Abstract
The ovarian steroid hormone progesterone is a major regulator of uterine function. The actions of this hormone is mediated through its cognate receptor, the progesterone receptor, Pgr. Ablation of the Pgr has shown that this receptor is critical for all female reproductive functions including the ability of the uterus to support and maintain the development of the implanting mouse embryo. High density DNA microarray analysis has identified direct and indirect targets of Pgr action. One of the targets of Pgr action is a member of the Hedgehog morphogen Indian hedgehog, Ihh. Ihh and members of the Hh signaling cascade show a coordinate expression pattern in the mouse uterus during the preimplantation period of pregnancy. The expression of Ihh and its receptor Patched-1, Ptc1, as well as, down stream targets of Ihh-Ptch1 signaling, such as the orphan nuclear receptor COUP-TF II show that this morphogen pathway mediates communication between the uterine epithelial and stromal compartments. The members of the Ihh signaling axis may function to coordinate the proliferation, vascularization and differentiation of the uterine stroma during pregnancy. This analysis demonstrates that progesterone regulates uterine function in the mouse by coordinating the signals from the uterine epithelium to stroma in the preimplantation mouse uterus.
I. Introduction
During the process of embryo implantation, the uterus must undergo a transformation into a “receptive” state, in which the blastocysts are able to form intimate physical and physiological contact with the endometrium. The primary factors that stipulate endometrial receptivity are the ovarian steroids, estrogen (E2) and/or progesterone (P4). These steroids act primarily through their respective cognate nuclear receptors, the estrogen receptor (Esr) and the progesterone receptor (Pgr). The molecular mechanisms coordinated by the ovarian steroids in the endometrium during the estrus cycle and in early pregnancy have been understood in mouse, where the use of genetic engineering of animal models has facilitated the ability of researchers to investigate these actions. During normal pregnancy in mice, a pre-ovulatory E2 surge stimulates uterine epithelial cell proliferation at 0.5 day post-coitus (dpc). At 1.5dpc, large numbers of epithelial cells undergo apoptosis due to the diminished E2. Rising P4 levels from the newly formed corpora luteum at 2.5dpc initiate uterine stromal cell proliferation. The combined actions of P4 and nidatory E2 continues to stimulate uterine stromal proliferation to bring about the uterus to the receptive state for implantation (Huet-Hudson et al., 1989). Although the critical roles of E2 and P4 have long been known, the downstream effectors, and the molecular mechanism utilized to regulate the transcription of target genes, remains relatively unexplored. The use of expression screening techniques, including microarray analysis, as well as, gene targeting technologies has begun to identify downstream effectors of the steroid hormones. This review will focus on the molecular mechanisms by which P4 regulates murine uterus function.
II. Progesterone Receptor
The ovarian steroid hormone progesterone (P4) is a critical regulator for the establishment and maintenance of pregnancy. Well characterized functions of P4 include regulating uterine receptivity to blastocyst attachment, inducing stromal cell proliferation and differentiation, regulating epithelial cell proliferation, and coordinating uterine and embryonic interactions on a morphological and molecular level. Most of the physiological affects of P4 are mediated through its receptor, the progesterone receptor (Pgr). Pgr belongs to the nuclear receptor superfamily and shares structural similarities and key functional domains common to this group (Tsai and O'Malley, 1994, Evans, 1988). The three major functional domains of Pgr, like all nuclear receptors, are the ligand binding domain (LBD), the DNA binding domain (DBD), and an activation domain (AF). The LBD confers specificity to the receptor for a particular ligand or molecule that regulates its transcriptional activation. The DBD determines which DNA sequences the receptor will recognize while the AFs link the transcriptional activity of the receptors to the core transcriptional complexes (Ribeiro et al., 1995). Pgr is encoded in one gene and exists as several isoforms with the most well characterized being PRA, PRB, and PRC. These isoforms arise from the alternate translation start sites in the Pgr gene. The human PRA isoform differs from the PRB isoform in that it lacks the first 164 amino acids contained in PRB, whereas the PRC isoform contains a truncated DBD and a full length LBD (Wei et al., 1996).
Mechanism of Action
The mechanisms by which the Pgr regulates the transcription of target genes is an area of active research. As shown in Figure 1, the steroid hormone receptors can be activated by several mechanisms. In the ligand-dependent mechanism, receptors can be activated by the binding of P4 to the LBD of P Pgr (Figure 1a). In the absence of ligand, the Pgr is associated with in a large complex of proteins including Heat shock proteins (Hsp) and FK506-binding proteins (FKBPs) in the cytoplasm and are transcriptionally inactive. Upon ligand binding, the receptors undergo a conformational change, are released from the chaperone complex, and translocate into the nucleus (Tsai and O'Malley, 1994). Another mechanism by which Pgr can be activated is through a ligand-independent mechanism (Figure 1a) (Power et al., 1992). The ligand-independent activation of the receptor results from crosstalk with membrane receptor signaling that results in the activation of kinases and phosphorylation of the receptor. The ligand-independent transcription of Pgr can be due to a variety of signaling pathways. The first study to demonstrate ligand independent transcription of Pgr showed that cAMP (8-bromo-cAMP), in a cAMP-dependent protein kinase (PKA) dependent manner, can phosphorylate the receptor and activate the gene (Denner et al., 1990). Subsequently, PKA has been implicated in Pgr mediated endometrial functions, such as decidualization loss of uterine quiescence near term (Brar et al., 1997, Ku and Sanborn, 2002). Dopamine can also activate Pgr in a ligand independent manner both in cell transfection systems (Power et al., 1991), as well as, in vivo in the mouse brain (Mani et al., 1994). Non-ligand bound Pgr has been shown to be critical for a dopamine dependent lordosis response in female mice (Mani et al., 1996) Finally, cyclin A/Cyclin-dependent kinase-2 (Cdk2) has been shown to phosphorylate Pgr and potentiate ligand independent signaling (Zhang et al., 1997, Pierson-Mullany and Lange, 2004). Since P4 is known to stimulate proliferation of the endometrium, these results may be important in elucidating Pgr action in the uterus. Through either the ligand dependent or independent mechanisms, phosphorylated Pgr translocates to the nucleus and is able to bind as a homodimer to cis-acting progesterone response elements (PREs) that are usually present in the 5'-flanking region of specific genes to stimulate expression of target genes. Although the perfect inverted repeat consensus sequence for a PRE was identified as AGGACA(nnn)TGTCCT, most progesterone-responsive genes have imperfect palindromes or do not have recognizable PREs. To date, only a few genes containing full inverted repeat PRE elements have been characterized (Cheng et al., 2001, Gao et al., 2000, Matsui et al., 2002, Moore et al., 1997, Slater et al., 1988, Lee et al., 2003). Many in vitro studies have shown that the Pgr may also regulate genes through crosstalk with other transcription factors, such as Sp1, NF-κB , and C/EBP-β or half-sites of the palindromic sequence (Tsuchiya et al., 2003, Gao et al., 2001, Tang et al., 2002, Leonhardt et al., 2003, Christian et al., 2002, Mueller et al., 2003). Upon the binding of the receptor to the PRE, the activated receptor then interacts with coactivators to accelerate the formation or increase the stability of the pre-initiation complex to activate transcription.
Figure 1.
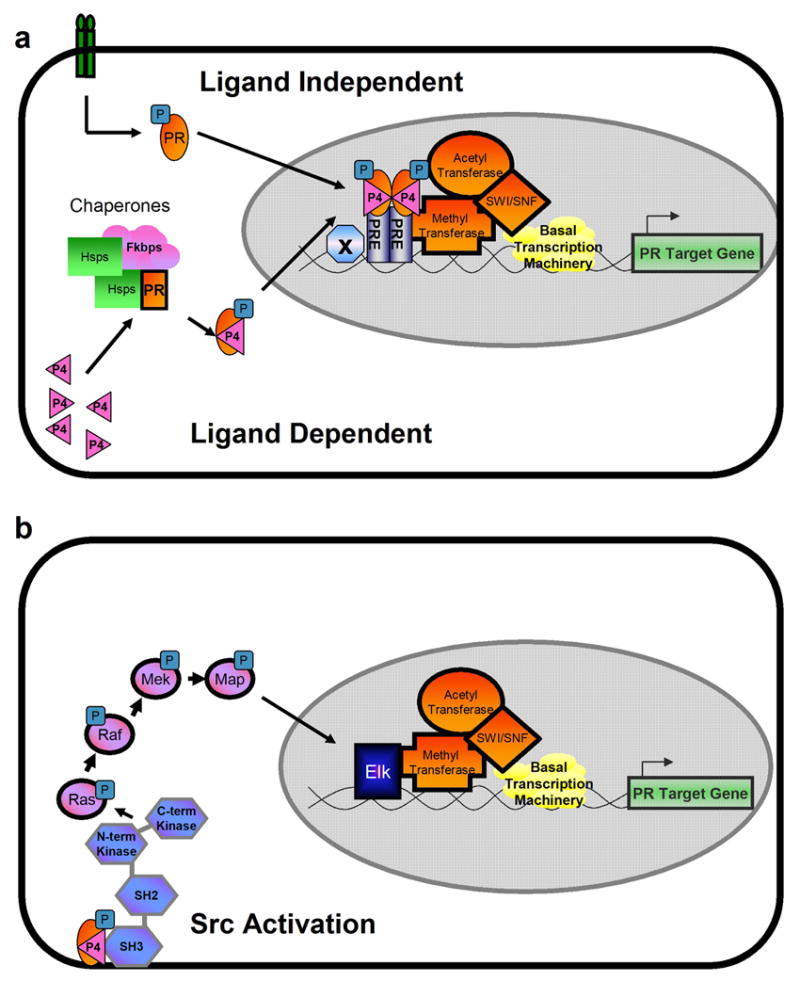
Mechanisms of Pgr Action. (a) Ligand independent and ligand dependent activation of Pgr (b) Pgr activation of kinase cascades through interaction with Src kinase.
The most recently discovered mechanism by which Pgr activates transcription is through its ability to activate the Src/Ras/Raf/mitogen-activated protein (MAP) kinase signaling cascade (Figure 1b). Cytoplasmic Pgr interacts directly with a proline rich motif located at the N-terminus Src homology 3 (SH3) domain of Src tyrosine kinase of the receptor (Boonyaratanakornkit et al., 2001). This mechanism may contribute to the central role that Src kinases have in the regulation of cellular proliferation, differentiation, and motility. The ability of Pgr to activate kinase cascades shows that Pgr is not only capable of acting as a transcription factor, but can directly activating signaling pathways from the cytoplasm.
Animal models that affect Pgr signaling
A. Pgr Knockout Models
Genetic ablation of both PRA and PRB in mice (PRKO) results in pleiotropic reproductive abnormalities, including behavioral defects, failure to ovulate, failure of the uterus to support implantation (demonstrated by a lack of a decidual response), and defects in mammary gland branching and glandular development (Lydon et al., 1995). Subsequently, selective ablation of specific isoforms has demonstrated that the individual Pgr isoforms are responsible for distinct and non-overlapping functions. The selective ablation of PRA in mice (PRAKO) demonstrates that PRA is the major mediator of P4 signaling in the mouse reproductive tract. Similar to the PRKO mice, these mice fail to undergo a decidual reaction from the administration of exogenous hormones and have a greatly reduced ovarian function (Mulac-Jericevic et al., 2003) Additionally, the PRAKO mice demonstrate that selective activation of PRB in the uterus resulted in an abnormal P-dependent induction of epithelial proliferation, in contrast to the customary P4 inhibition of E2-induced epithelial proliferation. This gain of PRB-dependent proliferative activity by removal of PRA indicates that PRA is required not only to inhibit E-induced hyperplasia of the uterus, but also to limit potentially adverse proliferative effects of the PRB. Selective ablation of PRB (PRBKO) showed no overt uterine phenotype, but resulted in reduced mammary gland morphogenesis (Mulac-Jericevic et al., 2000). Therefore, PRA and PRB, are the predominant forms of Pgr in the uterus and mammary glands, respectively, and have discrete and non-overlapping functions. The role of a third isoform of Pgr, PRC, has not been fully investigated in the murine uterus. The PRC isoform is proposed to be an inhibitory isoform since it contains a truncated DBD and a full length LBD (Wei et al., 1996, Wei et al., 1997). Recent evidence has suggested a role for PRC in parturition. Its upregulation by NF-κB on the Pgr promoter may inhibit Pgr activity and lead to a loss of uterine quiescence (Condon et al., 2005).
B. Fkbp4
In the absence of P4, Pgr interacts with molecular chaperones in the cytoplasm. This interaction is critical to maintain the functionality and competence of the receptors to bind P4 and subsequently activate gene transcription. Fkbp4 belongs to a subclass of immunophillin proteins (FKBPs) that was originally discovered as a component of the non-ligand bound steroid receptor chaperone complex. Heat shock protein 90 (Hsp90) serves as an adapter protein between Pgr and Fkbp4. Although the role of Hsp90 for maintenance of receptor function has been established, until recently, very little was known about the role of other chaperones. Fkbp4 catalyzes conformational changes in protein structure with a peptidyl-prolyl cis-trans isomerase domain (Davies and Sanchez, 2005). Several lines of evidence led to the discovery that Fkbp4 null females are completely infertile due to the inability to attain uterine receptivity. First, in vitro evidence demonstrates that Fkbp4 potentates Pgr transcriptional activity (Barent et al., 1998). Additionally, Fkbp4 is regulated in a distinct spatiotemporal pattern during the peri-implantation period. At 1 dpc, it is primarily expressed in the uterine epithelium, with expression expanding into the stroma by 4 dpc. During implantation, Fkbp4 is localized in the decidualizing stroma cells surrounding the newly formed implantation sites. Finally, in Hoxa10−/− mice, which are known to have endometrial defects, Fkbp4 is decreased (Daikoku et al., 2005). Fkbp4 null females exhibit reduced uterine Pgr transcriptional activity, and reduced expression of known targets of Pgr including, Amphiregulin (Areg), Indian Hedgehog (Ihh), and Hoxa10.(Tranguch et al., 2005) The functional dissection of the chaperone complex holds further promise as the function of other FKBP family members, such as Fkbp5 which is regulated by Pgr and modulates Pgr function (Hubler et al., 2003), Fkbp3 which associates with histone deacetylases (Yang et al., 2001), and Frap1 which controls cell size and proliferation (Murakami et al., 2004) has yet to be elucidated.
C. Src knockout mice
As previously mentioned, Pgr can directly activate kinase in the cytoplasm by interaction with Src kinase. Src protein tyrosine kinases are 52–62 kDa proteins composed of six distinct functional regions: the Src homology (SH) 4 domain, the unique region, the SH3 domain, the SH2 domain, the catalytic domain, and a short negative regulatory region. Src has been shown to regulate multiple signaling pathways including proliferation, differentiation, and angiogenesis, which are all critical processes for decidualization (Thomas and Brugge, 1997). Additionally, immunohistochemical studies revealed that active Src kinase is strongly expressed in the decidua. Src null mice showed no apparent decidual response, and the uterus lacked expression of known decidual markers. This result clearly demonstrates that Src activity is indispensable for an appropriate P4 induced decidualization (Shimizu et al., 2005). Additionally, in human endometrial stromal cells, the kinase activity of c-Src was increased during in vitro decidualization. These affects are clearly hormone dependent, as withdrawal of E2 and P4 reduced c-Src kinase activity to the basal level and also changed the pattern of tyrosine phosphorylation to the unstimulated state. These results further corroborate the phenotype in the mouse and establish the importance of hormone mediated Src kinase activation in decidualization across species (Maruyama et al., 1999).
III. Coregulators
The identification of coregulators that form a functional link between the activated receptors and transcription complex to affect transcriptional regulation is an active field of research. To date, over 200 coregulators have been identified through genetic or biochemical screens as reviewed by Mani (Mani, 2006). These proteins consist of coactivators and corepressors that enhance or inhibit gene transcription. Because coactivators are often rate-limiting for receptor activation, the relative expression level of coactivators and corepressors determines the appropriate cell-specific response to the presence of ligand.
Mechanism of Action
Upon the binding of the receptor to the PRE, the activated receptor then interacts with coactivators to accelerate the formation or increase the stability of the pre-initiation complex to activate transcription. The coactivators do not serve as a functional link between the activated receptor to the basal transcription machinery, but possess enzymatic activities that are necessary for efficient gene expression. These include acetyltransferase proteins, such as CBP/p300, pCAF, p160s; the steroid receptor coactivator [SRC] members, SRC1, SRC2, and SRC3; the ATP-coupled chromatin-remodeling SWI-SNF complex; methyltransferases, such as coactivator-associated arginine (R) methyltransferase-1 (CARM1) and PRMT-1/2; and ubiquitin ligases, such as E6-AP and Rsp5 as reviewed by Smith (Smith and O'Malley, 2004). Conversely, non-ligand bound receptors can repress basal transcription of target genes through corepressors N-CoR (nuclear receptor corepressor) and SMRT (silencing mediator of repressed transcription) (Wagner et al., 1998).
The coactivators of the steroid receptor (SRC) family were the first identified coactivators. In a yeast two-hybrid system, SRC-1 was identified as a protein that interacts with and enhances Pgr transcriptional activity, as well as, other nuclear receptors, without altering the basal activity (Onate et al., 1995). Subsequently, two more SRC family members SRC-2 [transcriptional intermediary factor 2 (TIF2)/GR-interacting protein 1 (GRIP-1)] and SRC-3 [(ACTR/pCIP/receptor associated coactivator (RAC3)/TRAM-1/amplified in breast cancer 1 (AIB1)] have been identified (McKenna et al., 1999). This family of coactivators bind to a coactivator-binding groove within the LBD of Pgr via an NR box motif (LxxLL, where L = leucine and x is any amino acid) (Heery et al., 1997). The mechanism of action, physiological affects, and identification of new coactivators remains an active area of research.
Coregulator Knockout models
A. SRC-1
SRC-1 null female mice are fertile and viable presumably due to the overlap in function and expression patterns among coactivators. Additionally, expression of SRC-2 was increased in the SRC-1 null mutant, partially compensating for the loss of SRC-1 function in target tissues. However, the SRC-1-null females do exhibit partial hormone resistance in P4 target tissues; as manifested by a decreased growth in response to decidual stimuli when stimulated with E2 and P4. Together, this data suggests that SRC-1 modulating the actions of Pgr in the mouse uterus may be compensated by the presence of other coactivators.
B. SRC-2
In contrast to SRC-1, SRC-2 is required during pregnancy. Although the SRC-2 null females are able to implant and the decidual reaction appears histologically normal, litter sizes of SRC-2 null females are drastically reduced. The reduction is presumably due to the marked increase of in utero embryonic resorptions observed between E12.5 and E18.5 if pregnancy in SRC-2-null females. Accordingly, SRC-2 null females often displayed a marked placental hypoplasia with decreased numbers of trophoblastic trabeculae and embryonic capillaries in the labyrinthine region (Gehin et al., 2002).
C. Other Coactivators
Knockout studies of other coactivators have given inconclusive results on their necessity for P4 mediated action in the uterus. The SRC-3 mouse has no apparent defects in uterine phenotype. Other coactivator knockouts, such as CBP, P300, and CARM-1 are embryonic or perinatal lethal, thus precluding further study (Yadav et al., 2003). Disruption of the maternal copy of E6-AP is correlated with Angelman syndrome, a genetic neurological disorder (Jiang et al., 1998). Although E6-AP ablation has been shown to impair the response of the uterus to exogenous estrogen, the effect on P4-mediated action has yet to be shown (Smith et al., 2002). The corepressor knockouts have also given little information. Targeted ablation of N-CoR leads to embryonic lethality (Jepsen et al., 2000), and expression of a dominant negative Smrt leads to numerous abnormalities in other animal systems (Malartre et al., 2006).
Identification of P4 Target Genes
Through candidate gene approaches, only a handful of P4-regulated genes had previously been identified. Recently, high-density DNA microarray technology has accelerated our ability to identify P4-regulated genes in the uterus. Various microarray approaches have been taken to identify P4 regulated genes including pregnancy, antagonist treatment, and exogenous P4 treatment.
Yoshioka et al. provided one of the first microarray based investigations of gene expression around the time of implantation by attempting to identify genes with differential expression between the preimplantation (d 3.5) and postimplantation (d 5.0) stages. Of the 6500 genes examined, changes were detected in 399 genes. The expression of 192 genes increased and that of 207 genes decreased in the transition from the preimplantation to the postimplantation phase. In order to avoid contamination of uterine tissue with that of embryonic tissue, the uterine lumen was washed with PBS and scraped with a scalpel blade (Yoshioka et al., 2000).
Reese et al. refined the microarray performed by Yoshioka, et al. This group compared gene expression profiles between implantation and inter-implantation sites on 3.5 dpc, as well as, the differences in the uterine gene expression profile of P4 treated, delayed implantation mice against those in which delayed implantation was terminated by E2 treatment. In this manner, they sought to determine which genes are expressed specifically at implantation sites, and under maternal hormonal control. They reported 36 up-regulated and 27 down-regulated genes at the implantation site and 128 up-regulated and 101 down-regulated genes upon termination of delayed implantation by E2. Taken together, these results yielded up-regulation of 27 genes both at the implantation site and during active implantation (Reese et al., 2001).
Taking another approach to identify P4 regulated genes, Cheon et al. performed microarray analysis by treating female mice at 2.5 dpc of pregnancy with the antiprogestin RU486 and investigated changes in gene expression 24 hours later. This approach identified the impact of withdrawal of the P4 signaling axis on gene expression and identified 148 target genes (Cheon et al., 2002).
Jeong, et al. took the most direct approach to define the molecular pathways regulated by the P4-Pgr signaling axis. Wild-type and PRKO mice were ovariectomized and then treated with vehicle or P4 every 12 h. Mice were killed either 4 h after the first injection, to identify genes that are directly regulated by the P4-Pgr axis, or after the fourth injection of P4 (40 hrs), to find indirect targets of the P4-Pgr axis. By utilizing the PRKO mice, the effect of P4 and Pgr both alone and in combination could be measured. At the 4 h time point, 139 genes were found to be up-regulated by P4 and Pgr, while 96 genes were found to be down-regulated. Earlier, this approach was used on a low density array and identified Indian Hedgehog (Ihh) as a Pgr target gene I (Takamoto et al., 2002, Jeong et al., 2005). Conversely, the major change in gene expression after chronic P4 treatment was a down regulation of genes (Jeong et al., 2005).
These microarrays have been useful in determining target genes of P4, as well as, genes implicated in implantation. A significant amount of overlapping targets common to the majority of the arrays, including with known P4 target genes, such as Areg, Muc-1, and Fst, serves to validate the differential approaches taken by these investigators. However, these various microarray approaches all have potential problems and pitfalls. Physical disruption of the uterine epithelium to remove embryonic tissue, as performed by Yoshioka et al, would likely result in different gene expression profiles. Reese et al. circumvented this problem, as blastocysts are present both in the delayed implantation, as well as, in the E2 delay-terminated group. However, in the study of implantation sites, since the implanting blastocysts were in the implantation sites, but not in the inter-implantation sites, these genes may be of embryonic origin. Further complications in interpretation occurs as further microarrays by this group shows distinct differences between the gene expression of activated and dormant blastocysts (Hamatani et al., 2004). Cheon et al. utilized RU486. Although RU486 is mostly known as an antiprogestin, it is also an antagonist of glucocorticoid and androgen receptors. Additionally, this study is measuring the impact of P4 withdrawal, and not P4 gene activation, and therefore, may omit targets transiently activated by P4. Validation of their results with the PRKO mouse was necessary to address these issues. Finally, Jeong et al. directly tested P4-Pgr regulated genes. However, the results of this array do not elucidate which genes are temporally correlated with implantation.
The Ihh Signaling Axis
Indian Hedgehog (Ihh) has been identified as a rapidly induced target of P4 that is also dependent on PR (Takamoto et al., 2002, Jeong et al., 2005). Ihh is a member of the Hedgehog family of diffusible morphogens that have been shown to be critical regulators of development in Drosophila (Lee et al., 1992) and in mammals. In mammals Hedgehog family members include, Sonic hedgehog (Shh), Ihh, and Desert hedgehog (Dhh). Ihh is the only member of this family expressed in the mouse uterus (Matsumoto et al., 2002). Ihh has been shown to regulate the development of mammalian tissues including: bone and cartilage (Lanske et al., 1996), the ovary (Wijgerde et al., 2005), the mammary gland (Lewis et al., 1999), the gastrointestinal tract (Ramalho-Santos et al., 2000), the pancreas (Hebrok et al., 2000), the sebaceous glands (Niemann et al., 2003), the retina (Perron et al., 2003), and blood vessels (Dyer et al., 2001). The signaling pathway for Ihh is shown in Figure 2. Once secreted, Ihh, as do all Hedgehog proteins, undergo autocatalytic cleavage to form amino terminal peptides, which are modified by the addition of a cholesterol moiety and palmitoylation, and participate in both short- and long-range paracrine signaling (Pepinsky et al., 1998, Porter et al., 1996). The amino terminal Hedgehog interacts with a 12-span transmembrane receptor protein, Patched (Ptc) (Ingham et al., 1991). The interaction between Hedgehog and Ptc relieves Ptc-mediated inhibition of the activity of a G protein-coupled seven-span transmembrane protein, Smoothened (Smo). Smo then begins a signal cascade allowing factors, such as the Gli family of transcription factors, to translocate to the nucleus to activate the transcription of target genes (Alcedo et al., 1996). A number of components are necessary to integrate the signal from Smo to the Gli transcription factors including the serine-threonine kinase Fused (Fu), Suppressor of fused (Sufu), and the microtubule binding kinesin-like molecule Costal 2 (Cos2). Known downstream targets of Ihh signaling are Ptc-1, Gli1, Gli2, and COUP-TF II, (Takamoto et al., 2002, Matsumoto et al., 2002).
Figure 2.
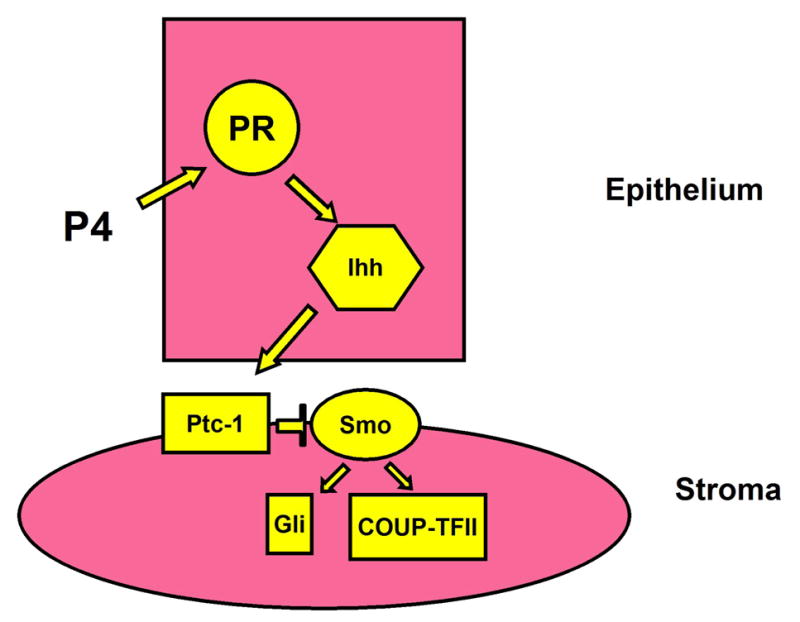
Model of Ihh action mediating epithelial-stromal communication in the uterus.
Expression of the Ihh signaling axis in murine uterus
Since Ihh is a diffusible morphogen, one must look at the cell specific expression of Ihh and members of its signaling cascade in the endometrium to understand where Ihh may be acting in the uterus. In the uterus, Ihh is expressed in the luminal and glandular epithelium (Takamoto et al., 2002). Known downstream targets of Ihh signaling including Ptc-1 , Gli1, Gli2, and COUP-TF II are expressed in the stroma, suggesting that Ihh may be involved in uterine epithelial regulation of stromal cell function (Takamoto et al., 2002, Matsumoto et al., 2002). Additionally, Gli3 is found in the epithelium on 0.5 dpc, and then strongly upregulated in the subluminal stroma at 3.5 dpc (Matsumoto et al., 2002). Figure 2 summarizes the expression of the Ihh signaling axis during the pre-implantation period. The expression of the Ihh signaling axis is also temporally coordinated in the mouse uterus during early pregnancy. Figure 3 shows the Quantitative RT-PCR analysis of the expression pattern of Ihh, Ptc and COUP-TF II in the mouse uterus during pseudopregnancy, as well as, in the uterus during an exogenous hormone treatment regimen that in conjunction with uterine trauma, mimics changes in the uterus during the peri-implantation period. This hormonal regimen delivers high levels of E2, as found in the proestrus/estrus junction, and then high levels of P4 with low levels of E2 to mimic the effect of the developing corpus luteum during the pre-implantation period. During natural pseudopregnancy, Ihh peaks in expression at day 2.5 post coital with Ptc1 and COUP-TF II increasing 1day later. During artificial decidualization, Ihh peaks early, 6 hrs after the first injection of P4 and E2 (Figure 3), reconfirming the rapid induction of Ihh in response to P4. As in the pregnant uterus, Ihh target genes, Ptc1 and COUP-TF II increase 1day later at the 30 hr time point (Figure 3). This temporal and spatial pattern of expression indicates that Progesterone acts on the uterine epithelium to cause Ihh stimulation of uterine stroma cell in preparation of the endometrium for implantation.
Figure 3.

Quantitative RT-PCR analysis of the expression of Ihh, Ptc and COUP-TF II in pseudopregnany and during an exogenous hormone treatment regimen. Pseudopregnancy: Female mice were mated with vasectomized male mice and collected daily. Presence of the vaginal plug after mating is 0.5dpc. The arrow represents the window of implantation. Endocrine Stimulation of Decidualization: Ovariectomized mice treated with 3 daily injections of 100 ng E2 per mouse. After 2 days rest, mice were then treated with daily injections of 1 mg P4 and 6.7 ng E2 per mouse for 3 days.,s.c. One uterine horn was traumatized by a needle scratch on the anti-mesometrial lumen 6 hrs after the last injection. The contralateral horn was not traumatized and served as a control. Mice were given daily injections of 1mg P4 and 6.7 ng E2 per mouse each day following the trauma, s.c. 6hrs after each injection of 1 mg P4 and 6.7 ng E2, mice were sacrificed and uteri were collected. The arrow represents the time at which the decidual trauma was given.
Genetic Mouse models Hedgehog signaling
The role of Ihh and members of its signaling axis have been investigated by gene ablation studies using homologous recombination in embryonic stem cells. Ihh null mice either die before birth presumably to defects in yolk sac vasculargenesis, or shortly after birth due to considerable skeletal malformations (St-Jacques et al., 1999). Ptc1 null mice die during early embryogenesis with overgrown neural tubes (Goodrich et al., 1997). Smo null animals arrest at early somite stages with defects in heart development, an open gut, cyclopia, and an absence of left/right asymmetry (Zhang et al., 2001). Mice homozygous for a disrupted Gli1 allele are viable and appear to be normal (Park et al., 2000). Gli2 mutants have numerous abnormalities including skeletal defects, abnormal lungs, and lack a floor plate and adjacent ventral intermediate region cells in the spinal cord (Mo et al., 1997, Motoyama et al., 1998, Ding et al., 1998, Matise et al., 1998). These phenotypes comprise a subset of the abnormalities found in Shh null animals, demonstrating that Gli2 is a major mediator of Shh signaling. Mice homozygous for a disrupted Gli3 allele die either embryonically or perinataly and demonstrate a wide range of defects including polydactyl and dorsal nervous system defects that are associated with ectopic Shh expression. Additionally, numerous studies using double mutants of two Gli family members have shown that they have partially overlapping affects dependent on the specific tissue and developmental time point. For example, studies utilizing double mutants of Gli1 and Gli2 demonstrate that Gli1 and Gli2, but not Gli3, have extensive overlapping functions in patterning the lungs and CNS (Park et al., 2000). On the other hand, Gli2 and Gli3 serve redundant functions during skeletal development (Mo et al., 1997). The clearly spatiotemporally controlled expression of the Gli family members in the uterus suggests that Gli1 and Gli2, which have similar expression patterns, may have overlapping function, while epithelial expression of Gli3 in the absence of Hedgehog signaling at ovulation may serve as an additional mechanism to finely control Hedgehog signaling in the uterus.
Recently, null mutations of the genes that mediate the signal from Smo to the Gli family of transcription factors have been made. Sufu knockouts are early embryonic lethal and show strong similarities with Ptch1 knockouts including neural tube defects (Svard et al., 2006). On the other hand, ablation of Fu leads to normal organogenesis during development, but early lethality within the first weeks of life. Therefore, Fu is only required postnatally, and has been implicated in the homeostasis of secretory and ciliated cells (Chen et al., 2005, Merchant et al., 2005). Targeted disruption of COUP-TF II gene results in early embryonic lethality due to defects in angiogenesis and heart development (Pereira et al., 1999). Hedgehog signaling is difficult to study in the murine uterus due to the early lethality exhibited by many of the null mutant mice. Supporting the hypothesis that Ihh signaling may be critical for uterine function, analysis of COUP-TF II +/− females shows significantly reduced fecundity due to both ovarian and uterine defects. Analysis of uterine function demonstrated a reduced response to an experimentally induced decidual cell reaction indicating that the ability of the uterus to support embryo implantation was reduced (Takamoto et al., 2005).
Genetic ablation of members of the Ihh signaling pathway have identified important physiological processes, including cell proliferation, differentiation and vascularization are regulated by this pathway. However, due to the embryo lethal nature of these knockouts, the role of these factors in uterine biology make it difficult to investigate. In order to study the function of these genes in the adult uterus, it will be necessary to create models that ablate gene expression in a tissue specific manner. A uterine specific Cre recombinase would make it possible to ablate genes in the uterus. At this time, a uterine specific Cre recombinase has yet to be developed. However, a mouse model in which Cre recombinase under the control of the endogenous PR promoter allows the specific ablation of genes in tissues that express Pgr. This mouse model demonstrates high levels of recombination throughout the uterus, in the corpus luteum of the ovary, and selected cellular populations in the pituitary and mammary gland (Soyal et al., 2005). Potential problems with this mouse model would include disruption of the pituitary/ovarian axis that may affect uterine function.
Conclusion
Progesterone acting through its receptor is an important regulator of uterine function. Identification of molecular pathways regulated by progesterone is important to understand the molecular mechanisms regulating uterine function. One morphogen pathway, the Hedgehog signaling pathway, has been identified as a potential pathway coordinating communication between the epithelium and stromal components of the endometrium. Members of his pathway have been shown to regulate proliferation, differentiation and vascularization in other tissues and through development. Tissue specific ablation of members of this signaling axis would be helpful in identifying the role of this cascade in uterine function.
Acknowledgments
This work was supported by NICHD/NIH as part of the Cooperative Program on Trophoblast-Maternal Tissue Interactions (U01HD042311) (to F.J.D); the Reproductive Biology Training Grant (5 T32 HD07165) (to K.L.); RO1-CA77530 and the Susan G. Komen Award BCTR0503763 (to J.P.L.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALCEDO J, AYZENZON M, VON OHLEN T, NOLL M, HOOPER JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86:221–32. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- BARENT RL, NAIR SC, CARR DC, RUAN Y, RIMERMAN RA, FULTON J, ZHANG Y, SMITH DF. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–54. doi: 10.1210/mend.12.3.0075. [DOI] [PubMed] [Google Scholar]
- BOONYARATANAKORNKIT V, SCOTT MP, RIBON V, SHERMAN L, ANDERSON SM, MALLER JL, MILLER WT, EDWARDS DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–80. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- BRAR AK, FRANK GR, KESSLER CA, CEDARS MI, HANDWERGER S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6:301–7. doi: 10.1007/BF02820507. [DOI] [PubMed] [Google Scholar]
- CHEN MH, GAO N, KAWAKAMI T, CHUANG PT. Mice deficient in the fused homolog do not exhibit phenotypes indicative of perturbed hedgehog signaling during embryonic development. Mol Cell Biol. 2005;25:7042–53. doi: 10.1128/MCB.25.16.7042-7053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG KW, CHENG CK, LEUNG PC. Differential role of PR-A and -B isoforms in transcription regulation of human GnRH receptor gene. Mol Endocrinol. 2001;15:2078–92. doi: 10.1210/mend.15.12.0739. [DOI] [PubMed] [Google Scholar]
- CHEON YP, LI Q, XU X, DEMAYO FJ, BAGCHI IC, BAGCHI MK. A genomic approach to identify novel progesterone receptor regulated pathways in the uterus during implantation. Mol Endocrinol. 2002;16:2853–71. doi: 10.1210/me.2002-0270. [DOI] [PubMed] [Google Scholar]
- CHRISTIAN M, POHNKE Y, KEMPF R, GELLERSEN B, BROSENS JJ. Functional association of PR and CCAAT/enhancer-binding protein beta isoforms: promoter-dependent cooperation between PR-B and liver-enriched inhibitory protein, or liver-enriched activatory protein and PR-A in human endometrial stromal cells. Mol Endocrinol. 2002;16:141–54. doi: 10.1210/mend.16.1.0763. [DOI] [PubMed] [Google Scholar]
- CONDON JC, HARDY DB, KOVARIC K, MENDELSON CR. Upregulation of the Progesterone Receptor (PR)-C Isoform in Laboring Myometrium by Activation of NF-{kappa}B May Contribute to the Onset of Labor through Inhibition of PR Function. Mol Endocrinol. 2005 doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- DAIKOKU T, TRANGUCH S, FRIEDMAN DB, DAS SK, SMITH DF, DEY SK. Proteomic analysis identifies immunophilin FK506 binding protein 4 (FKBP52) as a downstream target of Hoxa10 in the periimplantation mouse uterus. Mol Endocrinol. 2005;19:683–97. doi: 10.1210/me.2004-0332. [DOI] [PubMed] [Google Scholar]
- DAVIES TH, SANCHEZ ER. Fkbp52. Int J Biochem Cell Biol. 2005;37:42–7. doi: 10.1016/j.biocel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- DENNER LA, WEIGEL NL, MAXWELL BL, SCHRADER WT, O'MALLEY BW. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–3. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- DING Q, MOTOYAMA J, GASCA S, MO R, SASAKI H, ROSSANT J, HUI CC. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125:2533–43. doi: 10.1242/dev.125.14.2533. [DOI] [PubMed] [Google Scholar]
- DYER MA, FARRINGTON SM, MOHN D, MUNDAY JR, BARON MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–30. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- EVANS RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO J, MAZELLA J, SEPPALA M, TSENG L. Ligand activated hPR modulates the glycodelin promoter activity through the Sp1 sites in human endometrial adenocarcinoma cells. Mol Cell Endocrinol. 2001;176:97–102. doi: 10.1016/s0303-7207(01)00450-6. [DOI] [PubMed] [Google Scholar]
- GAO J, MAZELLA J, TANG M, TSENG L. Ligand-activated progesterone receptor isoform hPR-A is a stronger transactivator than hPR-B for the expression of IGFBP-1 (insulin-like growth factor binding protein-1) in human endometrial stromal cells. Mol Endocrinol. 2000;14:1954–61. doi: 10.1210/mend.14.12.0564. [DOI] [PubMed] [Google Scholar]
- GEHIN M, MARK M, DENNEFELD C, DIERICH A, GRONEMEYER H, CHAMBON P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–37. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODRICH LV, MILENKOVIC L, HIGGINS KM, SCOTT MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- HAMATANI T, DAIKOKU T, WANG H, MATSUMOTO H, CARTER MG, KO MS, DEY SK. Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc Natl Acad Sci U S A. 2004;101:10326–31. doi: 10.1073/pnas.0402597101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBROK M, KIM SK, ST JACQUES B, MCMAHON AP, MELTON DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–13. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- HEERY DM, KALKHOVEN E, HOARE S, PARKER MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–6. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- HUBLER TR, DENNY WB, VALENTINE DL, CHEUNG-FLYNN J, SMITH DF, SCAMMELL JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–7. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- HUET-HUDSON YM, ANDREWS GK, DEY SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–90. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- INGHAM PW, TAYLOR AM, NAKANO Y. Role of the Drosophila patched gene in positional signalling. Nature. 1991;353:184–7. doi: 10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- JEONG JW, LEE KY, KWAK I, WHITE LD, HILSENBECK SG, LYDON JP, DEMAYO FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- JEPSEN K, HERMANSON O, ONAMI TM, GLEIBERMAN AS, LUNYAK V, MCEVILLY RJ, KUROKAWA R, KUMAR V, LIU F, SETO E, HEDRICK SM, MANDEL G, GLASS CK, ROSE DW, ROSENFELD MG. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102:753–63. doi: 10.1016/s0092-8674(00)00064-7. [DOI] [PubMed] [Google Scholar]
- JIANG YH, ARMSTRONG D, ALBRECHT U, ATKINS CM, NOEBELS JL, EICHELE G, SWEATT JD, BEAUDET AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- KU CY, SANBORN BM. Progesterone prevents the pregnancy-related decline in protein kinase A association with rat myometrial plasma membrane and A-kinase anchoring protein. Biol Reprod. 2002;67:605–9. doi: 10.1095/biolreprod67.2.605. [DOI] [PubMed] [Google Scholar]
- LANSKE B, KARAPLIS AC, LEE K, LUZ A, VORTKAMP A, PIRRO A, KARPERIEN M, DEFIZE LH, HO C, MULLIGAN RC, ABOU-SAMRA AB, JUPPNER H, SEGRE GV, KRONENBERG HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–6. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- LEE JJ, VON KESSLER DP, PARKS S, BEACHY PA. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71:33–50. doi: 10.1016/0092-8674(92)90264-d. [DOI] [PubMed] [Google Scholar]
- LEE KY, OH GT, KANG JH, SHIN SM, HEO BE, YUN YW, PAIK SG, KRISINGER J, LEUNG PC, JEUNG EB. Transcriptional regulation of the mouse calbindin-D9k gene by the ovarian sex hormone. Mol Cells. 2003;16:48–53. [PubMed] [Google Scholar]
- LEONHARDT SA, BOONYARATANAKORNKIT V, EDWARDS DP. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids. 2003;68:761–70. doi: 10.1016/s0039-128x(03)00129-6. [DOI] [PubMed] [Google Scholar]
- LEWIS MT, ROSS S, STRICKLAND PA, SUGNET CW, JIMENEZ E, SCOTT MP, DANIEL CW. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126:5181–93. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- LYDON JP, DEMAYO FJ, FUNK CR, MANI SK, HUGHES AR, MONTGOMERY CA, JR, SHYAMALA G, CONNEELY OM, O'MALLEY BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- MALARTRE M, SHORT S, SHARPE C. Xenopus embryos lacking specific isoforms of the corepressor SMRT develop abnormal heads. Dev Biol. 2006;292:333–43. doi: 10.1016/j.ydbio.2006.01.007. [DOI] [PubMed] [Google Scholar]
- MANI SK. Signaling mechanisms in progesterone-neurotransmitter interactions. Neuroscience. 2006;138:773–81. doi: 10.1016/j.neuroscience.2005.07.034. [DOI] [PubMed] [Google Scholar]
- MANI SK, ALLEN JM, CLARK JH, BLAUSTEIN JD, O'MALLEY BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265:1246–9. doi: 10.1126/science.7915049. [DOI] [PubMed] [Google Scholar]
- MANI SK, ALLEN JM, LYDON JP, MULAC-JERICEVIC B, BLAUSTEIN JD, DEMAYO FJ, CONNEELY O, O'MALLEY BW. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–37. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- MARUYAMA T, YOSHIMURA Y, YODOI J, SABE H. Activation of c-Src kinase is associated with in vitro decidualization of human endometrial stromal cells. Endocrinology. 1999;140:2632–6. doi: 10.1210/endo.140.6.6933. [DOI] [PubMed] [Google Scholar]
- MATISE MP, EPSTEIN DJ, PARK HL, PLATT KA, JOYNER AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–70. doi: 10.1242/dev.125.15.2759. [DOI] [PubMed] [Google Scholar]
- MATSUI D, SAKARI M, SATO T, MURAYAMA A, TAKADA I, KIM M, TAKEYAMA K, KATO S. Transcriptional regulation of the mouse steroid 5alpha-reductase type II gene by progesterone in brain. Nucleic Acids Res. 2002;30:1387–93. doi: 10.1093/nar/30.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUMOTO H, ZHAO X, DAS SK, HOGAN BL, DEY SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245:280–90. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- MCKENNA NJ, XU J, NAWAZ Z, TSAI SY, TSAI MJ, O'MALLEY BW. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J Steroid Biochem Mol Biol. 1999;69:3–12. doi: 10.1016/s0960-0760(98)00144-7. [DOI] [PubMed] [Google Scholar]
- MERCHANT M, EVANGELISTA M, LUOH SM, FRANTZ GD, CHALASANI S, CARANO RA, VAN HOY M, RAMIREZ J, OGASAWARA AK, MCFARLAND LM, FILVAROFF EH, FRENCH DM, DE SAUVAGE FJ. Loss of the serine/threonine kinase fused results in postnatal growth defects and lethality due to progressive hydrocephalus. Mol Cell Biol. 2005;25:7054–68. doi: 10.1128/MCB.25.16.7054-7068.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MO R, FREER AM, ZINYK DL, CRACKOWER MA, MICHAUD J, HENG HH, CHIK KW, SHI XM, TSUI LC, CHENG SH, JOYNER AL, HUI C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–23. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- MOORE MR, ZHOU JL, BLANKENSHIP KA, STROBL JS, EDWARDS DP, GENTRY RN. A sequence in the 5' flanking region confers progestin responsiveness on the human c-myc gene. J Steroid Biochem Mol Biol. 1997;62:243–52. doi: 10.1016/s0960-0760(97)00036-8. [DOI] [PubMed] [Google Scholar]
- MOTOYAMA J, LIU J, MO R, DING Q, POST M, HUI CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–7. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- MUELLER MD, VIGNE JL, PRITTS EA, CHAO V, DREHER E, TAYLOR RN. Progestins activate vascular endothelial growth factor gene transcription in endometrial adenocarcinoma cells. Fertil Steril. 2003;79:386–92. doi: 10.1016/s0015-0282(02)04577-6. [DOI] [PubMed] [Google Scholar]
- MULAC-JERICEVIC B, LYDON JP, DEMAYO FJ, CONNEELY OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULAC-JERICEVIC B, MULLINAX RA, DEMAYO FJ, LYDON JP, CONNEELY OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–4. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M, ICHISAKA T, MAEDA M, OSHIRO N, HARA K, EDENHOFER F, KIYAMA H, YONEZAWA K, YAMANAKA S. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol. 2004;24:6710–8. doi: 10.1128/MCB.24.15.6710-6718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEMANN C, UNDEN AB, LYLE S, ZOUBOULIS CH C, TOFTGARD R, WATT FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11873–80. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONATE SA, TSAI SY, TSAI MJ, O'MALLEY BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–7. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- PARK HL, BAI C, PLATT KA, MATISE MP, BEEGHLY A, HUI CC, NAKASHIMA M, JOYNER AL. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–605. doi: 10.1242/dev.127.8.1593. [DOI] [PubMed] [Google Scholar]
- PEPINSKY RB, ZENG C, WEN D, RAYHORN P, BAKER DP, WILLIAMS KP, BIXLER SA, AMBROSE CM, GARBER EA, MIATKOWSKI K, TAYLOR FR, WANG EA, GALDES A. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998;273:14037–45. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- PEREIRA FA, QIU Y, ZHOU G, TSAI MJ, TSAI SY. The orphan nuclear receptor COUP-TF II is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–49. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRON M, BOY S, AMATO MA, VICZIAN A, KOEBERNICK K, PIELER T, HARRIS WA. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development. 2003;130:1565–77. doi: 10.1242/dev.00391. [DOI] [PubMed] [Google Scholar]
- PIERSON-MULLANY LK, LANGE CA. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24:10542–57. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER JA, YOUNG KE, BEACHY PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–9. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- POWER RF, CONNEELY OM, O'MALLEY BW. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992;13:318–23. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- POWER RF, MANI SK, CODINA J, CONNEELY OM, O'MALLEY BW. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991;254:1636–9. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- RAMALHO-SANTOS M, MELTON DA, MCMAHON AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–72. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- REESE J, DAS SK, PARIA BC, LIM H, SONG H, MATSUMOTO H, KNUDTSON KL, DUBOIS RN, DEY SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem. 2001;276:44137–45. doi: 10.1074/jbc.M107563200. [DOI] [PubMed] [Google Scholar]
- RIBEIRO RC, KUSHNER PJ, BAXTER JD. The nuclear hormone receptor gene superfamily. Annu Rev Med. 1995;46:443–53. doi: 10.1146/annurev.med.46.1.443. [DOI] [PubMed] [Google Scholar]
- SHIMIZU A, MARUYAMA T, TAMAKI K, UCHIDA H, ASADA H, YOSHIMURA Y. Impairment of decidualization in SRC-deficient mice. Biol Reprod. 2005;73:1219–27. doi: 10.1095/biolreprod.105.041616. [DOI] [PubMed] [Google Scholar]
- SLATER EP, CATO AC, KARIN M, BAXTER JD, BEATO M. Progesterone induction of metallothionein-IIA gene expression. Mol Endocrinol. 1988;2:485–91. doi: 10.1210/mend-2-6-485. [DOI] [PubMed] [Google Scholar]
- SMITH CL, DEVERA DG, LAMB DJ, NAWAZ Z, JIANG YH, BEAUDET AL, O'MALLEY BW. Genetic ablation of the steroid receptor coactivator-ubiquitin ligase, E6-AP, results in tissue-selective steroid hormone resistance and defects in reproduction. Mol Cell Biol. 2002;22:525–35. doi: 10.1128/MCB.22.2.525-535.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH CL, O'MALLEY BW. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr Rev. 2004;25:45–71. doi: 10.1210/er.2003-0023. [DOI] [PubMed] [Google Scholar]
- SOYAL SM, MUKHERJEE A, LEE KY, LI J, LI H, DEMAYO FJ, LYDON JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- ST-JACQUES B, HAMMERSCHMIDT M, MCMAHON AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVARD J, HEBY-HENRICSON K, PERSSON-LEK M, ROZELL B, LAUTH M, BERGSTROM A, ERICSON J, TOFTGARD R, TEGLUND S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–97. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- TAKAMOTO N, KURIHARA I, LEE K, DEMAYO FJ, TSAI MJ, TSAI SY. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19:2299–308. doi: 10.1210/me.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAMOTO N, ZHAO B, TSAI SY, DEMAYO FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–48. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- TANG M, MAZELLA J, GAO J, TSENG L. Progesterone receptor activates its promoter activity in human endometrial stromal cells. Mol Cell Endocrinol. 2002;192:45–53. doi: 10.1016/s0303-7207(02)00111-9. [DOI] [PubMed] [Google Scholar]
- THOMAS SM, BRUGGE JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- TRANGUCH S, CHEUNG-FLYNN J, DAIKOKU T, PRAPAPANICH V, COX MB, XIE H, WANG H, DAS SK, SMITH DF, DEY SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–31. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI MJ, O'MALLEY BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–86. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA S, TANAKA S, SUGIMOTO Y, KATSUYAMA M, IKEGAMI R, ICHIKAWA A. Identification and characterization of a novel progesterone receptor-binding element in the mouse prostaglandin E receptor subtype EP2 gene. Genes Cells. 2003;8:747–58. doi: 10.1046/j.1365-2443.2003.00672.x. [DOI] [PubMed] [Google Scholar]
- WAGNER BL, NORRIS JD, KNOTTS TA, WEIGEL NL, MCDONNELL DP. The nuclear corepressors NCoR and SMRT are key regulators of both ligand- and 8-bromo-cyclic AMP-dependent transcriptional activity of the human progesterone receptor. Mol Cell Biol. 1998;18:1369–78. doi: 10.1128/mcb.18.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI LL, HAWKINS P, BAKER C, NORRIS B, SHERIDAN PL, QUINN PG. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol. 1996;10:1379–87. doi: 10.1210/mend.10.11.8923464. [DOI] [PubMed] [Google Scholar]
- WEI LL, NORRIS BM, BAKER CJ. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J Steroid Biochem Mol Biol. 1997;62:287–97. doi: 10.1016/s0960-0760(97)00044-7. [DOI] [PubMed] [Google Scholar]
- WIJGERDE M, OOMS M, HOOGERBRUGGE JW, GROOTEGOED JA. Hedgehog signaling in mouse ovary: Indian hedgehog and desert hedgehog from granulosa cells induce target gene expression in developing theca cells. Endocrinology. 2005;146:3558–66. doi: 10.1210/en.2005-0311. [DOI] [PubMed] [Google Scholar]
- YADAV N, LEE J, KIM J, SHEN J, HU MC, ALDAZ CM, BEDFORD MT. Specific protein methylation defects and gene expression perturbations in coactivator-associated arginine methyltransferase 1-deficient mice. Proc Natl Acad Sci U S A. 2003;100:6464–8. doi: 10.1073/pnas.1232272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG WM, YAO YL, SETO E. The FK506-binding protein 25 functionally associates with histone deacetylases and with transcription factor YY1. Embo J. 2001;20:4814–25. doi: 10.1093/emboj/20.17.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIOKA K, MATSUDA F, TAKAKURA K, NODA Y, IMAKAWA K, SAKAI S. Determination of genes involved in the process of implantation: application of GeneChip to scan 6500 genes. Biochem Biophys Res Commun. 2000;272:531–8. doi: 10.1006/bbrc.2000.2818. [DOI] [PubMed] [Google Scholar]
- ZHANG XM, RAMALHO-SANTOS M, MCMAHON AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–92. [PubMed] [Google Scholar]
- ZHANG Y, BECK CA, POLETTI A, CLEMENT JPT, PRENDERGAST P, YIP TT, HUTCHENS TW, EDWARDS DP, WEIGEL NL. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11:823–32. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]


