 pg/ml) in BALB/c mice in comparison to CD-1 mice at day 2 (135 pg/ml) and at day 4 (281 pg/ml). The difference between CXCL9 and CXCL10 values from BALB/c and CD-1 mice at day 2 were significantly different (P < 0.01).
Fig. 2.
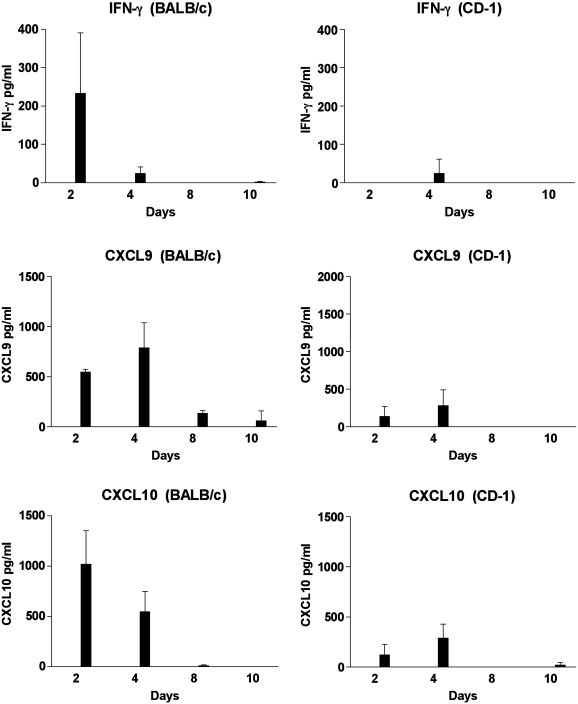
Identification of IFN-γ, CXCL9 and CXCL10 in the sera of MHV infected mice by EIA. Sera from 3 to 4 mice were tested at each time point. IFN-γ, CXCL9 and CXCL10 were not detected in the sera from mock injected or untreated (control) BALB/c and CD-1 mice.
The analysis of IL-10 and IL-12 in sera from these mice is shown in Fig. 3 . IL-10 was not detected in the sera from mock injected or untreated mice. In virus infected BALB/c mice IL-10 was detected at day 2, 4 and 8 PI whereas; in virus infected CD-1 mice IL-10 was only detected at day 2 PI. In contrast, similar low levels of IL-12 were detected in sera from mock injected and untreated mice. In untreated or mock injected BABL/c mice, IL-12 levels ranged from 104 to 120 pg/ml, whereas, in untreated or mock injected CD-1 mice, IL-12 levels ranged from 57 to 85 pg/ml. The IL-12 levels detected in BALB/c mice at day 2 (437 pg/ml) and day 4 (539 pg/ml) were higher than those detected in CD-1 mice at day 2 (120 pg/ml and day 4 (289 pg/ml).
Fig. 3.
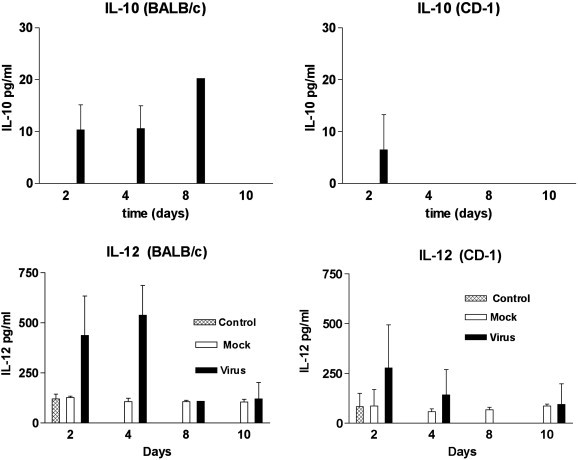
Identification of IL-10 and IL-12 in the sera of MHV infected mice by EIA. Sera from 3 mice were tested at each time point. IL-10 was not detected in the sera from mock injected or untreated (control) BALB/c and CD-1 mice.
3.3. Early (day 1–4) serum analysis IFN-γ, CXCL9 and CXCL10
The data described in Fig. 2 indicate that there are significant differences at day 2 PI in the levels of IFN-γ, CXCL9 and CXCL10 in virus infected BALB/c and CD-1 mice. Clearly, to better evaluate earlier time points, further analysis of these cytokines and chemokines was required. Therefore, we next injected the animals as previously described and harvested sera at day 1, 2, 3 and 4 for analysis. The data presented in Fig. 4 , demonstrated that sera levels of IFN-γ, CXCL9 and CXCL10 were present earlier and at higher concentrations in BALB/c mice in comparison to CD-1 mice. Serum IFN-γ levels were significantly elevated in each of the four days in virus infected BALB/s mice compared to mock injected mice (day 1,2,3 P < 0.001, day 4 P < 0.01). Peak levels of IFN-γ were detected at days 2 and 3 PI. In contrast, serum IFN-γ levels were not significantly elevated in virus infected CD-1 mice compared to mock injected mice (P = 0.03).
Fig. 4.
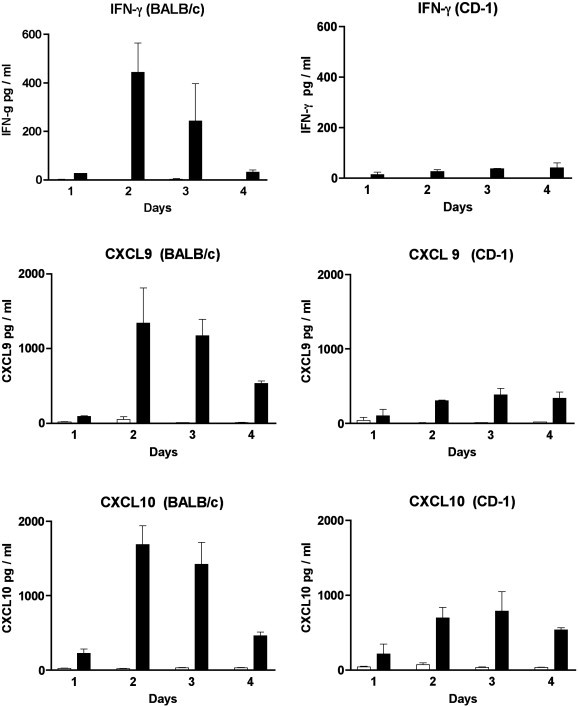
Identification of IFN-γ, CXCL9 and CXCL10 in the sera of MHV infected mice by EIA. Sera from 3 to 4 mice were tested at each time point (day 1,2,3 and 4). MHV infected mice are represented by the black bars while mock injected mice are represented by the white bars.
Serum CXCL10 levels were significantly elevated in each of the four days in virus infected BALB/c mice compared to mock injected mice (day 1 and 3 P = 0.01; day 2 and 4 P = 0.001). Again peak levels of CXCL10 in BALB/c mice were seen at days 2 and 3. In contrast, serum CXCL10 levels were significantly elevated only on day 4 in virus infected CD-1 mice compared to mock injected mice (P = 0.001). Serum CXCL9 levels were significantly elevated in each of the four days in virus infected BALB/c compared to mock injected mice (days 1 and 2 P < 0.01, days 3 and 4 P < 0.0001). Serum CXCL9 levels were significantly elevated on days 2, 3 and 4 in CD-1 mice compared to mock injected mice (days 2 and 3 P < 0.001, day 4 P < 0.01).
In summary, serum levels of IFN-γ, CXCL10 and CXCL9 detected at day 2 and 3 in virus infected BALB/c mice were compared to serum levels detected at this time in CD-1 mice. A statistically significant difference was observed (IFN-γ, P < 0.0001; CXCL10, P = 0.005; CXCL9, P = 0.0008). It is of interest to note that the peak levels of cytokines and chemokines on day 2 occurs one day prior to the infiltration of mononuclear cells into the retina (Fig. 1).
3.4. Chemokine gene expression in retinal tissue
IFN-γ is produced by T cells and NK cells and subsequently acts on resident cells to produce CXCL9 and CXCL10. We therefore evaluated the gene transcripts of CXCL9 and CXCL10 in isolated retinas from untreated, mock injected and virus infected BALB/c and CD-1 mice. Real time PCR analysis of retina mRNA revealed that gene expression for CXCL9 and CXCL10 was up regulated in both strains of mice following virus infection (Fig. 5 ). However, the relative gene expression for both chemokines was dramatically higher in BALB/c mice in comparison to CD-1 mice. For example, at day 1 PI, CXCL10 was augmented 7.4× in BALB/c mice compared to 2.3× in CD-1 mice. Likewise, at day 1 PI, CXCL9 was augmented 50× in BALB/c mice compared to 17× in CD-1 mice. These studies demonstrate that elevated chemokine transcripts in the retina correlate with serum chemokine levels in virus infected BALB/c and CD-1 mice.
Fig. 5.
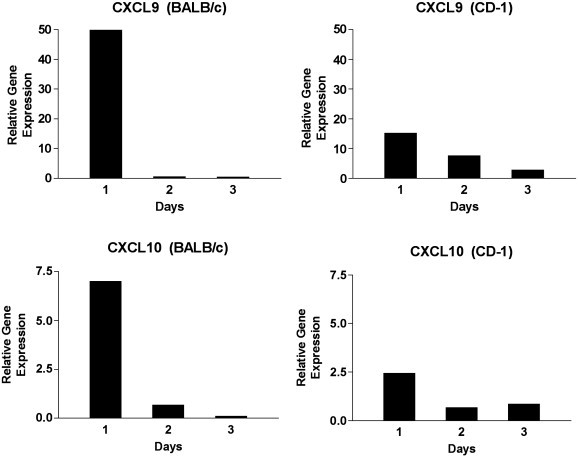
Real time RT-PCR analysis of CXCL9 and CXCL10 in mouse retina tissue. Retinas were dissected from enucleated eyes on days 1,2 and 3 PI and six to 12 retinas were pooled. The relative gene expression was determined by comparison with serially diluted standards followed by normalization to GAPDH levels and represented as a ratio.
3.5. Analysis of IFN-α and IFN-β in serum as marker of innate immunity
The type 1 IFNs, are a critical component of the innate immune response. In order to determine if there were a difference in innate immune responses between BALB/c and CD-1 mice following JHM virus infection, we evaluated serum IFN-α and IFN-β levels (Fig. 6 ). IFN-α was not detected in the sera from untreated (control) or mock injected mice. Peak levels of IFN-α were detected in the sera from virus infected mice at day 2. The levels of IFN-α produced were not significantly different between the two mouse strains. Low levels of IFN-β (4 to 5 pg/ml) were detected in some control and mock injected mice. Again, peak levels of IFN-β were detected in the sera from virus infected mice at day 2. Although higher levels of IFN-β were detected in BALB/c mice, this was not significantly different than the levels detected in CD-1 mice. It of interest to note that the production of IFN-α, IFN-β and IFN-γ all reached peak levels at day 2 PI, early during the course of the infection. This indicates that all 3 IFNs contribute to the innate immune response in MHV infection in the retina.
Fig. 6.
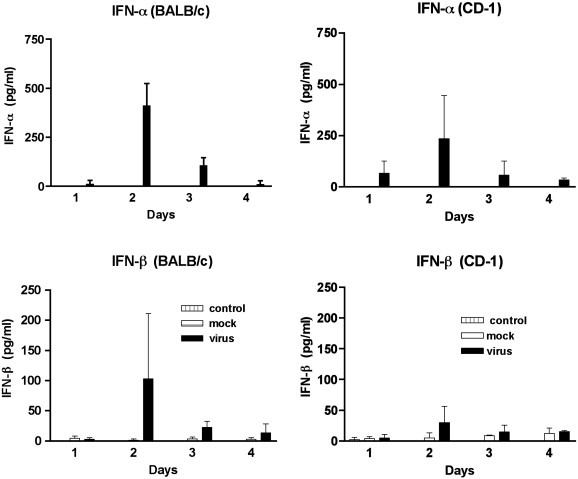
Identification of IFN-α and IFN-β in the sera of MHV infected mice by EIA. Sera from 3 mice were tested at each time point. MHV infected mice are represented by the black bars, mock injected mice are represented by the white bars and control mice are represented by the hashed bars.
4. Discussion
The findings presented herein demonstrate that an augmented innate immune response in BALB/c mice resulted in the production of elevated levels of cytokines and chemokines. High levels of IFNs and IFN inducible chemokines, CXCL9 and CXCL10, were detected in the serum and elevated levels of CXCL9 and CXCL10 gene expression were observed in retinal tissue. Although these cytokines and chemokines were demonstrated in the retinal degeneration resistant CD-1 mice, these molecules were present in significantly lower levels. The cytokine and chemokine levels peaked in each case on day 2 PI, one day prior to the infiltration of immune cells into the retina. Since these cytokines and chemokines perform critical roles in infection control and autoimmune responses, these studies contribute to our understanding of possible pathogenic mechanisms in this virus triggered retinal degeneration.
The data obtained in this study indicated that IFN-α, IFN-β, and IFN-γ all act in concert as a part of the innate immune response to MHV virus infection in the retina. This outcome was not expected and is strikingly different than what is reported in many other virus systems, including other MHV infection. In the MHV CNS model systems, IFN-α and IFN-β gene expression were detected on day 3 and IFN-γ gene expression was observed later, on day 7 (Bergmann et al., 2006). Based on the literature, we would speculate that in ECOR leukocytes and dendritic cells serve as the source of IFN-α while fibroblasts and epithelial cells may produce IFN-β. Recently, we have shown that the human retinal pigment epithelial (RPE) cells contain high levels of TLR3 and are a potent source of IFN-β within the retina (Kumar et al., 2004). It is of interest to note that the RPE cell is the first cell in which the virus replicates within the retina and hence these RPE cells may represent an important source of IFN-β. To date, the major cell types responsible for IFN-γ production are specifically sensitized T cells and NK cells (Pestka et al., 2004). Since we are detecting IFN-γ at day 2 PI, it is highly likely that the NK cell is the source of IFN-γ. In fact, in MHV infection of the CNS, NK cell recruitment occurs at day 2 in response to CXCL10 (Trifilo et al., 2004). In ECOR the type I IFNs, IFN-α and IFN-β, are produced at similar levels in both BALB/c and CD-1 mice. In contrast, significantly more IFN-γ is produced early in BALB/c mice that are infected with MHV.
In this model system, MHV proteins are first seen at day 3 within the RPE cell. Prior to this time we cannot detect viral antigens within the retina. Moreover, we do not see inflammatory cells within the retina until day 3. Macrophages are the predominant infiltrating cells. CD4 T cells are first seen at day 4 and CD8 T cells are first seen at day 6. Anti-viral neutralizing antibodies are first detected in the sera at day 6. In this report, we show that IFN-α, β, γ are all detected within the sera at day 2 PI. We detect high levels of IFN-γ and lower levels of IFN α, β. Therefore, the IFNs are detected at a time point prior to the time that we detect either new virus production or the components of the adaptive immune response (anti-neutralizing antibody, infiltrating cells). Therefore, based on these observations, we suggest that the IFNs act in concert as part of the innate immune response. Our findings are consistent with those of other investigators in which the IFNs have been reported to participate in the innate immune response (Hiscott, 2007, Wang et al., 2007).
The presence of CXCL9 and CXCL10 has been implicated as critical components in disease. In fact, several studies identified these molecules as key components of host responses in virus infections and in autoimmune diseases. CXCL9 production was also shown to be important in Theiler's virus infection (Ure et al., 2005). Neutralization of CXCL9 in a mouse CNS model system resulted in enhanced virus expression and pathology. Recent studies from Zingernagle's laboratory demonstrated that CXCL9 generation in the liver is required for autoimmune liver damage (Lang et al., 2006). In this animal model system the presence of highly activated liver-specific effector CD8 T cells alone were not sufficient to induce immune destruction. However, TLR-3 activation induced IFN-α and TNF-α and these molecules up-regulated expression of CXCL9 within the liver. This sequence of events resulted in enhanced CD8 T cell infiltration and subsequent immune destruction in the liver. These studies clearly demonstrated that if CXCL9 was not expressed within the liver, these mice did not develop autoimmune liver damage. Thus, CXCL9 was the key factor that contributed to tissue destruction. In this study we demonstrated that dramatically higher levels of CXCL9 are expressed within retinal tissue in retinal degeneration susceptible mice. It is possible that localized CXCL9 expression within the retina may be a key factor in the retinal degenerative process.
Studies on CXCL10 have demonstrated the importance of this molecule in virus infections, graft rejection and in autoimmunity. The administration of CXCL10 with antigens results in the induction of protective T cell responses to a number of pathogens and may represent a potent adjuvant effect (Krathwohl and Anderson, 2006). Moreover, CXCL10 has been shown to augment disease activity in murine models of experimental autoimmune encephalomyelitis, autoimmune sialadenitis, diabetes and adjuvant arthritis (Harasawa et al., 2006, Klein et al., 2005, Morimoto et al., 2004, Salomon et al., 2002). CXCL10 production has been reported to be a critical host response to Toxoplasma gondii infection and to virus infections of the CNS caused by MHV, HSV and West Nile virus (Khan et al., 2000, Bergmann et al., 2006, Carr et al., 2003, Klein et al., 2005). This chemokine has also been demonstrated to be important in the inflammatory responses seen in the lung in SARS patients and in the liver in chronic Hepatitis C patients (Mihm et al., 2003, Tang et al., 2005).
Intense study of the SARS coronavirus during the past 5 years has provided critical insight into key factors in the clinical and pathological features of SARS (Chen and Subbarao, 2007, Lo et al., 2006). Despite the fact that there is evidence for multiple organ involvement, the pathology of fatal cases of SARS is dominated by diffuse alveolar damage. Virus triggered cytopathic damage alone cannot explain the pathogenesis of SARS. A number of studies indicate that patients with a more vigorous immune response are at risk for poor outcome (Lo et al., 2006). One of the major contributors to this response may be chemokines. Serum levels of several chemokines, including CXCL9, CXCL10 and CCL2 are increased in SARS patients (Huang et al., 2005, Jiang et al., 2005, Wong et al., 2004). In fact, serum concentrations of CXCL10 detected early after infection have been identified as an independent prognostic indicator of disease outcome (Tang et al., 2005). A second factor that may contribute to poor outcome in patients with a more robust immune response is autoimmune reactivity (Lo et al., 2006). In the early phase of the disease, autoantibodies directed against lung epithelial cells have been observed (Yang et al., 2005). These studies suggest that immune-mediated damage triggered by coronavirus is due in part to the intensity of immune reactivity, the activation of cytokines and chemokines and the possible participation of autoimmune reactivity.
It is of interest to note that IL-10 was produced at higher levels and for longer times in the sera from BALB/c mice. IL-10 is produced by B cells and a subset of CD4 T regulatory cells and is known to be a potent down-regulator of IFN-γ production and autoimmune responses (Blenman et al., 2006, Tran et al., 2006). Since it is produced in the mice with high levels of IFN-γ, it is possible that IL-10 production is triggered in an attempt to down-regulate the robust IFN-γ response in BALB/c mice.
In this model system, sufficient levels of IFN-α, β, γ and the chemokines, CXCL9 and CXCL10, are produced to provide adequate protection from MHV spread and subsequent death. The studies presented here identify that one of the elements that IFNs contribute to host defense is the retinal production of CXCL9 and CXCL10. CXCL9 expression is a key factor in the development of autoimmune liver damage and may be one of the key factors in retinal tissue damage in retinal degeneration susceptible mice. We have recently demonstrated specific T cell reactivity to MHV in ECOR (unpublished data) and the presence of these chemokines may be important in that they may provide help in the migration of these cells to the retina for viral clearance. Additional studies are in progress to evaluate a direct role for chemokines in the degenerative process. One approach may be to delete chemokine production but this may be challenging due to the nature of the virus replication and spread. We know that eliminating the IFN-γ response results in increased virus titers and increased mortality in ECOR. The mice do not survive the acute phase (Hooks, 2003). Moreover, studies with MHV in the CNS also have demonstrated that loss of IFN-γ or CXCL10 results in increased virus replication and mortality (Dufour et al., 2002; Liu and Lane, 2001; Parra et al., 1999). Clearly, IFN-γ and the IFN-inducible chemokines are critical factors in infectious virus clearance. Therefore, strategies to down-regulate these molecules without eliminating IFN-γ and CXCL9 and CXCL10 will be necessary to evaluate pathogenic mechanisms of retinal degeneration in ECOR.
In conclusion, the presented experiments reveal that IFN-α, IFN-β and IFN-γ act in concert as part of the innate immune response to MHV infection in the retina. In this model system we have clearly identified a significant difference in the time course and levels of IFNs and chemokines in retinal degeneration susceptible BALB/c mice compared to the retinal degeneration resistant CD-1 mice. We speculate that early in the course of the retinal virus infection, the virus may initiate the progression to autoimmunity by inducing a robust immune response, high levels of IFNs, retinal tissue expression of CXCL9 and CXCL10 and the subsequent attraction of self-reactive T cells into the retina. Which component or combination of these components that induce the retinal degenerative process is not known. Additional studies are in progress to determine the key components of this system that can reverse the degenerative process.
References
- Bergmann C.C., Lane T.E., Stohlman S.A. Coronavirus infection of the central nervous system: host-virus stand-off. Nat. Rev., Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenman K.R., Duan B., Xu Z., Wan S., Atkinson M.A., Flotte T.R., Croker B.P., Morel L. IL-10 regulation of lupus in the NZM2410 murine model. Lab. Invest. 2006;86:1136–1148. doi: 10.1038/labinvest.3700468. [DOI] [PubMed] [Google Scholar]
- Carr D.J., Chodosh J., Ash J., Lane T.E. Effect of anti-CXCL10 monoclonal antibody on herpes simplex virus type 1 keratitis and retinal infection. J. Virol. 2003;77:10037–10046. doi: 10.1128/JVI.77.18.10037-10046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Subbarao K. The immunobiology of SARS. Annu. Rev. Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- Christen U., McGavern D.B., Luster A.D., von Herrath M.G., Oldstone M.B. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J. Immunol. 2003;171:6838–6845. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- Coelho A.L., Hogaboam C.M., Kunkel S.L. Chemokines provide the sustained inflammatory bridge between innate and acquired immunity. Cytokine Growth Factor Rev. 2005;16:553–560. doi: 10.1016/j.cytogfr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Dufour J.H., Dziejman M., Liu M.T., Leung J.H., Lane T.E., Luster A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195–3204. doi: 10.4049/jimmunol.168.7.3195. (Apr 1) [DOI] [PubMed] [Google Scholar]
- Harasawa H., Yamada Y., Hieshima K., Jin Z., Nakayama T., Yoshie O., Shimizu K., Hasegawa H., Hayashi T., Imaizumi Y., Ikeda S., Soda H., Soda H., Atogami S., Takasaki Y., Tsukasaki K., Tomonaga M., Murata K., Sugahara K., Tsuruda K., Kamihira S. Survey of chemokine receptor expression reveals frequent co-expression of skin-homing CCR4 and CCR10 in adult T-cell leukemia/lymphoma. Leuk. Lymphoma. 2006;47:2163–2173. doi: 10.1080/10428190600775599. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Holmes K. Coronaviruses. In: Knipe D., Howley P., editors. vol. 1. Lipincott Williams & Wilkins; Philadelphia: 2001. pp. 1187–1203. (Fields Virology). [Google Scholar]
- Hooks J.J., Chan C.C., Detrick B. Identification of the lymphokines, interferon-gamma and interleukin-2, in inflammatory eye diseases. Invest. Ophthalmol. Vis. Sci. 1988;29:1444–1451. [PubMed] [Google Scholar]
- Hooks J.J., Percopo C., Wang Y., Detrick B. Retina and retinal pigment epithelial cell autoantibodies are produced during murine coronavirus retinopathy. J. Immunol. 1993;151:3381–3389. [PubMed] [Google Scholar]
- Hooks J.J., Tso M.O., Detrick B. Retinopathies associated with antiretinal antibodies. Clin. Diagn. Lab. Immunol. 2001;8:853–858. doi: 10.1128/CDLI.8.5.853-858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks J.J., Wang Y., Detrick B. The critical role of IFN-gamma in experimental coronavirus retinopathy. Invest. Ophthalmol. Vis. Sci. 2003;44:3402–3408. doi: 10.1167/iovs.02-1106. [DOI] [PubMed] [Google Scholar]
- Hooper L.C., Chin M.S., Detrick B., Hooks J.J. Retinal degeneration in experimental coronavirus retinopathy (ECOR) is associated with increased TNF-alpha, soluble TNFR2 and altered TNF-alpha signaling. J. Neuroimmunol. 2005;166:65–74. doi: 10.1016/j.jneuroim.2005.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J., Zhou C., Wu Z., Zhong S., Liu J., Luo W., Chen T., Qin Q., Deng P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- Khan I.A., MacLean J.A., Lee F.S., Casciotti L., DeHaan E., Schwartzman J.D., Luster A.D. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity. 2000;12:483–494. doi: 10.1016/s1074-7613(00)80200-9. [DOI] [PubMed] [Google Scholar]
- Klein R.S., Lin E., Zhang B., Luster A.D., Tollett J., Samuel M.A., Engle M., Diamond M.S. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komurasaki Y., Nagineni C.N., Wang Y., Hooks J.J. Virus RNA persists within the retina in coronavirus-induced retinopathy. Virology. 1996;222:446–450. doi: 10.1006/viro.1996.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl M.D., Anderson J.L. Chemokine CXCL10 (IP-10) is sufficient to trigger an immune response to injected antigens in a mouse model. Vaccine. 2006;24:2987–2993. doi: 10.1016/j.vaccine.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Kumar M.V., Nagineni C.N., Chin M.S., Hooks J.J., Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004;153:7–15. doi: 10.1016/j.jneuroim.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang K.S., Georgiev P., Recher M., Navarini A.A., Bergthaler A., Heikenwalder M., Harris N.L., Junt T., Odermatt B., Clavien P.A., Pircher H., Akira S., Hengartner H., Zinkernagel R.M. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Invest. 2006;116:2456–2463. doi: 10.1172/JCI28349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.T., Lane T.E. Chemokine expression and viral infection of the central nervous system: regulation of host defense and neuropathology. Immunol. Res. 2001;24(2):111–119. doi: 10.1385/IR:24:2:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A.W., Tang N.L., To K.F. How the SARS coronavirus causes disease: host or organism? J. Pathol. 2006;208:142–151. doi: 10.1002/path.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff B., Luster A. Chemokines and chemokine receptor analysis. In: Detrick B., Hamilton R., Folds J., editors. vol. 7. ASM Press; Washington, DC: 2006. pp. 371–384. (Manual of Molecular and Clinical Laboratory Immunology). [Google Scholar]
- Mihm S., Schweyer S., Ramadori G. Expression of the chemokine IP-10 correlates with the accumulation of hepatic IFN-gamma and IL-18 mRNA in chronic hepatitis C but not in hepatitis B. J. Med. Virol. 2003;70:562–570. doi: 10.1002/jmv.10431. [DOI] [PubMed] [Google Scholar]
- Morimoto J., Yoneyama H., Shimada A., Shigihara T., Yamada S., Oikawa Y., Matsushima K., Saruta T., Narumi S. CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis. J. Immunol. 2004;173:7017–7024. doi: 10.4049/jimmunol.173.11.7017. [DOI] [PubMed] [Google Scholar]
- Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Parra B., Hinton D.R., Marten N.W., Bergmann C.C., Lin M.T., Yang C.S., Stohlman S.A. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 1999;162:1641–1647. [PubMed] [Google Scholar]
- Pestka S., Krause C.D., Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Robbins S.G., Hamel C.P., Detrick B., Hooks J.J. Murine coronavirus induces an acute and long-lasting disease of the retina. Lab. Invest. 1990;62:417–426. [PubMed] [Google Scholar]
- Salomon I., Netzer N., Wildbaum G., Schif-Zuck S., Maor G., Karin N. Targeting the function of IFN-gamma-inducible protein 10 suppresses ongoing adjuvant arthritis. J. Immunol. 2002;169:2685–2693. doi: 10.4049/jimmunol.169.5.2685. [DOI] [PubMed] [Google Scholar]
- Tang N.L., Chan P.K., Wong C.K., To K.F., Wu A.K., Sung Y.M., Hui D.S., Sung J.J., Lam C.W. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin. Chem. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E.H., Azuma Y.T., Chen M., Weston C., Davis R.J., Flavell R.A. Inactivation of JNK1 enhances innate IL-10 production and dampens autoimmune inflammation in the brain. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13451–13456. doi: 10.1073/pnas.0601155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilo M.J., Montalto-Morrison C., Stiles L.N., Hurst K.R., Hardison J.L., Manning J.E., Masters P.S., Lane T.E. CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J. Virol. 2004;78:585–594. doi: 10.1128/JVI.78.2.585-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ure D.R., Lane T.E., Liu M.T., Rodriguez M. Neutralization of chemokines RANTES and MIG increases virus antigen expression and spinal cord pathology during Theiler's virus infection. Int. Immunol. 2005;17:569–579. doi: 10.1093/intimm/dxh236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinores S.A., Wang Y., Vinores M.A., Derevjanik N.L., Shi A., Klein D.A., Detrick B., Hooks J.J. Blood–retinal barrier breakdown in experimental coronavirus retinopathy: association with viral antigen, inflammation, and VEGF in sensitive and resistant strains. J. Neuroimmunol. 2001;119:175–182. doi: 10.1016/S0165-5728(01)00374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace G.R., John Curnow S., Wloka K., Salmon M., Murray P.I. The role of chemokines and their receptors in ocular disease. Prog. Retin. Eye Res. 2004;23:435–448. doi: 10.1016/j.preteyeres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Wang Y., Detrick B., Hooks J.J. Coronavirus (JHM) replication within the retina: analysis of cell tropism in mouse retinal cell cultures. Virology. 1993;193:124–137. doi: 10.1006/viro.1993.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Burnier M., Detrick B., Hooks J.J. Genetic predisposition to coronavirus-induced retinal disease. Invest. Ophthalmol. Vis. Sci. 1996;37:250–254. [PubMed] [Google Scholar]
- Wang J.P., Kurt-Jones E.A., Finberg R.W. Innate immunity to respiratory viruses. Cell. Microbiol. 2007;9:1641–1646. doi: 10.1111/j.1462-5822.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W., Wu A.K., Ip W.K., Lee N.L., Chan I.H., Lit L.C., Hui D.S., Chan M.H., Chung S.S., Sung J.J. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.H., Huang Y.H., Chuang Y.H., Peng C.M., Wang L.C., Lin Y.T., Chiang B.L. Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection. J. Med. Virol. 2005;77:1–7. doi: 10.1002/jmv.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]


