Abstract
The synthesis of the bridged A-nor-paclitaxel 4 has been achieved from paclitaxel in a key test of the T-Taxol conformational hypothesis. Although the unbridged A-nor-paclitaxel 3 is essentially non-cytotoxic, the bridged analog 4 is strongly cytotoxic. This result provides strong evidence for the T-Taxol conformation as the bioactive tubulin-binding conformation of paclitaxel.
The natural product paclitaxel (PTX) (Taxol™, 1) is a clinically approved drug for several tumor malignancies,1 and its chemistry and biology have been investigated extensively.2 It acts by promoting the polymerization of tubulin to stabilized microtubules, leading to apoptotic cell death,3–4,5,6 and its clinical activity is believed to be directly related to this microtubule-binding activity.6
Since the bioactivity of PTX is intimately connected with its tubulin-assembly properties, and since tubulin assembly is initiated by the non-covalent binding of paclitaxel to tubulin, the nature of this binding is a matter of great interest. In one scenario, a knowledge of the tubulin-binding conformation of paclitaxel would enable the design of simple non-taxoid compounds with comparable binding affinity and bioactivity.
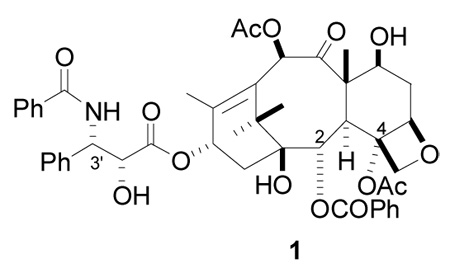
Although the taxane ring system of paclitaxel is relatively rigid, the compound has flexible side chains at C2, C4, C10, and notably at C13. As a result, there are many possible conformations available for binding to tubulin. Two different paclitaxel models of the tubulin-binding conformation were proposed based on NMR observables and molecular modeling. A “nonpolar” conformation was put forth on the basis of solution NMR investigations in nonpolar solvents,7–9 while similar studies in polar solvents were interpreted to favor a bound “polar” conformation.10- Although most of the reports assumed a single conformation, deconvolution of PTX in CDCl314 and D2O/DMSO-d615 makes it clear that the molecule adopts 9–10 conformations, no one of which achieves a population above 30%.
A second approach focused on tubulin-bound paclitaxel in the solid state. Application of REDOR NMR provided F-13C distances of 9.8 and 10.3 Å between the fluorine of a 2-(p-fluorobenzoyl)PTX and the C3′ amide carbonyl and C3′ methine carbons, respectively.16 A related examination reported a distance of 6.5 Å between the fluorines of 2-(p-fluorobenzoyl)-3′-(p-fluorophenyl)-10-acetyldocetaxel; and, like the first solid state study, proposed the polar form to be tubulin-bound.17,18,19
The “polar” and “nonpolar” conformations have inspired several elegant synthetic studies designed to generate constrained analogs which maintain these conformations, but none of these constrained analogs have shown tubulin-polymerization or cytotoxic activities equal to or greater than those of PTX itself. Various compounds designed to mimic the “polar” conformation were either inactive20 or less active than PTX.21 Analogs based on the “nonpolar” conformation were also less active than PTX.22–25
A more fruitful approach stems from the electron crystallographic (EC) structure of αβ-tubulin stabilized by Zn2+ and PTX. It provides a detailed view of subunit structure, but at 3.7 Å resolution leaves the PTX conformation unresolved.26 A model derived from intersection of PTX NMR and X-ray conformations with the EC density led to the proposal of T-Taxol as the bound conformation.27 A concurrent EC refinement28 at 3.5 Å resolution provided additional confidence in the model. Following these developments, a T-like conformer with a reorganized C-13 side chain (PTX-NY) was proposed as an alternative binding model.29,30 However, this conformer is inconsistent with the EC density.31,32
We report here an indirect method to establish the nature of the tubulin-taxane binding conformation more firmly. As described in more detail elsewhere33 we previously designed and prepared several bridged analogs such as 2 based on the T-Taxol or butterfly conformation. The latter juxtaposes the C3′-phenyl and C4-OAc groups and encouraged the construction of taxanes with bridges linking these positions. Three of the analogs have tubulin-assembly and cytotoxic activities superior to those of paclitaxel, providing strong support for the T-Taxol conformation.33,34 Nonetheless, the actual improvement in bioactivity is relatively modest, varying from a 22-fold increase in cytotoxicity in the A2780 bioassay to a factor of 1.4 in the case of the PC3 prostate cancer cell line.33
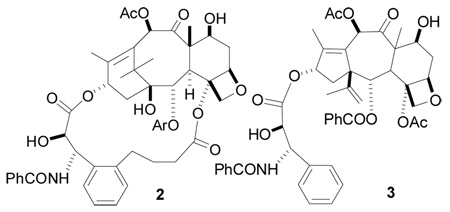
Tubulin-assembly activity appears to be less sensitive to structural variations than cytotoxicity, but 2 had an ED50 for tubulin polymerization of 0.21 µM as compared with paclitaxel’s 0.50 µM.33 We thus elected to carry out a rigorous test of the T-Taxol hypothesis by determining the effect of bridging on the bioactivity of the much less active starting compound A-nor-paclitaxel (3).
A-nor-paclitaxel, or (1α)-15(16)-anhydro-11(15→1)-abeotaxol (3), is an A-ring contracted paclitaxel analog that was first reported by us in 1991.35 Biological studies revealed that 3 was much less cytotoxic than paclitaxel towards the KB cell line, but that it retained ability to inhibit tubulin disassembly at a level about one third that of paclitaxel.36 A preliminary molecular modeling study showed that the rearranged A-nor-baccatin core of A-nor-paclitaxel has a similar conformation to that the baccatin core of paclitaxel. As a result, a number of A-nor-paclitaxel analogs with modifications on the C-1 isopropenyl moiety and the C-2 benzoyl group were prepared. A few of these analogs showed enhanced tubulin binding activities, in some cases to the same level as that of paclitaxel, but none possessed comparable cytotoxicity.37,38 Interestingly, unlike PTX, where some modifications on the C-2 benzoyl group increase tubulin-assembly activity, the same modifications on the C-2 benzoyl group of A-nor-paclitaxel uniformly decrease tubulin-assembly activity.38 Based on this information, the bridged compound 4 provides an excellent critical test of the T-Taxol hypothesis, since any cytotoxicity observed would represent a significant increase in bioactivity due to the imposed conformational lock.
We investigated two separate approaches to the synthesis of 4. The first approach involved the synthesis of the A-nor-baccatin III 5 and subsequent attachment of a modified side chain and olefin metathesis to form the bridge. This approach was selected because of its flexibility, since it could in principle be adapted to prepare several compounds with different chain lengths.
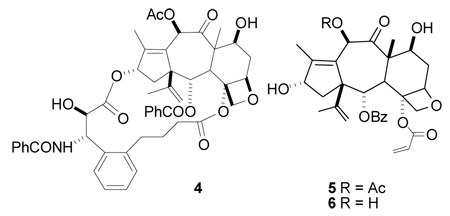
The approach failed, however, at the point of the conversion of the 10-deacetyl derivative 6 to 5. We thus elected to pursue the simpler direct route of conversion of a preformed bridged derivative to its A-nor analog.
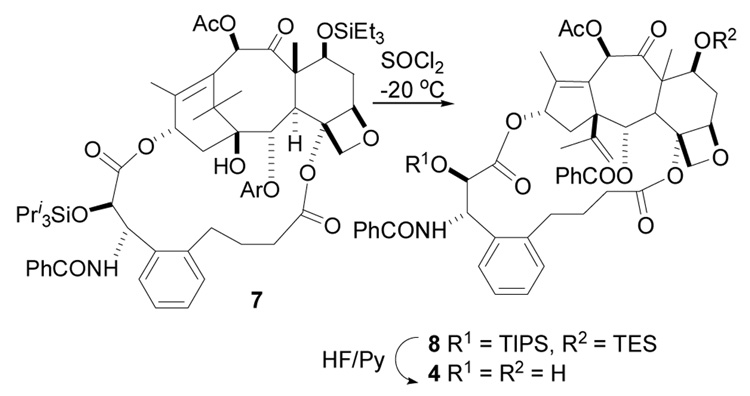
The known compound dihydrobritaxel-5 was prepared as previously described33 and converted to the silyl protected compound 7. This was then treated with SOCl2 at −20°C to give the A-nor derivative 8 (Scheme 1). The resulting compound was then deprotected to yield the desired product 4. The unbridged A-nor-paclitaxel 3 was also prepared as previously described35 for comparison purposes.
Scheme 1.
Synthesis of 4
PTX and compounds 3 and 4 were evaluated for tubulin-assembly capacity, tubulin binding ability and cytotoxicity against the A2780 and PC3 cell lines (Table 1). Impressively, the bridged A-nor-paclitaxel 4 binds to GMPcPP microtubules with slightly greater affinity than PTX itself and is more efficacious by a factor of 2 in lowering the critical concentration of tubulin-GDP. The enhanced tubulin polymerization activity (ED50) of compound 4 is probably due to an increase in both the ligand’s efficacy and its affinity for tubulin (Table 1).
Table 1.
Bioactivity of paclitaxel analogs
| cmpd | tubulin polym ED50 (µM) | crit. Tb conc., µMa | Ka × 107, M−1b | A2780 cytotox IC50 (nM) | PC3 cytotox. IC50 (nM) |
|---|---|---|---|---|---|
| 1 | 0.87 ± 0.15 | 2.0 ± 0.15 | 5.48 ± 0.22 | 2.4 ± 0.2 | 4.7 ± 2.6 |
| 2c | 0.21 ± 0.09 | 0.35 ± 0.06 | NDd | 0.56 ± 0.05 | 3.1 |
| 3 | NDd | ~ 3e | <0.02 | >2000 | >8300 |
| 4 | 0.46 ± 0.08 | 0.99 + 0.18 | 8.84 ± 0.61 | 89 ± 9 | 13.8 ± 7.1 |
GDP-tubulin, determined as described in Supporting Information.
GMPcPP microtubules, determined as described in Supporting Information.
Data from reference 33
Not detemined.
GTP-tubulin; the value for PTX under these conditions is 0.37 µM3
The unbridged A-nor-analog 3 was only weakly active in all of the assays performed. For example, no inhibition of fluorescent PTX binding to GMPcPP microtubules was observed at concentrations of 3 up to 40 µM (curves shown in Supplementary Material). The association constant for the binding of 3 to microtubules was therefore estimated to be at least two orders of magnitude less that that of PTX. The more active tubulin-GTP rather than tubulin-GDP was required to measure critical concentration. The critical concentration of tubulin-GTP in the presence of 1:1 molar ratio of 3 was > 3µM, which is at least eight times the value determined for PTX under these conditions (0.37µM).3
In cytotoxicity assays, the analog 4 shows significant cytotoxic activity to the A2780 and the PC3 cell lines, although it is still less active than PTX; in the PC3 cell line it is threefold less cytotoxic than PTX (1). In contrast, the unbridged A-nor-paclitaxel 3 is essentially non-cytotoxic, with IC50 values too weak to be determined.
These results indicate that a bridged A-nor-paclitaxel which can maintain a “T-taxol” conformation also retains all of paclitaxel’s tubulin-assembly activity and much of its cytotoxicity. This work thus offers further evidence for the significance of the T-taxol conformation for tubulin binding and tubulin assembly.
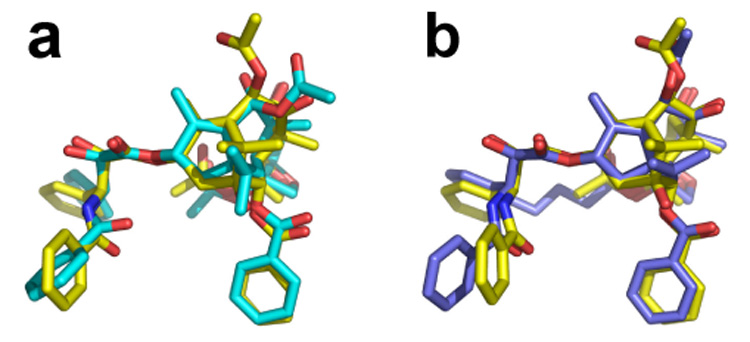
In an attempt to understand the origin of the differences between A-nor PTX 3 and bridged A-nor PTX 4, we performed extensive conformational searches for these structures and located 464 and 613 fully-optimized conformations, respectively. The latter were examined for the presence of the polar, nonpolar, PTX-NY and T-Taxol conformers. Bridged 4 delivered 61 T-form minima, while acyclic 3 yielded 75 such conformers. In each of the latter cases, one of the top two fits provides a very close match to the structure of T-Taxol (Figure 1). By comparison, neither the polar nor the PTX-NY types are present in the conformers of 4, although the acyclic set (3) contains them. In addition, the nonpolar form of 4 docks poorly into the binding site (See SI). Accordingly, we give the latter three rotamers no further consideration.
Figure 1.
Superposition of the centroids of the phenyl rings of fully-optimized T-forms of the A-nor PTX analogs and those of the corresponding rings of T-Taxol (yellow); a) acyclic 3 (cyan) (rms 0.35 Å); b) bridged 4 (blue) (rms 0.31 Å).
To compare the compounds in the taxoid binding site, both 3 and 4 were docked into β-tubulin with the Glide protocol using flexible ligand docking and the OPLS force field. Both were found to readily adopt the T-form (see SI). The MMFF/GBSA(H2O) energy differences between the docked structures and the respective conformational search global minima for the A-nor analogs were computed to show that the energy gap (ΔΔE) is 1.7 kcal/mol smaller for bridged 4 than acyclic 3. With the AMSOL/ SM5.4PDA/CM1 aqueous solvation model,39 we estimated that the free energies of solvation for the two docked structures favor the bridged A-nor-taxol by 0.6 kcal/mol, reducing ΔΔE to 1.1 kcal/mol.
To place the latter combination energy difference in the context of the biological data of Table 1, we note that bridged 4 is at least 23 and 600 fold more active than 3 in the A2780 and PC3 cytotoxicity assays, respectively (2000/89 and 8300/13.8). If we assume the IC50’s can be roughly equated with the Ki’s,40 then ΔΔG = −RTln[Ki(4)/Ki(3)]at 298 K is 1.8 and 3.8 kcal/mol, respectively. These numbers are in reasonable agreement with the calculated value of 1.1 kcal/mol. They suggest that the source of the activity of 4 vs. 3 resides in the relative strain energy associated with binding to tubulin in the T-Taxol conformation offset by a small desolvation penalty.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health (Grant CA-69571) and we are grateful for this support. We thank William Bebout in the Department of Chemistry, Virginia Polytechnic Institute and State University, for mass spectroscopic determinations.
Footnotes
Supporting Information Available Detailed synthetic procedures for the synthesis of the bridged A-nor-paclitaxel 4 and A-nor-baccatin III 5, 1H and 13C NMR spectra of compound 4, tubulin-GTP polymerization by compound 3, tubulin binding curves for 1, 3, and 4, and conformational searching, conformer extraction and binding site docking for 3 and 4. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Rowinsky EK. Ann. Rev. Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]; (b) Crown J, O'Leary M. Lancet. 2000;355:1176–1178. doi: 10.1016/S0140-6736(00)02074-2. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kingston DGI. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. Boca Raton: CRC Press; 2005. pp. 89–122. [Google Scholar]; (b) Ojima I, Kuduk S, Chakravarty S. Adv. Med. Chem. 1999;4:69–124. [Google Scholar]
- 3.Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz SB. Trends Pharmacol. Sci. 1992;13:134–136. doi: 10.1016/0165-6147(92)90048-b. [DOI] [PubMed] [Google Scholar]
- 5.Jordan MA, Toso RJ, Thrower D, Wilson L. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blagosklonny MV, Fojo T. Int. J. Cancer. 1999;83:151–156. doi: 10.1002/(sici)1097-0215(19991008)83:2<151::aid-ijc1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Dubois J, Guenard D, Gueritte-Voeglein F, Guedira N, Potier P, Gillet B, Betoeil J-C. Tetrahedron. 1993;49:6533–6544. [Google Scholar]
- 8.Williams HJ, Scott AI, Dieden RA, Swindell CS, Chirlian LE, Francl MM, Heerding JM, Krauss NE. Can. J. Chem. 1994;72:252–260. [Google Scholar]
- 9.Cachau RE, Gussio R, Beutler JA, Chmurny GN, Hilton BD, Muschik GM, Erickson JW. Supercomput Appl. High Perform. Comput. 1994;8:24–34. [Google Scholar]
- 10.Vander Velde DG, Georg GI, Grunewald GL, Gunn CW, Mitscher LA. J. Am. Chem. Soc. 1993;115:11650–11651. [Google Scholar]
- 11.Paloma LG, Guy RK, Wrasidlo W, Nicolaou KC. Chem. Biol. 1994;1:107–112. doi: 10.1016/1074-5521(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 12.Ojima I, Chakravarty S, Inoue T, Lin S, He L, Horwitz SB, Kuduk SC, Danishefsky SJ. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4256–4261. doi: 10.1073/pnas.96.8.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojima I, Kuduk SD, Chakravarty S, Ourevitch M, Begue J-P. J. Am. Chem. Soc. 1997;119:5519–5527. [Google Scholar]
- 14.Snyder JP, Nevins N, Cicero DO, Jansen J. J. Am. Chem. Soc. 2000;122:724–725. [Google Scholar]
- 15.Snyder JP, Nevins N, Jimenez-Barbero, Cicero D, Jansen JM. unpublished. [Google Scholar]
- 16.Li Y, Poliks B, Cegelski L, Poliks M, Gryczynski Z, Piszczek G, Jagtap PG, Studelska DR, Kingston DGI, Schaefer J, Bane S. Biochemistry. 2000;39:281–291. doi: 10.1021/bi991936r. [DOI] [PubMed] [Google Scholar]
- 17.Ojima I, Inoue T, Chakravarty S. J. Fluorine Chem. 1999;97:3–10. [Google Scholar]
- 18.The X-ray crystal structure of polar PTX (Ref. 19) utilized in Ref. 16 shows an F---F distance of 4.8 Å when the para-hydrogens of the phenyl rings at the C-2 and C-3′ positions are replaced with fluorines (r(C-F) = 1.33 Å)
- 19.Mastropaolo D, Camerman A, Luo Y, Brayer GD, Camerman N. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6920–6924. doi: 10.1073/pnas.92.15.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boge TC, Wu Z-J, Himes RH, Vander Velde DG, Georg GI. Bioorg. Med. Chem. Lett. 1999;9:3047–3052. doi: 10.1016/s0960-894x(99)00522-3. [DOI] [PubMed] [Google Scholar]
- 21.Ojima I, Lin S, Inoue T, Miller ML, Borella CP, Geng X, Walsh JJ. J. Am. Chem. Soc. 2000;122:5343–5353. [Google Scholar]
- 22.Ojima I, Geng X, Lin S, Pera P, Bernacki RJ. Bioorg. Med. Chem. Lett. 2002;12:349–352. doi: 10.1016/s0960-894x(01)00747-8. [DOI] [PubMed] [Google Scholar]
- 23.Geng X, Miller ML, Lin S, Ojima I. Org. Lett. 2003;5:3733–3736. doi: 10.1021/ol0354627. [DOI] [PubMed] [Google Scholar]
- 24.Querolle O, Dubois J, Thoret S, Dupont C, Guéritte F, Guénard D. Eur. J. Org. Chem. 2003:542–550. [Google Scholar]
- 25.Querolle O, Dubois J, Thoret S, Roussi F, Montiel-Smith S, Guéritte F, Guénard D. J. Med. Chem. 2003;46:3623–3630. doi: 10.1021/jm030770w. [DOI] [PubMed] [Google Scholar]
- 26.Nogales E, Wolf SG, Downing KH. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 27.Snyder JP, Nettles JH, Cornett B, Downing KH, Nogales E. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5312–5316. doi: 10.1073/pnas.051309398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe J, Li H, Downing KH, Nogales E. J. Mol. Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 29.Geney R, Sun L, Pera P, Bernacki RJ, Xia S, Horwitz SB, Simmerling CL, Ojima I. Chem.& Biol. 2005;12:339–348. doi: 10.1016/j.chembiol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 30.The T-Taxol conformer proposed by Geney, Ojima and colleagues was named “REDOR-Taxol”.(ref 29). However, since there are upwards of 100 PTX conformations that meet the REDOR constraints including T-Taxol (Ref 31,Ref 32), we refer to the Geney-Ojima model as New York paclitaxel (PTX-NY)
- 31.Johnson SA, Alcaraz A, Snyder JP. Org. Lett. 2005;7:5549–5552. doi: 10.1021/ol051780p. [DOI] [PubMed] [Google Scholar]
- 32.Alcaraz AA, Mehta AK, Johnson SA, Snyder JP. J. Med. Chem. 2006;49:2478–2488. doi: 10.1021/jm051119r. [DOI] [PubMed] [Google Scholar]
- 33.Ganesh T, Guza RC, Bane S, Ravindra R, Shanker N, Lakdawala AS, Snyder JP, Kingston DGI. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10006–10011. doi: 10.1073/pnas.0403459101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metaferia BB, Hoch J, Glass TE, Bane SL, Chatterjee SK, Snyder JP, Lakdawala A, Cornett B, Kingston DGI. Org. Lett. 2001;3:2461–2464. doi: 10.1021/ol016124d. [DOI] [PubMed] [Google Scholar]
- 35.Samaranayake G, Magri NF, Jitrangsri C, Kingston DGI. J. Org. Chem. 1991;56:5114–5119. [Google Scholar]
- 36.Yuan H, Kingston DGI, Long BH, Fairchild CA, Johnston KA. Tetrahedron. 1999;55:9089–9100. [Google Scholar]
- 37.Chordia MD, Kingston DGI, Hamel E, Lin CM, Long BH, Fairchild CA, Johnston KA, Rose WC. Bioorg. Med. Chem. 1997;5:941–947. doi: 10.1016/s0968-0896(97)00035-7. [DOI] [PubMed] [Google Scholar]
- 38.Kingston DGI, Chaudhary AG, Chordia MD, Gharpure M, Gunatilaka AAL, Higgs PI, Rimoldi JM, Samala L, Jagtap PG, Giannakakou P, Jiang YQ, Lin CM, Hamel E, Long BH, Fairchild CR, Johnston KA. J. Med. Chem. 1998;41:3715–3726. doi: 10.1021/jm980229d. [DOI] [PubMed] [Google Scholar]
- 39.Hawkins GD, Cramer CJ, Truhlar DG. J. Phys. Chem. 1996;100:19824–19839. [Google Scholar]
- 40.(a) Cheng Y-C, Prusoff WH. Biochem. Pharm. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]; (b) Cheng HC. J. Pharm. Tox. Methods. 2002;46:61–71. doi: 10.1016/s1056-8719(02)00166-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.