Abstract
Background
Despite the fact that adolescent rats have repeatedly been found to consume more ethanol than adult rats in a variety of ethanol access paradigms, the exact cause of the increase in ethanol consumption during adolescence is not known. One possibility is that age differences in sensitivity to ethanol's rewarding effects may contribute to the elevated intake seen among adolescents. Human studies have shown that autonomic effects of ethanol, particularly ethanol-induced tachycardia, are correlated with the positive hedonic properties of the drug and, hence, may serve as a biomarker for reward.
Methods
In this experiment, a limited-access self-administration paradigm was used to examine ethanol's autonomic effects in outbred male adolescent and adult Sprague-Dawley rats under circumstances likely to reveal the rewarding value of ethanol. Results: The results indicated that voluntary ethanol consumption was greater in adolescent than adult rats and that only adolescents consumed enough of the saccharinsweetened ethanol solution to show a tachycardic effect greater than that seen in response to saccharin alone.
Conclusions
To the extent that these tachycardic properties of ethanol are associated with the rewarding/hedonic properties of ethanol as previously reported in humans, these findings support the suggestion that adolescent animals may have found the ethanol-containing solution to be more rewarding than the saccharin solution. A similar effect was not seen in adults, findings consistent with the notion that adult rats may not consume enough ethanol under these circumstances to experience its positive rewarding properties.
The adolescent period is associated with increased ethanol intake relative to adults in both humans (Johnston et al., 2003) and rodents (Brunell & Spear, 2000; Doremus et al., 2005). Although the exact cause of this elevation in ethanol consumption is not known. Past research has indicated that adolescent rats are less sensitive than adult rats to many effects of ethanol that may serve as cues to moderate ethanol intake. For example, adolescents show less motor impairment (Silveri & Spear, 2001; White et al., 2002), sedation (Silveri & Spear, 1998) and dysphoria (Shram et al., 2005) than adults after ethanol administration. At the same time, adolescents have been shown to be more sensitive than adults to certain restricted effects of ethanol, including ethanol-induced social facilitation (Varlinskaya & Spear, 2002) and disruptions in memory (Markwiese et al., 1998).
It is possible that an attenuated sensitivity to various aversive effects of ethanol may serve as a permissive factor to enhance ethanol consumption during adolescence; indeed in a recent extensive review of the literature, aversive effects of ethanol were found to be inversely correlated with ethanol intake across a wide variety of genetic models varying in intake levels (Green & Grahame, 2008). It is also possible that adolescent animals may vary from adults in their sensitivity to the rewarding effects of ethanol, potentially predisposing them to greater consumption – a possibility to be examined in the present study. On the one hand, it has been suggested that adolescents may be relatively insensitive to the rewarding effects of ethanol, as well as other stimuli, when compared to adults. As a result, adolescents may require greater exposure to a rewarding stimulus in order to experience the same hedonic benefit as would be experienced by adults (Bjork et al., 2004; Spear, 2000). In contrast, increased use of ethanol and other drugs by adolescents has also been postulated to reflect a heightened sensitivity to reward (Chambers, Taylor & Potenza, 2003; Ernst et al., 2005; Galvan et al., 2006; Laviola et al., 2003). There has been little emphasis to date, however, on exploring potential age differences in ethanol hedonics.
In studies using adult human volunteers, ethanol-induced increases in heart rate (HR) have repeatedly been found to correlate significantly and positively with subjective measures of ethanol's rewarding value (Conrod, Pihl & Vassileva, 1998; Conrod, Peterson & Pihl, 2001; Holdstock & De Witt, 2001; Holdstock, King & de Witt, 2000). Much of this research has shown that subjects with a family history of alcoholism show reliable and consistent tachycardic responses to moderate ethanol consumption (Barrett, Brunelle & Pihl, 2004; Conrod, Pihl & Ditto, 1995; Conrod et al., 1997; Peterson et al., 1993, 1996; Newlin & Thomson, 1999), tachycardia that is more marked than in subjects without a family history of alcoholism (Conrod, Pihl & Ditto, 1995). Furthermore, this ethanol-induced tachycardia has been found to correlate with striatal dopamine release (Boileau et al., 2003) and can be blocked by administration of naltrexone (Peterson et al., 2006). These studies conducted in humans support the suggestion that ethanol-induced tachycardia and autonomic input to the heart may be related to the hedonic effects of the drug and hence may provide a useful endophenotype to identify populations at risk for alcoholism (e.g. Schuckit, 2000).
To date, only a few studies have examined potential age differences in ethanolinduced tachycardia between non-selected adolescent and adult rats (for discussion of adolescent (30 day-old) or adult (90 day-old) P rats, see Bell et al., 2006). In one study, no differences were observed between these two ages in their tachycardic responses to ethanol when administered via vapor inhalation in sessions that occurred for four hours each day over seven consecutive days (Ristuccia & Spear, 2005). However, the inhalation protocol used in that study maintained the rats at relatively high (160-200 mg/dl) blood ethanol contents (BECs) that may have been beyond the potentially appetitive range. Consequently, a follow-up study was conducted in which ethanolinduced tachycardia was assessed after injection of a range of ethanol doses, the highest of which produced BECs only slightly higher than those seen after vapor inhalation (200-250 mg/dl) (Ristuccia & Spear, submitted). In contrast to the results of the inhalation study, no evidence of ethanol-specific tachycardia was observed in adolescents after injection of any dose of ethanol, despite clear evidence of dose-related tachycardia in adults. Thus, the ontogenetic pattern of HR response to ethanol appears to depend partly on administration route, in that adolescent rats appeared less sensitive than adults to this effect of injected ethanol but the two ages appeared equivalently sensitive when ethanol was administered via vapor inhalation.
How relevant the data from either of these studies is to the issue of age differences in hedonic effects of ethanol is arguable, especially considering the potential interference that high doses of ethanol may have on baroreflex sensitivity (see Bell et al., 2002 for discussion). In addition, forced-administration paradigms may not accurately assess the rewarding value of ethanol, given past research showing that reward is maximized in situations where the drug is self- rather than experimenter-administered (Ackroff & Sclafani, 2001; Li et al., 2001). Furthermore, self-administered drugs, including ethanol, have also been associated with greater activation of reward-related brain regions than drugs that were forcibly administered (Bachtell et al., 1999; Dworkin, Co & Smith, 1995; Hemby et al., 1997; Porrino et al., 1998).
In light of these findings, use of a self-administration procedure may be especially desirable in work to assess possible age differences in autonomic and physiological measures that may reflect the hedonic properties of ethanol. Previous research has established that outbred adolescent rats generally consume two to three times more ethanol than adults in numerous homecage two-bottle choice paradigms (Brunell & Spear, 2005; Doremus et al., 2005), including a two-hour limited access procedure using sweetened 6% ethanol (Ristuccia, Brunell & Spear, 2005). In this work using a two-hour limited access paradigm, although overall g/kg ethanol consumption differed significantly across age, BEC collected at the end of the two hour session on the fourth and seventh access days did not, with these post-session BECs (approximately 33-36 mg/dl) being within the range shown to support conditioned place preference in both adolescents and adults (Philpot, Badanich & Kirstein, 2003; Matsuzawa, Suzuki & Misawa, 2000).
Whereas ethanol-induced increases in HR have been suggested to serve as a proxy for ethanol's rewarding properties (e.g. Assad et al., 2003), there is also some evidence to suggest that under some circumstances, the decline in body temperature (BT) that occurs after ethanol administration may contribute to ethanol's aversive effects. Exposure to ethanol in an environment with a low ambient temperature facilitated acquisition of ethanol-induced conditioned taste aversions, with the opposite pattern evident when ethanol exposure occurred in a high ambient temperature (Cunningham, Niehus & Bachtold, 1992). These findings suggest that the greater expression of hypothermia may have enhanced the aversive properties of the drug. However, these findings are not ubiquitous, given that other studies found that the magnitude of ethanol-induced hypothermia does not always correlate significantly with acquisition of conditioned taste aversions (Broadbent, Muccino & Cunningham, 2002; Cunningham et al., 1991). On the other hand, most studies examining age differences in ethanol-related hypothermia have used administration protocols producing BECs much higher than those seen after two-hour access (Ristuccia, Brunell & Spear, 2005). Thus, although it is questionable as to whether hypothermic responses to ethanol would emerge using the self-administration protocol in the current study, this autonomic measure was also monitored in the present study as a potential index of the aversive properties of ethanol.
In Exp. 1 of the present study, age differences in ethanol's effects on heart rate and body temperature in response to a two hour voluntary access period were examined. Blood and brain ethanol contents were examined in Exp. 2 at the time point of the greatest HR response to ethanol during the intake session to determine whether age differences in ethanol pharmacokinetics might have contributed to the observed ontogenetic patterns of autonomic effects. In light of previous findings of reduced ethanol-induced tachycardia in adolescents and the potential implications of that finding on the sensitivity of adolescent rats to ethanol's rewarding value, it was hypothesized that adolescent animals would show less ethanol-induced tachycardia than adult rats during self-administration, an indication of attenuated sensitivity to ethanol's rewarding value in adolescence. The results did not support this hypothesis.
Experiment 1: Assessment of Heart Rate, Body Temperature, and Activity during Limited-Access Ethanol Consumption
Methods
Subjects
Both of the experiments described here used adolescent and adult male rats of the outbred Sprague-Dawley strain bred in an AAALAC-accredited vivarium at Binghamton University (N=32 for Exp. 1, N=16 for Exp. 2). Litters were culled on the first day after birth (postnatal day [P] 1) to six males and four females whenever possible. Male offspring were weaned on P21 and housed in pairs with same-sex littermates under a 14/10 hour light/dark cycle (lights on at 0700hrs) until the time of testing as described below; female offspring were used in other projects. No more than one animal per litter was used in each condition. Animals were given ad lib access to food and water until the water restriction period began (as described below). All procedures used in the following experiments were approved by the Binghamton University Institutional Animal Care and Use Committee (IACUC).
Procedure
The experiment was an 8 Day (baseline + 7 days of ethanol exposure) × 2 Age (adolescent or adult) × 2 Experimental Solution (6% ethanol with 0.1% saccharin or 0.1% saccharin alone) design, with eight animals used in each experimental condition (N=32). This ethanol concentration has been used previously in work utilizing a limited access procedure and was found to produce post-session BECs in the moderately intoxicating range (see Eckardt et al, 1998) at both ages immediately following the two hour access period (Ristuccia, Brunell & Spear, 2005). In order to ensure accurate measurements of each individual animal's ethanol intake over days, all animals were isolated at the time of surgery and remained so throughout the experiment. No isocaloric control solutions were provided because past research has indicated that adolescent and adult ethanol consumption is not calorically-driven (Doremus et al., 2005).
On P25 or P65, adolescent and adult rats were implanted with model #TA10ETA-F20 telemetry probes (Data Sciences International; St Paul, MN) to monitor HR, BT and activity throughout the experiment. Animals were first anesthetized with isoflurane, and then the body of the probe inserted through a small incision in the abdomen and sutured to the peritoneum. Next, the two wire leads for measuring HR were fed through the peritoneum with a 17-gauge needle and tunneled subcutaneously to two locations on opposite sides of the heart where the tip of each lead was sutured into place within the muscle.
After surgery, animals were transferred to the telemetry recording room where their cages were placed on top of RLA1020 receiving plates (Data Sciences International; St Paul, MN) and given three days to recover. On the last day of the recovery period, the probes were magnetically activated so that baseline measurements could be taken the next day. All animals were given an additional water supplement (adolescents approximately 10 ml, adults – 13 ml) on this day and throughout the water restriction period at the conclusion of each day's two-hour access session. In pilot work, this amount of additional fluid (approximately 95 ml/kg for adolescents and 38 ml/kg for adults) was found to foster post-surgical recovery as well as to permit continued weight gain in adolescents and weight maintenance in adults without significantly decreasing fluid access during the solution access period at either age relative to animals given no supplemental water. Two-hour access sessions were conducted in the animal's home cage between 0900 and 1100 hrs daily. During the first session (the baseline day), only water was presented to each animal. Thereafter, the seven-day two-bottle exposure period began, with access to both water and one of the experimental solutions occurring each day. The position of each solution (i.e. left or right) was alternated each day of solution access. Animals were weighed prior to each day's fluid access period between the 0820 and 0830 hrs samples, and bottles were placed on the cages at 0855 hrs, just prior to the 0900 hrs sample. On baseline day and throughout the experiment, telemetry data were sampled for thirty seconds once every ten minutes for the entire 24-hour period and then averaged over thirty minute time bins. In order to distinguish binned data from a data point collected at specific time during the day, the latter are accompanied by an “hrs” designation. In contrast, binned data do not have an “hrs” designation and are identified using the first sample included in that bin (i.e. the 0830 bin includes data from the 0830, 0840 and 0850 hrs sampling intervals). Missing telemetry data points were replaced by the means of the samples immediately before and after the lost point. When too many samples were missing to calculate a mean, the point was left blank.
Data Analysis
Ethanol intake (g of ethanol/kg of body weight) was analyzed using a 7 Day × 2 Age analysis of variance (ANOVA), with day treated as a repeated measure. In addition, daily solution and water (ml/kg) consumption, as well as solution preference ((ml of experimental solution – ml of water) / ml of total fluid intake) * 100) and body weight gain (previous day's weight (g) - current day's weight (g)) were analyzed in all animals by means of 7 Day × 2 Age × 2 Experimental Solution (ethanol or saccharin) repeated measures ANOVAs, with day treated as a repeated measure.
Telemetry data were examined during two target intervals, both of which were composed of a series of 30-minute time bins. Analyzing the data using ANOVAs targeted toward specific components of the ethanol response profile permitted assessment of changes in the animals' responses across the days of the exposure period without making more comparisons than prudent given the statistical power of the design (see Ristuccia & Spear, 2005 for further discussion). Separate 8 Day × 2 Age × 2 Experimental Solution × 7 or 4 Time ANOVAs were conducted for each interval, with the length of the time interval (i.e. number of the time bins) varying across analyses depending on the time period of interest. The first assessment interval was set to examine effects of ethanol prior to and during the limited access period (i.e., the seven 30 min. time blocks from 0800-1100), while the second period of interest assessed recovery from these effects after the bottles have been removed (i.e., the four 30 min. time blocks from 1130-1300). Prior to binning the activity data, an estimation of the total number of counts that occurred during the entire ten minute epoch between samples was made. Counts/epoch were calculated using the following formula: (total counts/30)*600.
Where data was found to violate the assumption of homogeneity of variance (determined using Levene's test), nonparametric tests were used (Mann-Whitney U). In all ANOVAs, post hoc differences between groups were assessed using Fisher's LSD tests. No significant effects of solution emerged at either age in the analysis of the recovery period (1130-1300) data and, hence, these analyses are not reported below. An additional analysis was conducted to examine the dark phase of the diurnal cycle. However because no reliable effects of solution were observed in either age during this period, these data will also not be reported.
Results
Body Weight & Intake
Body weight and intake data are summarized (collapsed across Day) in Table 1. As expected, adolescent rats generally gained more weight than adult rats, an age difference that was significant on all days except for Days 2, 3 and 6 [Day × Age interaction: F(7, 196)=9.79, p<.001]. There were no significant effects or interactions of the solutions consumed. Ethanol intake was greater in adolescent than adult animals, an effect that did not interact significantly with Day [Age effect: F(1, 14)=12.35, p<.01]. Solution consumption was also higher in adolescent than adult animals on all days of access, with saccharin-consuming adolescents having higher intake than all other groups [Age × Solution interaction: F(1, 28)=10.75, p<.05]. Adolescents also consumed more water than adults [Age effect: F(1, 28)=22.33, p<.001]. No effects of age or solution emerged in the analysis of solution preference, although when data were collapsed across age and solution, a reliable preference for the experimental solutions over water (i.e. a preference score greater than zero) was observed on all days except for Day 1 [Day effect: F(6, 168)=2.40, p<.05] (data not shown).
Table 1.
Body Weight and Fluid Intake
| Body Weight Gain (g) |
Ethanol Intake (g/kg) |
Solution Intake (ml/kg) |
Water Intake (ml/kg) |
Solution Preference (%) |
||
| Adolescent |
Ethanol |
5.9±1.1* |
1.75±0.19* |
36.0±2.28** |
63.4±5.08* |
5.56±1.13 |
| Saccharin |
5.7±1.4* |
-- |
65.4±3.43** |
57.7±7.98* |
5.44±1.45 |
|
| Adult | Ethanol |
0.8±2.3 |
1.01±0.08 |
20.8±1.85 |
31.4±1.51 |
0.39±5.44 |
| Saccharin | 1.1±2.6 | -- | 43.8±2.42 | 30.5±2.72 | 1.03±2.81 | |
indicate significant main effects of Age
indicates significant Age × Solution interaction
Heart Rate (HR)
Significant differences in HR between adolescent and adult rats that did not interact with solution were evident prior to and during the two-hour access session (0800 to 1100 analysis), with adolescents having significantly higher HRs than adults in all time bins despite some fluctuations across time in animals of both ages [Time × Age interaction: F(6, 168)=4.14, p<.001]. Because of these consistent baseline differences in HR between adolescent and adult rats, the HR data were analyzed separately at each age.
The results of these analyses indicated some evidence for ethanol-specific tachycardia in the adolescent animals but not in adults. Although animals in both the ethanol-and the saccharin-consuming groups showed increases in HR after weighing and during the two-hour access session relative to the time period immediately prior to these manipulations, adolescents with access to ethanol showed higher HR change scores at the onset of the access period relative to the pre-access bin than those with access to saccharin solution. Adult rats, on the other hand, showed a similar rise in HR from the immediate pre-access period regardless of which solution was consumed.
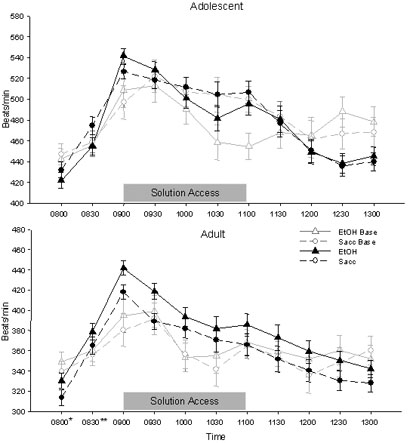
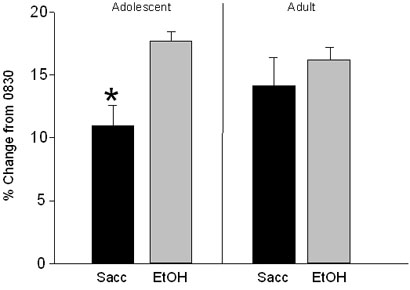
HR data during each time bin from 0800 to 1300 hrs for each group on the baseline day (when animals were given water alone during the fluid access period) as well as when averaged across the seven days of access to the experimental solutions are shown in Figure 1. Data are presented in this manner because, although HR on the solution access days differed from that seen on the baseline day, there were no significant interactions of day and solution in the analyses at either age. In both adolescent and adult rats, onset of the fluid access period on the baseline day as well as on the experimental days was associated with an increase in HR at 0900 relative to the preaccess 0830 bin [Main effect of Time: adolescents: F(6, 84)=75.96, p<.001; adults: F(6, 84)=64.34, p<.001]. Analysis of the HR responses to access to the experimental solutions per se, however, was complicated among the adolescent animals by HR differences between the two solution groups that were evident immediately prior to the access period, with adolescents in the ethanol access group having lower HRs than those with access to saccharin solution at 0830 (and again later in the access period [1030]) [Time × Solution interaction: F(6, 84)=2.52, p<.05]. Because of this pre-access difference between solution groups, HR data of each adolescent rat during the initial bin of the access period (0900) were transformed into percent change from that animal's HR in the pre-access bin (0830) using the following formula: (HR at 0900 – HR at 0830) / HR at 0830) * 100. This analysis again revealed a significant effect of solution, with adolescents given access to ethanol showing a greater percent change in HR at the onset of the access period than those adolescents given access to saccharin [Solution effect: F(1, 14)=14.47, p<.01] (see Figure 2). Although it may appear so in Figure 1, no significant main effect or interaction of solution emerged in the overall ANOVA of adult HR. However, data from adult rats at 0900 were also analyzed as change scores from 0830 to maintain consistency in analysis strategy across age. No effect of solution emerged among the adults in this analysis either.
Figure 1.
HR data from adolescent (top) and adult (bottom) rats with access to both solutions in Exp. 1 (represented as mean ± SEM beats/min). The gray lines represent HR on the baseline day in the individual solution access groups while the black lines represent the mean of all seven solution access days. The solution access period spanned the bins from 0900 to 1100, with pre-access weighing occurring at 0830.
Figure 2.
HR data from Exp. 1 during the 0900 time bin taken as percent change from the pre-access bin (0830). Data are collapsed across day (mean ± SEM). * indicates a significant difference between the saccharin- and ethanol-consuming adolescents.
Body Temperature (BT)
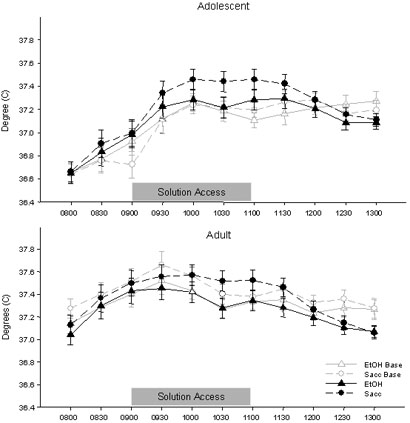
In the overall analysis of BT, significant age differences emerged that did not interact with solution. During the initial period of analysis (0800-1100), temperatures of the adolescent animals were lower than those of adults during most time bins, an age difference that was most pronounced on early assessment days and became somewhat attenuated over days [Day × Time × Age interaction: F(42, 1176)=1.62, p<.01]. Because of these prevalent age differences in BT, the data from adolescents and adults were again analyzed separately.
When the two ages were analyzed separately, only the adolescent rats showed differences in BT across solution access groups, while adult rats showed no effect of solution on BT. Among the younger animals, ethanol-consuming rats had slightly lower temperatures than those with saccharin access, although this difference was largely driven by increasing temperatures in the saccharin-consuming animals over days.
There was a trend for BTs of ethanol-consuming adolescents to be lower than those of saccharin-consuming adolescents during the second half of the two-hour access session (1000 and 1030), although the interaction did not reach statistical significance [Time × Solution interaction: p=.07]. These effects appeared to be driven by an increase in the temperatures of the saccharin-consuming animals over days that was not evident among the ethanol-consuming adolescents [Day × Solution interaction: F(7, 98)=3.20, p<.001] (data not shown). No indications of solution effects on body temperature were seen among the adult animals.
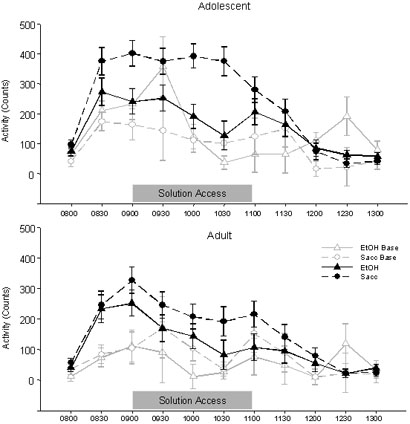
Activity
The ANOVA of activity counts derived from the telemetry probes from 0800 to 1100 indicated that, as expected, adolescent rats were more active than adult rats [Age effect: F(1,28)=7.90, p<.01]. As with HR and BT because of these reliable age differences in baseline activity between adolescent and adult rats, separate analyses were conducted at each age. These analyses revealed statistically significant effects of ethanol on activity only in adolescents, with ethanol consumption decreasing activity among adolescent animals during the two-hour access period. Specifically, between 0800 and 1100, there was a statistically significant effect of solution on activity among the adolescents [Solution effect: F(1, 14)=7.04, p<.05], but not adults, with activity lower in adolescents given access to ethanol (190.22 ± 28.75 counts/epoch) compared to those with access to saccharin (302.50 ± 31.05 counts/epoch). This effect appears to have been driven largely by time bins later in the access period, although the Time × Solution interaction did not reach significance [p=.06].
Experiment 2: Assessment of Blood and Brain Ethanol Content during the Peak Heart Rate Response
Methods
In Exp. 1, adolescent animals showed a greater ethanol-specific tachycardic response than adults, but only during the first thirty minutes of solution access. However, because blood collection would interfere with telemetry recordings, no blood ethanol content (BEC) data was collected in that experiment and, consequently, it was unclear from that study the extent to which the observed differences in HR responses to ethanol may have been related to differences in the amount of ethanol exposure between adolescent and adult animals. Therefore, in this experiment, BEC and brain ethanol content (BrEC) were assessed after thirty minutes of solution access in animals of both ages.
Procedure
Animals in this experiment underwent a two-hour limited-access procedure similar to those in Exp. 1, although no surgery was performed. After the water-only baseline day, adolescents and adults in Exp. 2 underwent two days of ethanol access. After 30 min of ethanol access on the second day, animals were euthanized by decapitation. Trunk blood and whole brains were collected and immediately frozen at −80°C for later assessment of ethanol content using gas chromatography. For analysis, frozen blood samples were thawed and ethanol content was determined using an HP5890 Series II Gas Chromatograph and HP7694E autosampler (Hewlett Packard; Wilmington, DE). Peak areas under the curve were compared to known standards using HP3365 Chemstation software. For analysis of brain samples, 2.0 ml of cold distilled water was added for each gram of brain tissue. Samples were then homogenized at 5°C and analyzed using the same assay procedures as blood samples (see Silveri & Spear, 2000).
Data Analysis
Ethanol intake (g/kg) on the second day of fluid access was calculated identically as in Exp. 1. Intake data at each age were compared using a t-test. Because Levene's test indicated that both BEC and BrEC data sets violated the assumption of homogeneity of variance, the data were analyzed by means of Mann-Whitney U tests. In addition, correlations were conducted between each measure of ethanol content and g/kg intake data.
Results
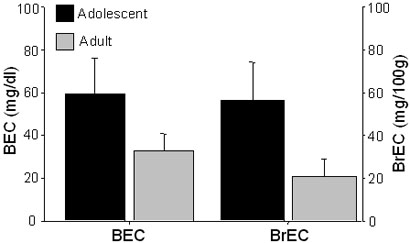
Adolescent rats consumed more ethanol than adult rats in the first 30 min of the two-hour access session (1.1 ± 0.23 vs. 0.36 ± 0.06 g/kg) [t(14)=2.87, p<.05]. Similarly, ethanol content was higher in both tissues in adolescents than adults (Figure 5), although Mann-Whitney U tests did not indicate significant differences between the ages in either blood or brain ethanol content (p>.05). Despite the lack of statistically significant differences, ethanol content in each compartment correlated highly with the amount of ethanol consumed (BEC: R=.89, p<.001; BrEC: R=.83, p<.001).
Figure 5.
Blood and brain ethanol content from animals consuming ethanol for 30 minutes in Exp. 2. Data are represented as mean ± SEM mg of ethanol/dl of blood or /100mg of brain.
Discussion
In these experiments, the two-hour limited access model of ethanol consumption produced an age-related pattern of ethanol consumption similar to that seen in numerous other studies (e.g. Brunell & Spear, 2005; Doremus et al., 2005). Adolescent rats were found to consume both more total g/kg ethanol over the entire session as well as more ethanol than adult animals during the first thirty minutes of fluid access, similar to the findings frequently reported in studies examining age differences in self-administration of ethanol in rats. Although caution is always necessary when comparing across experiments, it is nevertheless interesting that during the thirty min session of Exp. 2, adolescents drank an amount of ethanol that was approximately 63% of that consumed by adolescents over the two hour period in Exp. 1. Adults, on the other hand, drank an amount during the thirty min session that was equivalent to only 35% of what was consumed over two hours by the adults in Exp. 1. Thus, in the first thirty minutes of access, adolescent rats appeared to consume the majority of the ethanol that they would drink during the session, whereas adult ethanol consumption was spread more evenly across the solution access period. This finding is important to consider when examining the telemetry data from Exp. 1 because it suggests that the time period in which the greatest ethanol effect on HR is seen also coincides with the period in which the most ethanol is consumed by outbred adolescent rats – enough ethanol to elevate their blood ethanol levels to 50-60 mg/dl, well within the intoxicating range (Eckardt et al., 1998).
The results of Exp. 1 indicated that among non-selected Sprague-Dawley rats, some limited evidence of an ethanol-specific HR response to ethanol was evident in adolescents, with no comparable effect in adults. Adolescent animals showed a transient ethanol-related increase in HR during the initial part of the limited-access session that was greater than the HR response to the saccharin solution. Furthermore, this elevated HR response to ethanol coincided with the time period of greatest ethanol intake and, presumably reflects the ascending limb of the BEC curve during the fluid access session. This finding is particularly important considering that cardiac responses to ethanol in human volunteers have been found to correlate most strongly with stimulant effects of the drug during the ascending limb of the BEC curve (Brunelle, Barrett & Pihl, 2007). Ethanol-consuming adult rats, on the other hand, did not show an increase in HR greater than that seen in animals with saccharin access. It is important to note that BEC and BrEC both tended to be higher in adolescents than adults following the first thirty min of the intake session. Although not statistically different given the conservative nonparametric tests used, this seeming age difference in ethanol load confounds assessment of age differences in sensitivity to ethanol-induced tachycardia.
Thus, it is unclear whether adolescent and adult rats would differ in their tachycardic responses to ethanol if it had been self-administered in equal amounts across age, a situation that is difficult to accomplish given the consistency with which age differences in ethanol consumption emerge between adolescent and adult animals (e.g. Ristuccia, Brunell & Spear, 2005, 2007; Silveri & Spear, 2000, 2001; Varlinskaya & Spear, 2002; Varlinskaya, Spear & Spear, 1999; White et al., 2002). What can be concluded from these data, however, is that when using this two-bottle choice limitedaccess paradigm, only adolescents voluntarily consumed enough ethanol to experience a tachycardic effect of ethanol that exceeded the increase in HR seen with saccharin consumption, potentially reflecting a greater rewarding value of ethanol relative to saccharin among the younger animals. This increase in heart rate was most likely not a response to the caloric value of the ethanol solution, which may have the potential to be rewarding to food-deprived animals, because caloric intake has not been found to drive ethanol intake in adolescent rats in past research (Doremus et al., 2005). Similarly, the increased HR of the ethanol-consuming adolescents cannot be accounted for by an increased physical activity as no corresponding increase in locomotion was recorded in those animals.
No evidence of tolerance was seen to the tachycardic effect of ethanol in this experiment. This is not surprising, however, given the relatively short period of ethanol exposure that was used. Prior work using a seven day exposure period likewise found no evidence of tolerance to ethanol-induced tachycardia in either adolescents or adults (Ristuccia & Spear, 2005). Other experiments have only seen slight reductions in HR after long periods of self-administration in rats (Abdel-Rahman, Dar & Wooles, 1985; Bell at al., 2002), access periods not suitable for developmental work.
Unlike the HR findings, little evidence of ethanol-induced hypothermia was seen at either age, most likely because the amounts of ethanol consumed were too low to induce a significant decrease in BT. Despite a trend for lower BT in ethanol- than saccharin-consuming adolescent rats, this difference was largely driven by an increase across days in the BT of the animals with saccharin access. It seems unlikely that this difference was the result of a post-surgical impairment of body temperature regulation in the ethanol-consuming animals given that there were no differences in body weight gain between the two solution groups, suggesting a normal recovery from surgery in animals from both groups. Rather, given that activity was decreased during the consumption period in the animals with access to ethanol relative to those with access to saccharin, the lower BT of ethanol-consuming adolescents was most likely a result of the decreased activity brought on by ethanol's sedative properties. To the extent that ethanol-induced hypothermia has been shown to be related to the aversive properties of the drug (e.g. Cunningham, Niehus & Bachtold, 1992), these data could be interpreted to suggest that the animals did not experience negative ethanol hedonics after consumption.
Overall, however, robust sedative effects of ethanol were difficult to detect, particularly in adult animals, seemingly at least in part because of the low levels of activity commonly found among isolate-housed animals in their homecages during the light phase of the diurnal cycle (e.g. see Ristuccia & Spear, 2004). Consequently, ethanol consumption may have appeared to have little influence on adult activity in Exp. 1 because of this floor effect, with ethanol-induced decreases in activity only detected in adolescent rats (who, consistent with previous work, showed higher levels of homecage activity when compared to adults, both when isolate-housed as in this experiment and even more so when housed socially [Ristuccia & Spear, 2004]).
Assuming that the correlational relationship between tachycardia and ethanol reward that has been demonstrated in humans is also evident in rodents, the results of the present study suggest that non-selected adolescent rats consume enough ethanol to potentially experience a positive hedonic effect that is greater than that seen in response to a sweetened control solution, whereas adults will not in the limited-access paradigm used in the present studies. Although the reason why adult outbred rats do not appear to consume enough to experience positive hedonics specific to ethanol within this testing situation are not entirely clear, one possibility is that the greater sensitivity of the adults to various ethanol effects, such as its sedative (Silveri & Spear, 1998), dysphoric (Shram et al., 2005) or behaviorally-suppressing (Varlinskaya & Spear, 2002) effects, may prevent them from consuming enough ethanol to get to the point that it becomes pleasurable for them. In contrast, the lower sensitivity of adolescent animals to these cues might permit the younger animals to consume more ethanol and, hence, to derive positive hedonic effects from the drug.
Whether there are age differences in ethanol's positive hedonic effects per se remains to be directly determined. While the experiments conducted here indicate that a potential difference in ethanol hedonics exists between non-selected adolescent and adult rats, future studies may shed more light on this question by directly measuring ethanol's rewarding effects, both behaviorally and neurobiologically. Furthermore, relating the findings of such an experiment with those of this study may help to validate the use of autonomic assessments as an animal model of reward. Such studies may prove challenging to conduct using a self-administration paradigm in which adolescents typically consume more ethanol than adults, but may provide potentially exciting insights into the mechanisms that drive the marked increase in ethanol consumption seen during adolescence.
Figure 3.
BT data from adolescent (top) and adult (bottom) rats with access to both solutions in Exp. 1 (represented as mean ± SEM degrees C). The gray lines represent BT on the baseline day in the individual solution access groups while the black lines represent the mean of all seven solution access days. The solution access period spanned the bins from 0900 to 1100, with pre-access weighing occurring at 0830.
Figure 4.
Activity data from adolescent (top) and adult (bottom) rats with access to both solutions in Exp. 1 (represented as mean ± SEM counts). The gray lines represent activity on the baseline day in the individual solution access groups while the black lines represent the mean of all seven solution access days. The solution access period spanned the bins from 0900 to 1100, with pre-access weighing occurring at 0830.
Acknowledgments
This work was supported by NIH grants F31 AA16048 to R.C. Ristuccia and R37 AA12525 to L.P. Spear.
References
- Abdel-Rahman A-RA, Dar MS, Wooles WR. Effect of chronic ethanol administration on arterial baroreceptor function and pressor and depressor responsiveness in rats. J Pharmacol Exp Ther. 1985;232:194–201. [PubMed] [Google Scholar]
- Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric infusion of ethanol in rats. Pharmacol Biochem Beh. 2001;68:327–338. doi: 10.1016/s0091-3057(00)00467-6. [DOI] [PubMed] [Google Scholar]
- Assaad JM, Pihl RO, Seguin JR, Nagin D, Vitaro F, Carbonneau R, Tremblay RE. Aggressiveness, family history of alcoholism, and the heart rate response to alcohol intoxication. Exp Clin Psychopharm. 2003;11:158–166. doi: 10.1037/1064-1297.11.2.158. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang Y-M, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produced brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–36. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Webster AA, Lumeng L, Li T, Mcbride WJ, Murphy JM. Heart rate and motor-activating effect of orally self-administered ethanol in alcohol-preferring (P) rats. Alcoholism Clin Exp Res. 2002;26:1162–1170. doi: 10.1097/01.ALC.0000024126.59174.2C. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescent: Similarities and differences from young animals. J Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad J-M, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Brunell SC, Spear LP. The effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcoholism Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Barrett SP, Pihl RO. Psychostimulant users are sensitive to the stimulant properties of alcohol as indexed by alcohol-induced cardiac reactivity. Psychol Addict Beh. 2006;20:478–483. doi: 10.1037/0893-164X.20.4.478. [DOI] [PubMed] [Google Scholar]
- Brunelle C, Barrett SP, Pihl RO. Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Pharmacol Biochem Behav. 2007;22:437–443. doi: 10.1002/hup.866. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcoholism Clin Exp Res. 1997;21:140–149. [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Ditto B. Autonomic reactivity and alcohol-induced dampening in men at risk for alcoholism and men at risk for hypertension. Alcoholism Clin Exp Res. 1995;19:482–489. doi: 10.1111/j.1530-0277.1995.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcoholism: Clin Exp Res. 1998;22:585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Pihl RO. Reliability and validity of alcoholinduced heart rate increase as a measure of sensitivity to the stimulant properties of alcohol. Psychopharmacol. 2001;157:20–30. doi: 10.1007/s002130100741. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Howard MA, Gill SJ, Rubenstein M, Low MJ, Grandy DK. Ethanol-conditioned place preference is reduced in dopamine D2 receptordeficient mice. Pharmacol Biochem Beh. 2000;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus JS, Bachtold JF. Ambient temperature effects on taste aversion conditioned by ethanol: Contribution of ethanol hypothermia. Alcoholism Clin Exp Res. 1992;16:1117–1124. doi: 10.1111/j.1530-0277.1992.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcoholism Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Co C, Smith JE. Rat brain neurotransmitter turnover rates altered during withdrawal from chronic cocaine administration. Brain Res. 1995;682:116–126. doi: 10.1016/0006-8993(95)00327-m. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcoholism Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in response to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risktaking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: Is free choice drinking related to the reinforcing effects of ethanol. Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during responsedependent and response-independent cocaine administration in the rat. Psychopharmacol. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Williams-Hemby L, Whitlow C, Bowen C, Samson HH. Metabolic mapping of the effects of oral alcohol self-administration in rats. Alcoholism Clin Exp Res. 1998;22:176–182. [PubMed] [Google Scholar]
- Holdstock L, King AC, de Witt H. Subjective and objective response to ethanol in moderate/heavy and light social drinkers. Alcoholism Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in responses to ethanol and D-amphetamine: A within-subject study. Alcoholism Clin Exp Res. 2001;25:540–548. [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2003: Volume I Secondary school students (NIH Publication No. 04-5507) Bethesda, MD: National Institute on Drug Abuse; 2003. [Google Scholar]
- Laviola G, Macri S, Morely-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Li TK, Spanagel R, Colombo G, McBride WJ, Porrino LJ, Suzuki T, Rodd-Hendricks ZA. Alcohol reinforcement and voluntary ethanol consumption. Alcoholism Clin Exp Res. 2001;25:117s–126s. doi: 10.1097/00000374-200105051-00021. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcoholism Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Ethanol, but not the anxiolytic drugs buspirone and diazepam, produced a conditioned place preference in rats exposed to conditioned fear stress. Pharmacol Biochem Beh. 2000;65:281–288. doi: 10.1016/s0091-3057(99)00224-5. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Séguin JR, Finn PR, Stewart SH. Heart-rate reactivity and alcohol consumption among sons of male alcoholics and sons of nonalcoholics. J Psychiatry Neurosci. 1993;18:190–198. [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Pihl RO, Gianoulakis C, Conrod P, Finn PR, Stewart SH, Bruce KR. Ethanol-induced change in cardiac and endogenous opiate function and risk for alcoholism. Alcoholism Clin Exp Res. 1996;20:1542–1552. doi: 10.1111/j.1530-0277.1996.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: Age-related changes in the rewarding and aversive effects of alcohol. Alcoholism Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent ethanol sensitivity: Hypothermia and acute tolerance. Ann NY Acad Sci. 2004;1021:445–447. doi: 10.1196/annals.1308.061. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Brunell SC, Spear LP. Limited access ethanol consumption in adolescent and adult rats. Dev Psychobio. 2005;47:125. [Google Scholar]
- Ristuccia RC, Spear LP. Autonomic responses to ethanol in adolescent and adult rats: A dose-response analysis. submitted. [DOI] [PMC free article] [PubMed]
- Ristuccia RC, Spear LP. Age differences in sensitivity and tolerance to the autonomic effects of ethanol. Alcoholism Clin Exp Res. 2005;29:1809–1820. doi: 10.1097/01.alc.0000183010.72764.cd. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Genetics and the risk for alcoholism. Am J of Addiction. 2000;9:103–109. doi: 10.1080/10550490050173172. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Chau V, Li Z, Lê Age differences in the aversive properties of alcohol. Alcoholism Clin Exp Res Supplement to. 2005;29:16A. [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcoholism Clin Exp Res. 1998;20:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: Observations when equating ethanol perturbation across age. Alcoholism Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: Role of familiarity of the test situation. Alcoholism Clin Exp Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Beh. 2002;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]