Abstract
Noradrenergic projections from the locus coeruleus (LC) project to the olfactory bulb (OB), a cortical structure implicated in odor learning and perceptual differentiation among similar odorants. We tested the role of OB noradrenaline (NA) in short-term olfactory memory using an animal model of LC degeneration coupled with intrabulbar infusions of NA. Specifically, we lesioned cortical noradrenergic fibers in mice with the noradrenergic neurotoxin N-Ethyl-N-(2-chloroethyl)-2-bromobenzylamine hydrochloride (DSP4) and measured the effects on an olfactory habituation/spontaneous discrimination task. DSP4-treated mice failed to habituate to repeated odor presentations, indicating that they could not remember odors over the five-minute intertrial interval. We then infused NA bilaterally into the OBs of both DSP4-treated and nonlesioned control animals at two concentrations (10−3M and 10−5M, 2 ul/side). In DSP4-treated animals, NA administration at either concentration restored normal habituation and spontaneous discrimination performance, indicating that noradrenergic neuromodulation mediates these aspects of perceptual learning and that its efficacy does not require activity-dependent local regulation of NA release. Functional OB learning mechanisms may be necessary for normal odor recognition and differentiation among physically similar odorants.
Keywords: Noradrenaline, norepinephrine, olfaction, learning, behavioral pharmacology
Introduction
Noradrenergic neuromodulatory fibers originating from the locus coeruleus (LC) innervate multiple sensory cortical regions and are implicated in perceptual learning. Deficiencies in this noradrenergic regulation are associated with the progression of degenerative dementias including Alzheimer’s disease (AD) and Parkinson’s disease (Marien, Colpaert, & Rosenquist, 2004). Multiple allocortical regions associated with olfactory processing, including the piriform and entorhinal cortices and the hippocampus, receive noradrenergic inputs from the LC (Fallon, Koziell, & Moore, 1978; Fallon & Moore, 1978). In particular, the olfactory bulb (OB) receives substantial LC noradrenergic projections (McLean, Shipley, Nickell, Aston-Jones, & Reyher, 1989; Shipley, Halloran, & de la Torre, 1985) that strongly influence olfactory learning (Brennan, Schellinck, de la Riva, Kendrick, & Keverne, 1998; Kaba & Keverne, 1988; Levy, Gervais, Kindermann, Orgeur, & Piketty, 1990; Sullivan, Zyzak, Skierkowski, & Wilson, 1992; Yuan, Harley, Darby-King, Neve, & McLean, 2003). However, the specific roles of bulbar NA neuromodulation in adult olfactory learning remain unclear.
In this study, we investigated the role of bulbar NA in short term implicit odor memory using mice with a chemically lesioned noradrenergic system along with nonlesioned controls. Lesions were induced by injections of the specific noradrenergic neurotoxin, N-Ethyl-N-(2-chloroethyl)-2-bromobenzylamine hydrochloride (DSP4). DSP4 induces an immediate depletion of cortical NA levels and a massive retrograde degeneration of LC axons within 2–4 weeks after application, while sparing noradrenergic projections to structures such as the brainstem and basal forebrain (Bacon, Bondi, Salmon, & Murphy, 1998; Fritschy & Grzanna, 1989). We infused one of two concentrations of NA, or saline vehicle, bilaterally into the olfactory bulbs of DSP4-lesioned mice and nonlesioned controls and measured the performance of each of these six experimental groups in an olfactory habituation/spontaneous discrimination task (Cleland, Morse, Yue, & Linster, 2002; Mandairon et al., 2006). Unlike nonlesioned controls, DSP4-lesioned mice failed to habituate to repeated odor presentations, and consequently also failed to discriminate between the habituated odorant and a novel test odorant. The direct infusion of NA into the OB of DSP4-lesioned mice counteracted this effect; animals infused with NA habituated normally to repeated odor presentations and regained normal spontaneous discrimination performance. Spontaneous discrimination in control mice was also modulated by the bulbar infusion of NA. Although extrabulbar diffusion of NA to other brain regions cannot be conclusively excluded, these results suggest that noradrenergic neuromodulation in the OB is required for olfactory habituation, a fundamental indicator of implicit short-term memory underlying basic olfactory discrimination capacities.
Methods
Animals
Thirteen age-matched adult male CD-1 mice (Charles River, Wilmington, MA, USA) served as subjects. The mice were group-housed (2–4 per cage) before cannulation surgery and singly-housed thereafter, and were kept on a reversed 12:12 light cycle (dark period: 9:00 – 21:00 daily). Food and water were continuously available. All experiments were carried out under a protocol approved by the Cornell University Institutional Animal Care and Use Committee in accordance with NIH guidelines.
Experimental timecourse
The day of the first injection was termed Day 1 (D1). On D1, and again on D8, six mice were injected intraperitoneally (i.p.) with 50 mg/kg N-Ethyl-N-(2-chloroethyl)-2-bromobenzylamine hydrochloride (DSP4; Sigma-Aldrich, St. Louis, USA) dissolved in 0.2 ml sterile 0.9% sodium chloride solution vehicle (Baxter Healthcare Corporation, Deerfield, IL, USA). This dosage produces extensive, persistent degeneration of noradrenergic neurons in the rat LC (Fritschy & Grzanna, 1989) and is considered the minimum required to deplete at least 80% of cortical noradrenaline (Heneka et al., 2006; Marien et al., 2004). Indeed, cortical NA concentrations and the number of TH-positive neurons in the LC are still significantly reduced even six months after two spaced DSP4 treatments at this dosage (Heneka et al., 2006). Seven additional mice were injected i.p. with an identical volume of vehicle only on the same schedule (nonlesioned controls). Between D9 and D31, all thirteen mice were implanted with OB cannulae (see Surgery) and were given at least 10 days for complete recovery (D32 to D41). Behavioral studies began on D42; on any given day, mice received an intrabulbar infusion of high-concentration NA, low-concentration NA, or saline vehicle; behavioral testing began 20 minutes later in order for the drug to take effect and to ensure full recovery from the light isoflurane anesthesia used to facilitate intrabulbar drug administration. All experiments were repeated in full using four different odor sets. Each mouse was tested every two days to maintain their interest in the task. At D137, after completion of all behavioral tests, the mice were sacrificed by overdose and perfused transcardially (Figure 1A).
Figure 1.
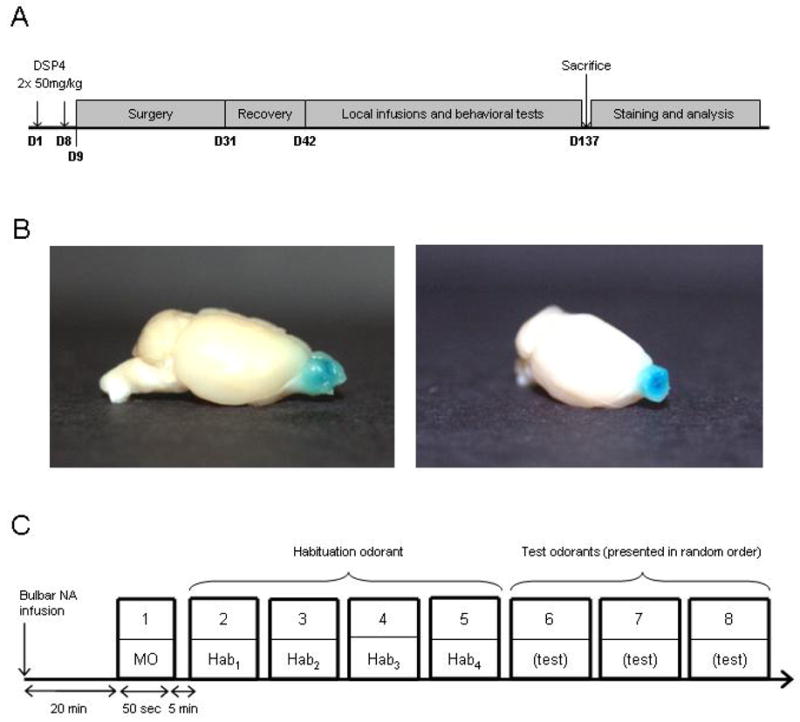
Experimental design. A. Experimental timecourse. Mice were injected with DSP4 (50 mg/kg) or saline vehicle on the first and eighth days of the study (D1, D8) and were implanted with infusion cannulae in the olfactory bulb on or after the ninth day (D9). After recovery from implantation surgery, mice were behaviorally tested. Prior to each behavioral session, one of two dosages of NA, or saline vehicle, was infused into the OB. At the end of behavioral testing, the brains were removed, frozen, and sectioned for immunohistochemical analysis. B. Bilateral infusions of 2 ul methylene blue dye (1 mg/ml) per OB result in diffusion throughout the olfactory bulbs without appreciable spread to neighboring structures. Left panel. Sagittal view of intact brain with stained OBs. Right panel. Three-quarter view of the right OB in frontal section. C. Habituation/spontaneous discrimination task. Each session consisted of 8 trials: one trial with mineral oil (MO) and four successive presentations of a habituation odorant (Hab) followed by presentation of three test odorants (Test) in a pseudorandom order. The duration of each odorant presentation was 50 seconds; trials were separated by 5 minute intertrial intervals.
Surgery
Mice were injected i.p. with 0.05 mg/kg atropine 10 min before general anesthesia (80 mg/kg ketamine + 6 mg/kg xylazine, 62.5μl/50 g body weight, i.p.). Anesthesia was supplemented during surgery with 1/3 of the original dose of ketamine when necessary. Mice were placed in a stereotaxic apparatus, a 32%-Novalsan/2% chlorhexidine disinfectant solution and Xylocaine (4% lidocaine) were topically applied to the skin above the nasal region, and an incision was made over the skull. The exposed skull was quickly cleaned with hydrogen peroxide (50%) and washed with sterile saline solution. Two holes were drilled above the OBs at the following stereotaxic coordinates: AP +5.0 mm, ML ±0.5 mm from bregma. The dura mater was broken with a needle, and two 22-gauge guide cannulae (double cannula assembly for mice, Plastics One, Roanoke, VA) were inserted into the holes, protruding to a depth of 1.5 mm. The guide cannulae were closed with stylets. Immediately after the surgery, and 24 and 48h later if needed, mice received an analgesic (ketoprofen, 2 mg/kg, i.p.) and warm saline solution injections for rehydration and anesthetic clearance (250 μl, i.p.). Mice recovered for a minimum of 10 days during which their weights were recorded to help ensure normal recovery.
Shaping
Behavioral experiments were preceded by a period during which the mice were shaped so as to habituate them to the experimental procedure. For shaping, mice were placed into a standard Plexiglas mouse cage (14×24 cm) separated into two equal chambers (A and B) by an opaque, removable wall. The mouse was placed into chamber A. 60 μl of mineral oil was placed onto a piece of filter paper contained within a mesh tea ball, which was introduced into chamber B. The intervening wall was then removed and the mouse entered chamber B, after which the wall was replaced and the 50-second trial begun. At the end of the trial, the mouse was removed into chamber A for a five-minute intertrial interval. During each trial, the time that the mouse spent investigating the tea ball was measured (investigation was defined as active sniffing with the nose less than 1 cm from the tea ball). After each day’s work, mice were replaced in their home cage.
Shaping consisted of 4 subsequent trials each day, for four days. On two of these days, trials were preceded by a brief isoflurane gas anesthesia mimicking that required for intrabulbar drug infusions (see Behavioral testing); on the other days, mice were not anesthetized. No effect of the anesthesia on normal habituation to repeated stimulus presentations was observed (data not shown).
Behavioral testing
After shaping, mice were utilized in a standard olfactory habituation/spontaneous discrimination task (Cleland et al., 2002; Mandairon et al., 2006). This task measures nonassociative odor memory as well as the breadth of cross-habituation among structurally similar odorants, specifying the degree of spontaneous discrimination among similar odorants unaltered by a history of reinforcement.
A total of six experimental groups were employed in a 2×3 matrix design. The first axis (2) comprised DSP4-lesioned mice (n=6) and nonlesioned controls (n=7); the second (3) reflected the three drug solutions bilaterally infused into the OB before behavioral testing. Specifically, mice were lightly anesthetized with 2% isoflurane in oxygen, after which 2 μl of either 10−3 M NA (high-NA), 10−5 M NA (low-NA), or plain saline (vehicle) were infused into each OB at a constant rate of 2 μl/min using a syringe pump, followed by 1 min diffusion time before the infusion cannula was removed. Drug concentrations were derived from previous studies (Mouly, Elaagouby, & Ravel, 1995); the infusion volume was chosen to adequately perfuse the OB while minimizing extrabulbar diffusion (Figure 1B; (Doucette, Milder, & Restrepo, 2007). Noradrenaline was purchased from Sigma-Aldrich (St. Louis, MO, USA); 0.9% NaCl sterile saline solution, pH 5.0, was purchased from Baxter Healthcare (Deerfield, IL, USA). Drug infusions were counterbalanced among mice such that each animal received each drug treatment a roughly equal number of times. Behavioral studies were performed 20 minutes after infusion to permit drug diffusion within the OB and ensure complete recovery from isoflurane anesthesia.
All behavioral experiments were conducted during the afternoon (13:00–19:00) during the animals’ dark cycle (which ran from 9:00 – 21:00 daily). Each animal was assigned a code number; experimenters were blind to animals’ lesion status (DSP4 or control) but not to their intrabulbar drug infusion status. Cross-habituation testing for measurement of spontaneous discrimination was conducted using the same apparatus described above (Shaping), except that odorants diluted in mineral oil, rather than plain mineral oil, were applied to the filter paper within the tea ball (Table 1; see Odorants). Each habituation/spontaneous discrimination test included eight 50 sec trials with 5 min intertrial intervals (ITIs; Figure 1C). During the first trial the tea-ball contained plain mineral oil to habituate the animals to the experimental context. Trials 2 – 5 comprised the four habituation trials during which the same habituation odorant was repeatedly presented (denoted as Hab1 to Hab4). During trials 6 – 8, three sequentially similar test odorants (Table 1) were presented in a pseudorandom order. During each trial, the time that the mouse spent investigating each odor was measured with a stopwatch.
Table 1.
Odor sets used for the habituation/spontaneous discrimination task. Numerical values depict vol/vol liquid dilutions in mineral oil theoretically producing a vapor phase partial pressure of 1.0 Pa.
| Prehabituation | Hab1 – Hab4 | C+1 | C+2 | C+4 | |
|---|---|---|---|---|---|
|
Odor set 1 :
carboxylic acids |
Mineral oil |
Acetic acid (C2)
7.9 × 10−5 |
Propionic acid (C3)
3.3 × 10−4 |
Butyric acid (C4)
1.3 × 10−3 |
Hexanoic acid (C6)
1.5 × 10−2 |
|
Odor set 2 :
acetate esters |
Mineral oil |
Ethyl acetate (C2)
1.7 × 10−5 |
Propyl acetate (C3)
6.3 × 10−5 |
Butyl Acetate (C4)
2.2 × 10−4 |
Hexyl acetate (C6)
2.3 × 10−3 |
|
Odor set 3 :
aldehydes |
Mineral oil |
Butanal (C4)
1.8 × 10−5 |
Pentanal (C5)
6.6 × 10−5 |
Hexanal (C6)
2.2 × 10−4 |
Octanal (C8)
1.5 × 10−3 |
|
Odor set 4 :
ketones |
Mineral oil |
2-butanone (C4)
1.5 × 10−5 |
2-pentanone (C5)
5.3 × 10−5 |
2-hexanone (C6)
1.8 × 10−4 |
2-octanone (C8)
1.7 × 10−3 |
Odorants
Experiments were repeated using four distinct odor sets, each comprising a homologous series of sequentially similar aliphatic molecules (carboxylic acids, acetate esters, aldehydes, and ketones) with unbranched carbon chains varying from two to six (acids, esters) or four to eight (aldehydes, ketones) carbons in length. These odorants have been demonstrated to be perceptually similar to one another in proportion to their structural similarity (Cleland & Narla, 2003). All odorants were diluted in mineral oil so as to obtain an approximate vapor-phase partial pressure of 1.0 Pa (Table 1; (Cleland et al., 2002; Cleland & Narla, 2003)). Odorant dilutions were prepared simultaneously at least 24 hours before use, stored at 4°C between experiments, and returned to room temperature at least 1 hour before testing. The test odorants differed from the habituation odor in each set by their carbon chain lengths; specifically, the three test odorants were 1, 2, and 4 carbons different in length from the habituation odor in each odor set (depicted as C+1, C+2, and C+4 respectively).
Tyrosine hydroxylase (TH) immunohistochemistry
Mice were overdosed with an anesthetic mixture of ketamine (240 mg/kg) and xylazine (18 mg/kg) and perfused transcardially with 50 ml/mouse of fixative (4% paraformaldehyde in phosphate buffer, pH 7.4). Brains were removed, kept in fixative for one night, and then cryoprotected by 5 days of immersion in 20% sucrose in phosphate buffer. The brains were then snap frozen in isopentane cooled with liquid nitrogen (−50°C) and stored at −20°C.
Frontal 14 μm sections of the OB were cut on a cryostat for analysis. Sections were incubated in phosphate-buffered saline (PBS) for 15 min and then in 0.1% Triton for 30 min at room temperature (RT). To block nonspecific binding, sections were then incubated for 1 hour at RT in a blocking solution containing 5% goat serum, 2% bovine serum albumin, and 0.1% Triton in PBS, after which they were incubated overnight at 4°C in rabbit anti-TH primary antibody diluted in the same blocking solution (1:500; Institut J. Boy, Reims, France). Finally, sections were incubated for 2 hours at RT in goat anti-rabbit Alexa 488 secondary antibody (1:200; Molecular Probes, Eugene, OR, USA) and coverslipped with Vectashield (Vector Labs, Burlingame, CA, USA).
Data analysis
Investigation times in seconds were analyzed using repeated measures ANOVA with trial number (for habituation) or test odorant (for spontaneous discrimination) as the within-subjects factor and experimental group (i.e., lesion status combined with drug infusion) as a between-subjects factor, followed by post hoc pairwise comparisons (Fisher’s least significant difference; LSD). Habituation was generally identified by a significant reduction in investigation time between trials 2 and 5 (Hab1 and Hab4), whereas spontaneous discrimination was identified as a significantly increased investigation time during presentation of a test odorant (trials 6 – 8) compared to the last habituation trial (trial 5; Hab4), based on the degree of structural and perceptual odor dissimilarity (Cleland et al., 2002; Linster & Hasselmo, 1999). The significance level was set at α = 0.05.
A habituation index and a spontaneous discrimination index were also calculated for each test series. The habituation index was calculated as 1 – (Hab4/Hab1) where Hab4 was the investigation time of the odor during the last habituation trial and Hab1 the investigation time during the first presentation of the habituation odor. This index approaches 0 when Hab4 is similar to Hab1 (no habituation) and approaches 1 when Hab4 is much smaller than Hab1 (substantial habituation). Similarly, the discrimination index was defined as 1 – (Hab4/Test) where Test was the investigation time of the test odorant (in trial 6, 7, or 8 as appropriate; Figure 1C) and Hab4 was the investigation time during the last habituation trial. The discrimination index approaches 0 when Test is similar to Hab4 (no discrimination) and 1 when Test is much larger than Hab4 (high discrimination). These indices enable comparison of habituation and spontaneous discrimination performance independently of overall variations in investigation time. To assess the effect of intrabulbar NA infusions on the habituation index in both DSP4-lesioned animals and nonlesioned controls, an ANOVA with DSP4 treatment (DSP4, vehicle) and intrabulbar drug infusion (vehicle, low-NA, high-NA) as main effects was performed, followed by post hoc pairwise comparisons (Fisher LSD). To similarly assess the effect of NA infusions on the discrimination index, ANOVA testing with DSP4 treatment, drug infusion, and test odorant (C+1, C+2 and C+4) as main effects was performed, again followed by post hoc pairwise comparisons.
Frontal sections of the OB from DSP4-treated and control animals were simultaneously processed for TH immunolabeling (Figure 5A). Photomicrographs were taken on a Zeiss Axioplan with a 40× objective (excitation 519 nm; emission 488 nm) and analyzed with image analysis software (Morpho Expert, Explora Nova, La Rochelle, France). The total area occupied by TH-positive fibers was measured on a series of micrographs of the granule cell layer of randomly-selected sections from both DSP4-injected and control animals (10 images per animal; 2 animals per experimental group). Area measurements were calculated automatically in Morpho Expert by a simple luminance thresholding algorithm (Figure 5B); a single threshold was defined and used for all images. Results are expressed as the ratio between the TH+ labeled area and the total area of the micrograph (Figure 5C). Differences were analyzed by t-test and the significance level was set at α=0.05.
Figure 5.

Noradrenaline immunohistochemistry. A. TH fluorescent immunostaining of the granule cell layer of the OB. DSP4 injection significantly reduced the density of noradrenergic fibers compared to controls. Scale bar: 50μm. B. Upper two panels. Results from automatic thresholding analysis of the same two images shown in A. The above-threshold areas scored as TH+ are indicated in red. The luminance threshold defining positive staining was the same for all images analyzed. Lower panel. The negative control was drawn from a control animal in which the tissue was processed normally except that incubation with the primary antibody was omitted. No regions in negative control images were above threshold. C. Quantification of immunolabeling. The proportion of the OB granule cell layer staining positively for TH is significantly lower in DSP4-treated animals than in control animals. Asterisk denotes a significant difference; p < 0.05.
Results
The habituation/spontaneous discrimination task used in these experiments incorporates two behavioral tests. First, analysis of habituation indicates whether animals form a memory for a presented odor over the course of repeated trials, enabling them to progressively reduce their investigation of a known odor source. Second, analysis of spontaneous discrimination measures how selective this memory is with respect to variations in stimulus quality -- i.e., the extent to which structurally and perceptually similar test odorants are behaviorally differentiated from the habituated odorant. Sequential perceptual similarity correlates with structural similarity in homologous aliphatic odorant series such as those used herein (Cleland et al., 2002; Cleland & Narla, 2003). Measurements of spontaneous discrimination, also known as cross-habituation, are not to be confused with motivated discrimination studies that assess animals’ maximum discriminative capacity (Cleland et al., 2002; Linster, Johnson, Morse, Yue, & Leon, 2002). Habituation and spontaneous discrimination results were analyzed separately; in both cases, analyses of raw data were followed by analyses of habituation and spontaneous discrimination indices that enable clear comparison of overall effects while minimizing the impact of intrinsic activity level differences among animals.
Normal habituation and spontaneous discrimination performance
Control mice (nonlesioned control animals that received intrabulbar vehicle infusions before testing) exhibited normal habituation to repeated odorant presentations (significant effect of trial number, F(3, 103) = 16.812; p < 0.001). Post hoc multiple comparisons testing further showed that investigation times during habituation trials 2, 3 and 4 were significantly lower than during the first trial (Figure 2A). Discrimination performance was also normal; mice differentiated significantly between moderately similar odorants (significant effect of test odorant, F(3, 97) = 6.038; p < 0.001; Figure 2B).
Figure 2.
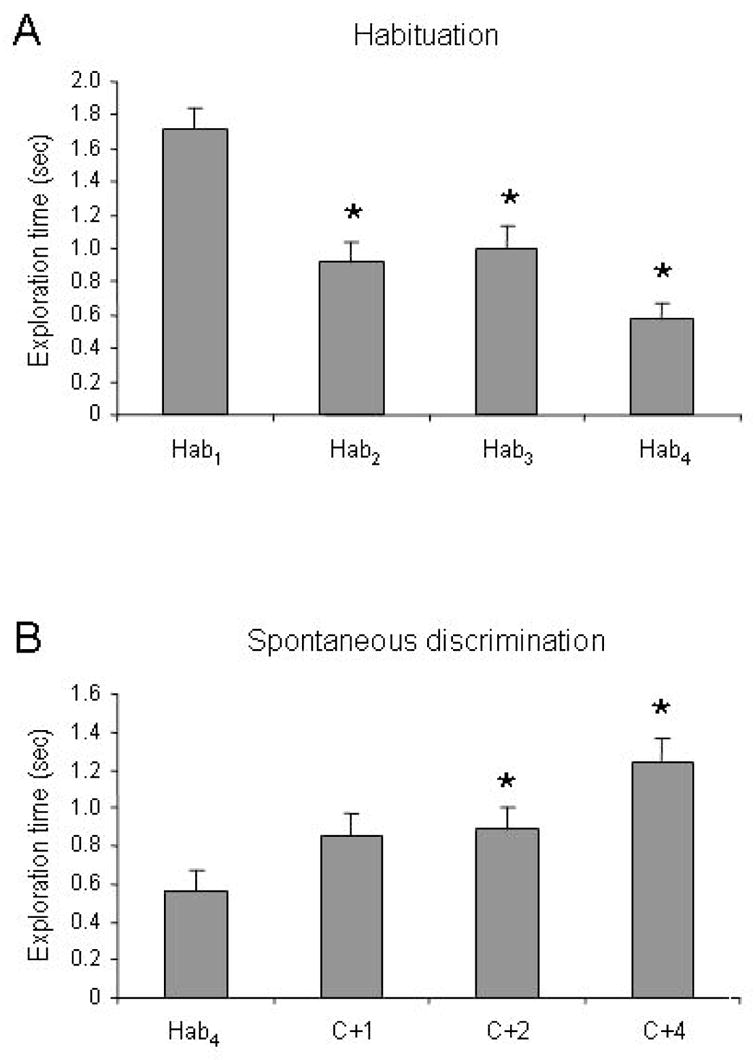
Behavioral responses in nonlesioned, vehicle-infused control animals. Ordinates indicate time spent actively investigating the scented tea ball. A. Normal habituation. Control animals habituated to repeatedly-presented odors over the course of four successive presentations. Asterisks indicate significant reductions in investigation times with respect to the first presentation (Hab1). B. Normal spontaneous discrimination. Control animals significantly discriminated sufficiently dissimilar test odorants from the habituated odorant; specifically, they responded to the test odorants C+2 and C+4 to a significantly greater extent than they did the habituation odorant. Asterisks indicate significant increases in investigation times with respect to the fourth habituation trial (Hab4). Error bars denote standard error.
Noradrenergic effects on habituation
Analysis of variance with trial number across the four habituation trials (Hab1 – Hab4) as a within-subjects factor and experimental group (DSP4/vehicle, DSP4/low-NA, DSP4/high-NA, nonlesioned/vehicle, nonlesioned/low-NA, nonlesioned/high-NA) as a between-subjects factor indicated significant effects of both trial number (Wilks’ lambda; F(3, 474) = 48.884; p < 0.001) and experimental group (F(5, 158) = 9.142; p < 0.001) as well as a significant interaction (F(15, 474) = 3.547; p < 0.001). This indicates that the progression of habituation across successive trials was affected by the experimental group – i.e., by DSP4 lesion and/or intrabulbar drug infusion.
Multiple comparisons testing between the first and the last habituation trials showed that nonlesioned control mice successfully habituated in all cases, irrespective of the intrabulbar drug infusion (vehicle, low-NA, or high-NA; p < 0.001 in all cases; Figure 3A). However, pairwise comparisons also showed that nonlesioned mice treated with the higher dosage of bulb-infused NA habituated significantly more than did mice treated with the lower NA dosage (p < 0.01) or vehicle only (p < 0.01), showing that sufficiently increased levels of bulbar NA could still enhance habituation in animals with fully functional noradrenergic systems.
Figure 3.

Effect of intrabulbar NA infusions on habituation. A. Effect of NA infusions in nonlesioned control mice. Habituation was significant under all three drug conditions (vehicle only, 10−5 M NA and 10−3 M NA). Ordinates indicate mean investigation times in seconds; error bars denote standard error. Asterisks denote significant differences in investigation times between Hab1 and Hab4. B. Effect of NA infusions in DSP4-lesioned mice. DSP4-lesioned mice failed to habituate to repeated odorant presentations when infused with vehicle only, but habituated normally when either concentration of NA was infused into the olfactory bulbs. C. Effects of NA infusions on the habituation index in nonlesioned controls. Intrabulbar infusion of NA at either concentration effected no significant changes in habituation performance. D. Effects of NA infusions on the habituation index in DSP4-lesioned mice. The degree of habituation increased significantly when NA was infused into the OBs prior to odorant presentation. Asterisks indicate significant increases in the habituation index with respect to DSP4-lesioned, vehicle-infused mice; double asterisks indicate significant differences in the habituation index with respect to nonlesioned, vehicle-infused controls.
DSP4-lesioned animals receiving no exogenous NA (i.e., receiving OB infusions of vehicle only) habituated significantly differently from control animals (pairwise comparisons between DSP4 and nonlesioned animals with saline infusions; p < 0.001); specifically, DSP4-lesioned animals injected with vehicle did not habituate at all (comparison of Hab1 and Hab4; p > 0.05). In contrast, DSP4-lesioned animals receiving intrabulbar infusions of either a low or high dosage of NA habituated normally – i.e., similarly to nonlesioned, vehicle-infused mice (pairwise comparison between groups; p > 0.05 in both cases). The degree of habituation was significant in both of these groups (comparison of Hab1 and Hab4; p < 0.01; Figure 3B), indicating that the infusion of NA into the OB rescued the capacity to form odor memories and exhibit habituation.
Analysis of the habituation index (see Methods) confirmed that DSP4 lesions of the noradrenergic system impair olfactory habituation to stimuli presented at 5 min ITIs. Analysis of variance with DSP4 treatment (DSP4-lesioned, nonlesioned control) and drug infusion (vehicle, low-NA, high-NA) as separate main effects revealed significant effects of DSP4 treatment (F(1,127) = 7.195; p < 0.01) and drug dosage (F(2,127) = 8.171; p < 0.001) as well as a significant interaction (F(2,127) = 10.203; p < 0.001). Further analyses demonstrated that the effects of intrabulbar NA infusions were significant only in DSP4-lesioned animals (F(2,63) = 13.275; p < 0.01) and not in nonlesioned controls (F(2, 64) = 1.742; p > 0.05; Figure 3C, D). Only DSP4-lesioned, vehicle-infused animals differed from vehicle-infused controls in habituation performance (p < 0.001), reiterating that local infusions of NA into the OB were sufficient to restore normal olfactory habituation memory in animals with disabled noradrenergic systems.
Noradrenergic effects on spontaneous discrimination
Analysis of variance with test odorant (Hab4, C+1, C+2, C+4) as a within-subjects factor and experimental group (DSP4/vehicle, DSP4/low-NA, DSP4/high-NA, nonlesioned/vehicle, nonlesioned/low-NA, nonlesioned/high-NA) as a between-subjects factor showed significant effects of both test odorant (Wilks’ lambda; F(3, 420) = 11.548; p < 0.001) and experimental group (F(5, 140) = 3.723; p < 0.01) as well as a significant interaction (F(15, 420) = 2.098; p < 0.01). This indicates that DSP4 lesion and/or intrabulbar drug infusion significantly altered the pattern of spontaneous discrimination among habituated and test odorants.
Among nonlesioned mice, pairwise comparisons showed that infusions of either the lower or the higher dosages of NA had no effect on discrimination abilities compared to vehicle-infused controls (p > 0.05), although comparison of the individual responses within each of these groups may suggest an incipient effect (Figure 4A). Among DSP4-lesioned mice, those infused only with vehicle exhibited significantly different spontaneous discrimination profiles compared to untreated control mice (nonlesioned mice with vehicle infusions; p < 0.01), whereas those infused with either dosage of NA exhibited profiles statistically identical to those of untreated controls. These results demonstrate that, as was the case for habituation, local infusion of NA into the OB rescues normal spontaneous discrimination performance in DSP4-lesioned animals. Multiple comparisons testing of investigation times confirmed that DSP4-lesioned mice infused only with vehicle investigated each of the three sequentially similar test odorants (C+1, C+2, C+4) to the same extent as they did the habituation odor during the last habituation trial (Hab4; p > 0.05 in all cases), whereas mice infused with NA exhibited significant spontaneous discriminations between Hab4 and individual test odorants (Figure 4B).
Figure 4.

Effect of intrabulbar NA infusions on spontaneous odor discrimination. A. Effect of NA infusions in nonlesioned control mice. Spontaneous discrimination between the habituated odorant and modestly dissimilar test odorants was significant under all three drug conditions (vehicle only, 10−5 M NA and 10−3 M NA). Ordinates indicate mean investigation times in seconds; error bars denote standard error. Asterisks denote significant differences in investigation times between Hab4 and each test odorant (C+1, C+2, C+4). B. Effect of NA infusions in DSP4-lesioned mice. DSP4-lesioned mice did not spontaneously discriminate test odorants when infused with vehicle only, but discriminated normally when either concentration of NA was infused into the olfactory bulbs. C. Effects of NA infusions on the discrimination index in nonlesioned controls. Intrabulbar infusion of NA at the lower concentration effected no changes in discrimination performance, but the higher NA concentration modestly improved discrimination of relatively dissimilar test odorants (C+4). Section symbols indicate significant increases in the discrimination index across all three test odorants with respect to vehicle-infused controls within the same group (nonlesioned). D. Effects of NA infusions on the discrimination index in DSP4-lesioned mice. Spontaneous discrimination was significantly improved by infusion of NA at either dosage. Section symbols indicate significant increases in the discrimination index across all three test odorants with respect to vehicle-infused mice within the same group (DSP4-lesioned); double section symbols indicate significant differences in the discrimination index across all three test odorants with respect to nonlesioned, vehicle-infused controls.
Analysis of the discrimination index (see Methods) confirmed that DSP4 lesions of the noradrenergic system impair the spontaneous discrimination of structurally and perceptually similar test odorants from a repeatedly presented habituation odorant. Analysis of variance with DSP4 treatment, drug infusion, and test odorant as separate main effects revealed significant effects of both DSP4 treatment (F(1, 283) = 16.748; p < 0.001) and drug infusion (F(2, 283) = 7.377; p < 0.001) but no significant interaction between these two effects (F(2, 283) = 1.700; p > 0.05) and no significant effect of test odorant (F(2, 283) = 0.186; p > 0.05). Further analyses demonstrated that the effects of intrabulbar NA infusions on spontaneous discrimination performance were significant in nonlesioned controls (F(2, 150) = 5.239; p < 0.01) as well as in DSP4-lesioned animals (F(2, 142) = 3.794; p < 0.05), indicating that local infusion of NA into the OB affected spontaneous discrimination in both groups (Figure 4C, D). Post hoc analyses showed, however, that in nonlesioned controls the effects of intrabulbar NA infusion were only significant at the higher dosage (p < 0.05 for high NA; p > 0.05 for low NA; Figure 4C), whereas in DSP4-lesioned animals both concentrations of NA had significant effects on spontaneous discrimination (p < 0.05 for both NA concentrations; Figure 4D). Furthermore, DSP4-lesioned animals infused with either dosage of NA performed similarly to nonlesioned, vehicle-infused controls (p > 0.05). In summary, infusion of even a low dosage of NA into the olfactory bulbs enables normal odor discrimination capabilities as it restores the capacity for habituation disrupted by DSP4 lesioning of the noradrenergic system, whereas in control animals infusion of a higher dosage of NA is required to significantly enhance the capacity for odor discrimination.
Summary of behavioral results
These results show that lesions of cortical noradrenergic fibers by intraperitoneal injection of DSP4 prevent the formation of olfactory habituation memory and reduce spontaneous discrimination among chemically related odorants. Bilateral infusion of NA into the olfactory bulbs is sufficient to rescue both odor memory formation and normal spontaneous discrimination performance at levels comparable to untreated animals, suggesting that bulbar NA plays a crucial role in these processes.
Histological analysis
To measure the efficacy of the noradrenergic lesioning induced by DSP4 administration, OBs from DSP4-treated and nonlesioned control mice were processed for TH expression after behavioral testing was complete. In the granule cell layer of the OB, TH immunoreactivity was found exclusively in noradrenergic fibers originating from the LC since there is no centrifugal dopaminergic innervation of the OB (reviewed in (Cleland & Linster, 2003)) and intrinsic dopaminergic periglomerular neurons do not project to the granule cell layer (McLean & Shipley, 1988). We found that the network of noradrenergic fibers in the granular cell layer of the OBs appeared much sparser in DSP4-lesioned animals than in nonlesioned controls (Figure 5). Quantification of the area occupied by TH-positive fibers within the granule cell layer (see Methods) confirmed that DSP4-lesioned animals exhibited a significant reduction in TH expression in the OB granule cell layer compared to nonlesioned controls (p < 0.05).
Discussion
Lesions of the noradrenergic system prevented olfactory habituation in our experiments, indicating a failure of the lesioned mice to remember or recognize a repeatedly presented odorant. Specifically, DSP4-treated mice did not show the normal progressive reduction in the time spent investigating an odorant repeatedly presented for 50 sec at 5 min intervals. This deficit was not due to a general reduction in exploratory behavior (Lapiz, Mateo, Durkin, Parker, & Marsden, 2001), since DSP4-treated and nonlesioned control animals investigated the odorant to the same extent during the first trial (Figure 3A,B). In contrast, a previous study observed only a weak and inconsistent effect of DSP4 treatment in a comparable task using urinary chemical cues (Guan, Blank, & Dluzen, 1993). Direct comparison of these results with the present study is difficult given their substantial differences. For example, Guan and colleagues injected rats only once with DSP4, rather than twice as in the present study, which may not have depleted NA levels to an extent sufficient to yield significant behavioral effects. Unfortunately, their observation of a ~40% reduction in OB NA levels as measured by HPLC roughly 1 week after DSP4 injection is difficult to compare usefully to our measurement of a ~50% reduction in NA immunoreactivity within the OB granule cell layer measured roughly 18 weeks after the first of two DSP4 injections. There were also differences in subject species, as well as in task parameters; for example, Guan and colleagues presented urine odors directly to rats, holding the odorant near their noses until they expressed disinterest for 2 seconds, a task feature that may influence the salience of presented odorants in comparison with our protocol. Modest changes in task parameters can substantially alter underlying neuronal mechanisms; for example, presenting odor stimuli at an accelerated timescale alters the location, pharmacology, selectivity, and duration of olfactory memory (McNamara, Magidson, Linster, Wilson, & Cleland, 2008). Whereas odorant presentation times in Guan et al. (1993) differed from those in the present study, the ITI durations were comparable (6 min vs. 5 min).
The observed habituation impairments in the present study were presumably due to the depletion of NA resulting from DSP4-induced lesions of the noradrenergic system. At the end of behavioral testing, roughly 18 weeks after the first DSP4 injection, immunohistochemical assessment of TH expression revealed that DSP4 had induced significant reductions in the density of TH+ (noradrenergic) fibers in the granule cell layer of the OB, in concordance with results from previous studies (Fritschy & Grzanna, 1989; Guan et al., 1993). This result also suggests that compensatory sprouting of NA fibers, a potential long term effect of DSP4 administration (Fritschy & Grzanna, 1992), had not yet occurred. The paucity of TH+ fibers suggests a strongly reduced bulbar neuromodulatory capacity in DSP4-lesioned mice. In concordance with this conclusion, we demonstrated that the direct infusion of NA into the OB was sufficient to restore normal habituation performance to DSP4-lesioned mice. That is, the infusion of reasonable concentrations of NA into the OB – without sophisticated activity-dependent local regulation of its release –sufficed to restore animals’ capacity to remember odorants in such a way as to habituate normally to their repeated presentation. This again concurs with data demonstrating that habituation memory for odorants presented at this timescale is dependent on NMDA-dependent neuronal mechanisms located within the olfactory bulb (McNamara et al., 2008).
Spontaneous discrimination among odorants, also referred to as cross-habituation to clearly distinguish it from rewarded discrimination tasks (Cleland et al., 2002; Linster et al., 2002), is assessed by measuring the progressive increase in investigation times of novel odorants in proportion to their structural and perceptual dissimilarity from a habituated odorant that elicits little or no investigation. DSP4-lesioned mice failed to habituate to the habituation odorant over repeated trials, complicating this assessment of their discrimination capacity. It remains to be seen whether DSP4-lesioned mice could, for example, learn to discriminate such odor pairs in a motivated discrimination task, addressing the question of how critical this form of habituation memory is to performance in distinctly different olfactory learning and memory paradigms. It is, however, clear that restoration of bulbar NA by direct infusion restored not only the animals’ capacity to habituate, but also their normal spontaneous discrimination performance.
A related study (Doucette et al., 2007) has shown that rewarded odor discrimination by mice in a go/no-go task is impaired by the intrabulbar infusion of both alpha- and beta-adrenergic receptor antagonists (although not by either antagonist alone, suggesting an overlapping functional role for the two receptor classes). However, this discrimination impairment was restricted to extremely similar odor pairs; no impairment in discrimination was observable between pairs of odors sufficiently dissimilar to be spontaneously discriminated, such as those used in the present study. This superficial inconsistency with our results may arise from any of several factors. First, the go/no-go task employed by Doucette and colleagues consisted of 2.5 sec odor presentations alternating with ~6 sec intertrial intervals, a timescale at which the olfactory memory traces underlying behavioral habituation are located primarily within the piriform cortex and not the olfactory bulb (McNamara et al., 2008). Second, the go/no-go task is essentially a rewarded discrimination task, which is fundamentally different from nonrewarded cross-habituation (spontaneous discrimination) tasks such as the present study (Cleland et al., 2002). Finally, of course, there are potential efficacy differences between the effects of acute intrabulbar pharmacological antagonists and the global noradrenergic lesions mediated by DSP4 administration. These two studies should not be directly contrasted, but rather taken together to assess the role of bulbar NA neuromodulation in mediating differently structured and motivated olfactory learning tasks.
Noradrenergic dysfunction is implicated in degenerative dementias including Alzheimer’s and Parkinson’s diseases; indeed, DSP4 administration models Alzheimer’s-induced noradrenergic degeneration closely, affecting most noradrenergic fibers originating in the LC but sparing the few fibers projecting from cell groups A1 and A2 to the thalamus, hypothalamus, and preoptic areas (Heneka et al., 2006; Marien et al., 2004). Olfactory models of these disorders are receiving increasing attention at several levels from the physiological to the clinical. For example, Alzheimer’s patients develop diagnostic olfactory deficiencies at early stages of the disease, including odor identification impairments (Devanand et al., 2000; Hawkes, 2006; Koss, Weiffenbach, Haxby, & Friedland, 1988; Lehrner, Brucke, Dal-Bianco, Gatterer, & Kryspin-Exner, 1997; Serby, Larson, & Kalkstein, 1991) and threshold alterations (Bacon et al., 1998; Lehrner et al., 1997; Murphy, Gilmore, Seery, Salmon, & Lasker, 1990). The onset of these deficits likely corresponds with progressive tissue degeneration in the olfactory bulb (OB), the first cortical area to exhibit characteristic tau pathology in AD progression (Kovacs, Cairns, & Lantos, 2001). In transgenic mouse models of AD pathology, tau protein overexpression in Tα1–3RT mice results in olfactory dysfunction (Macknin, Higuchi, Lee, Trojanowski, & Doty, 2004), whereas Tg2576 mice, which overexpress a mutant human amyloid precursor protein linked to AD (Hsiao et al., 1996), exhibit precocious LC degeneration accompanied by olfactory habituation impairments (Guerin, Sacquet, Mandairon, Jourdan, & Didier, 2007). Bulbar dysfunction underlying impairments in habituation and other olfactory behaviors is thus a promising reduced model system for studying the functional degenerations underlying progressive dementia.
In conclusion, the results presented here reveal a necessary role for NA in olfactory habituation and spontaneous discrimination processes and implicate the OB in the mediation of the olfactory memory mechanisms underlying these processes, although effects arising from the extrabulbar circulation of infused NA cannot be ruled out. In addition, we show that the olfactory dysfunction attributable to this bulbar NA deficit resembles that observed in a mouse model of AD (Guerin et al., 2007; Marien et al., 2004), consistent with models suggesting that such noradrenergic mechanisms play a role in the etiology of AD (Marien et al., 2004). Finally, we demonstrate that replacement of NA within the OB by direct infusion suffices to counteract these lesion-induced olfactory deficits. These results suggest a possible neural basis for the olfactory identification deficits predictive of AD, and may serve as a reduced model system for understanding the physiological mechanisms underlying the progressive deficits in cortical function that characterize the degenerative dementias.
Acknowledgments
Supported by CNRS, European Chemoreception Research Organization, Région Rhône-Alpes and MENRT fellowships to DG and NIDCD grant CRCNS DC008702 to CL. We are grateful to Olga Escanilla for whole-mount photography and to Drs M. Marien and A.M. Mouly for discussions and helpful advice.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/bne/
References
- Bacon AW, Bondi MW, Salmon DP, Murphy C. Very early changes in olfactory functioning due to Alzheimer’s disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci. 1998;855:723–731. doi: 10.1111/j.1749-6632.1998.tb10651.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Schellinck HM, de la Riva C, Kendrick KM, Keverne EB. Changes in neurotransmitter release in the main olfactory bulb following an olfactory conditioning procedure in mice. Neuroscience. 1998;87(3):583–590. doi: 10.1016/s0306-4522(98)00182-1. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Linster C. Central olfactory processing. In: Doty RL, editor. Handbook of olfaction and gustation. 2. New York: Marcel Dekker; 2003. pp. 165–180. [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116(2):222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Narla VA. Intensity modulation of olfactory acuity. Behav Neurosci. 2003;117(6):1434–1440. doi: 10.1037/0735-7044.117.6.1434. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. Am J Psychiatry. 2000;157(9):1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem. 2007;14(8):539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180(3):509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol. 1978;180(3):533–544. doi: 10.1002/cne.901800309. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Immunohistochemical analysis of the neurotoxic effects of DSP-4 identifies two populations of noradrenergic axon terminals. Neuroscience. 1989;30(1):181–197. doi: 10.1016/0306-4522(89)90364-3. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Restoration of ascending noradrenergic projections by residual locus coeruleus neurons: compensatory response to neurotoxin-induced cell death in the adult rat brain. J Comp Neurol. 1992;321(3):421–441. doi: 10.1002/cne.903210309. [DOI] [PubMed] [Google Scholar]
- Guan X, Blank JL, Dluzen DE. Role of olfactory bulb norepinephrine in the identification and recognition of chemical cues. Physiol Behav. 1993;53(3):437–441. doi: 10.1016/0031-9384(93)90136-4. [DOI] [PubMed] [Google Scholar]
- Guerin D, Sacquet J, Mandairon N, Jourdan F, Didier A. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Adv Otorhinolaryngol. 2006;63:133–151. doi: 10.1159/000093759. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Ramanathan M, Jacobs AH, Dumitrescu-Ozimek L, Bilkei-Gorzo A, Debeir T, Sastre M, Galldiks N, Zimmer A, Hoehn M, Heiss WD, Klockgether T, Staufenbiel M. Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J Neurosci. 2006;26(5):1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Kaba H, Keverne EB. The effect of microinfusions of drugs into the accessory olfactory bulb on the olfactory block to pregnancy. Neuroscience. 1988;25(3):1007–1011. doi: 10.1016/0306-4522(88)90053-x. [DOI] [PubMed] [Google Scholar]
- Koss E, Weiffenbach JM, Haxby JV, Friedland RP. Olfactory detection and identification performance are dissociated in early Alzheimer’s disease. Neurology. 1988;38(8):1228–1232. doi: 10.1212/wnl.38.8.1228. [DOI] [PubMed] [Google Scholar]
- Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport. 2001;12(2):285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- Lapiz MD, Mateo Y, Durkin S, Parker T, Marsden CA. Effects of central noradrenaline depletion by the selective neurotoxin DSP-4 on the behaviour of the isolated rat in the elevated plus maze and water maze. Psychopharmacology (Berl) 2001;155(3):251–259. doi: 10.1007/s002130100702. [DOI] [PubMed] [Google Scholar]
- Lehrner JP, Brucke T, Dal-Bianco P, Gatterer G, Kryspin-Exner I. Olfactory functions in Parkinson’s disease and Alzheimer’s disease. Chem Senses. 1997;22(1):105–110. doi: 10.1093/chemse/22.1.105. [DOI] [PubMed] [Google Scholar]
- Levy F, Gervais R, Kindermann U, Orgeur P, Piketty V. Importance of beta-noradrenergic receptors in the olfactory bulb of sheep for recognition of lambs. Behav Neurosci. 1990;104(3):464–469. doi: 10.1037//0735-7044.104.3.464. [DOI] [PubMed] [Google Scholar]
- Linster C, Hasselmo ME. Behavioral responses to aliphatic aldehydes can be predicted from known electrophysiological responses of mitral cells in the olfactory bulb. Physiol Behav. 1999;66(3):497–502. doi: 10.1016/s0031-9384(98)00324-2. [DOI] [PubMed] [Google Scholar]
- Linster C, Johnson BA, Morse A, Yue E, Leon M. Spontaneous versus reinforced olfactory discriminations. J Neurosci. 2002;22(16):6842–6845. doi: 10.1523/JNEUROSCI.22-16-06842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknin JB, Higuchi M, Lee VM, Trojanowski JQ, Doty RL. Olfactory dysfunction occurs in transgenic mice overexpressing human tau protein. Brain Res. 2004;1000(1–2):174–178. doi: 10.1016/j.brainres.2004.01.047. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Ferretti CJ, Stack CM, Rubin DB, Cleland TA, Linster C. Cholinergic modulation in the olfactory bulb influences spontaneous olfactory discrimination in adult rats. Eur J Neurosci. 2006;24(11):3234–3244. doi: 10.1111/j.1460-9568.2006.05212.x. [DOI] [PubMed] [Google Scholar]
- Marien MR, Colpaert FC, Rosenquist AC. Noradrenergic mechanisms in neurodegenerative diseases: a theory. Brain Res Brain Res Rev. 2004;45(1):38–78. doi: 10.1016/j.brainresrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Postmitotic, postmigrational expression of tyrosine hydroxylase in olfactory bulb dopaminergic neurons. J Neurosci. 1988;8(10):3658–3669. doi: 10.1523/JNEUROSCI.08-10-03658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CK. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol. 1989;285(3):339–349. doi: 10.1002/cne.902850305. [DOI] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15(3):117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly AM, Elaagouby A, Ravel N. A study of the effects of noradrenaline in the rat olfactory bulb using evoked field potential response. Brain Res. 1995;681(1–2):47–57. doi: 10.1016/0006-8993(95)00280-4. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol Aging. 1990;11(4):465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Serby M, Larson P, Kalkstein D. The nature and course of olfactory deficits in Alzheimer’s disease. Am J Psychiatry. 1991;148(3):357–360. doi: 10.1176/ajp.148.3.357. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res. 1985;329(1–2):294–299. doi: 10.1016/0006-8993(85)90537-2. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res Dev Brain Res. 1992;70(2):279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Darby-King A, Neve RL, McLean JH. Early odor preference learning in the rat: bidirectional effects of cAMP response element-binding protein (CREB) and mutant CREB support a causal role for phosphorylated CREB. J Neurosci. 2003;23(11):4760–4765. doi: 10.1523/JNEUROSCI.23-11-04760.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


