Abstract
Fecal hormone assays provide a powerful tool for noninvasive monitoring of endocrine status in wild animals. In this study we validated a protocol for extracting and measuring glucocorticoids in free-living and captive Belding’s ground squirrels (Spermophilus beldingi). We first compared two commonly used extraction protocols to determine which performed better with commercially available antibodies. We next verified the preferred extraction method by correlating circulating and fecal glucocorticoid measures from a group of individuals over time. For this comparison, we used both a cortisol and a corticosterone antibody to determine which had greater affinity to the fecal metabolites. Cortisol was the primary circulating glucocorticoid, but both hormones were present in well above detectable concentrations in the blood, which does not occur in other sciurids. In addition, the cortisol antibody showed greater binding with the fecal extracts than did the corticosterone antibody. Finally, we used adrenocorticotropic hormone and dexamethasone challenges to demonstrate that changes in adrenal functioning are reflected in changing fecal corticoid levels. These results suggest that our extraction protocol provides a fast, reliable assay of stress hormones in free-living ground squirrels without the confounding influence of short-term rises in glucocorticoid concentrations caused by handling and restraint stress and that it can facilitate ecological and evolutionary studies of stress in wild species.
Introduction
Steroid hormones from the adrenal glands regulate many facets of an organism’s homeostasis, including responses to environmental perturbations. In addition to regulating energy storage and circadian rhythms, adrenal glucocorticoids protect the body during and after the stress response (here defined as the cumulative physiological reactions triggered by unpredictable events). Stress-induced functions of glucocorticoids include increasing available glucose, improving cardiovascular tone, and inhibiting gastrointestinal, reproductive, and immune systems. The associated cascade of hormones through the blood, known as the hypothalamic-pituitary-adrenal (HPA) axis, is activated by a wide variety of environmental and social stressors, in particular, exposure to novelty and lack of predictability or controllability of important events (for a review see Sapolsky 1992).
Behavioral ecologists are interested in how the stressors faced by free-living animals affect their physiology and their reproductive success. Acute stress is triggered by unpredictable events such as a predator attack, sudden severe weather, or agonistic social interactions, whereas chronic stress stems from long-term adverse environmental conditions such as unpredictable decreased food supply or low or high social status (Morton and Sherman 1978; Kotrschal et al. 1998; Lima 1998; Cavigelli 1999; Hubbs et al. 2000; Creel 2001; Goymann et al. 2001; Abbott et al. 2003; Sands and Creel 2004), and both types of stress can have deleterious effects on survival or reproduction. Knowledge of stress hormones is also important from a conservation perspective in order to evaluate, for example, management strategies, population declines, relocation or reintroduction, or the impact of habitat disturbance (Boonstra and Singleton 1993; Graham and Brown 1996; Wasser et al. 1997; Wingfield et al. 1997; Mashburn and Atkinson 2004; von der Ohe et al. 2004). Research on stress in free-living animals has been aided greatly by the development of techniques to monitor glucocorticoids noninvasively, often using fecal corticoid metabolites. Hormone samples can be acquired with minimal or no contact with study animals, reducing human impact on the populations and eliminating transient increases in circulating glucocorticoid levels as a result of handling (see Whitten et al. 1998 for a review).
The tribe Marmotini of the subfamily Sciurinae, which includes marmots, prairie dogs, and ground squirrels, has long been the focus of behavioral ecological research (Murie and Michener 1984), with studies on dispersal, mating strategies, antipredator behaviors, nepotism, population dynamics, and circannual cycles, among others. These species exhibit a wide range of social systems and live in very diverse habitats. Because they are also sedentary and diurnal, these animals are excellent study species for investigations of behavioral endocrinology in the field. However, to date there has been limited work on sciurid endocrinology, and it has all involved blood sampling (Boswell et al. 1994; Arnold and Dittami 1997; Boonstra and McColl 2000; Millesi et al. 2000; Nunes et al. 2000; Boonstra et al. 2001; Hackländer et al. 2003). Here we validate an extraction protocol for fecal glucocorticoids in Belding’s ground squirrels (Spermophilus beldingi) that may be generalizable to other species of ground-dwelling squirrels.
Spermophilus beldingi are diurnal social rodents that live in alpine and subalpine habitats throughout the Sierra Nevada and southern Cascade mountains. They are socially active aboveground between April and August and hibernate for the remainder of the year, with females producing just one litter each summer. Spermophilus beldingi experience a variety of potential stressors, both acute and chronic. Their predators include coyotes (Canis latrans), badgers (Taxidea taxus), long-tailed weasels (Mustela frenata), and various species of raptors (Buteo, Accipiter, Falco). Predators evoke two types of alarm calls from adult squirrels, each of which elicits a different rapid physiological and behavioral response in listeners (Mateo 1996a, 1996b). Other sources of stress include attempted infanticide, agonism during mating competition, low food availability, and exposure to unfamiliar locations and conspecifics during natal or breeding dispersal (Sherman and Morton 1984; J. M. Mateo, personal observation). Spermophilus beldingi live in heterogeneous habitats ranging from sagebrush to high alpine meadows, so the particular stressors they encounter depend in part on their local ecology. Our current research focuses on geographic differences in microhabitats and predation pressures experienced by Belding’s ground squirrels and how these differences affect their antipredator behaviors. In particular we are examining how stress hormones mediate not only their responses to predators but also their acquisition of antipredator strategies. Preliminary data suggest that natural selection has favored hormonal profiles during early development that facilitate rapid learning of locally adapted responses (J. M. Mateo, unpublished data).
Research on adrenal functioning in ground-dwelling squirrels has included adrenal gland weights of shot animals and measurements of blood glucocorticoid levels in live animals. Many species secrete both cortisol and corticosterone; in round-tailed (Spermophilus tereticaudus) and California (Spermophilus beecheyi) ground squirrels, corticosterone is reported to be the major circulating glucocorticoid (Vanjonack et al. 1975; Petrovic and Janic-Sibalic 1976; but see Adams 1972). For golden-mantled (Spermophilus lateralis and Spermophilus saturatus), arctic (Spermophilus parryii), and Columbian (Spermophilus columbianus) ground squirrels, as well as yellow-pine chipmunks (Tamias amoenus, a species of ground-dwelling squirrel in a different genus), cortisol is the main circulating glucocorticoid (Boswell et al. 1994; Boonstra and McColl 2000; Hubbs et al. 2000; Kenagy and Place 2000; Place and Kenagy 2000; Boonstra et al. 2001; K. E. Holekamp, private communication). Note, however, that researchers have used a variety of methods to quantify sciurid glucocorticoids, preventing generalization across species. Glucocorticoids have not yet been quantified for S. beldingi, but in this species adrenal glands are largest about 1 mo after emergence from hibernation (while females are lactating) and decrease sharply in size during the remainder of the active season (McKeever 1963). Sex differences in hormone concentrations and patterns of hormonal changes during the season vary by species (Boswell et al. 1994; Kenagy and Place 2000; Place and Kenagy 2000; Boonstra et al. 2001). In general, glucocorticoids are excreted in urine or feces primarily as conjugated or unconjugated metabolites of varying polarity (Graham and Brown 1996; Monfort et al. 1998; Whitten et al. 1998; Goymann et al. 1999; Wallner et al. 1999) and are probably hydroxysteroids in ground-dwelling squirrels (Florant and Weitzman 1980).
As part of a long-term project on stress and predation pressure in free-living S. beldingi, we sought to verify that fecal concentrations of glucocorticoid metabolites accurately reflect physiological functioning of the HPA axis. We first compared two widely used extraction protocols to determine which method was better for quantifying S. beldingi fecal corticoids (study 1). We next evaluated whether fecal metabolite levels reflect unmanipulated circulating concentrations of glucocorticoids (study 2). Finally, we determined whether short-term changes in circulating glucocorticoid concentration are reflected in fecal samples. We administered exogenous corticotrophin (adrenocorticotropin hormone [ACTH], a glucocorticoid stimulant) or dexamethasone (DEX; a glucocorticoid agonist) and looked for associated elevations or suppressions in fecal corticoid measures during subsequent days (study 3). Because both cortisol and corticosterone are produced by most mammalian species (Bentley 1976), we used commercial antibodies to measure circulating levels of both glucocorticoids within individuals. In addition, we compared the effectiveness of both antibodies in binding to fecal metabolites and thus in serving as a proxy for circulating glucocorticoid concentrations. To our knowledge, this is the first direct comparison of how cortisol and corticosterone antibodies respond to serum and fecal extracts from the same animal.
Material and Methods
Animals
We collected fecal and serum samples from adult Spermophilus beldingi during the summers of 2002 and 2004 at and near the Sierra Nevada Aquatic Research Laboratory (SNARL; near Mammoth Lakes, CA; administered by the University of California, Santa Barbara). For study 1, fecal samples were collected from free-living adults at a site in Rock Creek Canyon, Mono County, California (see Mateo 1996a for details). For studies 2 and 3, S. beldingi were collected from various populations within 100 km of SNARL and were housed individually in a laboratory building at SNARL in standard plastic cages (38 cm × 33 cm × 18 cm; solid sides and bottom, wire top) with pine shavings and paper towels for bedding material. Animals were given ≈15 g Purina mouse chow (#5015) per animal per day and water ad lib. and were occasionally provided with vegetables and sunflower seeds. The building was maintained on a 13L : 11D schedule, with temperature regulated by a combination of a heater and automatic fans. These studies were approved by Cornell University’s Center for Research Animal Resources (4/20/00; 00-32), University of Chicago’s Institutional Animal Care and Use Committee (11/26/02; 71255), and University of California, Santa Barbara’s Animal Resource Center (3/30/00; 4-00-532) and adhere to standards set forth by the National Institutes of Health for animal research.
Fecal Collection
Ground squirrels typically defecate when handled, so to acquire fecal samples, animals were held with a gloved hand over a clean table or bucket. If animals did not immediately defecate, they were placed in a clean trap set inside a clean plastic bucket until they defecated. Fecal pellets were collected immediately with clean tweezers and transferred to polypropylene microcentrifuge tubes (Cole Parmer, Vernon Hills, IL). Feces contaminated with urine (i.e., visibly wet and/or in a pool of urine) were discarded (see Cavigelli et al. 2005). Tweezers, traps, tables, and buckets were cleaned with Cide-All germicidal detergent (Chemifax, Santa Fe Springs, CA). Samples were stored immediately at −15°C and then transferred to −80°C storage at the end of the field season (4–6 wk later). All fecal pellets collected from an individual during a given sampling period were thoroughly mixed before extraction.
Serum Collection
For blood collection, an animal’s home cage was removed from the cage rack, and the animal was grabbed by hand and lightly anesthetized with isoflurane inhalant (10–20 s of exposure). Blood (300–400 μL) was collected from a femoral vein, typically within 3 min of moving an animal’s cage (range 100–270 s, with 85% of samples collected within 200 s of initial movement). An increase in circulating glucocorticoid concentrations in response to stress is not detectable in rodents for approximately 2–3 min (Sapolsky 1992); therefore, our blood collections likely represent baseline values. Ground squirrels were then returned to their home cage and monitored until recovery from anesthesia (within a few minutes). Samples were refrigerated for 1 h before being centrifuged, and serum was stored at −15° C for 4–6 wk, after which it was stored at −80° C until assayed.
Fecal Corticoid Extraction
Methods 1 and 2
These two methods involved a variant of the rapid extraction procedure developed by Rupert Palme (Palme et al. 2000; R. Palme, private communication). Samples were thawed and placed in a drying oven (95°C) for 4–6 h to evaporate the water. Dried feces were crushed, and 0.2 g was weighed into a microcentrifuge tube. Next, 1.5 mL of 80% (method 1) or 100% (method 2) ethanol was added to each tube, which was briefly vortexed (≈3 s) and immediately centrifuged (2,500 g, 20 min). Supernatants were reserved and frozen (−80° C) until assayed. We included 100% ethanol in the study to determine whether it increased recovery of metabolites over 80% ethanol (Teskey-Gerstl et al. 2000) and to facilitate comparison with method 3.
Method 3
Fecal steroids were extracted using previously published methods (Wasser et al. 1994). Briefly:, frozen samples were thawed, dried overnight in a centrifugal evaporator, and crushed into a dustlike material, and then 0.2 g was weighed into a 15-mL centrifuge tube. Next, 10 mL of 100% ethanol was added to each sample, and tubes were boiled in a water bath for 20 min. Upon removal from the bath, tubes were centrifuged for 15 min, and the supernatant was poured off into a glass tube. Then 5 mL of ethanol was added to each tube, and tubes were vortexed for 1 min and recentrifuged for 15 min. Supernatants were combined in the appropriate tube and evaporated under air. Dried extracts were reconstituted with 1 mL methanol and stored at −80° C until assayed.
Hormone Assays
We used commercially available double-antibody 125I-corticosterone and 125I-cortisol Corticote radioimmunoassay (RIA) kits (ICN Biomedicals, Costa Mesa, CA; MP Biomedicals, Irvine, CA). For fecal samples in studies 2 and 3a analyzed with the Corticote kit, 25 μL of steroid-free human serum (ICN Biomedicals) was added to each sample tube to provide a medium similar in protein content to the serum standards and S. beldingi serum samples (R. Gilmartin, private communication). All samples were assayed in duplicate and reanalyzed if the coefficient of variation between duplicates exceeded 20%. Two control samples, each made by pooling fecal extracts from five animals, were analyzed in every assay (the “low” pool, approximately 60%–70% binding, and the “high” pool, approximately 20%–30% binding). Based on repeated analyses (n = 5) of the low and high pools, intra- and interassay coefficients of variation for the assays were 5.05% and 7.34%, respectively, for the low pool, and 7.22% and 6.25%, respectively, for the high pool. Serum glucocorticoid concentrations are presented as nanograms per milliliter. Fecal corticoid concentrations were corrected for dilution ratios and divided by the proportion of isotope recoveries and are expressed as nanograms per gram of dry feces.
Study 1: Comparison of Extraction Protocols
To evaluate two commonly used extraction methods, we first compared the three protocols described above (methods 1–3). We assessed each method for parallelism, accuracy, and recovery efficiency. Donors were free-living animals collected from one site (n = 4 males and 1 female); the males had regressed testes, and the female was lactating. Feces were collected during morning observations, typically between 0800 and 1100 hours. These five individuals each produced enough feces for three extractions, one with each method. From each extraction method we created a pooled sample. Thus, each individual’s feces were extracted with each of the three methods, and pooled samples from five individuals for each method were then created.
Parallelism
To determine the parallelism of fecal extracts, the pooled samples were serially diluted with the steroid diluent provided with the corticosterone RIA kit (1 : 2 to 1 : 1,024 for corticosterone antibody) or with the zero standard provided with the cortisol RIA kit (4 : 1 to 1 : 1,024 for cortisol antibody) to compare the slope of the antibody binding to that of the standards supplied with the RIA kits. ANCOVA was used to test whether sample slopes were parallel to the standard slopes. Methods 1–3 were assessed with the corticosterone antibody; method 1 was assessed with the cortisol antibody after we determined it was the preferred extraction method (see “Results”).
Accuracy
To determine whether the extract medium interfered with antibody binding, recovery of a known concentration of glucocorticoids was calculated in the presence of fecal extract containing endogenous hormone. Twenty-five microliters of the corticosterone or cortisol standard provided with the RIA kits (0–0.125 ng and 0–15 μg, respectively) was added to separate tubes, each containing 25 μL of the pooled extract. Extracts were diluted 1 : 200 with the steroid diluent provided with the corticosterone kit for the corticosterone antibody and left undiluted for the cortisol antibody. Methods 1–3 were assessed with the corticosterone antibody and methods 1 and 2 with the cortisol antibody.
Recovery efficiency
To determine if extraction methods 1 and 3 had similar procedural recovery, we spiked dry fecal samples (0.2 g) with a known amount of 125I-corticosterone or 125I-cortisol. Fecal samples from 12 free-living adult S. beldingi were used for each method and for each steroid antibody.
Study 2: Relationship between Basal Concentrations of Serum and Fecal Glucocorticoids
The results of study 1 indicated that the steroid extraction procedures and antibodies available with the commercial RIA kits for cortisol and corticosterone provide a means for measuring glucocorticoid-metabolite levels in feces of Belding’s ground squirrels. Our next goal was to determine whether these fecal measures accurately reflect circulating glucocorticoid concentrations (as they do in some birds, carnivores, ungulates, and primates; Graham and Brown 1996; Palme and Mostl 1997; Wasser et al. 1997; Boinski et al. 1999; Cavigelli 1999). This step was important to confirm that our extraction methods detected fecal metabolites functionally related to circulating glucocorticoids. If an animal secretes significant quantities of both cortisol and corticosterone, one may be predominant in the blood while the other is more readily metabolized and/or excreted in feces. Such results may be a consequence of the range of metabolite binding to each RIA-kit antibody or may reflect the fact that one of the two hormones is preferentially excreted in feces or urine in a given study species. To identify the better commercial RIA antibody for binding to glucocorticoid metabolites in ground-squirrel feces, we used both cortisol and corticosterone antibodies. This study also allowed us to determine the primary circulating glucocorticoid secreted by ground squirrels (see also Boonstra et al. 2001 for a discussion of measurement techniques and predominant glucocorticoids in ground-dwelling squirrels).
Blood and feces were collected four times from each of 16 temporarily captive adult S. beldingi (n = 11 males and 5 females). The samples were collected during the postreproductive season (after mating, gestation, and lactation) to minimize the effects of sex differences and seasonal variation on HPA functioning (McKeever 1963; Boswell et al. 1994; Hubbs et al. 2000; Kenagy and Place 2000; Boonstra et al. 2001). Males had regressed testes, and females were not lactating (i.e., had no swollen or extended nipples).
Animals were trapped from four populations and housed at SNARL in the laboratory building for at least 10 d before the study began. Rates of alarm calling and retreat into nest boxes in response to humans decline quickly during the first 10 d, and the behaviors of animals do not change significantly after this time (J. M. Mateo and S. A. Cavigelli, personal observation). Furthermore, in other species, glucocorticoid concentrations increase after animals are placed in captive housing or transferred to a different housing environment, but the levels return to baseline within 10 d (Hansen and Damgaard 1993; Manzo et al. 1994; Hennessy et al. 1997). Based on these observations we expected that the stress induced by captivity would be attenuated before the study started, with little change thereafter.
Serum glucocorticoids are secreted in a circadian fashion, with highest levels at the beginning of the active period and declining over the course of the day. Because individual variation in glucocorticoid levels is most pronounced during the nadir (Whitten et al. 1998), all blood samples were collected between 1700 and 1900 hours. Freshly excreted feces were also collected at that time of day, 24 h before blood collection. We collected feces before blood to avoid potential spikes in fecal corticoid levels resulting from handling, restraint, and venipuncture. Although fecal and blood samples within a given sampling period represent different time points of circulating corticoids, corticoid levels within each sample type remained fairly constant across the study period (see “Results”). This allowed us to average the values of each sample type for each individual and then examine the data for correlations between serum and fecal corticoid levels. Thus, during a 17-d period, animals were sampled once every 5 d, for a total of four fecal and four blood samples for each animal. We sampled animals four times rather than just once to obtain a better representation of the variation in fecal and serum levels.
Glucocorticoid metabolites were extracted from feces using method 1 with 80% ethanol. Fecal extracts were diluted 1 : 5 with the kit’s steroid diluent and serum samples diluted 1 : 10 before assaying with the corticosterone antibody, and all samples within a sample type were assayed together in random order. A subset of serum and fecal samples (n = 7 males and 4 females that had sufficient serum remaining) was analyzed with the cortisol RIA kit. To determine which concentration approximated 50% binding with the Corticote kit, we conducted a pilot study with serial dilutions (1 : 2 to 1 : 1,024 using the steroid diluent provided with the corticosterone kit) with a serum sample pooled from five captive individuals and a fecal sample pooled from the same five individuals. Concentrations were barely detectable at 1 : 2 dilution (equal amounts extracted sample and diluent), so both sample types were subsequently assayed without dilution (but with steroid-free serum in fecal samples to control for protein differences).
The fecal and serum glucocorticoid values for each individual were averaged and then analyzed with Pearson correlations (data did not depart significantly from normality) followed by simple linear regression to test the significance of the slope of the line (t-statistic). Because of sex differences in metabolism of glucocorticoids in some rodents (Eriksson and Gustafsson 1970; Touma et al. 2003; Cavigelli et al. 2005), we examined the relationship between serum and fecal corticoids separately by sex with ANOVA. We also used ANOVA to examine possible differences in corticoid levels as a function of population of origin. Changes in both serum and fecal concentrations over time were analyzed with repeated-measures ANOVA.
Study 3: Sensitivity of Fecal Hormone Assay to ACTH and DEX Challenges
The final validation procedure involved monitoring fecal corticoid levels following exogenous administration of ACTH, DEX, and saline (as a control). Although the results of study 2 revealed a correlation between basal concentrations of serum corticoids and fecal metabolites, it was necessary to demonstrate a temporal correlation between changes in adrenal activity and changes in glucocorticoid-metabolite levels in feces. In large mammals, an increase in fecal metabolite levels can be detected approximately 24–48 h after an ACTH challenge (Graham and Brown 1996; Monfort et al. 1998; Goymann et al. 1999; Wasser et al. 2000; Brown et al. 2001; Millspaugh et al. 2002; Wielebnowski et al. 2002; Mashburn and Atkinson 2004; Young et al. 2004). In smaller mammals (<150 g), fecal glucocorticoid-metabolite levels typically increase within 24 h of stimulation of the adrenal glands or injection of labeled glucocorticoids (e.g., 6–12 h in house mice, deer mice, and red-backed voles [Harper and Austad 2000]; 8–24 h in common marmosets [Bahr et al. 2000]; 20–44 h in black-footed ferrets [Young et al. 2001]; 30 h in chinchillas [Ponzio et al. 2004]).
We conducted two replicates of this study, which we call studies 3a and 3b. Animals from study 2 were used in study 3a (ACTH: n = 3 males and 2 females; DEX: n = 3 males and 2 females; saline: n = 4 males and 1 female). Individually housed animals had been in captivity for ≈30 d before sampling, which began 2 d after cages were cleaned. Cages were opened in the morning for feeding, but otherwise the animals were left undisturbed except for fecal collection. Feces were collected daily between 1600 and 1800 hours, starting 48 h before injection and continuing for 96 h after injection. Day of injection is referred to as day 0, with fecal collection beginning on day 2 and continuing to day 4.
On day 0 of fecal collection, each ground squirrel was given an injection of either (1) 4 IU/kg of a synthetic ACTH preparation intramuscularly (ACTH group; Cortrosyn suspended in gelatin; Wedgewood Pharmacy, Sewell, NJ), (2) 1.25 mg/kg dexamethasone subcutaneously (DEX group; Wedgewood Pharmacy; doses following Boonstra and McColl 2000 and Bamberg et al. 2001), or (3) 0.1 mL 0.90% NaCl subcutaneously (saline group). Treatments occurred between 1600 and 1700 hours in order to control for time-of-day effects on metabolism of ACTH and DEX (see also Touma et al. 2003). Although neither ACTH nor DEX had saline as a vehicle, the saline group served as a control for the handling and injection stress of treatment. For use with the corticosterone kits, fecal samples were diluted 1 : 5 with RIA diluent; for use with the cortisol kits, they were left undiluted. In study 3a, for each hormone, samples were analyzed over two assays, with all seven samples for each individual run in random order within an assay.
Study 3b was conducted with higher dosages, a larger sample size, and a more frequent sampling schedule. Animals in this study were nonreproductive adults derived from four populations (ACTH: n = 6 males and 3 females; DEX: n = 8 males and 2 females; saline: n = 4 males and 1 female). For this replicate, all preparations were given subcutaneously (ACTH: 200 μg/kg; DEX: 2 mg/kg; saline: 0.1 mL 0.90% NaCl). Eight fecal samples from each individual were collected over 78 h, typically once every 12 h at 0700 and 1900 hours, starting with hour 0, when injections were administered. Extracts were measured only with the cortisol antibody, and because no direct comparison was made with serum samples or with a corticosterone antibody, steroid-free serum was not added to the fecal samples. All samples were analyzed over two assays, with all eight samples for each individual run in random order within an assay.
For both replicates, animals were assigned to groups using a random number generator, with sexes assigned separately to ensure nearly equal distribution across the groups. Fecal glucocorticoid measures were analyzed with a repeated-measures ANOVA separately for each treatment (data did not depart significantly from normality). Animals were not weighed, except on injection day 0, in order to minimize handling time and potential resulting stress responses.
Results
Study 1: Comparison of Extraction Methods
Corticosterone antibody
Fecal samples with antibody binding on the nonlinear portion of the standard curve (<20% or >80% binding) were excluded. Serial dilutions yielded displacement curves parallel to standard corticosterone (F3, 11 =0.926, P > 0.40; Fig. 1a). When corticosterone was added to the pooled fecal samples, the majority of corticosterone was recovered (one-sample t-test on the slopes; t2 =0.911, P > 0.50; Fig. 2a). Method 1 (n =12 ) recovered an average of 90.92% ± 1.62% of added corticosterone. Method 3 recovered 91.99% ± 0.90% of added corticosterone (t11 =−0.747, P > 0.10).
Figure 1.
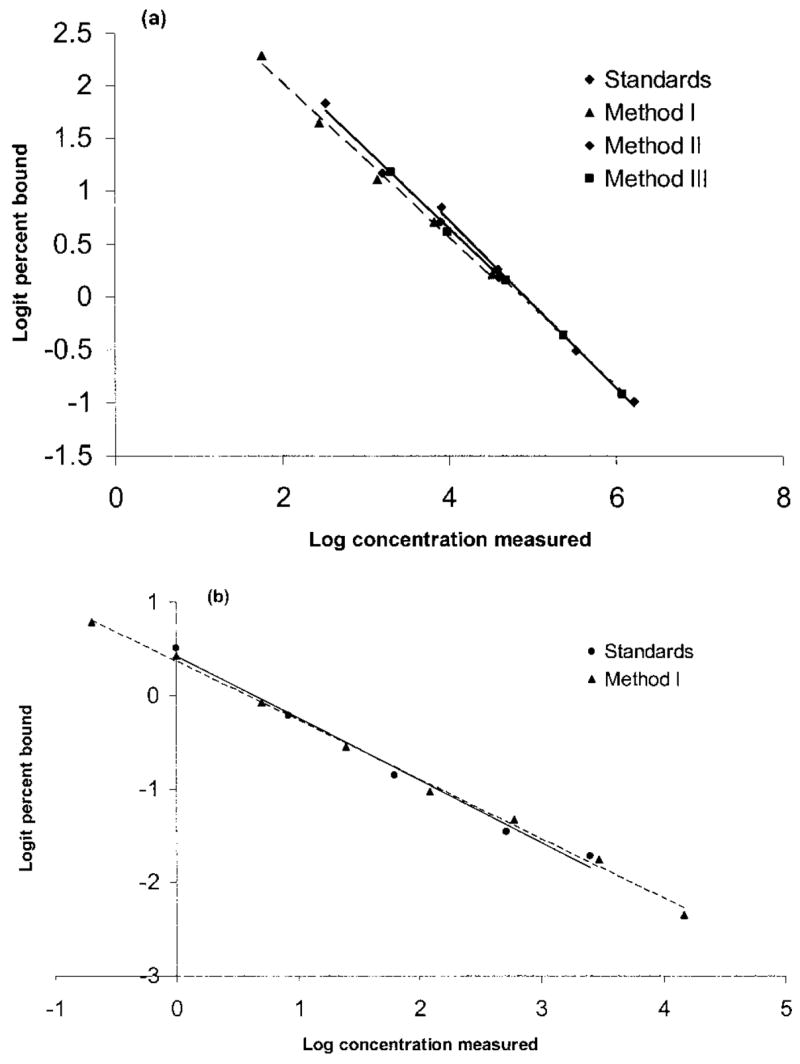
a, Parallelism of fecal corticoid-metabolite levels using three extraction protocols and a commercial corticosterone antibody. Standards (circles): y = −0.7938x + 3.9015, r2 = 0.9956; method 1 (triangles): y = −0.7327x + 3.4872, r2 = 0.9932; method 2 (diamonds): y = −0.7464x + 3.6413, r2 = 0.9905; method 3 (squares): y = −0.7471x + 3.6343, r2 = 0.999. b, Parallelism of fecal corticoid-metabolite levels using a commercial cortisol antibody. Standards (circles): y = −0.6665x + 0.4264, r2 = 0.9883; method 1 (triangles): y = −0.6367x + 0.3701, r2 = 0.9966.
Figure 2.
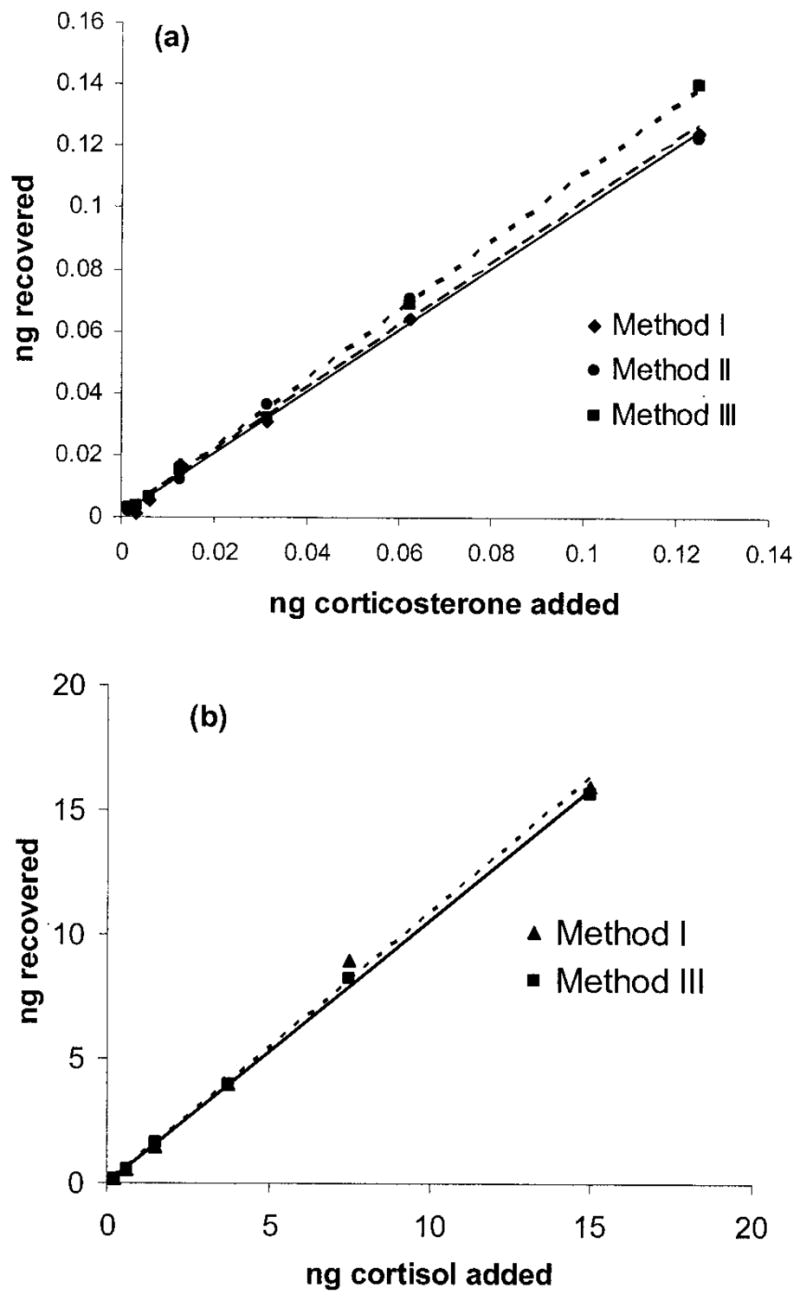
Accuracy of extraction procedures as assessed through quantitative recovery of exogenous corticoids from fecal extracts. a, Slopes of pooled samples extracted with three different protocols and spiked with standards provided with the corticosterone RIA kit. Method 1 (triangles): y = 0.9931x + 0.4752, r2 = 0.9979; method 2 (circles): y = 1.0002x + 0.3133, r2 = 0.993; method 3 (squares): y = 1.1118x + 0.0028, r2 = 0.9993. b, Slopes of pooled samples extracted with two different protocols and spiked with standards provided with the cortisol RIA kit. Method 1 (triangles): y = 1.0886x − 0.0012, r2 = 0.9954; method 3 (squares): y = 1.0556x + 0.0006, r2 = 0.9991.
Based on these results as well as several replicates of the parallelism and accuracy assays, we decided to use method 1 with 80% ethanol for the remaining extractions. Methods 1 and 2 are significantly less time-consuming than method 3 (approximately 25 min per sample vs. >5 h), and the extraction protocols produce extracts that perform equally well with the commercial corticosterone RIA kit. We found little difference between the results of methods 1 and 2, which suggested that the higher concentration of ethanol did not affect extraction efficiency. Following previously published extraction protocols (see “Methods 1 and 2” above), we therefore used 80% ethanol for the cortisol antibody in study 1 and for all extractions in studies 2 and 3.
Cortisol antibody
After determining that cortisol is the main circulating glucocorticoid (see study 2), we examined how well a commercial cortisol antibody performed with the fecal extracts. A serial dilution with the pooled extracts using method 1 yielded a displacement curve parallel to the cortisol standards (F 1, 9 =0.652, P > 0.40; Fig. 1b). When cortisol was added to the pooled fecal extracts using methods 1 and 3, the majority of it was recovered both times (Fig. 2b). As indicators of recovery efficiency, method 1 (n =12) recovered an average of 89.2% ± 1.50% of added cortisol, and method 3 recovered 83.90% ± 1.50% (t11 =−2.666, P < 0.03).
Study 2: Relationship between Basal Concentrations of Serum and Fecal Glucocorticoids
Corticosterone antibody
There was no significant positive correlation between serum corticosterone concentrations and the handling time required to draw blood from animals, which indicated that concentrations did not increase significantly between first movement of an animal’s cage and completion of blood collection (range 100–270 s; r =−0.069, P > 0.60, n =48) and therefore likely reflect baseline values. Fecal corticoid-metabolite levels correlated significantly with serum corticosterone levels: r =0.552, P < 0.03, n =16 (regression t =2.48, P < 0.01; Fig. 3a). There were no significant sex or population differences in either serum or fecal corticoid-metabolite concentrations. For each of the four samples per individual (blood and fecal collections every 5 d), serum and fecal corticoid concentrations generally correlated (sample 1: r =0.390, P < 0.20, n =13; sample 2: r =0.634, P < 0.05, n =10; sample 3: r =0.615, P < 0.04, n =12; sample 4: r = 0.559, P < 0.05, n =13). Individuals’ fecal metabolite concentrations did not change significantly across the study (repeated-measures ANOVA: F3, 39 =1.236, P > 0.30, n =14), and neither did serum corticosterone concentrations (F3, 33 =0.375, P > 0.70, n =12).
Figure 3.
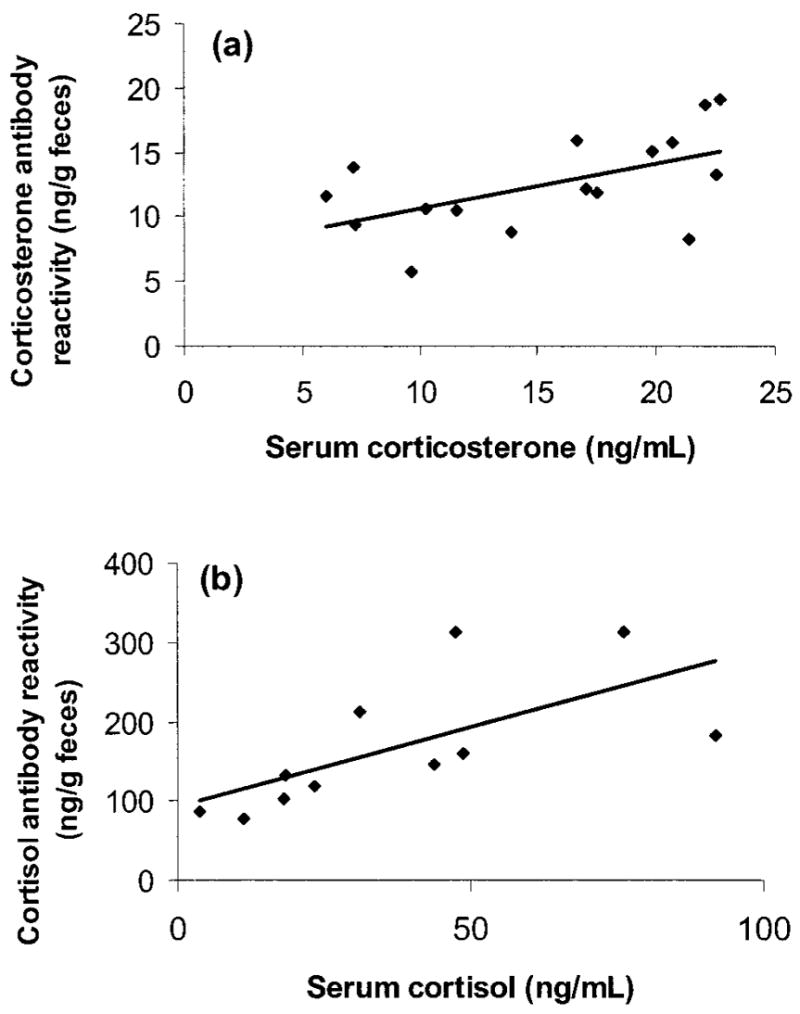
Correlation between serum and fecal corticoid values in captive Spermophilus beldingi, (a) measured with corticosterone antibody (n =48 samples from 16 animals; y =0.3458x + 7.2055) and (b) measured with cortisol antibody (n =24 samples from 11 animals; y =2.0055x + 92.088).
Cortisol antibody
There was no significant positive correlation between serum corticosterone concentrations and the handling time required to draw blood from animals, which indicated that concentrations did not increase significantly between first movement of an animal’s cage and completion of blood collection (range 100–270 s; r = −0.199, P > 0.40, n =24) and therefore likely reflect baseline levels. Fecal corticoid concentrations correlated significantly with serum cortisol concentrations: r =0.668, P < 0.03, n =11 (regression t =2.70, P < 0.05; Fig. 3b). There were no significant sex or population differences in either serum or fecal corticoid-metabolite concentrations. There were insufficient samples within each of the four sampling periods to examine changes in cortisol levels over time.
In Spermophilus beldingi, cortisol appears to be the predominant circulating glucocorticoid. In addition, the commercial cortisol antibody showed greater binding of metabolites in the fecal extracts than did the corticosterone antibody (paired t-tests; serum: t10 =3.284, P < 0.01; feces: t10 =6.35, P < 0.0001; Fig. 4).
Figure 4.
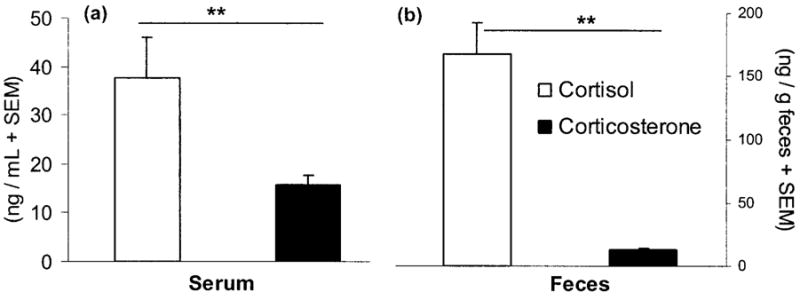
Mean (+SEM) serum (a) and fecal (b) corticoid concentrations in captive Spermophilus beldingi, measured with cortisol (open bars) and corticosterone (filled bars) antibodies. Lines over bars represent significant differences. Double asterisks indicate P < 0.01.
Study 3: Sensitivity of Fecal Hormone Assay to ACTH and DEX Challenges
Study 3a: Corticosterone antibody
Analyses of fecal metabolites were restricted to the 48-h period starting just before injection (labeled days 0–2). There was a nearly significant increase in corticoid-metabolite levels as a result of ACTH treatment: F2, 8 =3.192, P =0.09. One animal maintained high concentrations throughout the collection period (>3 SEM from the ACTH mean on all days; see Fig. 5a), and if that individual is omitted from the analysis, ACTH treatment resulted in a significant increase in corticosterone-metabolite levels from day 0 to day 1 and a decrease from day 2 to day 3 (overall F2, 6 =5.824, P < 0.04; Fig. 6a; post hoc two-tailed general linear contrasts, CMATRIX command in Systat, both P < 0.05). Similarly, DEX treatment tended to suppress corticoid excretion (F2, 8 =3.402, P =0.08). One animal had very high concentrations of corticosterone on day 0 for reasons that are unknown (it may have escaped briefly during fecal collection on the preceding day), with a value almost 4 SEM higher than the DEX mean on day 0 and values 2–3 SEM from the mean on all other days except days 3 and 4 (Fig. 5b). If that individual is eliminated from the analysis as an outlier, DEX led to a significant reduction in fecal corticosterone-metabolite levels (F2, 6 =53.564, P < 0.0001; Fig. 6a). Corticoid concentrations declined from day 0 to day 1 (P < 0.0001) and increased moderately from day 1 to day 2 (P =0.068). Saline treatment did not significantly influence concentrations of corticoids (F2, 8 =0.045, P > 0.90; Figs. 5c, 6a). There were no significant effects of sex, population, or treatment assignment on fecal corticoid concentrations on day 0. Similar results were obtained when days −2 to 0 were averaged as a baseline and compared to days 1 and 2.
Figure 5.
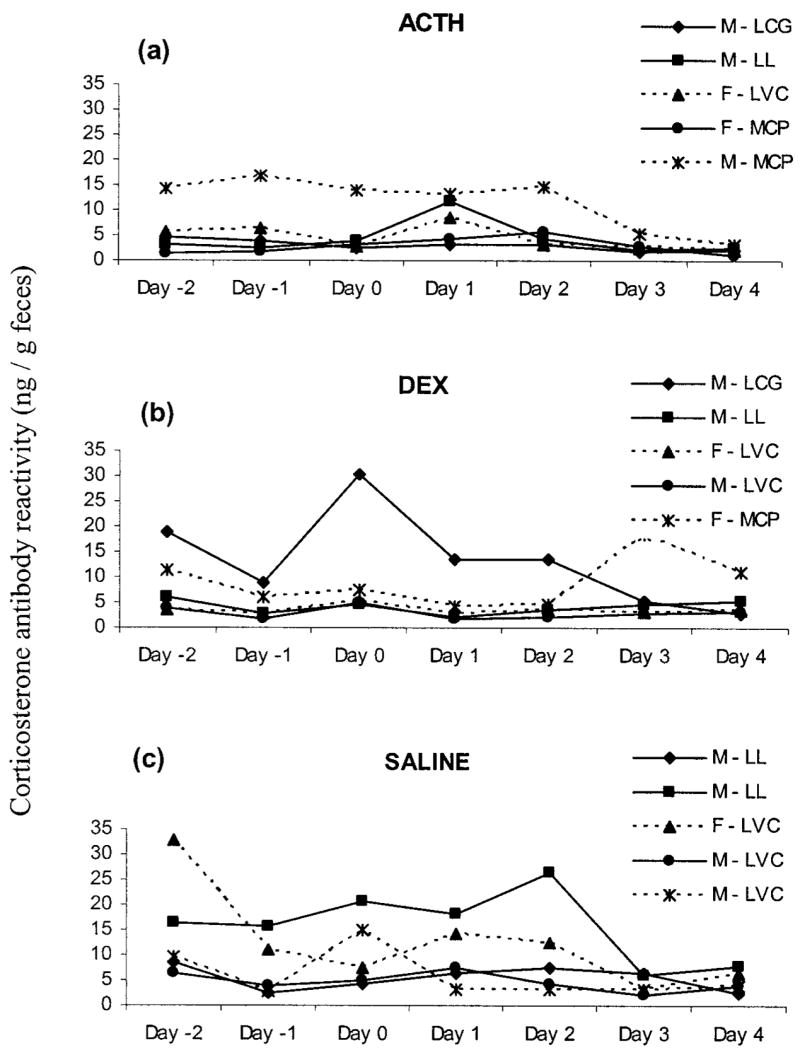
Fecal corticoid concentrations measured with corticosterone antibody of individual captive Spermophilus beldingi before and after injection with ACTH (a), DEX (b), or saline (c). Day 0 is the day of injection. Keys indicate the individual’s sex followed by its site of origin (LCG =Lundy Campground; LL =Lundy Lake; LVC =Lee Vining Campground; MCP =Mono County Park).
Figure 6.
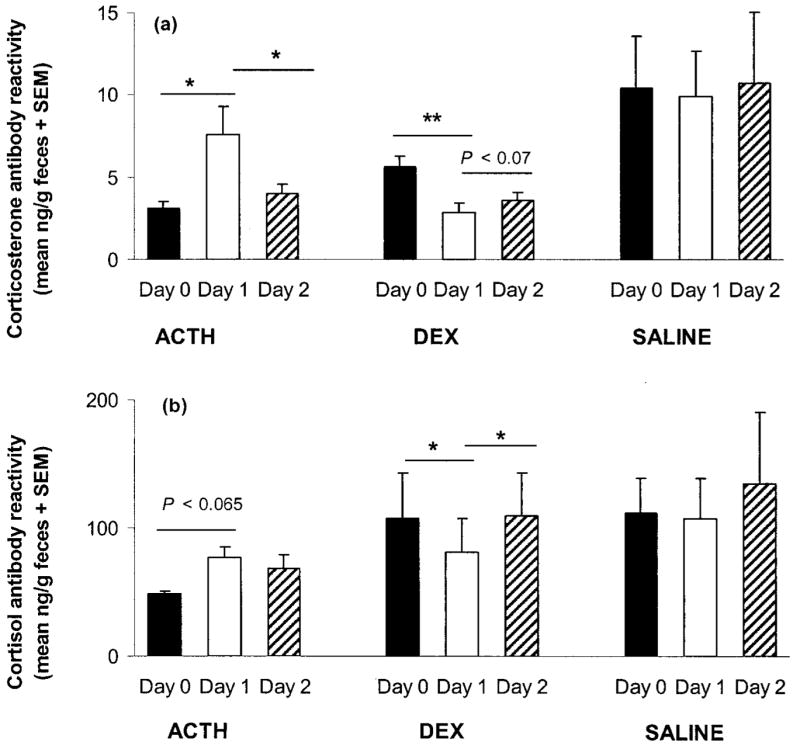
Mean (+SEM) fecal corticoid concentrations in captive Spermophilus beldingi treated with ACTH, DEX, or saline, (a) measured with corticosterone antibody and (b) measured with cortisol antibody. Day 0 is the day of injection. One outlier from the ACTH group and one from the DEX group were excluded from these figures (see text). Lines over columns indicate significant differences. Asterisks and double asterisks indicate P < 0.05 and P < 0.01, respectively.
Study 3a: Cortisol antibody
In general, results were similar to those of the corticosterone assay. Overall there was no significant increase in fecal corticoid concentrations as a result of ACTH treatment: F2, 8 =0.170, P > 0.80. As was evident for the corticosterone assay, one animal maintained high concentrations throughout the collection period (>3 SEM away from the ACTH group mean on all days except day 3; Fig. 7a), and with this individual omitted from the analysis there was a nearly significant increase in fecal corticoid concentrations following ACTH administration (F2, 6 =4.537, P < 0.065; Fig. 5b), with a moderate increase from day 0 to day 1. Similarly, DEX treatment led to lowered fecal corticoid concentrations after injection: F2, 8 =3.592, P < 0.08 (Fig. 6b). If the animal with unusually high concentrations on day 0 is eliminated from the analysis as an outlier (>3 SEM away from the ACTH group mean on all days; Fig. 7b), then DEX did have a significant influence on fecal corticoid concentrations (F2, 6 =9.793, P < 0.05). Fecal corticoid concentrations declined from day 0 to day 1 and increased moderately from day 1 to day 2 (both P < 0.05; Fig. 6b). Treatment with saline had no significant influence on concentrations of fecal corticoids (F2, 4 =1.099, P > 0.40; Figs. 7c, 5b; only three animals had sufficient fecal samples remaining for all 3 d of analysis). Again, there were no significant effects of sex, population, or treatment assignment on fecal corticoid concentrations on day 0. Similar results were obtained when days −2 to 0 were averaged as a baseline and compared to days 1 and 2.
Figure 7.
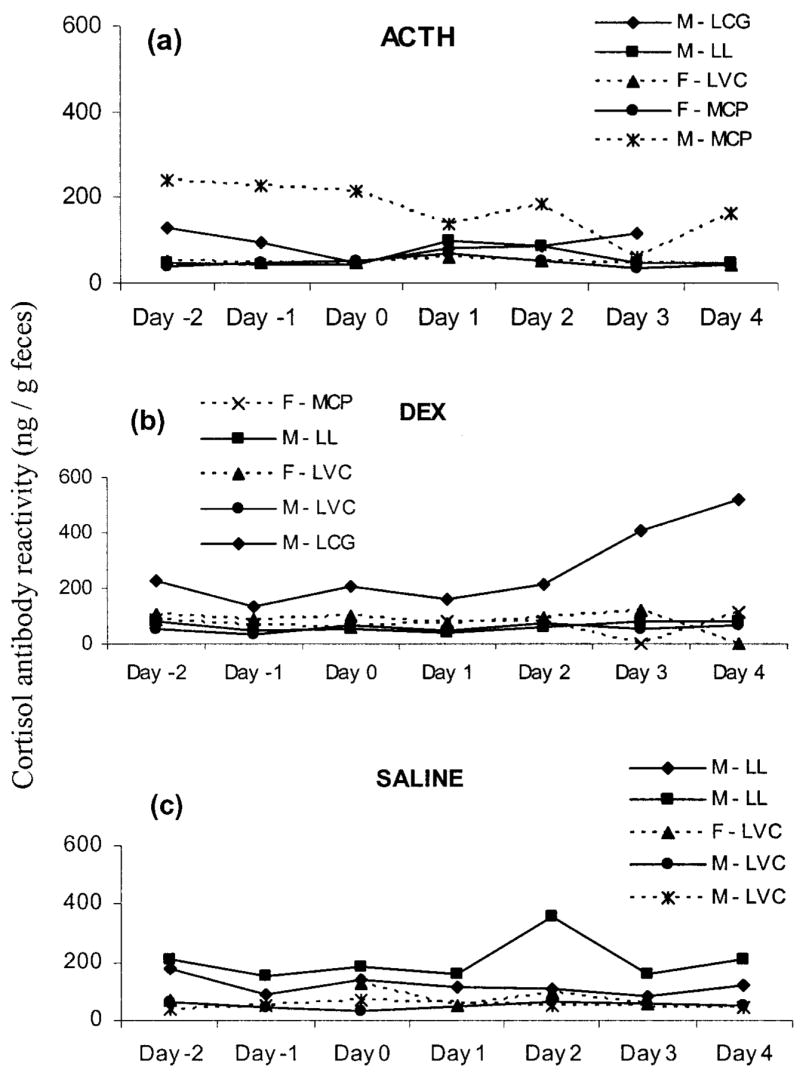
Fecal corticoid concentrations of individual captive Spermophilus beldingi measured with cortisol antibody before and after injection with ACTH (a), DEX (b), or saline (c). Day 0 is the day of injection. Keys indicate the individual’s sex followed by its site of origin (LCG =Lundy Campground; LL =Lundy Lake; LVC =Lee Vining Campground; MCP =Mono County Park).
Study 3b: Cortisol antibody
Fecal corticoid concentrations changed significantly over the sampling period as a result of ACTH administration (F7, 56 =2.687, P < 0.02; Fig. 8a). In particular, concentrations increased between hours 18 and 30 after injection and decreased between hours 30 and 66 (two-tailed general linear contrasts, CMATRIX command in Systat, both P < 0.05). DEX treatment significantly decreased concentrations of fecal corticoids (F7, 63 =3.841, P < 0.01; Fig. 8b), with values attenuating significantly from hours 6 to 18 and 30 to 42 and increasing from hours 66 to 78 (all P < 0.05). Animals injected with saline showed no significant changes in fecal corticoid concentrations (F 7, 28 =0.942, P > 0.40; Fig. 8c). There were no significant effects of sex, population, or treatment assignment on fecal corticoid concentrations at hour 0.
Figure 8.
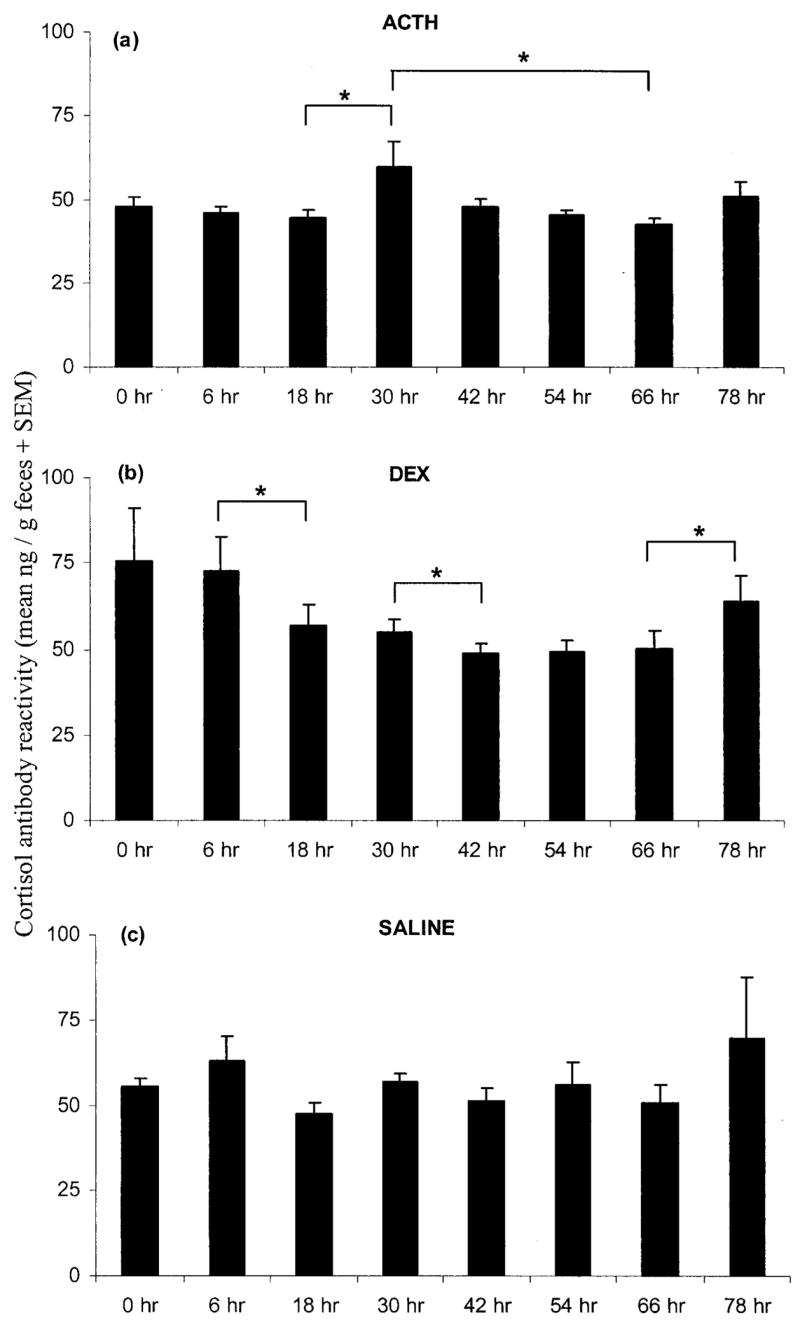
Mean (+SEM) fecal corticoid concentrations measured with cortisol antibody in captive Spermophilus beldingi treated with ACTH (a), DEX (b), or saline (c). Hour 0 is the time of injection, with sampling continuing every 6 or 12 h. Brackets over columns indicate significant differences between the two end columns. Asterisks indicate P < 0.05.
Discussion
The three studies described here present a physiological validation of methods for noninvasively assessing adrenal activity. Together they indicate that extraction of fecal glucocorticoid metabolites provides a measure that reflects circulating adrenal hormone levels in free-living Belding’s ground squirrels. We tested several methods for extracting steroid metabolites from feces and found that methods 1, 2, and 3 performed equally well when using the RIA corticosterone and cortisol antibodies, based on tests of parallelism, accuracy, and recovery (Figs. 1, 2). Given these results, and because method 1 is significantly quicker than method 3 (<0.5 h vs. >5 h), we chose to use method 1 for all subsequent extractions in this and other studies.
Excreted hormones represent cumulative hours of variation in circulating concentrations of hormones, so fecal concentrations may not always accurately reflect serum concentrations over time. Yet for both cortisol and corticosterone antibodies, we found that fecal metabolite concentrations correlated positively with serum concentrations (Fig. 3). We did not measure the concentrations of corticosteroid- or cortisol-binding globulins, so we do not know what percentage of the circulating glucocorticoids was free as opposed to bound (Boonstra and McColl 2000), which is information that would assist in evaluating the physiological effects of the two hormones. For serum, binding to the cortisol antibody was higher than binding to the corticosterone antibody (Fig. 4a). For feces, it was also important to determine which antibody binds better to excreted metabolites. In Spermophilus beldingi, the cortisol antibody showed greater affinity for the fecal glucocorticoid metabolites than did the corticosterone antibody (Fig. 4b). Indeed, there was a stronger correlation between serum and fecal cortisol measures than between corticosterone measures, which suggests that there are more cortisol metabolites in S. beldingi feces than there are corticosterone metabolites (although such a conclusion would have to be verified with high-performance liquid chromatography [HPLC] studies). Once it is determined that fecal hormone levels reliably reflect serum hormone levels (Graham and Brown 1996; Palme and Mostl 1997; Whitten et al. 1998; Boinski et al. 1999; Cavigelli 1999; this study), then non-invasive fecal monitoring can be used to compare basal levels across populations (which may differ in predation pressure, social stability, or food availability) or between environments (e.g., free-living vs. captive animals, breeding vs. overwintering sites).
Fecal hormone assays have revealed stress- or ACTH-induced changes in glucocorticoid levels in a variety of mammalian species (Graham and Brown 1996; Monfort et al. 1998; Goymann et al. 1999; Wallner et al. 1999; Millspaugh et al. 2002; Young et al. 2004; see also references in Whitten et al. 1998). We conducted two such “challenge” studies with S. beldingi to verify that changes in adrenal functioning are reflected temporally in fecal corticoid concentrations. In the first study, most, but not all, individuals showed an increase in fecal glucocorticoid concentrations following an ACTH challenge and a decrease following a DEX challenge, both evident within 24 h of treatment (Figs. 5, 7). In ACTH and DEX studies with hyenas, common marmosets, and ferrets, some nonresponsive individuals were also observed (Goymann et al. 1999; Saltzman et al. 2000; Young et al. 2001). A nonresponsive profile may reflect a history of chronic stress (e.g., competition for territories, unpredictable weather, or low food availability) or an alteration of glucocorticoid receptors. In this study, animals receiving saline did not show a transient rise in fecal corticoid levels as a result of injection, which suggests that the effects of ACTH and DEX were not simply the result of handling and injection stress. Fecal corticoid concentrations in study 3a were higher among the saline group than the ACTH or DEX groups, but the reason for this is unclear, since animals were assigned to groups randomly. Regardless, there was no significant difference in fecal corticoid concentrations on day 0 (the day of treatment) across the three groups, in part because of the high individual variation among the saline-group animals (Figs. 5c, 7c).
Because not all individuals responded to either ACTH or DEX treatment in the first challenge study, we repeated the study with higher dosages, more subjects, and more frequent sampling. In this replicate, individuals showed a clear change in fecal metabolite concentrations 30–66 h after injection with ACTH or 18–78 h after DEX administration (Fig. 8). Although the magnitude of S. beldingi responses to drug treatment was lower than that reported in other studies (Graham and Brown 1996; Monfort et al. 1998; Goymann et al. 1999; Wielebnowski et al. 2002; Mashburn and Atkinson 2004; Ponzio et al. 2004; Young et al. 2004), this may be simply a result of the particular dosages used. Here, ACTH increased fecal metabolite concentrations 0.5–2.5 times, and DEX reduced metabolite concentrations 0.25–0.5 times relative to baseline values, revealing modest but significant physiological responses to the treatments. These responses are similar to the moderate effects of ACTH reported for some ferrets, rhinoceroses, and chinchillas (Brown et al. 2001; Young et al. 2001; Ponzio et al. 2004). As additional studies utilize exogenous glucocorticoid stimulants or suppressors, patterns of differential response as a function of species, population, sex, or individual will become more clear (Hubbs et al. 2000; Teskey-Gerstl et al. 2000; Brown et al. 2001; Goymann et al. 2001; Hik et al. 2001; Young et al. 2001).
Our results indicate that S. beldingi produce both cortisol and corticosterone in detectable concentrations in serum and that commercial RIA kits detect products in feces that reflect these circulating cortisol and corticosterone concentrations. Spermophilus beldingi differ somewhat from other sciurids that have only one glucocorticoid in detectable concentrations in blood (Adams 1972; Boswell et al. 1994; Kenagy and Place 2000; Place and Kenagy 2000; Boonstra et al. 2001). Mammals produce both cortisol and corticosterone, with the former being the predominant circulating corticoid. Rats, mice, and some rabbits are an exception to this rule, since they lack the enzyme (17-hydroxylase) necessary to convert progesterone to cortisol (Bentley 1976). Despite the recent upsurge in studies of glucocorticoids in a wide range of taxonomic groups, few investigators have conducted formal comparisons of cortisol and corticosterone concentrations within a species, and to our knowledge this is the first study to examine the two glucocorticoids in both serum and feces.
Based on our results, one could measure either cortisol or corticosterone in S. beldingi (e.g., plasma corticosterone used by Nunes et al. 2002), but since the cortisol antibody showed greater binding of both serum cortisol and fecal corticoids, its use may yield greater resolution than use of the corticosterone antibody. That is, the cortisol antibody may be more likely to reveal increases or decreases in glucocorticoid concentrations resulting from social status, reproductive condition, or predation pressure, for example, or to reveal differences among experimental groups or among multiple free-living populations. From a methodological perspective, there are several issues one could examine to further understand the physiological functioning of ground-squirrel glucocorticoids, including quantification of free hormones and corticosteroid- and cortisol-binding globulin (Boonstra et al. 2001), the effects of storage and drying methods on fecal steroid measures (Khan et al. 2002; Terio et al. 2002; Beehner and Whitten 2004), preferred antibody choice (Goymann et al. 1999; Wasser et al. 2000), the effect of anesthesia on fecal concentrations (Mashburn and Atkinson 2004; Young et al. 2004), and use of HPLC and gas chromatography–mass spectrometry to identify specific fecal metabolites detected by radio- and enzymeimmunoassay techniques (Wasser et al. 2000; Bamberg et al. 2001; Touma et al. 2003; Mashburn and Atkinson 2004; Young et al. 2004; Cavigelli et al. 2005). Finally, the applicability of this extraction protocol to other steroid hormones, such as testosterone or estradiol, could be determined using the approach outlined here.
Measurement of stress hormones can be inherently difficult if the assessment procedure itself stresses an animal. Fecal quantification of adrenal hormones is a powerful alternative for noninvasive monitoring of free-living animals’ responses to environmental and social stressors. Short of catheterization in captivity, which can also be stressful, it is the only way to measure glucocorticoid levels without the confounding influence of increased stress caused by handling and restraint during blood collection. Our current studies use the methods validated here to examine developmental and geographic differences in free-living S. beldingi cortisol concentrations, in particular focusing on the role of stress in the learning of antipredator behaviors (McEwen and Sapolsky 1995; Mateo 1996a). Fecal corticoid assays will be especially useful when knowledge of physiological responses to stressors facilitates prediction or interpretation of behavioral responses to stressors, such as when testing hypotheses about population dynamics, predator-prey relationships, trade-offs between reproduction and survival, circannual rhythms of torpor and arousal, and conservation strategies. We encourage field researchers to consider adoption of this increasingly tractable approach to behavioral endocrinology.
Acknowledgments
We thank J. Bruck, M. Heintz, A. Janas, E. Malamud, M. Nelson, and C. Pitt for their assistance in collection of the samples, Rhonda Gilmartin and Bill Richardson for advice on the RIA kits, Steve Monfort and Rupert Palme for discussions of extraction protocols, and Nancy Peters and anonymous reviewers for comments on an earlier version of the manuscript. J.M.M. especially thanks S.A.C. for her patient tutoring in extraction and assaying techniques. This research was funded by the National Institutes of Health (grant R01 MH63921-01A1).
Literature Cited
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, et al. Are subordinates always stressed? a comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Adams L. Evaluation of an in vitro technique for quantitative assay of adrenocortical secretion in the California ground squirrel. J Wildl Dis. 1972;8:10–18. doi: 10.7589/0090-3558-8.1.10. [DOI] [PubMed] [Google Scholar]
- Arnold W, Dittami J. Reproductive suppression in male alpine marmots. Anim Behav. 1997;53:53–66. [Google Scholar]
- Bahr NI, Palme R, Moehle U, Hodges JK, Heistermann M. Comparative aspects of the metabolism and excretion of cortisol in three individual nonhuman primates. Gen Comp Endocrinol. 2000;117:427–438. doi: 10.1006/gcen.1999.7431. [DOI] [PubMed] [Google Scholar]
- Bamberg E, Palme R, Meingassner JG. Excretion of corticosteroid metabolites in urine and faeces of rats. Lab Anim. 2001;35:307–314. doi: 10.1258/0023677011911886. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Whitten PL. Modifications of a field method for fecal steroid analysis in baboons. Physiol Behav. 2004;82:269–277. doi: 10.1016/j.physbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Bentley PJ. Comparative Vertebrate Endocrinology. Cambridge University Press; New York: 1976. [Google Scholar]
- Boinski S, Swing SP, Gross TP, Davis JK. Environmental enrichment of brown capuchins (Cebus apella): behavioral and plasma and fecal cortisol measures of effectiveness. Am J Primatol. 1999;47:49–68. doi: 10.1002/(SICI)1098-2345(1999)48:1<49::AID-AJP4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Boonstra R, Hubbs AH, Lacey EA, McColl CJ. Seasonal changes in glucocorticoid and testosterone concentrations in free-living arctic ground squirrels from the boreal forest of the Yukon. Can J Zool. 2001;79:49–58. [Google Scholar]
- Boonstra R, McColl CJ. Contrasting stress response of male arctic ground squirrels and red squirrels. J Exp Zool. 2000;286:390–404. [PubMed] [Google Scholar]
- Boonstra R, Singleton GR. Population declines in the snowshoe hare and the role of stress. Gen Comp Endocrinol. 1993;91:126–143. doi: 10.1006/gcen.1993.1113. [DOI] [PubMed] [Google Scholar]
- Boswell T, Woods SC, Kenagy GJ. Seasonal changes in body mass, insulin, and glucocorticoids of free-living golden-mantled ground squirrels. Gen Comp Endocrinol. 1994;96:339–346. doi: 10.1006/gcen.1994.1189. [DOI] [PubMed] [Google Scholar]
- Brown JL, Bellen AC, Fouraker M, Wildt DE, Roth TL. Comparative analysis of gonadal and adrenal activity in the black and white rhinoceros in North America by non-invasive endocrine monitoring. Zoo Biol. 2001;20:463–486. [Google Scholar]
- Cavigelli SA. Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim Behav. 1999;57:935–944. doi: 10.1006/anbe.1998.1054. [DOI] [PubMed] [Google Scholar]
- Cavigelli SA, Monfort SL, Whitney TK, Mechref YS, Novotny M, McClintock MK. Frequent serial fecal corticoid measures from rats reflect circadian and ovarian corticosterone rhythms. J Endocrinol. 2005;184:153–163. doi: 10.1677/joe.1.05935. [DOI] [PubMed] [Google Scholar]
- Creel SR. Social dominance and stress hormones. Trends Ecol Evol. 2001;16:491–497. [Google Scholar]
- Eriksson H, Gustafsson J. Steroids in germfree and conventional rats: distribution and excretion of labeled pregnenolone and corticosterone in male and female rats. Eur J Biochem. 1970;15:132–139. doi: 10.1111/j.1432-1033.1970.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Florant GL, Weitzman ED. Diurnal and episodic pattern of plasma cortisol during fall and spring in young and old woodchucks (Marmota monax) Comp Biochem Physiol. 1980;66A:575–581. [Google Scholar]
- Goymann W, East ML, Wachter B, Honer OP, Mostl E, Van’t Hof TJ, Hofer H. Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyenas. Proc R Soc Lond B Biol Sci. 2001;268:2453–2459. doi: 10.1098/rspb.2001.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goymann W, Mostl E, Van’t Hof T, East ML, Hofer H. Noninvasive fecal monitoring of glucocorticoids in spotted hyenas, Crocuta crocuta. Gen Comp Endocrinol. 1999;114:340–348. doi: 10.1006/gcen.1999.7268. [DOI] [PubMed] [Google Scholar]
- Graham LH, Brown JL. Cortisol metabolism in the domestic cat and implications for non-invasive monitoring of adrenocortical function in endangered felids. Zoo Biol. 1996;15:71–82. [Google Scholar]
- Hackländer K, Möstl E, Arnold W. Reproductive suppression in female Alpine marmots, Marmota marmota. Anim Behav. 2003;65:1133–1140. [Google Scholar]
- Hansen SW, Damgaard BM. Behavioural and adrenocortical coping strategies and the effect on eosinophil leucocyte level and heterophil/lymphocyte-ratio in beech marten (Martes foina) Appl Anim Behav Sci. 1993;35:369–388. [Google Scholar]
- Harper JM, Austad SN. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool. 2000;73:12–22. doi: 10.1086/316721. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Davis HN, Williams MT, Mellott C, Douglas CW. Plasma cortisol levels of dogs at a county animal shelter. Physiol Behav. 1997;62:485–490. doi: 10.1016/s0031-9384(97)80328-9. [DOI] [PubMed] [Google Scholar]
- Hik DS, McColl CJ, Boonstra R. Why are Arctic ground squirrels more stressed in the boreal forest than in alpine meadows? Ecoscience. 2001;8:275–288. [Google Scholar]
- Hubbs AH, Millar JS, Wiebe JP. Effect of brief exposure to a potential predator on cortisol concentrations in female Columbian ground squirrels (Spermophilus columbianus) Can J Zool. 2000;78:578–587. [Google Scholar]
- Kenagy GJ, Place NJ. Seasonal changes in plasma glucocorticoids of free-living female yellow-pine chipmunks: effects of reproduction and capture and handling. Gen Comp Endocrinol. 2000;117:189–199. doi: 10.1006/gcen.1999.7397. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. Gen Comp Endocrinol. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Kotrschal K, Hirschenhauser K, Mostl E. The relationship between social stress and dominance is seasonal in greylag geese. Anim Behav. 1998;55:171–176. doi: 10.1006/anbe.1997.0597. [DOI] [PubMed] [Google Scholar]
- Lima SL. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Stud Behav. 1998;27:215–290. [Google Scholar]
- Manzo C, Zerani M, Gobbetti A, Di Fiore MM, Angelini F. Is corticosterone involved in the reproductive processes of the male lizard, Podarcis sicula sicula? Horm Behav. 1994;28:117–129. doi: 10.1006/hbeh.1994.1009. [DOI] [PubMed] [Google Scholar]
- Mashburn KL, Atkinson S. Evaluation of adrenal function in serum and feces of Steller sea lions (Eumetopias jubatus): influences of molt, gender, sample storage, and age on glucocorticoid metabolism. Gen Comp Endocrinol. 2004;136:371–381. doi: 10.1016/j.ygcen.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Mateo JM. The development of alarm-call response behaviour in free-living juvenile Belding’s ground squirrels. Anim Behav. 1996a;52:489–505. doi: 10.1006/anbe.1996.0446. [DOI] [PubMed] [Google Scholar]
- Mateo JM. Early auditory experience and the ontogeny of alarm-call discrimination in Belding’s ground squirrels (Spermophilus beldingi) J Comp Psychol. 1996b;110:115–124. doi: 10.1037/0735-7036.110.2.115. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- McKeever S. Seasonal changes in body weight, reproductive organs, pituitary, adrenal glands, thyroid gland, and spleen of the Belding ground squirrel (Citellus beldingi) Am J Anat. 1963;113:153–173. doi: 10.1002/aja.1001130111. [DOI] [PubMed] [Google Scholar]
- Millesi E, Huber S, Pieta K, Walzl M, Arnold W, Dittami J. Estrus and estrogen changes in mated and unmated free-living European ground squirrels. Horm Behav. 2000;37:190–197. doi: 10.1006/hbeh.2000.1574. [DOI] [PubMed] [Google Scholar]
- Millspaugh JJ, Washburn BE, Milanick MA, Beringer J, Hansen LP, Meyer TM. Non-invasive techniques for stress assessment in white-tailed deer. Wildl Soc Bull. 2002;30:899–907. [Google Scholar]
- Monfort SL, Mashburn KL, Brewer BA, Creel SR. Evaluating adrenal activity in African wild dogs (Lycaon pictus) by fecal corticosteroid analysis. J Zoo Wildl Med. 1998;29:129–133. [PubMed] [Google Scholar]
- Morton ML, Sherman PW. Effects of a spring snowstorm on behavior, reproduction, and survival of Belding’s ground squirrels. Can J Zool. 1978;56:2578–2590. [Google Scholar]
- Murie JO, Michener GR. The Biology of Ground-Dwelling Squirrels: Annual Cycles, Behavioral Ecology, and Sociality. University of Nebraska Press; Lincoln: 1984. [Google Scholar]
- Nunes S, Muecke EM, Holekamp KE. Seasonal effects of food provisioning on body fat, insulin, and corticosterone in free-living juvenile Belding’s ground squirrels (Spermophilus beldingi) Can J Zool. 2002;80:366–371. [Google Scholar]
- Nunes S, Muecke EM, Ross HE, Bartholomew PA, Holekamp KE. Food availability affects behavior but not circulating gonadal hormones in maternal Belding’s ground squirrels. Physiol Behav. 2000;71:447–455. doi: 10.1016/s0031-9384(00)00366-8. [DOI] [PubMed] [Google Scholar]
- Palme R, Mostl E. Measurement of cortisol metabolites in faeces of sheep as a parameter of cortisol concentration in blood. Int J Mamm Biol. 1997;62:192–197. [Google Scholar]
- Palme R, Robia C, Baumgartner W, Mostl E. Transport stress in cattle as reflected by an increase in faecal cortisol metabolite concentrations. Vet Rec. 2000;146:108–109. doi: 10.1136/vr.146.4.108. [DOI] [PubMed] [Google Scholar]
- Petrovic VM, Janic-Sibalic V. Adrenocortical control of phenylethanolamine-N-methyl transferase and mono-amine oxidase activity in the ground squirrel (Citellus citellus) during the summer. Gen Comp Endocrinol. 1976;29:492–497. doi: 10.1016/0016-6480(76)90032-0. [DOI] [PubMed] [Google Scholar]
- Place NJ, Kenagy GJ. Seasonal changes in plasma testosterone and glucocorticoids in free-living male yellow-pine chipmunks and the response to capture and handling. J Comp Physiol B. 2000;170:245–251. doi: 10.1007/s003600050282. [DOI] [PubMed] [Google Scholar]
- Ponzio MF, Monfort SL, Busso JM, Dabbene VG, Ruiz RD, de Cuneo MF. A non-invasive method for assessing adrenal activity in the chinchilla (Chinchilla lanigera) J Exp Zool. 2004;301:218–227. doi: 10.1002/jez.a.20030. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Prudom SL, Schultz-Darken NJ, Abbott DH. Reduced adrenocortical responsiveness to adrenocorticotropic hormone (ACTH) in socially subordinate female marmoset monkeys. Psychoneuroendocrinology. 2000;25:463–477. doi: 10.1016/s0306-4530(00)00003-2. [DOI] [PubMed] [Google Scholar]
- Sands JL, Creel S. Social dominance, aggression and fecal glucocorticoid levels in a wild population of wolves, Canis lupus. Anim Behav. 2004;67:387–396. [Google Scholar]
- Sapolsky RM. Neuroendocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral Endocrinology. MIT Press; Cambridge, MA: 1992. pp. 287–324. [Google Scholar]
- Sherman PW, Morton ML. Demography of Belding’s ground squirrels. Ecology. 1984;65:1617–1628. [Google Scholar]
- Terio KA, Brown JL, Moreland R, Munson L. Comparison of different drying and storage methods on quantifiable concentrations of fecal steroids in the cheetah. Zoo Biol. 2002;21:215–222. [Google Scholar]
- Teskey-Gerstl A, Bamberg E, Steineck T, Palme R. Excretion of corticosteroids in urine and faeces of hares (Lepus europaeus) J Comp Physiol B. 2000;170:163–168. doi: 10.1007/s003600050271. [DOI] [PubMed] [Google Scholar]
- Touma C, Sachser N, Mostl E, Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol. 2003;130:267–278. doi: 10.1016/s0016-6480(02)00620-2. [DOI] [PubMed] [Google Scholar]
- Vanjonack WJ, I, Scott M, Yousef MK, Johnson HD. Corticosterone plasma levels in desert rodents. Comp Biochem Physiol. 1975;51A:17–20. doi: 10.1016/0300-9629(75)90406-5. [DOI] [PubMed] [Google Scholar]
- von der Ohe CG, Wasser SK, Hunt KE, Servheen C. Factors associated with fecal glucocorticoids in Alaskan brown bears (Ursus arctos horribilis) Physiol Biochem Zool. 2004;77:313–320. doi: 10.1086/378139. [DOI] [PubMed] [Google Scholar]
- Wallner B, Mostl E, Dittami J, Prossinger H. Fecal glucocorticoids document stress in female Barbary macaques (Macaca sylvanus) Gen Comp Endocrinol. 1999;113:80–86. doi: 10.1006/gcen.1998.7183. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Bevis K, King G, Hanson E. Non-invasive physiological measures of disturbance in the northern spotted owl. Conserv Biol. 1997;11:1019–1022. [Google Scholar]
- Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol. 2000;120:260–275. doi: 10.1006/gcen.2000.7557. [DOI] [PubMed] [Google Scholar]
- Wasser SK, Monfort SL, Southers J, Wildt DE. Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) faeces. J Reprod Fertil. 1994;101:213–220. doi: 10.1530/jrf.0.1010213. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Brockman DK, Stavisky RC. Recent advances in noninvasive techniques to monitor hormone-behavior interactions. Yearb Phys Anthropol. 1998;41:1–23. doi: 10.1002/(sici)1096-8644(1998)107:27+<1::aid-ajpa2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Wielebnowski NC, Fletchall N, Carlstead K, Busso JM, Brown JL. Noninvasive assessment of adrenal activity associated with husbandry and behavioral factors in the North American clouded leopard population. Zoo Biol. 2002;21:77–98. [Google Scholar]
- Wingfield JC, Hunt K, Breuner C, Dunlap K, Fowler GS, Freed L, Lepson J. Environmental stress, field endocrinology, and conservation biology. In: Clemmons JR, Buchholz R, editors. Behavioral Approaches to Conservation in the Wild. Cambridge University Press; Cambridge: 1997. pp. 95–131. [Google Scholar]
- Young KM, Brown JL, Goodrowe KL. Characterization of reproductive cycles and adrenal activity in the black-footed ferret (Mustela nigripes) by fecal hormone analysis. Zoo Biol. 2001;20:517–536. [Google Scholar]
- Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL, Brown JL. Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol. 2004;137:148–165. doi: 10.1016/j.ygcen.2004.02.016. [DOI] [PubMed] [Google Scholar]


