Abstract
Objectives
The IL6 -174 promoter polymorphism impacts serum cytokine levels through transcriptional regulation. The objective of our study was to determine if -174 IL6 genotype influences survival in ovarian cancer.
Methods
The IL6 -174 polymorphism was assessed by direct DNA sequencing in lymphocyte DNA from 160 women with invasive ovarian, or peritoneal cancer patients. IL6 levels were measured in ascites and plasma in a subset of cases using colorimetric sandwich ELISA procedure. Overall survival was calculated according to the method of Kaplan and Meier. Cox regression analysis was used to evaluate the significance of individual variables in multivariate analysis. Chi-square or Fishers Exact was used to assess the significance of contingency tables.
Results
The IL6 -174 genotype frequencies of CC (19%), CG (50%), and GG (31%) were in Hardy-Weinberg equilibrium and were similar to published frequencies in Caucasian controls. There were no associations with IL6 -174 genotype and age, stage or optimal cytoreduction. Stage had a significant impact on survival (p=0.003). The IL6 -174 GG genotype was significantly associated with longer overall survival (median 131 months) compared to CC or CG (median 28 months, p=0.0007). In cox regression analysis using the covariates genotype (p=0.006) and stage (p=0.02), both were independently significant. Furthermore, there was no association found between IL6 levels in ascites or plasma, and genotype, stage, or overall survival.
Conclusions
The IL6 -174 GG genotype has a strong, independent, and favorable impact on survival for women with ovarian, and peritoneal carcinoma.
Introduction
IL6, a phosphorylated glycoprotein, is a proinflammatory cytokine that may serve as a growth regulator for both human ovarian surface epithelial cells and ovarian cancer cell lines [1]. The human IL6 gene on chromosome 7p21-24 has a common G/C polymorphism of the IL6 promoter region on position –174 upstream of the transcription start site that impacts serum cytokine levels [2, 3]. This polymorphism may result in inter-individual variation in transcription, expression, disease risk and underlying pathogenesis [4]. In the present study, we hypothesized that –174 IL6 genotype influences survival in ovarian, and peritoneal cancers.
Methods
Tissue was collected by the University of Washington Gynecologic Oncology Tissue Bank as approved by the Human Subjects Committee of the Institutional Review Board. Tumors were surgically staged according to the International Federation of Obstetrics and Gynecology (FIGO) criteria. Normal DNA was obtained from lymphocytes or other normal tissue in 160 invasive epithelial ovarian, or peritoneal cancer patients. All patients from the tissue bank were included that had invasive ovarian, or peritoneal carcinoma that had available paired normal DNA and were greater than 12 months from diagnosis (to allow for clinical follow-up).
We PCR-amplified the IL6 –174 polymorphism as previously described [8]. The amplicon was purified and sequenced using Big Dye terminator chemistry (Applied Biosystems, Foster City, CA) and an ABI 3100 DNA sequence detection system (Applied Biosystems).
Ascites and plasma samples were available on a subset of patients. IL6 levels were measured by colorimetric sandwich ELISA method using capture antibody and color developer. The absorbance was read on a plate reader at a wavelength of 450 nm.
Overall survival was calculated according to the method of Kaplan and Meier from the date of diagnosis to the date of death or last follow-up. Cox regression analysis was used to evaluate the significance of individual variables in multivariate analysis. Chi-square or Fishers Exact was used to assess the significance of contingency tables.
Results
The IL6 -174 genotype frequencies of CC (19%), CG (50%), and GG (31%) were in Hardy-Weinberg equilibrium and were similar to published frequencies in controls. There was no association with IL6 genotype and age, stage or optimal cytoreduction (Table 1). Over 90% of patients were Caucasian.
Table 1.
Characteristics of study sample by IL6-174 genotype
| Total | IL6-174CC | IL6-174CG | 1L6-174GG | |
|---|---|---|---|---|
| Age | ||||
| Range | 32–85 | 36–87 | 33–82 | |
| Median | 57 | 63 | 56 | |
| Site | ||||
| Ovary | 144 (90%) | 28 (93%) | 72 (90%) | 44 (88%) |
| Peritoneum | 16 (10%) | 2 (7%) | 8 (10%) | 6 (12%) |
| FIGO Stage* | ||||
| I | 11 (7%) | 1 (3%) | 7 (9%) | 3 (6%) |
| II | 5 (3%) | 1 (3%) | 3 (4%) | 1 (2%) |
| III | 116 (74%) | 20 (67%) | 55 (71%) | 41 (84%) |
| IV | 24 (16%) | 8 (27%) | 12 (16%) | 4 (8%) |
| Grade* | ||||
| 1 | 7 (4%) | 0 (0%) | 5 (6%) | 2 (4%) |
| 2 | 6 (4%) | 0 (0%) | 4 (5%) | 2 (4%) |
| 3 | 145 (92%) | 29 (100%) | 71 (89%) | 45 (92%) |
| Surgical Cytoreduction* | ||||
| Optimal | 101 (64%) | 13 (43%) | 53 (69%) | 35 (70%) |
| Suboptimal | 56 (36%) | 17 (57%) | 24 (31%) | 15 (30%) |
| Tumor Histology* | ||||
| Serous | 109 (68%) | 21 (70%) | 53 (66%) | 35 (70%) |
| Endometrioid | 12 (7%) | 1 (3%) | 8 (10%) | 3 (6%) |
| Mucinous | 3 (2%) | 1 (3%) | 2 (3%) | 0 (0%) |
| Clear Cell | 4 (2%) | 0 (0%) | 3 (4%) | 1(2%) |
| Mixed | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) |
| Other ** | 31 (20%) | 7 (24%) | 13 (16%) | 11(22%) |
| Total | 160 | 30(19%) | 80 (50%) | 50(31%) |
Data unavailable for stage (n=4), grade (n=2), cytoreduction (n=3)
Includes adenocarcinoma, carcinoma NOS, poorly differentiated NOS, small cell and malignant Brenner’s tumors
The IL6 -174 GG genotype was significantly associated with longer overall survival (median 131 months) compared to CC or CG (median 28 months, p=0.0007) (Figure 1a). Stage had a significant impact on survival (p=0.003) (Figure 1b). Optimal cytoreduction (p=0.71) and age (p=0.45) were not significantly associated with improved survival. In cox regression analysis using the covariates genotype (p=0.006) and stage (p=0.02), both were independently significant.
Figure 1.
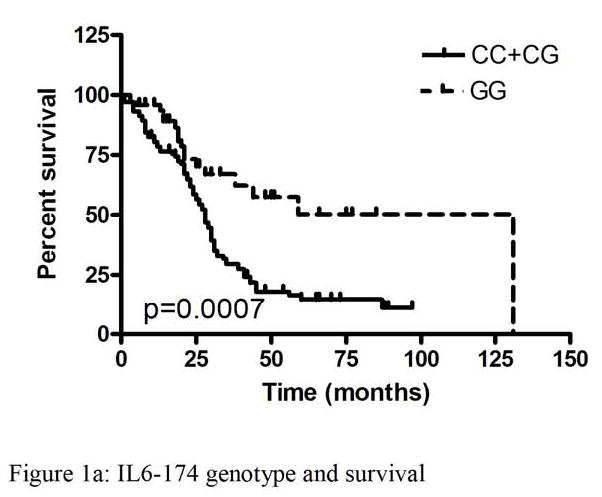

Kaplan-Meier analysis of overall survival of patients with ovarian cancer by IL6 polymorphism and stage
IL6 levels were measured in ascites and plasma in a subset of patients from our study. IL6 levels ranged from <0.8 to 200.6 pg/ml in plasma and from 2.1 to 7548.1 pg/ml in ascites. Ascites IL6 levels were significantly higher than in plasma (p<0.0001). There were no associations found between genotype and either plasma or ascites IL6 levels. Likewise, stage was not associated with IL6 levels in ascites or plasma. There was no survival difference with respect to ascites IL6 levels (p=0.59) or plasma IL6 levels (p=0.69) (Figure 2a-d).
Figure 2.
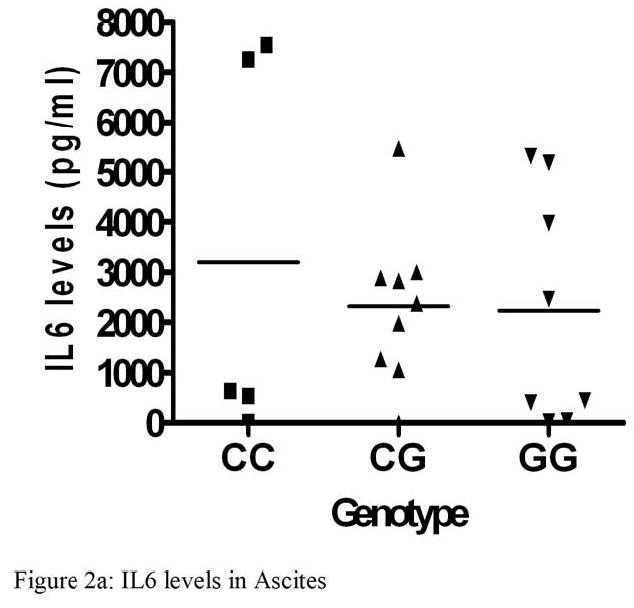

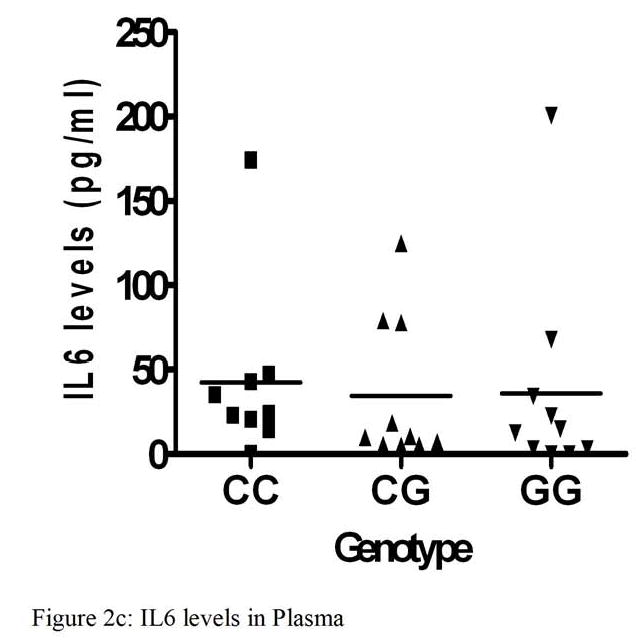

Interleukin 6 levels (pg/ml) in Ascites and Plasma and Kaplan-Meier analysis of overall survival of patients with ovarian cancer
Discussion
The IL6 -174 allele frequency in our study of mostly Caucasian women with ovarian, and peritoneal cancer was consistent with previously reported frequencies in Caucasian controls. [3]. Thus, risk of ovarian cancer does not appear to be influenced by IL6 -174 genotype.
Women with poor prognosis breast cancer who have the GG genotype at IL6 –174 have a shorter disease free survival compared with patients with at least one C allele (45% vs. 65%, p=0.002) [5]. In women with ovarian cancer, Hefler and colleagues reported that the IL6 -174 C allele correlated with early tumor stage, improved disease free survival and improved overall survival [6]. Surprisingly, we found that the IL6 -174 GG genotype has a strong, independent, and favorable impact on survival in epithelial ovarian and peritoneal cancer patients. Our contradictory findings in women with ovarian cancer may partially be explained by differences in our study population. Ninety percent of our study population had advanced stage ovarian cancer (stage III and IV) versus 53% of the Hefler subjects. Furthermore, there were more grade 3 tumors in our series (92% vs. 51% in the Hefler study). While the study populations are certainly different, it remains difficult to reconcile why completely opposite effects were observed. Further studies will be required to resolve this discrepancy.
The IL6 -174 G/C single nucleotide polymorphism is a functional alteration that affects gene transcription and subsequent serum levels of IL6 cytokine [3]. Higher serum IL6 levels has been associated with an increased tumor burden and higher volume of ascites, faster tumor progression, decreased effectiveness of chemotherapy, worse clinical disease status, increased relapse, and impaired survival in ovarian carcinoma [7,8,9,10,11]. Ovarian cancer cells produce significant levels of IL6 and express the soluble IL6 receptor (sIL-6R) [12]. This IL6R is expressed on murine endothelial cells derived from the ovary and mesentery as well as on human blood vessels within clinical specimens of human ovarian carcinoma [12]. IL6 elicits an angiogenic response from the microvascular endothelial cells [12]. Anti-sense IL6 oligonucleotides inhibit the proliferation of human ovarian-tumor cell lines with constitutive IL6 expression [13]. Therefore, IL6 levels could hypothetically influence survival in ovarian cancer patients.
Humphries and colleagues have associated the presence of –174 C allele with higher systolic blood pressure and more coronary heart disease events [14]. Whereas, in a variety of other diseases, the presence of the –174 G allele has been associated with higher IL6 levels [4] and poor outcome including colon cancer [15], graft versus host disease [16], juvenile arthritis [3] and Sjogren’s syndrome [17]. Thus, the association of –174 genotype with serum IL6 level is not uniform and may vary between different diseases.
We hypothesized that genotype could influence IL6 levels differentially in serum versus ascites, and local (ascites) versus systemic (serum) IL6 levels could have varying import based on stage. We measured IL6 levels in ascites and/or plasma in a subset of our patients. By analyzing IL6 in ascites (not peritoneal washings) we were selecting patients with more advanced stage. Within this selected group, there was no association found between IL6 levels and stage III versus IV disease. Although the sample size is small, there was no obvious association between IL6 genotype and either plasma or ascites levels. Thus, the association between increased survival and IL6 GG phenotype cannot be explained on the basis of alterations in systemic (plasma) or local (ascites) IL6 levels.
To our knowledge, direct measurements of serum and ascites IL6 levels in women with ovarian cancer have not been previously correlated with germline genotype and prognosis. Perhaps a larger sample size and direct measurement of IL6 in tumor tissues would clarify how the IL6 –174 genotype influences systemic and local IL6 levels in women with ovarian cancer. In our study of predominantly advanced stage cancers, the IL6 -174 GG genotype had an overall favorable impact on survival, but the origin of that effect remains uncertain.
Footnotes
Précis
The IL6 -174 GG genotype has a favorable impact on survival in ovarian, and peritoneal cancers.
This work was supported by NIH K08CA096610 (to EMS).
References
- 1.Syed V, Ulinski G, Mok SC, Ho SM. Reproductive hormone-induced, STAT3-mediated interleukin 6 action in normal and malignant human ovarian surface epithelial cells. Journal of the National Cancer Institute. 2002;94(8):617–29. doi: 10.1093/jnci/94.8.617. [DOI] [PubMed] [Google Scholar]
- 2.Bowcock AM, Kidd JR, Lathrop GM, Daneshvar L, May LT, Ray A, Sehgal PB, Kidd KK, Cavalli-Sforza LL. The human “interferon-beta/hepatocyte stimulating factor/interleukin-6” gene: DNA polymorphism studies and localization to chromosome 7p21. Genomics. 1988;1:8–16. doi: 10.1016/0888-7543(88)90152-8. [DOI] [PubMed] [Google Scholar]
- 3.Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. Journal of Clinical Investigations. 1998;102:1369–76. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terry CF, Loukaci V, Green FR. Cooperative Influence of Genetic Polymorphisms on Interleukin 6 Transcriptional Regulation. The Journal of Biological Chemistry. 2000;275(24):18138–44. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- 5.DeMichele A, Martin AM, Mick R, Gor P, Wray L, Klein-Cabral M, Athanasiadis G, Colligan T, Stadtmauer E, Weber B. Interleukin-6 -174G→C polymorphism is associated with improved outcome in high-risk breast cancer. Cancer Research. 2003;63:8051–6. [PubMed] [Google Scholar]
- 6.Hefler LA, Grimm C, Ackermann S, Malur S, Radjabi-Rahat AR, Leodolter S, Beckmann MW, Zeillinger R, Koebl H, Tempfer CB. An interleukin-6 gene promoter polymorphism influences the biological phenotype of ovarian cancer. Cancer Research. 2003;63:3066–8. [PubMed] [Google Scholar]
- 7.Plante M, Rubin SC, Wong GY, Federici MG, Finstad CL, Gastl GA. Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer. 1994;73(7):1882–8. doi: 10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Scambia G, Testa U, Panici PB, et al. Interleukin-6 and serum levels in patients with gynecological tumors. International Journal of Cancer. 1994;57:318–323. doi: 10.1002/ijc.2910570305. [DOI] [PubMed] [Google Scholar]
- 9.Scambia G, Testa U, Benedetti PP, Foti E, Martucci R, Gassucci A, Perillo A, Facchini V, Peschle C, Mancuso S. Prognostic significance of IL-6 serum levels in patients with ovarian cancer. British Journal of Cancer. 1995;71(2):354–6. doi: 10.1038/bjc.1995.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tempfer C, Zeisler H, Sliutz G, Haeusler G, Hanzal E, Kainz C. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecologic Oncology. 1997;67:27–30. doi: 10.1006/gyno.1997.4726. [DOI] [PubMed] [Google Scholar]
- 11.Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martionez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. American Journal of Obstetrics and Gynecology. 1991;164:1038–43. doi: 10.1016/0002-9378(91)90582-c. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Research. 2005;65(23):10794–800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson JM, Berek JS, Martinez-Maza O. Growth inhibition of ovarian-cancer cells induced by antisense IL-6 oligonucleotides. Gynecologic Oncology. 1993;49:8–15. doi: 10.1006/gyno.1993.1077. [DOI] [PubMed] [Google Scholar]
- 14.Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. The interleukin-6-174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. European Heart Journal. 2001;22:2243–52. doi: 10.1053/euhj.2001.2678. [DOI] [PubMed] [Google Scholar]
- 15.Belluco C, Olivieri F, Bonafe M, Giovagnetti S, Mammano E, Scalerta R, Ambrosi A, Franceschi C, Nitti D, List M. –174G>C polymorphism of interleukin 6 gene promoter affects interleukin 6 serum level in patients with colorectal cancer. Clinical Cancer Research. 2003;9:2173–76. [PubMed] [Google Scholar]
- 16.Cavet J, Dickinson AM, Norden J, Taylor PR, Jackson GH, Middleton PG. Interferon-γ and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood. 2001;98:1594–1600. doi: 10.1182/blood.v98.5.1594. [DOI] [PubMed] [Google Scholar]
- 17.Hulkkonen J, Pertovaara M, Antonen J, Pasternack A, Hurme M. Elevated interleukin-6 plasma levels are regulated by the promoter region polymorphism of the IL6 gene in primary Sjogren’s syndrome and correlate with the clinical manifestations of the disease. Rheumatology. 2001;40:656–661. doi: 10.1093/rheumatology/40.6.656. [DOI] [PubMed] [Google Scholar]


