Abstract
The heterogeneous progression to the development of prostate cancer (PCa) has precluded effective early detection screens. Existing prostate cancer screening paradigms have relatively poor specificity for cancer relative to other prostate diseases, commonly benign prostatic hyperplasia (BPH). A method for discrimination of BPH, HGPIN, and PCa urine proteome was developed through testing 407 patient samples using matrix assisted laser desorption-mass spectrometry time of flight (MALDI-TOF). Urine samples were adsorbed to reverse phase resin, washed, and the eluant spotted directly for MALDI-TOF analysis of peptides. The processing resolved over 130 verifiable signals of a mass range of 1000-5000 m/z to suggest 71.2% specificity and 67.4% sensitivity in discriminating PCa vs. BPH. Comparing BPH and HGPIN resulted in 73.6% specificity and 69.2% sensitivity. Comparing PCa and HGPIN resulted in 80.8% specificity and 81.0% sensitivity. The high throughput, low-cost assay method developed is amenable for large patient numbers required for supporting biomarker identification.
Keywords: MALDI-TOF, prostate cancer, HGPIN, BPH, urine peptide profiling, PSA
Introduction
Prostate cancer (PCa) remains the second most common malignant neoplasm diagnosed in the United States, next to skin cancer in men [1]. PCa is initiated through a cascade of asymptomatic events that phenotypically exhibit itself as high-grade prostatic intraepithelial neoplasia (HGPIN) [2, 3]. Such development may include inflammation as the possible sequence of proliferative inflammatory atrophy (PIA) that can eventually led to HGPIN and PCa [4, 5]. The existing screen for prostate cancer, digital rectal exam and serum prostate specific antigen (PSA), lacks specificity to distinguish PCa from benign prostate hyperplasia (BPH), HGPIN, or PIA resulting in unnecessary prostate biopsies, anxiety, discomfort and costs [6, 7].
New diagnostic biomarkers to clinically identify early-stage of PCa are needed to obviate unnecessary biopsies. The unique disease-tissue and its microenvironment [8] express proteins that are subjected to proteolytic activity resulting in peptides released into the circulation. The urine is uniquely the product of extensive filtration of low molecular weight molecules in the serum in an individual with normal renal function. Peptides in urine represent proteins that have been degraded in blood and based on previous studies, these degradation products may be important biomarkers [9, 10]. Others have reported having success in identifying proteins in urine as biomarkers for cancer progression [11].
Accordingly, we hypothesize that the urine proteome contains peptides that can be readily profiled by MALDI-TOF mass spectrometry for distinguishing patients with prostate cancer. To this end, we have developed a high throughput approach for MALDI-TOF analysis of urine, with subsequent biostatistical analysis to evaluate large number of specimens.
Materials and Methods
Study populations
MALDI-TOF profiling was performed in two study populations. The initial study population consisted of men seeking a diagnostic prostate biopsy in response to a suspicious PSA test or digital rectal exam. Eligible patients were approached for recruitment in one of several urology clinics under “The Tennessee Men Health Control” project. Each subject provided a spot urine sample prior to prostate biopsy, and also completed a structured research questionnaire to query demographics and other prostate cancer risk factors. Cancer or HGPIN status was determined by pathology chart review of biopsy results. In all, 89 men were diagnosed with PCa, 52 with HGPIN, and 125 controls were confirmed by biopsy without HGPIN or prostate cancer. Table 1 summarizes the age and PSA levels of the subjects. The second study population consisted of 103 prostate cancer patients and 38 men without a history of PCa. Urine was collected by catheter from prostate cancer patients during radical prostatectomy. Eighty-six patients had Gleason score sums of 5 - 7, while the remaining had a Gleason score of 8-10. Controls without PCa included 38 male patients reporting no prior history of cancer. All studies were approved by the Vanderbilt University institutional review board (IRB# 040575). Medical records were reviewed to exclude men with renal insufficiency, liver disease, HIV, hepatitis, hypoglycemia, or prior bladder surgery. Due to the age group of the populations in these studies (age 41 – 65), approximately 80% of men without HGPIN or cancer had BPH. Similarly, about 80% of HGPIN and cancer patients had BPH. None of the BPH patients in the study were under palliative medications (e.g. 5-alpha reductase inhibitors).
Table 1.
Characteristics of patients used in the study described in Figure 2, 3 and 4. It should be noted, that all patients were biopsy tested for the diagnosis provided. However, biopsies can miss PCa or HGPIN due to its highly focal nature.
| MEAN AGE | MEDIAN AGE | MEAN PSA | MEDIAN PSA | |
|---|---|---|---|---|
| PCa | 66 | 66 | 7.5 | 5.7 |
| HGPIN | 63 | 64 | 7.3 | 5.5 |
| BPH | 65 | 65 | 7.8 | 5.4 |
Urine storage and processing
Collected urine samples were stored in triplicate 1.8mL aliquots frozen at −80°C until analysis. The stored urine was later thawed and divided into three wells of deepwell 96 well plates (400μL each) for desalting and concentration. Urine peptides were desalted using a mixed bed (50 μL slurry) of both C8 and C18 reversed-phase resins (1:1 ratio, Alltech Biotechnology), in batch method through hydrophobic affinity. The resin was separated by centrifugation of the 96 well plates 5 min at 1000 × g. The unbound urine components were aspirated and the resin washed three times with 0.1% tri-fluoroacetic acid (Burdick and Jackson, Muskegon, WI). The peptides were eluted with 5 μL of 75% acetonitrile (EMD Chemicals Inc. Merk Damstadt, Germany). The eluant (5 μL) was transferred to another 96-well plate, were mixed with an equal volume of 10 mg/ml matrix (α-cyano-4-hydroxycinnamic acid, Aldrich Biotechnology) and spotted onto a MALDI target plate.
Mass spectrometry
Peptide profiles were analyzed with Applied Biosystems 4700 model TOF-TOF MALDI mass spectrometer equipped with a solid-state Nd:YAG laser operating at 200 Hz. Spectra were acquired in reflector mode geometry (giving resolution capability of multiple isotopes for each peptide). The acquired spectra were calibrated externally and the peak list was exported as ASCII files to be further processed. Spectral pre-processing was accomplished using ProTS Data™ (Biodesix, Steamboat Springs, CO). Spectral processing included correction of the baseline, noise estimation, normalization (according to total ion current), and peak picking. Only mono-isotopic signals were considered for the subsequent statistical analysis. The individual peak files were organized by employing a binning process. Peaks were binned together such that the number of peaks in a bin from different samples is maximized while the number of peaks in a bin from the same sample is minimized [12]. Particular windows or bins were of expanding proportion with peptide mass. Once the bin or window parameters were established, the m/z values of the samples were segregated corresponding to the assigned mass (bins).
HPLC fractionation and MS-MS sequencing
In order to identify the peptide sequences of the distinguishing peptides sufficient protein was required for sequencing. This was achieved through the pooling the acetonitrile eluant from C8/C18 batch processing of 73 PCa and 122 BPH individual patient urine samples. 50 μl of each of the pools were fractionated on a BioAdvantage C18 column (100 Å, 250 × 4.6 mm) by HPLC (Agilent 1100). 96 fractions 1 ml were collected through a linear gradient of 0% Acetonitrile : 100% water, 0.1% TFA to 50% acetonitrile : 50% water, 0.1% TFA. Each of the fractions was spotted on a MALDI target plate for determination the fractions containing the distinguishing four m/z values. The remainder of the respective fractions were reconstituted in 5 μl 70% formic acid and 20 μl 0.1% TFA for LC-MS-MS analysis on a Waters Q-TOF mass spectrometer. All MS/MS spectra were searched using automated Mascot algorithm. The HPLC fractions were also analyzed on MALDI-TOF-TOF (AB 4700, Applied Biosystems) mass spectrometer to try to gain additional information.
Statistical analysis
The main statistical analyses focus on the following two steps: selecting the important proteins that were differentially expressed among the histological groups to build a prediction model, and verifying the prediction model with leave-one-out resampling strategy.
The selection of significant proteins was based on the multiple statistical methods including, Kruskal-Wallis test, Fisher's exact test, permutation t-test, significance analysis of microarray (SAM), weighted gene analysis (WGA), the mutual-information scoring (Info Score), and Kolmogorov-Smirnov test, collectively termed the weighted flexible compound covariate method (WFCCM) [12, 13]. The cutoff points for each method were determined based on the multiplicity of the statistical tests perfomed as well as the prediction power of each method. This method was designed to combine the most significant proteins associated with the biologic status from each analysis method. In other words, the WFCCM is an extension of the compound covariate method which allows considering more than one statistical analysis method simultaneously and reducing the dimensionally of the problem utilizing the weighted sum of the important predictors.
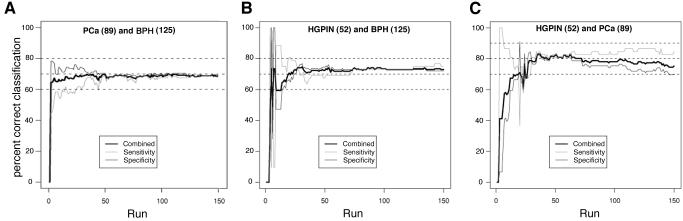
The class-prediction model was applied to determine whether the patterns of protein expression could be used to classify tissue samples into two classes according to the chosen parameter. The determination of misclassification rate was assessed using leave-one-out cross-validation class prediction method to estimate generalization error based on resampling. The classification rule selected by the WFCCM method is applied to the remaining dataset and the center of the two groups (e.g., HGPIN and PCa) were computed based on the top m/z values (bins) selected by the WFCCM method. The proportion of the data that are correctly classified is computed, and it is plotted in Figure 2 for each “run”
Figure 2.
The MADLI-MS data collected compare urine proteome of PCa, HGPIN, and BPH patients. A pair-wise comparisons of PCa and BPH subjects (A), as well as HGPIN patients with BPH (B) and PCa (C) patients were plotted to show the sensitivity and specificity as well as the overall probability of correct assignment (combined) based on increasing number of bins used in the analysis. The top 150 bins were chosen using leave one out cross-validation, the graph shows the approximate number of bins necessary to achieve optimal probability of correct assignment. The positive classification rate was determined to indicate correct classification rate (y axis) in relation to the number of bins required to achieve the score (x axis). The number of subjects in each cohort is indicated in parenthesis.
To test consistency between technicians and preparation methods, we use the number of nonzero entries as the response variable. We applied Wilcoxon Rank Sum Test to test if there are significant differences between two technicians and between two preparation volumes (Figure 1).
Figure 1.
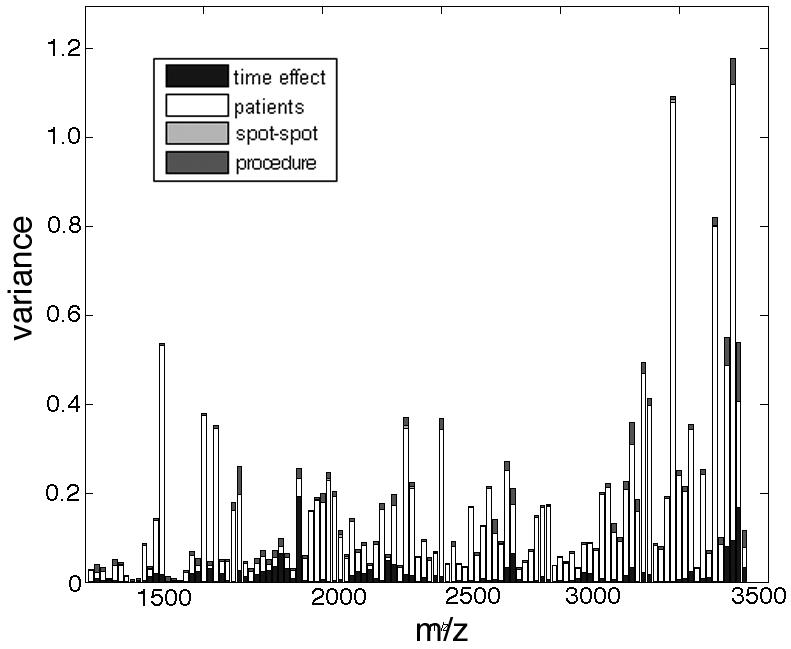
Analyses of the reproducibility from MALDI-TOF profiles have the urine proteome and supervised classification of PCa, HGPIN and control subjects. Urine from 3 normal subjects was processed 16 times each as described in the text. Each sample was spotted 3 times each and the spectra from the spotted samples analyzed by MALDI-TOF were assigned bins. The individual signals from each profile were compared and the variance for signals in a particular bin based on indicated parameters was plotted.
Results
Developing a method for MALDI-TOF profiling of urine
An obstacle to obtaining quality spectra (>130 verifiable monoisotopic signals between 1000-5000 m/z) was the removal of salts. After an initial comparison of a variety of methods including size exclusion centrifugation, ZipTip cartridges (Millipore), and anion exchange resin (Pierce), we determined that using a 50:50 mixture of C8 and C18 reverse phase resins (C8/C18 resin) demonstrated superior results (not shown). There are several reports demonstrating the use of C8 and C18 separately with different signals depending on the resin used, however none so far have combined 2 resin types [14]. This was the method chosen in the rest of the study for enriching for peptides in urine since they are optimally profiled by MALDI-TOF analysis.
To test reproducibility and signal stability, we initially desalted 400 μl of urine from three non-cancer subjects using the C8/C18 resin (62.5 μL/mL urine, Alltech Biotechnology) 16 times each. Samples were processed as described in the materials and methods section after initial collection and following 24 h incubation at room temperature. Each extraction was spotted in triplicate on the MALDI plate for analysis. The individual (288) peak files were organized by employing a standard binning process [12, 14]. The inter-patient spectra were dominant contributors of variance in the mass range tested (Figure 1). The incubation time effected peaks at 1977 and 3344 m/z uniquely and procedural variance was negligible. Thus individual patients could be differentiated despite differences in collection timing. Since sample collection procedures have been highlighted by others in past to contribute to variations in mass spectrometry analysis [15, 16], this was an important initial analysis performed to help determine the robustness of the method.
Distinguishing patients with PCa using MALDI-TOF profiling
We next applied our MALDI-TOF profiling strategy to distinguish patients diagnosed with PCa versus those with BPH and HGPIN. Not surprisingly, a number of the signals were statistically common to all the study populations (data not shown). Leave-one-out cross-validated class prediction method was used to estimate generalization error based on resampling in the analysis of the MALDI profiling of the “The Tennessee Men Health Control” study group (Figure 2). The study demonstrated 71.2% specificity and 67.4% sensitivity in discriminating PCa vs. BPH (Figure 2a). Comparing BPH and HGPIN resulted in 73.6% specificity and 69.2% sensitivity (Figure 2b). Comparing PCa and HGPIN resulted in 80.8% specificity and 81.0% sensitivity (Figure 2c). The patients diagnosed with BPH alone were controls, given that about 80% of HGPIN and PCa patients also had BPH.
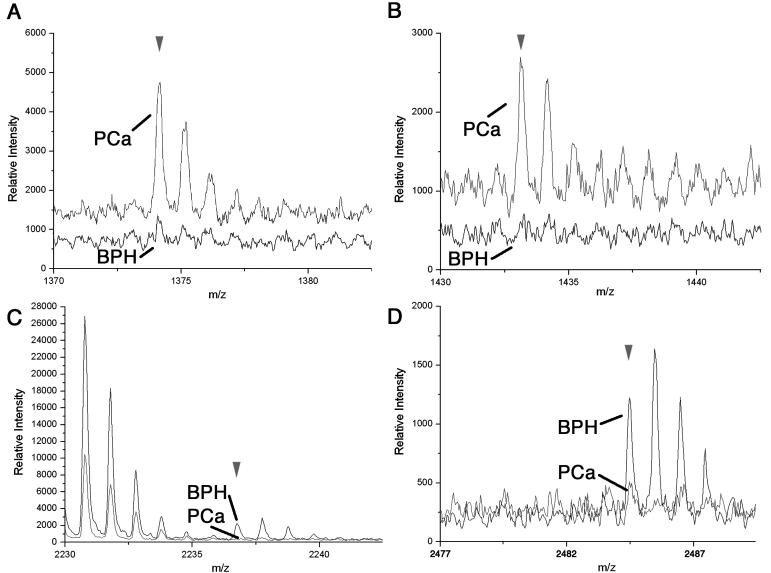
Then in an independent study, a class comparison with urine samples collected from prostate cancer patients during radical prostatectomy (n=103) and men with no history of cancer (n=38) were compared. In this study MALDI profiling of urine was sensitive enough to recognize differences between patients with and without cancer having 80% accuracy achieved through leave-one-out cross validation methods (data not shown). There were notable differences in the urine collected from the patient populations in the two studies, which included the combination of catheter and spot urine collection and difference in the non-cancer group of the second study and BPH in the first study. Nevertheless, we found four specific signals that were ranked as the top ten distinguishable features between the normal and PCa patient populations common to both the independent study populations. From the top ten PCa vs. control distinguishing signals in the first study determined by WFCCM, m/z values of 1373.1, 1433.5, 2236.3, and 2484.6 were also part of the distinguishing signals for PCa vs. control in the second independent study. These differentiating signals were verified by visual inspection of the MALDI-TOF profile using isotope peaks to support the authenticity of the signal from the noise. Figure 3 illustrates an overlay comparison of the mean MALDI profiles from the BPH and PCa subjects for the respective 1373.1, 1433.5, 2236.3, and 2484.6 m/z values. The 1373.1 m/z and 1433.5 m/z signals were greater in the PCa subjects (Figure 3 A, B). The m/z values of 2236.3, and 2484.6 were of greater intensity in the mean BPH subjects versus PCa subjects (Figure 3 C, D).
Figure 3.
Distinguishing MALDI-TOF signals that differentiate between PCa and BPH patients. Data collected in the reflectron mode enables isotopic resolution of the following m/z values (A) 1373.1, (B) 1433.5, (C) 2236.3, and (D) 2484.6. The BPH (black) and PCa (gray) mean spectra in each panel were overlaid. The arrowhead indicates the monoisotopic peak. In each panel the offset of the overlaid spectra is set at 0%, however the differing level of baseline noise in the particular regions of the spectra cause the offset to appear greater in some cases.
Identification of peptide biomarkers
To determine the identity of the distinguishing peptides, LC-MS/MS sequencing was performed. Adequate protein concentration and diversity in the two PCa or BPH samples was achieved through pooling 73 PCa and 122 BPH individual patient urine samples for subsequent fractionated on a C18 column through HPLC (Figure 4). The 96 fractions from each of the HPLC runs were evaluated by MALDI-TOF to identify the fraction containing the original four distinguishing m/z values (1373.1, 1433.5, 2236.3, and 2484.6 m/z). Then LC-MS/MS analysis was performed on doubly and triply charged species for each mass of interest. Due to the small mass of the individual peptides of interest only the 2484 m/z sample had a protein identified through five independent fragmentation peptides to uromodulin using the IPI human database with no enzymatic cleavage sites utilized. For the 1374 m/z value there was a doubly charged mass at 687.42 that required manual conversion to the singly charged ion for Mascot analysis. With only a single peptide fragment a manual exact match was made to semenogelin I isoform b preproprotein. The very nature of having a single fragment match makes this identification tentative, however, when identifying peptides at a small mass range is expected. The other two distinguishing peptides could not be identified through LC-MS/MS or MALDI TOF-TOF methodologies.
Figure 4.
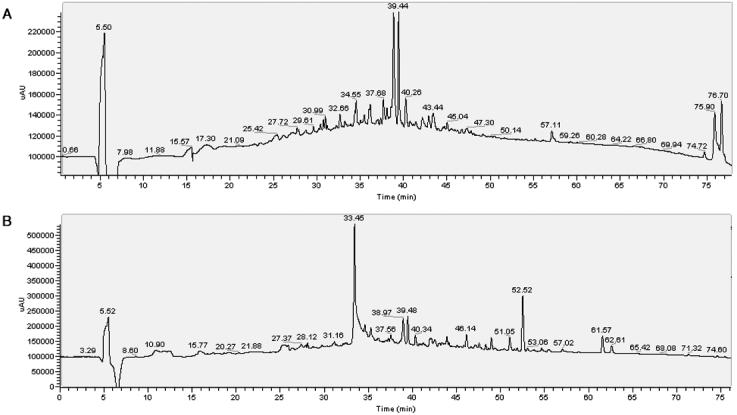
HPLC profiles of the BPH and PCa urine peptides were collected on a diode array detector (Agilent) at 280 nm. All the fractions were analyzed from the (A) BPH and (B) PCa patient sample pools by MALDI-TOF for the presence of the distinguishing peaks determined from the profiling studies. Fractions 68 (1373.1 m/z), 70 (2236.3 m/z and 1433.5 m/z), and 74 (2484.6 m/z) were found to contain the MALDI peaks of interest, thus subjected to subsequent sequencing by LC-MS/MS.
In the course of this study, we processed both spot urine samples and urine collected by catheter at the time of radical prostatectomy surgery. We found notable differences in the urine from the patients collected by catheter. Urine collected by catheter exhibited an added repeating peak signature with a 44 Da separation indicative of contaminating polyethylene glycol. The polyethylene glycol most likely is from the lubricant, Surgilube (Fougera, Melville, NY), used to facilitate insertion of the urethral catheter prior to surgery. In most cases this contaminant did not detract from the urine-specific peaks.
Discussion
A combination of markers is likely needed to for an early non-invasive detection for PCa. There are a number of promising biomarker mining reports analyzing serum, and urine to a lesser extent, for early detection of PCa [17-19]. Urine markers being evaluated currently for PCa diagnostic include PCA3DD3 [1], the use of GSTPi1, and other DNA methylation biomarkers which when combined may have higher accuracy [2, 3]. In this study we identified uromodulin and semenogelin I isoform b preproprotein as two biomarkers that can help enable distinction of PCa, and BPH. Semenogelin I (1373.1 m/z), is a seminal vesical derived protein found in the seminal fluid that is cleaved by PSA [20]. Semenogelin I, having greater intensity in the PCa patients in our study (Figure 3A), is associated with elevated immunoreactivity in hematologic malignancies and small cell lung cancer [21, 22]. Uromodulin (2484.6 m/z), also called Tamm–Horsfall protein) is the most abundant protein in normal human urine, was down regulated in PCa patient population tested (Figure 3D). Uromodulin is found to bind IL-α and TNF-α with apparent loss in expression in Wilms' tumour, mesoblastic nephroma, and bone metastasizing renal tumour [23, 24]. In comparison to the most studied PCa biomarker, PSA, serum levels between 4.1 - 9.9 ng/ml reported greater than 96% specificity and 20.5% to 22% level of sensitivity, respectively [25]. The patients screened herein, with a similar PSA distribution, provided a superior sensitivity yet lower specificity for prostate cancer. The prediction rate of the MALDI-TOF profiling results was not improved by the addition of PSA indices. Uniquely, urine profiling enabled differentiation of HGPIN patients, unattainable by PSA screening, to potentially limit unnecessary biopsies.
In conclusion the urine processing and MALDI-TOF analysis described enabled large patient cohorts, each yielding >100 verifiable signals, at a cost of approximately ten cents per sample.
Acknowledgments
We thank Robert J. and Helen C. Kleberg Foundation and Susan and Luke Simons (to NAB), NIH-Institutional Research and Academic Career Development Award (5K12GM068543, to AMK), Department of Defense (DAMD17-02-1-0139, to JHF), the NCI (CA98348, to JHF), the T.J. Martel Foundation, and a Vanderbilt-Ingram Cancer Center Support Grant (CA68485).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Bostwick DG, Qian J, Frankel K. The incidence of high grade prostatic intraepithelial neoplasia in needle biopsies. J Urol. 1995;154:1791–1794. [PubMed] [Google Scholar]
- 3.Sakr WA, Partin AW. Histological markers of risk and the role of high-grade prostatic intraepithelial neoplasia. Urology. 2001;57:115–120. doi: 10.1016/s0090-4295(00)00953-5. [DOI] [PubMed] [Google Scholar]
- 4.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 5.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 6.Stamey TA, Johnstone IM, McNeal JE, Lu AY, Yemoto CM. Preoperative serum prostate specific antigen levels between 2 and 22 ng./ml. correlate poorly with post-radical prostatectomy cancer morphology: prostate specific antigen cure rates appear constant between 2 and 9 ng./ml. J Urol. 2002;167:103–111. [PubMed] [Google Scholar]
- 7.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 8.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva J, Shaffer DR, Philip J, Chaparro CA, Erdjument-Bromage H, Olshen AB, Fleisher M, Lilja H, Brogi E, Boyd J, Sanchez-Carbayo M, Holland EC, Cordon-Cardo C, Scher HI, Tempst P. Differential exoprotease activities confer tumor-specific serum peptidome patterns. J Clin Invest. 2006;116:271–284. doi: 10.1172/JCI26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petricoin EF, Ornstein DK, Liotta LA. Clinical proteomics: Applications for prostate cancer biomarker discovery and detection. Urol Oncol. 2004;22:322–328. doi: 10.1016/j.urolonc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006;354:2621–2622. doi: 10.1056/NEJMc053410. [DOI] [PubMed] [Google Scholar]
- 12.Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, Roberts JR, Edgerton M, Gonzalez A, Nadaf S, Moore JH, Caprioli RM, Carbone DP. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet. 2003;362:433–439. doi: 10.1016/S0140-6736(03)14068-8. [DOI] [PubMed] [Google Scholar]
- 13.Shyr Y, KyungMann K. Weighted Flexible Compound Covariate Method for Classifying Microarray Data. In: Berrar D, editor. A Practical Approach to Microarray Data Analysis. Kluwer Academic Publishers; Norwell, MA, USA: 2002. pp. 186–201. [Google Scholar]
- 14.Villanueva J, Philip J, Entenberg D, Chaparro CA, Tanwar MK, Holland EC, Tempst P. Serum peptide profiling by magnetic particle-assisted, automated sample processing and MALDI-TOF mass spectrometry. Anal Chem. 2004;76:1560–1570. doi: 10.1021/ac0352171. [DOI] [PubMed] [Google Scholar]
- 15.West-Norager M, Kelstrup CD, Schou C, Hogdall EV, Hogdall CK, Heegaard NH. Unravelling in vitro variables of major importance for the outcome of mass spectrometry-based serum proteomics. J Chromatogr B Analyt Technol Biomed Life Sci. 2006 doi: 10.1016/j.jchromb.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 16.Gatlin CL, Eng JK, Cross ST, Detter JC, Yates JR., 3rd Automated identification of amino acid sequence variations in proteins by HPLC/microspray tandem mass spectrometry. Anal Chem. 2000;72:757–763. doi: 10.1021/ac991025n. [DOI] [PubMed] [Google Scholar]
- 17.Nagano K, Masters JR, Akpan A, Yang A, Corless S, Wood C, Hastie C, Zvelebil M, Cramer R, Naaby-Hansen S. Differential protein synthesis and expression levels in normal and neoplastic human prostate cells and their regulation by type I and II interferons. Oncogene. 2004;23:1693–1703. doi: 10.1038/sj.onc.1207297. [DOI] [PubMed] [Google Scholar]
- 18.Ornstein DK, Rayford W, Fusaro VA, Conrads TP, Ross SJ, Hitt BA, Wiggins WW, Veenstra TD, Liotta LA, Petricoin EF., 3rd Serum proteomic profiling can discriminate prostate cancer from benign prostates in men with total prostate specific antigen levels between 2.5 and 15.0 ng/ml. J Urol. 2004;172:1302–1305. doi: 10.1097/01.ju.0000139572.88463.39. [DOI] [PubMed] [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 20.Robert M, Gagnon C. Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55:944–960. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Wang Z, Liu H, Giles FJ, Lim SH. Pattern of gene expression and immune responses to Semenogelin 1 in chronic hematologic malignancies. J Immunother. 2003;26:461–467. doi: 10.1097/00002371-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues RG, Panizo-Santos A, Cashel JA, Krutzsch HC, Merino MJ, Roberts DD. Semenogelins are ectopically expressed in small cell lung carcinoma. Clin Cancer Res. 2001;7:854–860. [PubMed] [Google Scholar]
- 23.Su SJ, Yeh TM. The dynamic responses of pro-inflammatory and anti-inflammatory cytokines of human mononuclear cells induced by uromodulin. Life Sci. 1999;65:2581–2590. doi: 10.1016/s0024-3205(99)00527-5. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Marsden HB, Jasani B, Kumar P. Study of childhood renal tumours using a monoclonal antibody to Tamm-Horsfall protein. J Clin Pathol. 1987;40:1456–1462. doi: 10.1136/jcp.40.12.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, Parnes HL, Coltman CA., Jr. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. Jama. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]