Abstract
Janus kinase 2 (Jak2) protein tyrosine kinase plays an important role in interleukin-3– or granulocyte–macrophage colony-stimulating factor–mediated signal transduction pathways leading to cell proliferation, activation of early response genes, and inhibition of apoptosis. However, it is unclear whether Jak2 can activate these signaling pathways directly without the involvement of cytokine receptor phosphorylation. To investigate the specific role of Jak2 in the regulation of signal transduction pathways, we generated gyrase B (GyrB)–Jak2 fusion proteins, dimerized through the addition of coumermycin. Coumermycin induced autophosphorylation of GyrB–Jak2 fusion proteins, thus bypassing receptor activation. Using different types of chimeric Jak2 molecules, we observed that although the kinase domain of Jak2 is sufficient for autophosphorylation, the N-terminal regions are essential for the phosphorylation of Stat5 and for the induction of short-term cell proliferation. Moreover, coumermycin-induced activation of Jak2 can also lead to increased levels of c-myc and CIS mRNAs in BA/F3 cells stably expressing the Jak2 fusion protein with the intact N-terminal region. Conversely, activation of the chimeric Jak2 induced neither phosphorylation of Shc or SHP-2 nor activation of the c-fos promoter. Here, we showed that the GyrB–Jak2 system can serve as an excellent model to dissect signals of receptor-dependent and -independent events. We also obtained evidence indicating a role for the N-terminal region of Jak2 in downstream signaling events.
INTRODUCTION
The Janus kinase (Jak) family of cytoplasmic protein tyrosine kinases, composed of Jak1, Jak2, Jak3, and Tyk2, has a kinase domain in the C terminus (JH1), a pseudokinase domain (JH2), and a large N-terminal region with highly conserved Jak homology domains (JH3–JH7). The N-terminal region of the Jaks appears to be responsible for interactions with the β subunit of hematopoietic growth factor receptors, whereas the kinase domain is required for functional activity (Frank et al., 1995; Zhao et al., 1995; Chen et al., 1997). By bringing at least two Jak molecules in close proximity, Jaks become activated likely through transphosphorylation. Like other tyrosine kinases, phosphorylation of tyrosine residues within the activation loop of the kinase domain is probably an essential part of this activation (Feng et al., 1998). The activated Jaks subsequently phosphorylate tyrosine residues of cytokine receptors as well as a variety of substrates, most notably the Stat family of transcription factors (Ihle, 1996). Upon phosphorylation, Stats undergo dimerization and translocate to the nucleus where they bind to specific DNA sequence and enhance the transcription of specific genes (Darnell et al., 1994).
Granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 exert biological activities, such as cell proliferation, activation of early response genes, and inhibition of apoptosis, through heterodimeric receptors composed of α and β subunits (Arai et al., 1990). The α subunit is specific for each receptor (Gearing et al., 1989), whereas the β subunit (βc) is shared by all (Kitamura et al., 1991). We reported that IL-3 and GM-CSF stimulate multiple signal transduction pathways through distinct cytoplasmic domains of the βc (Sakamaki et al., 1992; Watanabe et al., 1993b). The membrane proximal region, which contains the box1 motif, is involved in the induction of c-myc as well as in cell proliferation. In addition to the membrane proximal region, tyrosine residues in the membrane distal region are required for activation of the MAP kinase cascade leading to c-fos promoter activation (Itoh et al., 1996, 1998). Experiments using dominant negative Jak2 revealed that all known activities of GM-CSF depend on the activation of Jak2 (Watanabe et al., 1996), and the binding of overexpressed Jak2 to the membrane proximal region of βc-containing box1 was noted in insect cells (Quelle et al., 1994). Because the activation of various signaling events by βc mutants indicated that the box1 region is essential for the activation of all the tested GM-CSF–induced phenomena (Itoh et al., 1996), GM-CSF may exert its activities through Jak2 activation, which binds to the box1 region of βc. Downstream of Jak2, there are probably at least two different types of signaling pathways, one depending on the tyrosine phosphorylation of βc and the other arising from Jak2, without involvement of a receptor domain. In this study we focused on the signals derived from Jak2.
To isolate the activation of this protein tyrosine kinase aside from other growth factor-induced signals, we introduced the coumermycin/gyrase B (GyrB) system (Farrar et al., 1996). Coumermycin acts as a natural dimerizer of GyrB, because it binds GyrB with a stoichiometry of 1:2. To generate a Jak2 molecule that could be induced to dimerize by coumermycin, we constructed chimeras involving GyrB and the entire Jak2 or GyrB fused to the Jak2 kinase region only. We report here that GyrB–Jak2 becomes activated in the presence of coumermycin, and the importance of the N-terminal region of Jak2 for downstream signaling events became evident.
MATERIALS AND METHODS
Reagents and Antibodies
The GyrB cDNA (pKS-GyrB) was kindly provided by Dr. R. Perlmutter (University of Washington, Seattle, WA). Cytokine-inducible SH2-containing protein (CIS) cDNA used as a probe in the Northern blot analysis was a gift from Dr. A. Yoshimura (Kurume University, Kurume, Japan). Coumermycin was obtained from Sigma Chemical (St. Louis, MO). G418 was a gift from Schering-Plough (Kenilworth, NJ). The anti-Jak2 antibody (C-20), mainly used for immunoprecipitation, anti-Stat1, anti-Stat3, anti-Stat4, anti-Stat5a, anti-Stat5b, anti-Stat6, and anti-Src homology 2-containing protein-tyrosine phosphatase (SHP-2) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-phosphotyrosine antibody (4G10), anti-Shc antibody, and anti-Jak2 antibody, used for Western blotting, were purchased from Upstate Biotechnology (Lake Placid, NY). Fetal calf serum (FCS) was purchased from Biocell Laboratories (Carson, CA). RPMI 1640 medium was purchased from Nikken BioMedical Laboratories (Kyoto, Japan). Recombinant murine IL-3 (mIL-3) expressed in silkworm, Bombyx mori, was purified as described elsewhere (Miyajima et al., 1987).
Construction of Plasmids
Jak2 cDNA was originally cloned into the pME18S vector, which has the SRα promoter (Takebe et al., 1988), as described (Watanabe et al., 1996). There are seven conserved regions in Jak family proteins, which are referred to as JH1–JH7 starting with the most C-terminal end (Ihle and Kerr, 1995). JH1 and JH2 correspond to kinase and pseudokinase domains, respectively. The cDNA for GyrB fused to the 5′ end of N terminus–truncated Jak2 containing JH2 and JH1 regions (GNJK) was constructed by replacing the JH7 to JH3 region (BstxI–MscI fragment blunt ended using T4 DNA polymerase) of pME18S-Jak2 with GyrB sequence (NotI–SpeI fragment blunt ended using the Klenow fragment) of pKS-GyrB. The cDNA for GyrB inserted between the JH3 and JH2 domains of full-length Jak2 (GIJW) was prepared by inserting GyrB (blunt-ended SacI-SpeI fragment from pKS-GyrB) into the MscI site of pME18S-Jak2. To construct the cDNA for GyrB fused to the N-terminus of whole Jak2 (GNJW), the coding region of GyrB was isolated from pKS-GyrB at NotI and EcoRV sites and blunt ended using the Klenow fragment. The fragment was then ligated into BstxI-digested pME18S-Jak2, which was also blunt ended using T4 DNA polymerase. All the constructs were verified by restriction enzyme digestion and by dideoxy sequencing using an automated sequencer (Applied Biosystems, Foster City, CA).
Cell Culture and Transfections
COS7 cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% FCS, 50 units/ml penicillin, and 50 μg/ml streptomycin. Transient transfection of plasmids into COS7 cells was done by using the DEAE-dextran method (Maniatis et al., 1982). A mIL-3–dependent proB cell line, BA/F3 (Palacios and Steinmetz, 1985), was maintained in RPMI 1640 medium supplemented with 5% fetal calf serum, 50 units/ml penicillin, 50 μg/ml streptomycin, and 0.25 ng/ml mIL-3. To obtain stable transfectants, BA/F3 cells were cotransfected with 13.5 μg of GNJK, GIJW, or GNJW plasmids together with 1.5 μg of pKU-2Neo vector, containing the neomycin resistance gene, by electroporation, as described (Watanabe et al., 1995a). After selection in 1 mg/ml G418 for ∼15 d, drug-resistant clones were screened for protein expression by Western blotting using anti-Jak2 antibody.
Immunoprecipitation and Western Blotting
Immunoprecipitation and Western blotting were done as described (Itoh et al., 1996). Briefly, cells (1 × 107 cells per sample) were harvested and lysed in 500 μl of ice-cold lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 100 μM Na3VO4, 50 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 1 μg/ml pepstatin A) for 1 h at 4°C. Cell lysates were incubated for 2 h at 4°C together with the indicated antibody and protein A-Sepharose beads (Pharmacia, Piscataway, NJ). The immunoprecipitates were separated on a 7% SDS-polyacrylamide gel and transferred electrophoretically to an Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, MA). After blocking with 5% bovine serum albumin (Fraction V, Sigma), the membrane was subjected to Western blot analysis with appropriate antibodies and visualized using an enhanced chemiluminescence detection kit (Amersham, Buckinghamshire, United Kingdom) as described previously (Watanabe et al., 1996).
Luciferase Assay
BA/F3 cells stably expressing various GyrB–Jak2 fusion proteins (1 × 106 cells per sample) were transfected with c-fos–luciferase DNA (Watanabe et al., 1993a) (1 μg per sample) or β-casein–luciferase (Wakao et al., 1994) (3 μg per sample) by electroporation, as described (Watanabe et al., 1995a). The cells were maintained in mIL-3 medium for ∼12 h, and then mIL-3 was depleted for 6 h, followed by stimulation with mIL-3 (1 ng/ml), coumermycin (1 μM), or novobiocin (1 μM) for 6 h, and then harvested. Protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL), and the luciferase activity was measured using a luminometer (model LB9501; Berthold Lumat, Tokyo, Japan) and a luciferase assay substrate (Promega, Madison, WI). All values are expressed relative to the protein concentration.
Cell Proliferation Assay
Cell proliferation was measured by [3H]thymidine incorporation, as described (Watanabe et al., 1993a). Briefly, cells were seeded into 96-well plates (1 × 104 cells per well) with various concentrations of coumermycin or mIL-3. After 24 h of culture, [3H]thymidine (1 μCi/well) was added, followed by incubation for another 3 h before harvest. Cells were harvested onto a glass fiber filter, and [3H]thymidine incorporation was measured using a filter counter (model 1450 MicroBeta; Wallac, Turku, Finland).
DNA Fragmentation Assay
To detect DNA fragmentation, 5 × 106 cells were cultured for 16 h in 5% FCS-containing RPMI 1640 medium, the same medium supplemented with 1 ng/ml mIL-3, or 1 μM coumermycin. Low-molecular-weight chromosomal DNA was isolated using the ApopLader Ex kit (Takara Biomedicals, Shiga, Japan) according to the manufacturer’s instructions and electrophoresed through a 2% agarose gel. DNA fragments were visualized by ethidium bromide staining.
Northern Blot Analysis
Northern blots were performed with mRNA prepared using the Fast Track 2.0 kit (Invitrogen, San Diego, CA). Briefly, 1 μg of mRNA was separated on a 1% agarose gel containing 6% formaldehyde and transferred onto a nylon membrane (Hybond-N, Amersham) by capillary blotting. The blots were hybridized with cDNA probes (c-myc, CIS, and glyceraldehyde-3-phosphate dehydrogenase genes) labeled by the Ready-To-Go kit (Pharmacia) using [α-32P]dCTP. The blotted membrane was visualized using a Fuji BAS-3000 image analyzer (Tokyo, Japan).
RESULTS
Construction and Expression of GyrB–Jak2 Fusion Proteins
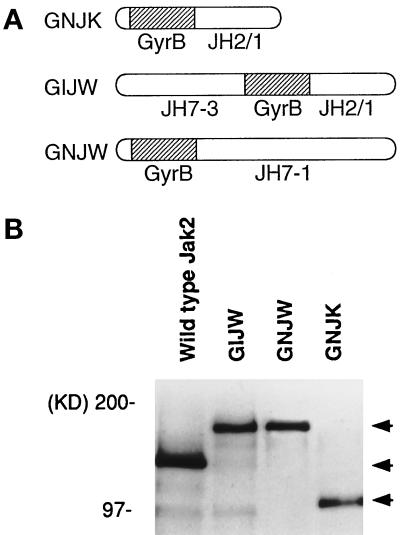
To examine the specific role of Jak2 in signal transduction, we used a coumermycin-induced chemical dimerization method (Farrar et al., 1996). We constructed three different fusion genes in which GyrB was fused to Jak2. Figure 1A shows a schematic diagram of fusion proteins GNJK (GyrB fused to the 5′ end of the JH2–JH1 fragment of Jak2), GIJW (GyrB is inserted between JH2 and JH3 of full-length Jak2), and GNJW (GyrB fused to the 5′ end of full-length Jak2). The size and level of fusion protein expression were examined in COS7 cells by immunoprecipitation followed by Western blotting using anti-Jak2 antibody. GNJK was detected at ∼100 kDa, and GIJW and GNJW migrated at ∼160 kDa, being consistent with the predicted molecular mass (Figure 1B). The constructs were then used to generate stable transfectants in BA/F3 cells. The sizes and levels of the transfected fusion proteins in BA/F3 stable cell lines were confirmed by immunoprecipitation followed by Western blotting, using a Jak2-specific antibody (Figure 2A, bottom panel).
Figure 1.
Expression of GyrB–Jak2 fusion proteins in COS7 cells. (A) Schematic diagram of the GyrB–Jak2 fusion protein constructs. Open bar, region from Jak2; hatched bar, GyrB sequence. In GNJK, GyrB is fused to the 5′ end of the JH2–JH1 region of Jak2. In GIJW and GNJW, GyrB is inserted between the JH3 and JH2 regions or fused at the N terminus of full length Jak2, respectively. (B) Expression of GNJK, GIJW, GNJW and the wild-type Jak2 (as control) were examined in COS7 cells. Immunoprecipitation followed by Western blotting was done using anti-Jak2 antibody. The positions of the expressed Jak2 and GyrB–Jak2 fusion proteins and molecular mass standards are shown.
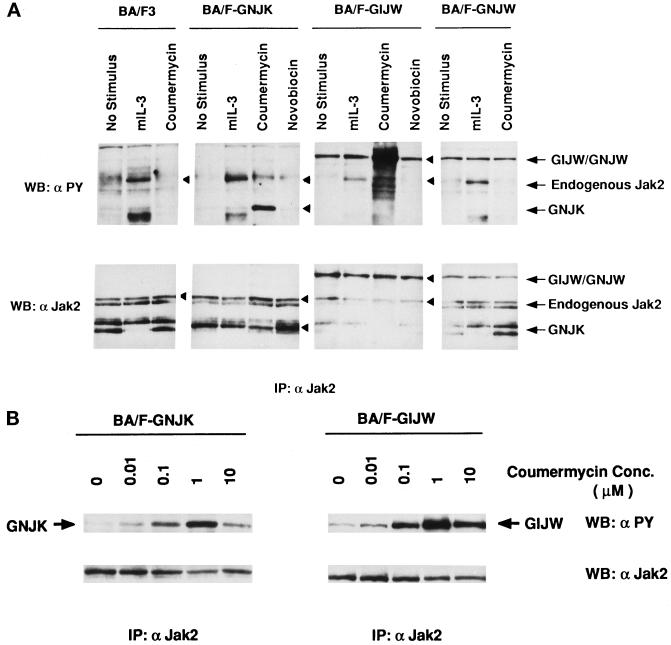
Figure 2.
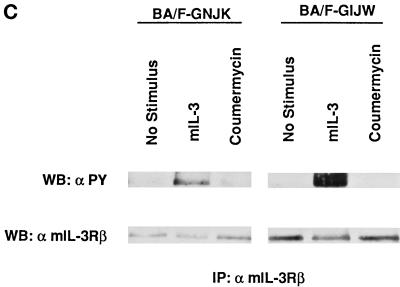
Coumermycin-induced tyrosine phosphorylation of GyrB–Jak2 fusion proteins in BA/F3 transfectants. (A) Tyrosine phosphorylation of GyrB–Jak2 in BA/F-GNJK, -GIJW, or -GNJW cells. The factor-depleted cells were stimulated with 1 ng/ml mIL-3, 1 μM coumermycin, or 1 μM novobiocin for 15 min. Jak2 immunoprecipitants were blotted with either anti-phosphotyrosine antibody (top panel, α PY) or anti-Jak2 antibody (bottom panel). The arrows indicate the 160-kDa GIJW/GNJW bands, the 130-kDa endogenous Jak2 band, and the 100-kDa GNJK band. (B) Dose response of coumermycin-induced tyrosine phosphorylation of GyrB–Jak2 proteins. The factor-depleted BA/F-GNJK and BA/F-GIJW cells were stimulated for 15 min with the indicated amounts of coumermycin. (C) Tyrosine phosphorylation of mIL-3 receptor β subunit. Immunoprecipitation was done using anti-mIL-3 receptor β subunit, and the membrane was blotted with either anti-phosphotyrosine antibody (top panel, α PY), or anti-β antibody (bottom panel).
Coumermycin-induced Tyrosine Phosphorylation of GyrB–Jak2 Fusion Proteins in BA/F3 Cells
Because tyrosine phosphorylation of Jak2 closely correlates with the tyrosine kinase activity of Jak2 (Witthuhn et al., 1993; Ihle, 1995; Nakamura et al., 1996), we first examined the tyrosine phosphorylation status of the GyrB–Jak2 fusion proteins by immunoprecipitation followed by Western blotting with anti-phosphotyrosine antibody (4G10). As shown in Figure 2A, addition of coumermycin induced tyrosine phosphorylation of the GNJK (100 kDa) and the GIJW (160 kDa) fusion proteins but not the GNJW fusion protein or the endogenous Jak2. The tyrosine-phosphorylated bands close to the position of endogenous Jak2 appearing in the 6th and 10th lanes were different from endogenous Jak2, because these bands migrated slightly slower than the endogenous Jak2. The monomeric coumarin antibiotic novobiocin did not induce GNJK and GIJW phosphorylation, as expected. The endogenous Jak2, but not the fusion proteins, was tyrosine phosphorylated after mIL-3 stimulation. In BA/F-GIJW and -GNJW cells, fusion proteins but not endogenous Jak2 were slightly tyrosine phosphorylated, even in the absence of mIL-3 or coumermycin, possibly because of overexpression of the fusion proteins. The tyrosine phosphorylation status of the GNJW fusion protein was not changed after coumermycin stimulation, possibly because the GyrB fused at the N terminus of the entire Jak2 molecule could not bring the kinase domains of two Jak2 molecules close enough to cross-phosphorylate each other after induction with coumermycin. Because GNJW is not phosphorylated in response to coumermycin, BA/F-GNJK and BA/F-GIJW cells were used for the following experiments.
We next examined the coumermycin dose dependence of GyrB–Jak2 phosphorylation. The maximum activation occurred at a concentration of 1 μM coumermycin (Figure 2B) in both BA/F-GNJK and BA/F-GIJW cells. The level of phosphorylation induced by 10 μM coumermycin was less than that induced by 1 μM coumermycin. These results are consistent with the idea that excess amounts of coumermycin may prevent dimer formation because of 1:1 rather than 1:2 stoichiometrical binding of coumermycin and fusion proteins. Taken together, these results suggest that coumermycin induces phosphorylation of the GyrB–Jak2 protein by dimerization. To analyze whether GyrB–Jak2 phosphorylates the endogenous mIL-3 receptor, we checked tyrosine phosphorylation of the mIL-3 receptor β subunit by immunoprecipitation followed by Western blotting using anti-phosphotyrosine antibody. As shown in Figure 2C, the mIL-3 receptor β subunit is tyrosine phosphorylated in response to stimulation with mIL-3 in both cells but is not phosphorylated by the addition of coumermycin. This result indicates that the coumermycin-induced activation of the GyrB–Jak2 fusion protein bypasses the receptor activation.
Stat5 but neither SHP-2 nor Shc Was Tyrosine Phosphorylated in BA/F-GIJW Cells
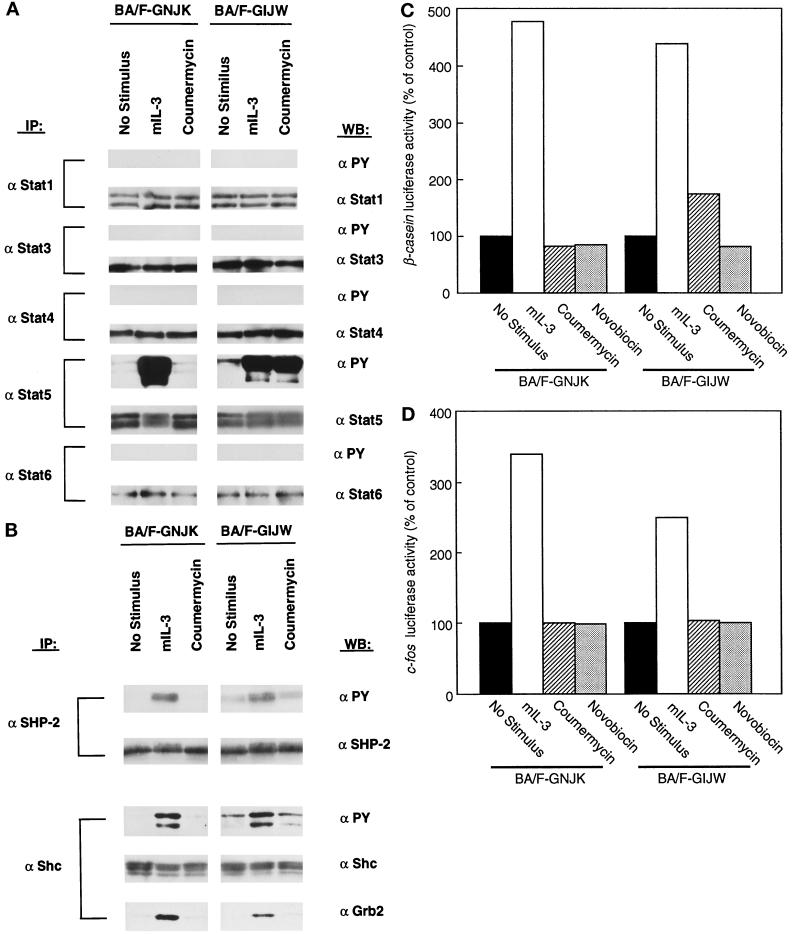
Because Jak2 is responsible for the phosphorylation of a variety of cellular proteins after IL-3 and GM-CSF stimulation (Watanabe et al., 1995b), we next attempted to identify molecules that were tyrosine phosphorylated after coumermycin-induced activation of GyrB–Jak2 fusion proteins. We first examined Stat5, which is phosphorylated after Jak2 activation by mIL-3 stimulation in BA/F3 cells (Mui et al., 1995). The tyrosine phosphorylation of Stat5 was investigated by immunoprecipitation, using anti-Stat5a and anti-Stat5b antibodies, followed by Western blotting with anti-phosphotyrosine antibody (Figure 3A). The phosphorylation was observed only in BA/F-GIJW cells, not in BA/F-GNJK cells. Therefore, the JH3–JH7 regions of Jak2, in addition to the kinase and pseudokinase domains, are involved in Stat5 phosphorylation. We also examined whether other members of Stats could be activated in these cells by immunoprecipitation using specific Stat antibodies followed by Western blotting with anti-phosphotyrosine antibody. We did not observe any phosphorylation of Stat1, Stat3, Stat4, and Stat6 after stimulation with mIL-3 or coumermycin in both BA/F-GNJK and BA/F-GIJW cells.
Figure 3.
Activation of signal molecules by the addition of coumermycin in BA/F3 transfectants. BA/F-GNJK and BA/F-GIJW cells were factor depleted for 6 h and stimulated with mIL-3 or coumermycin (1 μM) for 15 min. Cell lysates were immunoprecipitated with anti-Stat1, anti-Stat3, anti-Stat4, anti-Stat5, and anti-Stat6 antibodies (A) or anti-SHP-2 and anti-Shc antibodies (B) followed by immunoblotting with anti-phosphotyrosine or appropriate antibodies. Activation of β-casein (C) and c-fos promoter (D) was measured by transient transfection assay using β-casein–luciferase and c-fos–luciferase as reporter genes. BA/F-GNJK and BA/F-GIJW cells were transfected with either β-casein–luciferase or c-fos–luciferase, and luciferase activities induced by 1 ng/ml mIL-3 (□), 1 μM coumermycin (▧), or 1 μM novobiocin (▩) were analyzed as described in MATERIALS AND METHODS. The results are represented as a relative value of that of the unstimulated controls (▪). Experiments were done at least three times, and essentially the same results were obtained.
We next addressed the question of tyrosine phosphorylation of SHP-2 and Shc, because these have been implicated in the activation of the Ras–MAP kinase pathway leading to activation of the c-fos serum response element site (Watanabe et al., 1997). Stimulation by mIL-3 resulted in tyrosine phosphorylation of SHP-2 and Shc (Figure 3B), as expected. In contrast, coumermycin stimulation did not induce tyrosine phosphorylation of SHP-2 or Shc in either BA/F-GNJK cells or BA/F-GIJW cells. Reprobing the Shc immunoprecipitant membrane with an anti-Grb2 antibody revealed that the adapter protein Grb2, which forms a complex with Shc after mIL-3 stimulation (Itoh et al., 1998), could not be coimmunoprecipitated by coumermycin stimulation.
We further analyzed the functional activities of the Stat and MAP kinase cascades using a transient transfection analysis of β-casein and c-fos promoter fused to the luciferase gene. As shown in Figure 3C, β-casein–luciferase activity is induced in BA/F-GIJW but not in BA/F-GNJK cells in response to the addition of coumermycin. It is notable that the level of induction is far less than that observed with mIL-3 stimulation. Transient transfection assays using c-fos–luciferase as the reporter gene revealed that the c-fos promoter was not activated by stimulation with coumermycin in BA/F-GNJK or BA/F-GIJW cells (Figure 3D). Phosphorylated tyrosine residues of the cytoplasmic distal region of βc are essential for the recruitment and activation of both SHP-2 and Shc (Itoh et al., 1996, 1998). Taken together, these results indicate that the activation of GyrB–Jak2 fusion protein is insufficient to activate the Ras–MAP kinase pathway leading to c-fos promoter activation.
Coumermycin-induced Growth of BA/F-GIJW Cells
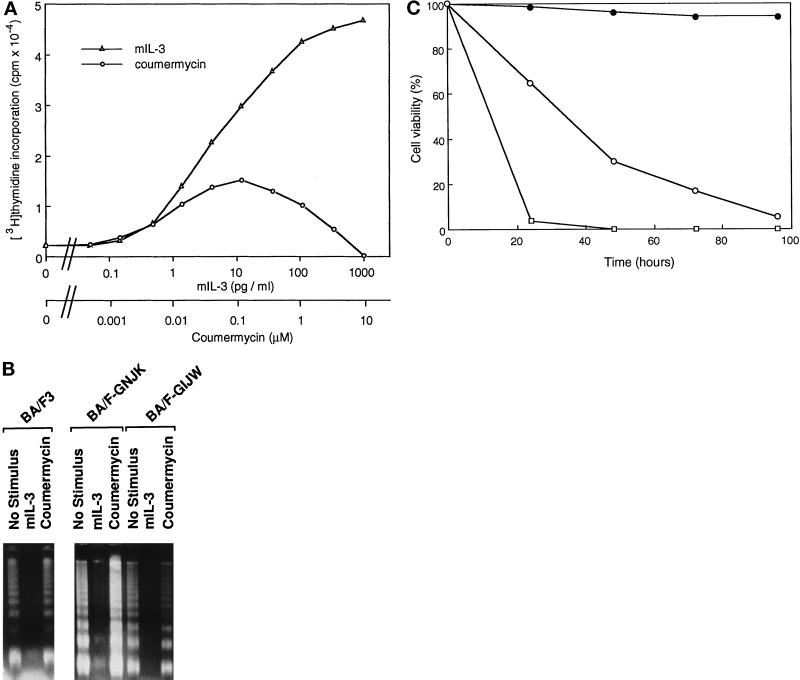
We reported that Jak2 plays an essential role in cell proliferation (Watanabe et al., 1996). To determine whether GyrB–Jak2 fusion proteins have growth-promoting function, we analyzed [3H]thymidine incorporation in response to coumermycin in BA/F-GNJK and -GIJW cells. Cells were washed with depletion media and cultured in various concentrations of coumermycin or mIL-3 for 24 h. In response to coumermycin stimulation, incorporation of [3H]thymidine was observed in BA/F-GIJW cells but not in BA/F-GNJK cells (our unpublished results). We next examined the coumermycin dose dependency for [3H]thymidine incorporation in BA/F-GIJW cells. As shown in Figure 4A, coumermycin-induced [3H]thymidine incorporation was observed even with 1.4 nM coumermycin and reached the maximum level at 0.12 μM coumermycin. The level of incorporation was lower than that observed in the case of mIL-3 stimulation, even with the optimal concentration of coumermycin. However, with higher doses of coumermycin there was a decline in activity, and the value returned close to the basal level with 10 μM coumermycin. These results further confirm that activation of GyrB–Jak2 proceeds through dimerization and that Jak2 activation can lead to proliferative signals.
Figure 4.
Effects of coumermycin on growth, apoptosis, and viability of BA/F3 cells. (A) [3H]Thymidine incorporation induced by mIL-3 or coumermycin in BA/F-GIJW cells. Factor-depleted cells were cultured in various concentrations of mIL-3 or coumermycin for 24 h and labeled with 1 μCi of [3H]thymidine for another 3 h. (B) Effect of coumermycin on DNA fragmentation induced by factor depletion. BA/F3, BA/F-GNJK, and BA/F-GIJW cells were cultured for 16 h in mIL-3 or coumermycin (1 μM) or factor-free medium. DNA fragmentation was examined by gel electrophoresis. (C) Effect of coumermycin on BA/F-GIJW cell viability as measured by trypan blue exclusion. Cells were cultured in depletion medium (□), mIL-3 medium (•), or coumermycin medium (○). Results are means for triplicate determinations.
We next tested the potential of GyrB–Jak2 fusion proteins to prevent apoptosis, measured by a chromosomal DNA fragmentation assay. Within 16 h of mIL-3 depletion, characteristic DNA fragmentation was observed in parental BA/F3 cells (Liu, Itoh, Arai, and Watanabe, unpublished data), and substantial DNA fragmentation was observed in both BA/F-GNJK and BA/F-GIJW cells by 16 h of factor depletion, as expected (Figure 4B). DNA fragmentation was prevented when the cells were cultured in the presence of mIL-3. In the presence of coumermycin, DNA fragmentation occurred even in BA/F-GIJW cells, which can stimulate [3H]thymidine incorporation. Therefore, the activation of GyrB–Jak2 fusion protein is insufficient to prevent factor depletion-induced apoptosis.
We next tested the long-term cell viability of the BA/F-GIJW cell in the presence of coumermycin by trypan blue exclusion. Although virtually no cell was viable after 48 h of factor depletion, the presence of coumermycin kept a small but significant population of cells viable for >72 h (Figure 4C). After 100 h of culture, no cell was viable, indicating that GIJW can sustain short-term but not long-term proliferation or cell survival.
Induction of c-myc and CIS mRNA by Coumermycin in BA/F-GIJW Cells
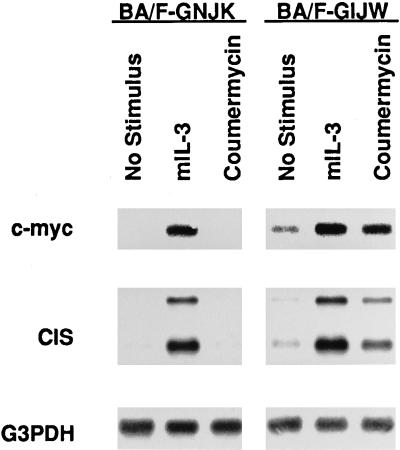
c-myc is induced by mIL-3 and human GM-CSF (hGM-CSF) stimulation in BA/F3 cells expressing the hGM-CSF receptor (Watanabe et al., 1993a). We reported previously that the membrane proximal region, including box1 and box2 of βc, is essential and sufficient to induce c-myc mRNA in BA/F3 cells (Watanabe et al., 1995a, 1993b). This region is also essential for GM-CSF–induced phosphorylation of Jak2, and dominant negative Jak2 suppressed c-myc induction by hGM-CSF (Watanabe et al., 1996). We next asked whether the activation of GyrB–Jak2 fusion proteins would lead to the induction of c-myc as determined by Northern blot analysis. As shown in Figure 5, coumermycin induced an increase in the level of c-myc mRNA expression in BA/F-GIJW but not BA/F-GNJK cells. The level of induction was slightly lower than that observed in the case of mIL-3 stimulation. CIS, a negative regulator of cytokine signals, is another immediate-early gene that may be a target of the Jak–Stat pathway (Yoshimura et al., 1995). Because the coumermycin-induced activation of GyrB–GIJW proved to be sufficient to activate Stat5, we next examined the induction of CIS in these cells by Northern blot analysis. The addition of mIL-3 resulted in the activation of the CIS gene in both BA/F-GNJK and BA/F-GIJW cells. Induction of CIS mRNA was observed in BA/F-GIJW but not in BA/F-GNJK cells by coumermycin stimulation.
Figure 5.
Coumermycin-dependent induction of c-myc and CIS mRNAs. BA/F-GNJK and BA/F-GIJW cells were factor depleted for 5 h and stimulated with 1 ng/ml mIL-3 or 1 μM coumermycin for 30 min. mRNA was extracted, and Northern blot analysis was performed using c-myc, CIS, and glyceraldehyde-3-phosphate dehydrogenase (G3PDH) as probes.
DISCUSSION
In the present study, we analyzed the mechanisms of Jak2 activation and its specific role in signal transduction pathways. For this we made use of GyrB–Jak2 chimeric proteins, which can be artificially dimerized by antibiotics without involving ligand–receptor systems. Because ligand binding to cytokine receptors apparently induces dimerization of the receptor subunits, leading to transactivation of associated Jak molecules (O’Shea, 1997), we speculated that Jak2 could possibly be activated by artificial forced dimerization. Such an approach has been successfully used to activate Raf-1 kinase, using a GyrB–Raf1 fusion protein (Farrar et al., 1996). We found that coumermycin induced phosphorylation of GyrB–Jak2 without the induction of endogenous Jak2 phosphorylation. Therefore, it is evident that dimerization or aggregation of Jak2 is important for its activation. Although GyrB–Jak2 was activated, the βc was not phosphorylated upon coumermycin stimulation. So, we speculate that the activation of Jak2 may not be sufficient to phosphorylate the βc chains. Also, SHP-2 and Shc were not tyrosine phosphorylated by coumermycin, possibly because the receptor βc was not phosphorylated. These observations support the findings that phosphorylated tyrosine residues of the cytoplasmic distal region of the βc are essential for phosphorylation of both SHP-2 and Shc (Itoh et al., 1996, 1998). Thus, we isolated signaling downstream of Jak2 from growth factor receptor–dependent signals.
We observed that GyrB–Jak2 specifically activated Stat5. No other members of Stats could be phosphorylated upon coumermycin stimulation, indicating that the fusion protein has some specificity in phosphorylating the cellular substrates. It is believed that activation of Stat by cytokine stimulation requires tyrosine residues of the cytokine receptor (Stahl et al., 1995; Ihle, 1996). Our findings imply that Stat5 can be activated without the involvement of receptor tyrosine residues. To support this view, it was reported that there may be two distinct pathways for Stat5 activation, one by creating tyrosine-phosphorylated docking sites at the cytokine receptor and the other by direct interaction between Jaks and Stat5 (Fujitani et al., 1997). We found the induction of Stat5 phosphorylation was diminished but not completely abrogated when all eight tyrosines of the βc were mutated (Itoh et al., 1998). It therefore seems feasible that in growth factor signaling, two distinct pathways act synergistically for the maximum activation of Stat5.
We reported the essential role of Jak2 in GM-CSF–dependent cell proliferation using dominant negative Jak2 (Watanabe et al., 1996). In this study, coumermycin-induced activation of GyrB–Jak2 can lead to cell proliferation in BA/F-GIJW cells. However, the level of incorporation of [3H]thymidine by coumermycin was lower than that observed in the case of mIL-3 stimulation, and proliferation was only short term. Using a series of βc mutants containing mutated tyrosine residues, we showed the requirement of receptor tyrosine residues to promote proliferation. Any single tyrosine residue maintains the ability to induce proliferation (Itoh et al., 1998); thus lack of cell proliferation in the present system may be explained by lack of receptor involvement. Factor depletion–induced apoptosis occurred even in the presence of coumermycin implied that GyrB–Jak2 activation is insufficient to prevent apoptosis. In an apparent contradiction with our present finding, a previous report (Sakai and Kraft, 1997) demonstrated that activation of CD16–Jak2-kinase domain fusion is sufficient to prevent cells from apoptosis and showed constitutive phosphorylation of Shc, which also has been implicated in transducing signals to the Ras–MAP kinase pathway. Our series of analysis of antiapoptosis activity of hGM-CSF using various βc mutants revealed that Jak2 activation through the box 1 region is essential, and that activation of either a tyrosine kinase inhibitor genistein-sensitive pathway or the MAP kinase cascade is sufficient to sustain cell viability (Liu, Itoh, and Watanabe unpublished data). Thus, the ability of the CD16–Jak2 chimera to prevent cells from apoptosis may be facilitated through the MAP kinase pathway which in downstream of Shc activation. The most prominent difference between GyrB–Jak2 and CD16–Jak2 may be in the subcellular localization of the fusion proteins. It is possible that although Jak2 alone is insufficient, with the help of other signaling molecules presumably located at the membrane, it can induce antiapoptotic signals. This hypothesis may also explain the lack of SHP-2 and Shc phosphorylation.
Our results show that c-myc and CIS are targets of Jak2 activation, because the presence of coumermycin led to an increase in the levels of c-myc and CIS mRNAs. However, it remains to be determined how the activation of Jak2 ultimately leads to the activation of c-myc. It has been reported that putative E2F binding sites at the P2 region of the c-myc promoter play an important role in mIL-3– or hGM-CSF–induced activation of c-myc (Watanabe et al., 1995a). It is therefore tempting to speculate that Jak2 activates the c-myc promoter by altering the composition of E2F complexes. But how Jak2 alters the composition of E2F complexes is not clear.
We also found that the N-terminal domains of Jak2 are required for the activation of Stat5 as well as for induction of c-myc and CIS genes, because they are induced only in BA/F-GIJW cells. Therefore, the N-terminal regions (JH3–JH7) of Jak2 thought to be important for binding of the cytokine receptor are also required for activation of some signaling events possibly by binding or interacting with other signaling molecules required for induction of such events. Future efforts toward defining the functional aspects of all the JH regions may elucidate mechanisms of signal transduction mediated by Jak2.
ACKNOWLEDGMENTS
We thank T. Itoh, A. Muto, M. Dahl, Y. Sakurai, and R. Liu for helpful discussion, Y. Aoki for excellent technical assistance, and M. Ohara for helpful comments.
REFERENCES
- Arai K, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Chen M, Cheng A, Chen Y-Q, Hymel A, Hanson EP, Kimmel L, Minami Y, Taniguchi T, Changelian PS, O’Shea JJ. The amino terminus of Jak3 is necessary and sufficient for binding to the common γ chain and confers the ability to transmit iterleukin 2-mediated signals. Proc Natl Acad Sci USA. 1997;94:6910–6915. doi: 10.1073/pnas.94.13.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Alberola-Ila J, Perlmutter RM. Activation of the raf-1 kinase cascade by coumermycin-induced dimerization. Nature. 1996;383:178–181. doi: 10.1038/383178a0. [DOI] [PubMed] [Google Scholar]
- Feng J, Witthuhn BA, Matsuda T, Kohlhuber F, Kerr IM, Ihle JN. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol Cell Biol. 1998;17:2497–2501. doi: 10.1128/mcb.17.5.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SJ, Yi W, Zhao Y, Goldsmith JF, Gilliland G, Jiag J, Sakai I, Kraft AS. Regions of the JAK2 tyrosie kinase required for coupling to the growth hormone receptor. J Biol Chem. 1995;270:14776–14785. doi: 10.1074/jbc.270.24.14776. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Hibi M, Fukada T, Takahashi-Tezuka M, Yoshida H, Yamaguchi T, Sugiyama K, Yamanaka Y, Nakajima K, Hirao T. An alternative pathway for STAT activation that is mediated by the direct interaction between JAK and STAT. Oncogene. 1997;14:751–761. doi: 10.1038/sj.onc.1200907. [DOI] [PubMed] [Google Scholar]
- Gearing DP, King JA, Gough NM, Nicola NA. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989;8:3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle JN. Cytokine receptor signalling. Nature. 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- Ihle JN, Kerr IM. Jaks and stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Itoh T, Muto A, Watanabe S, Miyajima A, Yokota T, Arai K. Granulocyte-macrophage colony-stimulating factor provokes Ras activation and transcription of c-fosthrough different modes of signaling. J Biol Chem. 1996;271:7587–7592. doi: 10.1074/jbc.271.13.7587. [DOI] [PubMed] [Google Scholar]
- Itoh T, Rui L, Arai K, Watanabe S. Definition of the role of tyrosine residues of the common β subunit regulating multiple signaling pathways of granulocyte-macrophage colony-stimulating factor receptor. Mol Cell Biol. 1998;18:742–752. doi: 10.1128/mcb.18.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Sato N, Arai K, Miyajima A. Expression cloning of the human IL-3 receptor cDNA reveals a shared β subunit for human IL-3 and GM-CSF receptors. Cell. 1991;66:1165–1174. doi: 10.1016/0092-8674(91)90039-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- Miyajima A, Schreurs J, Otsu K, Kondo A, Arai K, Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58:273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- Mui AL-F, Wakao H, O’Farrell A-M, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14:1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Chin H, Miyasaka N, Miura O. An epidermal growth factor receptor/Jak2 tyrosine kinase domain chimera induces tyrosine phosphorylation of stat5 and transduces a growth signal in hematopoietic cells. J Biol Chem. 1996;271:19483–19488. doi: 10.1074/jbc.271.32.19483. [DOI] [PubMed] [Google Scholar]
- O’Shea JJ. Jaks, STATs, cytokine signal transduction, and immunoregulation: are we there yet? Immunity. 1997;7:1–11. doi: 10.1016/s1074-7613(00)80505-1. [DOI] [PubMed] [Google Scholar]
- Palacios R, Steinmetz M. IL-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Quelle FW, Sato N, Witthuhn BA, Inhorn RC, Eder M, Miyajima A, Griffin J, Ihle JN. JAK2 associates with the βc chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994;14:4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai I, Kraft AA. The kinase domain of Jak2 mediates induction of Bcl-2 and delays cell death in hematopoietic cells. J Biol Chem. 1997;272:12350–12358. doi: 10.1074/jbc.272.19.12350. [DOI] [PubMed] [Google Scholar]
- Sakamaki K, Miyajima I, Kitamura T, Miyajima A. Critical cytoplasmic domains of the common β subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992;11:3541–3550. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994;13:2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Ishida S, Koike K, Arai K. Characterization of cis-regulatory elements of the c-mycpromoter responding to human GM-CSF or mouse interleukin 3 in mouse proB cell line BA/F3 cells expressing the human GM-CSF receptor. Mol Biol Cell. 1995a;6:627–636. doi: 10.1091/mbc.6.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Ito Y, Miyajima A, Arai K. Granulocyte macrophage colony stimulating factor dependent replication of polyoma virus replicon in hematopoietic cells: analyses of receptor signals for replication and transcription. J Biol Chem. 1995b;270:9615–9621. doi: 10.1074/jbc.270.16.9615. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Itoh T, Arai K. JAK2 is essential for activation of c-fos and c-mycpromoters and cell proliferation through the human granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. J Biol Chem. 1996;271:12681–12686. doi: 10.1074/jbc.271.21.12681. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kubota H, Sakamoto KM, Arai K. Characterization of cis-acting sequences and trans-acting signals regulating early growth response 1 and c-fospromoters through the granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. Blood. 1997;89:1197–1206. [PubMed] [Google Scholar]
- Watanabe S, Mui AL-F, Muto A, Chen JX, Hayashida K, Miyajima A, Arai K. Reconstituted human granulocyte-macrophage colony-stimulating factor receptor transduces growth-promoting signals in mouse NIH 3T3 cells: comparison with signalling in BA/F3 pro-B cells. Mol Cell Biol. 1993a;13:1440–1448. doi: 10.1128/mcb.13.3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Muto A, Yokota T, Miyajima A, Arai K. Differential regulation of early response genes and cell proliferation through the human granulocyte macrophage colony-stimulating factor receptor: selective activation of the c-fos promoter by genistein. Mol Biol Cell. 1993b;4:983–992. doi: 10.1091/mbc.4.10.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following EPO stimulation. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Ohkubo T, Kiguchi T, Jenkins NA, Gilbert DJ, Copeland NG, Hara T, Miyajima A. A novel cytokine-indicible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wagner F, Frank SJ, Kraft AS. The amino-terminal portion of the JAK2 protein kinase is necessary for binding and phosphorylation of the granulocyte-macrophage colony-stimulating factor receptor βc chain. J Biol Chem. 1995;270:13814–13818. doi: 10.1074/jbc.270.23.13814. [DOI] [PubMed] [Google Scholar]