Abstract
Aging is associated with an overall loss of function at the level of the whole organism that has origins in cellular deterioration. Most cellular components, including mitochondria, require continuous recycling and regeneration throughout the lifespan. Mitochondria are particularly susceptive to damage over time as they are the major bioenergetic machinery and source of oxidative stress in cells. Effective control of mitochondrial biogenesis and turnover, therefore, becomes critical for the maintenance of energy production, the prevention of endogenous oxidative stress and the promotion of healthy aging. Multiple endogenous and exogenous factors regulate mitochondrial biogenesis through the peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α). Activators of PGC-1α include nitric oxide, CREB and AMPK. Calorie restriction (CR) and resveratrol, a proposed CR mimetic, also increase mitochondrial biogenesis through activation of PGC-1α. Moderate exercise also mimics CR by inducing mitochondrial biogenesis. Negative regulators of PGC-1α such as RIP140 and 160MBP suppress mitochondrial biogenesis. Another mechanism involved in mitochondrial maintenance is mitochondrial fission/fusion and this process also involves an increasing number of regulatory proteins. Dysfunction of either biogenesis or fission/fusion of mitochondria is associated with diseases of the neuromuscular system and aging, and a greater understanding of the regulation of these processes should help us to ultimately control the aging process.
Introduction
Cells and tissues under demand for increased energy requirements respond by stepping up production of new mitochondria. In general, the regulation of mitochondrial biogenesis is expected to be influenced by changing energetic and physiological conditions. It is therefore not surprising that factors such as availability of nutrients, presence or absence of certain hormones, temperature, exercise, hypoxia, stress and aging have all been reported to impact the process of mitochondriogenesis (Annex, et al., 1991; Freyssenet, Berthon & Denis, 1996; Lee & Wei, 2005; Lee, et al., 2002; Nagino, et al., 1989; Wu, et al., 2007).
Energy-dependent cellular changes known to affect both mitochondrial function and number involve a complex set of factors that link energy requirements to gene regulation. There is a considerable body of literature describing some of these factors as well as their mechanisms of action. Early work on the biogenesis of mitochondria focused on studies performed in two model organisms, the yeast Saccharomyces cerevisiae (Linnane, et al., 1968; Trembath, et al., 1975a; Trembath, et al., 1975b; Vary, Edwards & Stewart, 1969; Vary, Stewart & Linnane, 1970; Watson, Haslam & Linnane, 1970) and the filamentous fungus Neurospora crasa (Beck & Greenawalt, 1976a; Beck & Greenawalt, 1976b; Beck & Greenawalt, 1976c). Later studies expanded the investigation of mitochondrial biogenesis to other systems, including mammals (Callen, Dennebouy & Mounolou, 1980; Mutvei, et al., 1989; Rosano & Jones, 1976; Van den Bogert, et al., 1988). However, despite this important body of information, the process by which eukaryotic cells increase mitochondrial mass and number under diverse physiological and pathological conditions is still poorly understood.
The aim of this review is to identify all the mechanisms currently understood to be involved in mitochondrial biogenesis and elucidate the interactions between the various factors participating in its modulation. We will also cover the current knowledge of the influence of mitochondrial biogenesis and fission/fusion on aging. Special attention will be paid to the role of mitochondrial recycling during neurodegeneration.
Regulation of mitochondrial biogenesis is complex
The complexity of mitochondrial biogenesis regulation cannot be understated; it involves changes in the expression of more than 1000 genes, the cooperation of two genomes, and alters the level of approximately 20% of cellular proteins. In the nucleus of the cell, the concerted regulation of such a large number of genes requires a common set of transcription factors able to orchestrate the interaction of the RNApol II complex with the various target promoters. Importantly, in addition to the nuclear genes (which encode more than the 95 % of mitochondrial proteins), mitochondriogenesis requires the participation of the mitochondrial genome, which is responsible for the production of the most hydrophobic proteins of the electron transport chain, as well as mitochondrial tRNAs and rRNAs. Although the most important regulatory steps of mitochondriogenesis appear to take place at the level of transcriptional regulation of nuclear genes (Lenka, et al., 1998; Scarpulla, 2002), it has been recently shown that transcription affecting both nuclear and mitochondrial genomes must be coordinated in order to produce new mitochondria (Roy, et al., 2007). The precise synchronization of two independent genomes located in separate subcellular compartments undoubtedly requires intricate molecular machinery, making the regulation of the resulting process a very complex task.
Hormones affect mitochondrial biogenesis
At the level of the organism, energetic homeostasis is tightly regulated by the release of hormones and the specific responses they elicit on their target cells. Both thyroid and steroid hormones such as glucocorticoids regulate the expression of most of the nuclear genes encoding mitochondrial proteins. Sex hormones have been shown to exert differential effects on the expression of various mitochondriogenic molecules. For example, in brown adipose tissue progesterone promotes while testosterone inhibits mitochondriogenesis by differentially modulating the expression of several transcription factors involved in this process (Rodriguez-Cuenca, et al., 2007). Other hormones, like adrenal steroids, play an important role in perinatal mitochondrial maturation and biogenesis in a tissue-specific manner (Prieur, Bismuth & Delaval, 1998).
In mammals, the most important factors involved in mitochondrial biogenesis are the thyroid hormones (Mutvei, et al., 1989). Treatment of rats with the thyroid hormone T4 produces hyperplasia and increases the number and mass of mitochondria in both liver (Wooten & Cascarano, 1980) and cardiac muscle (Goldenthal, Weiss & Marin-Garcia, 2004). Levels of T3 and T4 hormones have been regarded as important factors in the maintenance of proper mitochondriogenic rates during aging (Mutvei, et al., 1989). The effectiveness of thyroid hormones on specific tissues depends on the amount of receptors present at the site of action, and receptor levels can vary under different physiological conditions. For instance, T3 is able to increase mitochondrial biogenesis in oxidative rat muscle but not in glycolytic muscle, and this differential response correlates with lower amounts of TH receptor in the glycolytic tissue (Bahi, et al., 2005).
Transcription factors regulate mitochondrial biogenesis
At the molecular level, several transcription factors and cofactors are involved in the activation and regulation of mitochondrial biogenesis. These factors can be clustered in three main groups: ubiquitous transcription factors (SP1, YY1, CREB, MEF-2/E-box), nuclear respiratory factors (NRF-1, -2, REBOX/OXBOX, MT-1 to -4) and coactivators (PGC-1α, -1β, PRC) (Goffart & Wiesner, 2003). The exact contribution of each of these proteins to the generation of new mitochondria is rather difficult to dissect. These factors participate in a complex network that also includes hormone-induced signaling pathway components. Moreover, another set of transcription factors are involved in the metabolic adaptation to fasting such as the family of the peroxisome proliferator activated receptor (PPAR) and liver X receptor (LXR) that together with PGC-1α increase mitochondrial biogenesis and fatty acid catabolism.
Despite the complexity of the various signaling pathways that converge to regulate mitochondrial biogenesis, they all seem to share the common key component of the PGC-1 family of co-transcription factors. Specifically, has been shown to act as a common intracellular mediator during mitochondrial biogenesis induced by hormonal factors (Alaynick, 2008; Bahi, et al., 2005; Hsieh, et al., 2005; Nervina, et al., 2006; Weitzel, Iwen & Seitz, 2003; Zhang, et al., 2004). also seems to be a crucial factor in both the activation of the full program of mitochondriogenesis and in respiration. Its physiological importance is underscored by the fact that repression of PGC-1α by a mutant form of the huntingtin protein leads to mitochondrial dysfunction and neurodegeneration, whereas the overexpression of PGC-1α rescues cells from the deleterious effect of huntingtin (Cui, et al., 2006). PGC-1α appears to act as a master regulator of energy metabolism and mitochondrial biogenesis by integrating and coordinating the activity of multiple transcription factors, such as NRF-1, -2, PPARα and mtTFA (Puigserver, et al., 1998). PGC-1α has been shown to directly dock on some of these transcription factors (e.g. ERRx, NRF-1 and -2) and modulate their activities (Gleyzer, Vercauteren & Scarpulla, 2005; Schreiber, et al., 2004). Moreover, PGC-1α can act at DNA target sites to recruit additional co-activators such as the steroid receptor coactivator-1 (SRC-1), which through its histone acetyltransferase activity induces morphological alterations in the DNA making it more available to transcriptional machinery and thereby stimulating gene expression (Puigserver, et al., 1999).
Expression levels of PGC-1α are directly related to mitochondrial biogenesis activity. As a multi-responsive factor, many agents and events can regulate the levels of PGC-1α mRNA by activating different intracellular mediators (Figure 1). In a screening of more than 10,000 putative transcriptional regulators of this gene performed by Wu et al. (2006), the most potent activators found were members of the Transducer Of Regulated CREB (cAMP response element-binding protein)-binding protein (TORC) family, which are co-activators of CREB (Wu, et al., 2006). Moreover, an increase in muscle cytosolic calcium levels induced by exercise stimulates Calmodulin kinase (CamK(IV)) which then promotes PGC-1α expression through CREBP (Wu, et al., 2002). Calcineurin A also enhances PGC-1α expression in cardiac muscle through activation of Myocyte Enhancer Factor-2 (MEF-2) (Czubryt, et al., 2003). Furthermore, a mutant version of huntingtin is able to repress the transcription of PGC-1α by associating with its promoter and interfering with the CREB/TAF4-dependent transcription pathway (Cui, et al., 2006). Hence, these data indicate that the transcriptional regulation of PGC-1α is strongly modulated by CREB activity.
Figure 1. PGC-1α regulating network.
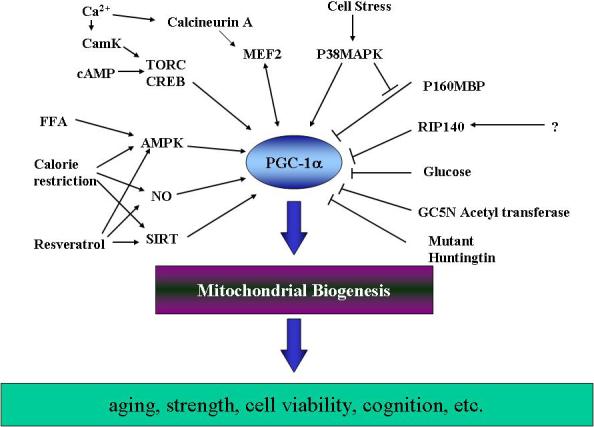
PGC-1α is at the center of a complex network of signals affected by metabolic, nutritional and environmental factors that modulate (e.g. through transcriptional and post-translational modifications) PGC-1α activity and thereby mitochondrial biogenesis. To date, very few negative regulators of PGC-1α have been found. However, increasing interest in this protein suggests the present regulatory network will grow yet more complex in the future.
Another important factor involved in the regulation of PGC-1α transcription is AMP-activated kinase (AMPK). This kinase, which is activated by an increase in intracellular AMP/ATP ratio, is a cellular energy sensor (Reznick, et al., 2007). AMPK also functions as an integrating factor that modulates several aging-associated processes, such as insulin resistance, obesity, and decreased fatty acid catabolism and mitochondrial biogenesis (Irrcher, et al., 2003). In primary muscle cells, AMPK mediates metabolic changes affecting glucose uptake, fatty acid oxidation and mitochondrial biogenesis by directly phosphorylating PGC-1α (Jager, et al., 2007; Winder, Taylor & Thomson, 2006). During food deprivation, an increase in the cellular AMP/ATP ratio results in activation of AMPK that initiates a signaling process that recruits mediators of fatty acid-dependent oxidative metabolism and mitochondrial biogenesis including PGC-1α, PPARδ and others (de Lange, et al., 2006).
Recently, nitric oxide (NO) was shown to regulate mitochondrial biogenesis through the transcriptional activation of (Leary & Shoubridge, 2003; Nisoli, et al., 2003). PGC-1α-mediated mitochondrial biogenesis independent of NO/cGMP stimulation has been reported (Wadley & McConell, 2007), however, NO and its derivatives are produced and consumed by mitochondria and can also stimulate mitochondrial biogenesis via cGMP-mediated upregulation of transcriptional factors (Brown, 2007). Increases in cGMP levels can also lead to the expression of the deacetylase SIRT1 (Nisoli, et al., 2005). SIRT1 is a NAD+-dependent deacetylase that can act to deacetylate and thereby increase its activity (Nisoli & Carruba, 2006). SIRT1 activation by resveratrol (3,5,4,9-trihydroxystilbene) has been recently implicated in the mitochondrial biogenesis induced by this polyphenol in cultures of liver and muscle cells (Baur, et al., 2006; Lagouge, et al., 2006). It appears that both NO and SIRT1 function as signaling mediators that link mitochondrial activity and transcriptional regulation at the nucleus leading to the expression of genes involved in mitochondrial biogenesis through the activation of PGC-1α.
In contrast to the growing list of PGC-1α-activating factors identified to date, little is known about cellular factors that act as negative regulators of PGC-1α. One of these negative regulators could be the transcriptional co-repressor RIP140, which interacts with many nuclear receptors including retinoic acid receptor and retinoid X receptor (RXR) in a ligand-dependent manner that affects heterodimer formation between RXR and PPAR (White, et al., 2008). Thus, RIP140 has been proposed as suppressor of mitochondrial biogenesis and oxidative metabolism in mammalian cells (Powelka, et al., 2006; Treuter, et al., 1998). However, this co-repressor has also been observed have a greater effect on PGC-1β than PGC-1α in adipocytes probably because of the relatively low levels of PGC-1α found in these cells (Powelka, et al., 2006). PGC-1α also contains a negative regulatory domain that attenuates its transcriptional activity, and the p160 myb binding protein (p160MBP) acts as a repressor of PGC-1α by binding to this regulatory region. This interaction is further regulated by p38 MAPK, which phosphorylates the inhibitory domain of PGC-1α, efficiently disrupting p160MBP-binding and releasing PGC-1α from its inhibition (Fan, et al., 2004). In summary, the confluence of several activating and repressing pathways on PGC-1α affects the complex system that coordinate the demand of energy in the cell with mitochondrial biogenesis.
As mentioned above, sirtuins act as major positive regulators of PGC-1α activity through deacetylation. It follows then that there should be an acetylase enzyme that serves to repress PGC-1α activity. Recently, Lerin et al. (2006) have demonstrated that, at least in the liver, there is a factor involved in the repression of PGC-1α by acetylation: the GCN5 acetyltransferase complex. Acetylation of PGC-1α by GCN5 results in a transcriptionally inactive protein that re-localizes from the promoter of regulated genes to nuclear foci.
Aging affects mitochondrial biogenesis
Among the plethora of biological phenomena affected by aging, the malfunction and decrease of biogenesis of mitochondria seem to exert some of the most potent effects on the organism. If biogenesis is affected, it is reasonable to expect mitochondrial turnover must be slower and the accumulation of modified lipids, proteins and DNA must also increase, further aggravating the situation resulting from the deficient activity of aged mitochondria.
The precise reason for the decrease in the rate of mitochondrial biogenesis during aging is currently unknown. However, it seems that both, extra- and intra-cellular regulatory factors of mitochondrial biogenesis are implicated. In fact, the pleiotropic signaling of mitochondria, H2O2 and NO diffusion to the cytosol is modified in aged animals and contributes to the decreased mitochondrial biogenesis in old animals (Navarro & Boveris, 2007). Moreover, hypothyroidism has often been described in the elderly (Finucane & Anderson, 1995; Ravaglia, et al., 2000).
Among the known intracellular regulators of mitochondrial biogenesis, AMPK activity appears to be one of the main factors associated with deficient mitochondrial biogenesis, insulin resistance and impaired lipid metabolism observed in aged cells (Qiang, et al., 2007; Reznick, et al., 2007). An increasing body of experimental data on AMPK's function in different organisms and experimental paradigms has positioned this kinase at a critical point within a complex signaling network that integrates metabolism and aging. AMPK seems to play a critical role in several important processes such as the control of insulin resistance, obesity, fatty acid oxidation and mitochondrial biogenesis. Therefore, its dysfunction during aging seems to be one of the key factors involved in the deficiency in mitochondrial activity and metabolism regulation during aging. Thus, chronic AMPK inactivation is linked to a marked decrease in mitochondrial biogenesis in aged animals (Reznick, et al., 2007). Moreover, reduced AMPK activity was recently reported to correlate with aging-related insulin resistance and insufficient intracellular fat oxidation (Qiang, et al., 2007). On the other hand, overexpression in C. elegans of the AMPK worm ortholog, aak-2, results in increased lifespan (Curtis, O'Connor & DiStefano, 2006). Thus, due to its wide-ranging impact, it has been proposed that chronic activation of AMPK might be a strategy for slowing aging (McCarty, 2004).
A variety of other strategies are currently been considered to alleviate deficits in mitochondrial activity and biogenesis during aging. For example, it has been observed that several conditions that promote survival such as vitamin E dietary supplementation, CR, polyphenols and moderate physical exercise ameliorate mitochondrial dysfunction in aging. In particular, CR, resveratrol and exercise have been shown to increase the activity of both PGC-1α and mitochondrial biogenesis. CR affects several of the main factors involved in mitochondrial biogenesis, leading to increased NO levels (Nisoli, et al., 2005) and PGC-1α activity (Lopez-Lluch, et al., 2006). However, it is not clear if CR affects AMPK function (Gonzalez, et al., 2004) although short term CR increases the phosphorylation and thereby the activity of AMPK in both young and old animals (Shinmura, Tamaki & Bolli, 2005). In C. elegans, CR bypasses the need for aak-2/AMPK activity during lifespan extension (Curtis, O'Connor & DiStefano, 2006).
In spite of its reported effects on mitochondrial biogenesis, it is not clear whether CR affects this process in all tissues or if its regulatory function is tissue-specific. In skeletal muscle, CR only affects mitochondrial activities in aged but not young animals (Baker, et al., 2006; Hepple, et al., 2006). The decline in oxidative capacity seen in skeletal muscle in aging is prevented in aged CR animals (Hepple, et al., 2006). Moreover, the slower decline in PGC-1α gene expression with aging found in CR animals suggests a better maintenance of mitochondrial biogenesis with aging (Baker, et al., 2006; Hepple, et al., 2006). In fact, it has been suggested that CR-mediated prevention of the age-associated decline in mitochondrial function in heart and skeletal muscles is a secondary effect resulting from the enhanced maintenance of mitochondrial biogenesis (Hepple, et al., 2006).
Recently we and others demonstrated that a CR mimetic, the polyphenol resveratrol, affects mitochondrial biogenesis in liver, muscle and brain (Baur, et al., 2006; Dasgupta & Milbrandt, 2007; Lagouge, et al., 2006). The effects of resveratrol are largely explained by its ability to activate SIRT-1, which subsequently deacetylates and activates PGC-1α. In fact, resveratrol is completely unable to modulate PGC1-α function in SIRT1−/− MEFs. (Lagouge, et al., 2006). However, in contrast with CR, resveratrol is clearly able to increase the activity of AMPK (Dasgupta & Milbrandt, 2007). Furthermore, the increase in lifespan found in C. elegans overexpressing Sir2.1 partially depends on the activity of the aak-2/AMPK protein (Curtis, O'Connor & DiStefano, 2006).
CR and resveratrol not only maintain mitochondrial biogenesis but also affect other aging-related processes such as insulin resistance and lipid metabolism. Both CR and resveratrol modify the activity of mitochondria by inducing a switch in the source of energy utilized by the organelle from glucose to lipids. It is clear that induction of fatty acid catabolism and inhibition of fatty acid synthesis is central to CR and resveratrol's effects on mitochondrial biogenesis. For example, inhibition of fatty acid synthase in the hypothalamus has been shown to increase the number of mitochondria in white and red (soleus) skeletal muscle by increasing the levels of malonyl-CoA (Cha, et al., 2006). The “malonyl-CoA signal” is rapidly transmitted to skeletal muscle by the sympathetic nervous system and increases fatty acid oxidation, uncoupling protein-3 (UCP3) expression, and thus energy expenditure. Interestingly, PGC-1α appears to play an important role in this pathway. Overexpression of PGC-1α in C2C12 muscle cells increases fatty acid oxidation by modifying and increasing the activity of the enzymes that support mitochondrial fatty acid oxidation, ATP synthesis, and thermogenesis (Cha, et al., 2006).
Other agents that display potential aging-modulating properties also seem to affect the axis that connects lipid catabolism and mitochondrial biogenesis. For instance, the growth hormone releasing peptide hexarelin is able to induce both mitochondrial biogenesis and lipid metabolism. Addition of this peptide to 3T3-L1 adipocytes resulted in the depletion of intracellular lipid pools in a CD34-dependent fashion (Rodrigue-Way, et al., 2007). Like resveratrol, hexarelin induced the mobilization of fatty acids toward the mitochondrial oxidative phosphorylation process. Also, as in other mechanisms involved in mitochondrial biogenesis, hexarelin induced known targets of PPARγ such as FATP, CPT-1, and F(1)-ATPase, suggesting a PPARγ-dependent activity.
Some of the beneficial effects mediated by CR, resveratrol or low levels of stress like moderate exercise can be associated with the new concept of hormesis. Hormetic responses trigger active, protective reactions with reparative capacities, including mitochondrial biogenesis and a higher turnover of aged and damaged organelles (Rohrbach, et al., 2006). The hormetic effect has been suggested to explain the effects of exercise on mitochondrial activity and biogenesis in aged muscle. In that regard, CR and exercise have been shown to produce similar results in muscle. Endurance training induces mitochondrial biogenesis in muscle (Freyssenet, Berthon & Denis, 1996; Short, et al., 2003), and a recent report by Menshikova et al. showed that in a small group of old humans exercise was able to enhance mitochondrial activity and biogenesis particularly in subsarcolemmal tissue (Menshikova, et al., 2006). Interestingly, exercise training induces immediate expression of PGC-1α and PRC, whereas other factors such as PPARβ/δ and FKHR only show increased levels a few hours after exercise. The changes induced by exercise on mitochondrial biogenesis seem to be independent of the age of the individual (Short, et al., 2003).
The role of mitochondrial fission/fusion dynamics in aging
In addition to PGC-1α-regulated mitochondrial biogenesis, another emerging factor involved in regulating the activity of mitochondria during aging is mitochondrial fission/fusion. Mitochondria are very dynamic organelles and maintain complex mechanisms for fusion and fission. These processes permit a constant remodeling of mitochondrial architecture, allowing morphological transitions from individual structures to complex tubular networks. This dynamic structure is regulated by proteins controlling fission, such as hFis1 and Drp1, and fusion, such as mitofusin 1 and 2 (MFN1 and 2) and OPA1. The correct function of these proteins appears to be critical for normal mitochondrial activity, and their dysregulation is associated with several pathologic conditions. For example, disrupting mutations of the opa1 gene cause optic atrophy (Alexander, et al., 2000) whereas mutations that affect MFN2 function are associated with Charcot-Marie-Tooth neuropathy type 2A (Verhoeven, et al., 2006). The activity of these proteins is essential for the maintenance of mtDNA, as has been demonstrated in yeast (Herlan, et al., 2003).
Recently the fission-related protein hFis1 has been associated with the process of senescence in mammalian cell cultures (Lee, et al., 2007). Depletion of hFis1 by RNA interference in mammalian cells induced dramatic changes in mitochondrial structure, including the enlargement and flattening of the organelle. These morphological changes were correlated with increased β-galactosidase activity and reduced cell growth, both of which are markers of cell senescence. Sustained elongation of mitochondria was linked to decreased mitochondrial membrane potential, increased generation of reactive oxygen species, and DNA damage. Restoration of hFis1 levels led to cell growth recovery and reduced β-galactosidase activity, confirming that hFis1 plays an important role in cell senescence. In summary, it is becoming increasingly clear that both the structure and dynamics of mitochondrial architecture are important factors involved in the cell senescence program, and therefore the role of proteins that regulate fission/fusion in aging merits further investigation.
Biogenesis of mitochondria and neurodegeneration
Maintenance of mitochondrial activity during aging seems to be a key factor in the development of age-associated neurodegenerative diseases. Recently it has been demonstrated that synaptic plasticity, and therefore neuronal remodeling, is correlated with an increase in mitochondria at the synaptic axon terminals in animals under behavior training (Briones, et al., 2005).
While dysfunction of mitochondrial biogenesis affects the whole organism during aging its effects are particularly deleterious at the level of the central nervous system (CNS). A decrease in mitochondrial biogenesis would reduce turnover of mitochondrial components resulting in the accumulation of oxidized lipids, proteins and DNA. Thus, it seems clear that maintaining a high turnover of mitochondria during aging would be critical to prevent the deleterious side effects of mitochondrial malfunction. PGC-1α, poised centrally in multiple pathways affecting mitochondrial biogenesis, should play a key role in this prevention. PGC-1α is especially abundant in embryonic and early postnatal forebrain and cerebellum, indicating the importance of this regulator in the developing CNS (Cowell, Blake & Russell, 2007). Thus, it is conceivable that higher activity levels of PGC-1α induced by CR could sustain neuronal health during aging by maintaining the mitochondrial turnover. Indeed, CR induction of PGC-1α has already been shown to preserve muscle cell function with age (Baker, et al., 2006; Hepple, et al., 2006). Furthermore, repression of PGC-1α activity by mutant huntingtin clearly leads to mitochondrial dysfunction and neurodegeneration whereas overexpression of PGC-1α rescues the activity of cells (Cui, et al., 2006).
CR and resveratrol have demonstrated the capacity to maintain neuronal activity in different situations by increasing mitochondrial biogenesis. CR induces the PKA-CREB system that is involved in the signaling pathway that mediates plasticity and survival in neurons after oxidative stress (Ryu, et al., 2005). On the other hand, resveratrol promotes neurite outgrowth and also stimulates mitochondrial biogenesis in a AMPK-dependent manner contributing to neuronal energy homeostasis and neuroprotection (Dasgupta & Milbrandt, 2007). Furthermore, high levels of SIRT1 have been found to protect cells against amyloid-β-induced ROS production and DNA damage (Qin, et al., 2006). Moreover, neurons affected by Alzheimer's or Huntington's diseases are rescued by the over-expression of SIRT1, which is induced either by CR or resveratrol (Pallas, et al., 2008).
Conclusion
Maintenance of mitochondrial activity and biogenesis capacity during aging seems to be a key factor in the prevention of the progression of age-related diseases affecting tissues including the muscle and CNS. Stimulation of the regulatory components of mitochondrial biogenesis, mainly PGC-1α and AMPK, emerges as a promising field of investigation to increase the quality of life in old people. To date, maintenance of an active lifestyle through exercise and social relationships, and a diet moderate in calories, seem to be the best ways to maintain mitochondrial activity and thereby cell viability and performance during aging. The use of promising compounds such as resveratrol in the future could positively affect healthy aging by the same mechanism.
Acknowledgement
We thank Dr. Robin Minor for her critical inputs and help with editing this review.
Footnotes
Publisher's Disclaimer: This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2−3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- Alaynick WA. Nuclear receptors, mitochondria and lipid metabolism. Mitochondrion. 2008 doi: 10.1016/j.mito.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26(2):211–5. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- Annex BH, Kraus WE, Dohm GL, Williams RS. Mitochondrial biogenesis in striated muscles: rapid induction of citrate synthase mRNA by nerve stimulation. Am J Physiol. 1991;260(2 Pt 1):C266–70. doi: 10.1152/ajpcell.1991.260.2.C266. [DOI] [PubMed] [Google Scholar]
- Bahi L, Garnier A, Fortin D, Serrurier B, Veksler V, Bigard AX, Ventura-Clapier R. Differential effects of thyroid hormones on energy metabolism of rat slow- and fast-twitch muscles. J Cell Physiol. 2005;203(3):589–98. doi: 10.1002/jcp.20273. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Betik AC, Krause DJ, Hepple RT. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. J Gerontol A Biol Sci Med Sci. 2006;61(7):675–84. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DP, Greenawalt JW. Biogenesis of mitochondrial membranes in Neurospora crassa during cellular differentiation: changes in oxidative phosphorylation and synthesis of mitochondrial phospholipids. J Gen Microbiol. 1976a;92(1):111–9. doi: 10.1099/00221287-92-1-111. [DOI] [PubMed] [Google Scholar]
- Beck DP, Greenawalt JW. Biogenesis of mitochondrial membranes in Neurospora crassa during cellular differentiation: ultrastructural changes accompanying differentiation. J Gen Microbiol. 1976b;92(1):97–110. doi: 10.1099/00221287-92-1-97. [DOI] [PubMed] [Google Scholar]
- Beck DP, Greenawalt JW. Biogenesis of mitochondrial membranes Neurospora crassa during cellular differentiation: ultrastructural changes accompanying differentiation. J Gen Microbiol. 1976c;92(1):97–110. doi: 10.1099/00221287-92-1-97. [DOI] [PubMed] [Google Scholar]
- Briones TL, Suh E, Jozsa L, Rogozinska M, Woods J, Wadowska M. Changes in number of synapses and mitochondria in presynaptic terminals in the dentate gyrus following cerebral ischemia and rehabilitation training. Brain Res. 2005;1033(1):51–7. doi: 10.1016/j.brainres.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–33. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- Callen JC, Dennebouy N, Mounolou JC. Development of the mitochondrial mass and accumulation of mtDNA in previtellogenic stages of Xenopus laevis oocytes. J Cell Sci. 1980;41:307–20. doi: 10.1242/jcs.41.1.307. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Blake KR, Russell JW. Localization of the transcriptional coactivator PGC-1alpha to GABAergic neurons during maturation of the rat brain. J Comp Neurol. 2007;502(1):1–18. doi: 10.1002/cne.21211. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional Repression of PGC-1[alpha] by Mutant Huntingtin Leads to Mitochondrial Dysfunction and Neurodegeneration. Cell. 2006;127(1):59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Curtis R, O'Connor G, DiStefano PS. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell. 2006;5(2):119–26. doi: 10.1111/j.1474-9726.2006.00205.x. [DOI] [PubMed] [Google Scholar]
- Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha ) and mitochondrial function by MEF2 and HDAC5. PNAS. 2003;100(4):1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SH, Rodgers JT, Puigserver P, Chohnan S, Lane MD. Hypothalamic malonyl-CoA triggers mitochondrial biogenesis and oxidative gene expression in skeletal muscle: Role of PGC-1alpha. Proc Natl Acad Sci U S A. 2006;103(42):15410–5. doi: 10.1073/pnas.0607334103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104(17):7217–22. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange P, Farina P, Moreno M, Ragni M, Lombardi A, Silvestri E, Burrone L, Lanni A, Goglia F. Sequential changes in the signal transduction responses of skeletal muscle following food deprivation. Faseb J. 2006;20(14):2579–81. doi: 10.1096/fj.06-6025fje. [DOI] [PubMed] [Google Scholar]
- Fan M, Rhee J, St-Pierre J, Handschin C, Puigserver P, Lin J, Jaeger S, Erdjument-Bromage H, Tempst P, Spiegelman BM. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18(3):278–89. doi: 10.1101/gad.1152204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane P, Anderson C. Thyroid disease in older patients. Diagnosis and treatment. Drugs Aging. 1995;6(4):268–77. doi: 10.2165/00002512-199506040-00002. [DOI] [PubMed] [Google Scholar]
- Freyssenet D, Berthon P, Denis C. Mitochondrial biogenesis in skeletal muscle in response to endurance exercises. Arch Physiol Biochem. 1996;104(2):129–41. doi: 10.1076/apab.104.2.129.12878. [DOI] [PubMed] [Google Scholar]
- Gleyzer N, Vercauteren K, Scarpulla RC. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol. 2005;25(4):1354–66. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart S, Wiesner RJ. Regulation and co-ordination of nuclear gene expression during mitochondrial biogenesis. Exp Physiol. 2003;88(1):33–40. doi: 10.1113/eph8802500. [DOI] [PubMed] [Google Scholar]
- Goldenthal MJ, Weiss HR, Marin-Garcia J. Bioenergetic remodeling of heart mitochondria by thyroid hormone. Mol Cell Biochem. 2004;265(1−2):97–106. doi: 10.1023/b:mcbi.0000044321.17680.a2. [DOI] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004;287(5):E1032–7. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Baker DJ, McConkey M, Murynka T, Norris R. Caloric restriction protects mitochondrial function with aging in skeletal and cardiac muscles. Rejuvenation Res. 2006;9(2):219–22. doi: 10.1089/rej.2006.9.219. [DOI] [PubMed] [Google Scholar]
- Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS. Processing of Mgm1 by the Rhomboid-type Protease Pcp1 Is Required for Maintenance of Mitochondrial Morphology and of Mitochondrial DNA. J. Biol. Chem. 2003;278(30):27781–27788. doi: 10.1074/jbc.M211311200. [DOI] [PubMed] [Google Scholar]
- Hsieh YC, Yang S, Choudhry MA, Yu HP, Rue LW, 3rd, Bland KI, Chaudry IH. PGC-1 upregulation via estrogen receptors: a common mechanism of salutary effects of estrogen and flutamide on heart function after trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2005;289(6):H2665–72. doi: 10.1152/ajpheart.00682.2005. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Adhihetty PJ, Sheehan T, Joseph AM, Hood DA. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am J Physiol Cell Physiol. 2003;284(6):C1669–77. doi: 10.1152/ajpcell.00409.2002. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1[alpha]. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Leary SC, Shoubridge EA. Mitochondrial biogenesis: which part of “NO” do we understand? Bioessays. 2003;25(6):538–41. doi: 10.1002/bies.10298. [DOI] [PubMed] [Google Scholar]
- Lee H-C, Wei Y-H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. The International Journal of Biochemistry & Cell Biology. 2005;37(4):822–834. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Lee HC, Yin PH, Chi CW, Wei YH. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J Biomed Sci. 2002;9(6 Pt 1):517–26. doi: 10.1007/BF02254978. [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong SY, Lim WC, Kim S, Park YY, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282(31):22977–83. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- Lenka N, Vijayasarathy C, Mullick J, Avadhani NG. Structural organization and transcription regulation of nuclear genes encoding the mammalian cytochrome c oxidase complex. Prog Nucleic Acid Res Mol Biol. 1998;61:309–44. doi: 10.1016/s0079-6603(08)60830-2. [DOI] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3(6):429–38. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Linnane AW, Lamb AJ, Christodoulou C, Lukins HB. The biogenesis of mitochondria, VI. Biochemical basis of the resistance of Saccharomyces cerevisiae toward antibiotics which specifically inhibit mitochondrial protein synthesis. Proc Natl Acad Sci U S A. 1968;59(4):1288–93. doi: 10.1073/pnas.59.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A. 2006;103(6):1768–73. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF. Chronic activation of AMP-activated kinase as a strategy for slowing aging. Med Hypotheses. 2004;63(2):334–9. doi: 10.1016/j.mehy.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–40. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutvei A, Husman B, Andersson G, Nelson BD. Thyroid hormone and not growth hormone is the principle regulator of mammalian mitochondrial biogenesis. Acta Endocrinol (Copenh) 1989;121(2):223–8. doi: 10.1530/acta.0.1210223. [DOI] [PubMed] [Google Scholar]
- Nagino M, Tanaka M, Nishikimi M, Nimura Y, Kubota H, Kanai M, Kato T, Ozawa T. Stimulated rat liver mitochondrial biogenesis after partial hepatectomy. Cancer Res. 1989;49(17):4913–8. [PubMed] [Google Scholar]
- Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292(2):C670–86. doi: 10.1152/ajpcell.00213.2006. [DOI] [PubMed] [Google Scholar]
- Nervina JM, Magyar CE, Pirih FQ, Tetradis S. PGC-1alpha is induced by parathyroid hormone and coactivates Nurr1-mediated promoter activity in osteoblasts. Bone. 2006;39(5):1018–25. doi: 10.1016/j.bone.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119(Pt 14):2855–62. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial Biogenesis in Mammals: The Role of Endogenous Nitric Oxide. Science. 2003;299(5608):896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Pallas M, Verdaguer E, Tajes M, Gutierrez-Cuesta J, Camins A. Modulation of sirtuins: new targets for antiageing. Recent Patents CNS Drug Discov. 2008;3(1):61–9. doi: 10.2174/157488908783421492. [DOI] [PubMed] [Google Scholar]
- Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, Czech MP. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116(1):125–36. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur B, Bismuth J, Delaval E. Effects of adrenal steroid hormones on mitochondrial maturation during the late fetal period. Eur J Biochem. 1998;252(2):194–9. doi: 10.1046/j.1432-1327.1998.2520194.x. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O'Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286(5443):1368–71. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp Mol Med. 2007;39(4):535–43. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Zhao W, Thiyagarajan M, MacGrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng H, Pedrini S, Gandy S, Sauve AA, Pasinetti GM. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281(31):21745–54. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Nesi B, Pratelli L, Savarino L, Cucinotta D, Cavalli G. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab. 2000;85(6):2260–5. doi: 10.1210/jcem.85.6.6627. [DOI] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5(2):151–6. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue-Way A, Demers A, Ong H, Tremblay A. A growth hormone-releasing peptide promotes mitochondrial biogenesis and a fat burning-like phenotype through scavenger receptor CD36 in white adipocytes. Endocrinology. 2007;148(3):1009–18. doi: 10.1210/en.2006-0975. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Monjo M, Gianotti M, Proenza AM, Roca P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab. 2007;292(1):E340–6. doi: 10.1152/ajpendo.00175.2006. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Niemann B, Abushouk AM, Holtz J. Caloric restriction and mitochondrial function in the ageing myocardium. Exp Gerontol. 2006;41(5):525–31. doi: 10.1016/j.exger.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Rosano TG, Jones DH. Developmental changes in mitochondria during the transition into lactation in the mouse mammary gland. J Cell Biol. 1976;69(3):573–80. doi: 10.1083/jcb.69.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Felty Q, Narayan S, Jayakar P. Signature of mitochondria of steroidal hormones-dependent normal and cancer cells: potential molecular targets for cancer therapy. Front Biosci. 2007;12:154–73. doi: 10.2741/2056. [DOI] [PubMed] [Google Scholar]
- Ryu H, Lee J, Impey S, Ratan RR, Ferrante RJ. Antioxidants modulate mitochondrial PKA and increase CREB binding to D-loop DNA of the mitochondrial genome in neurons. Proc Natl Acad Sci U S A. 2005;102(39):13915–20. doi: 10.1073/pnas.0502878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochimica et Biophysica Acta (BBA) - Gene Structure and Expression. 2002;1576(1−2):, 1–14. doi: 10.1016/s0167-4781(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Schreiber SN, Emter R, Hock MB, Knutti D, Cardenas J, Podvinec M, Oakeley EJ, Kralli A. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci U S A. 2004;101(17):6472–7. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K(+) channels in both young and aged hearts. J Mol Cell Cardiol. 2005 doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–96. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Trembath MK, Monk BC, Kellerman GM, Linnane AW. Biogenesis of mitochondria 36, The genetic and biochemical analysis of a mitochondrially determined cold sensitive oligomycin resistant mutant of Saccharomyces cerevisiae with affected mitochondrial ATPase assembly. Mol Gen Genet. 1975a;141(1):9–22. doi: 10.1007/BF00332375. [DOI] [PubMed] [Google Scholar]
- Trembath MK, Monk BC, Kellerman GM, Linnane AW. Biogenesis of mitochondria 40. Phenotypic suppression of a mitochondrial mutation by a nuclear gene in Saccharomyces cerevisiae. Mol Gen Genet. 1975b;140(4):333–7. [PubMed] [Google Scholar]
- Treuter E, Albrektsen T, Johansson L, Leers J, Gustafsson JA. A regulatory role for RIP140 in nuclear receptor activation. Mol Endocrinol. 1998;12(6):864–81. doi: 10.1210/mend.12.6.0123. [DOI] [PubMed] [Google Scholar]
- Van den Bogert C, Muus P, Haanen C, Pennings A, Melis TE, Kroon AM. Mitochondrial biogenesis and mitochondrial activity during the progression of the cell cycle of human leukemic cells. Exp Cell Res. 1988;178(1):143–53. doi: 10.1016/0014-4827(88)90385-0. [DOI] [PubMed] [Google Scholar]
- Vary MJ, Edwards CL, Stewart PR. The biogenesis of mitochondria. IX. Formation of the soluble mitochondrial enzymes malate dehydrogenase and fumarase in Saccharomyces cerevisiae. Arch Biochem Biophys. 1969;130(1):235–43. doi: 10.1016/0003-9861(69)90029-0. [DOI] [PubMed] [Google Scholar]
- Vary MJ, Stewart PR, Linnane AW. Biogenesis of mitochondria. XVII. The role of mitochondrial and cytoplasmic ribosomal protein synthesis in the oxygen-induced formation of yeast mitochondrial enzymes. Arch Biochem Biophys. 1970;141(2):430–9. doi: 10.1016/0003-9861(70)90159-1. [DOI] [PubMed] [Google Scholar]
- Verhoeven K, Claeys KG, Zuchner S, Schroder JM, Weis J, Ceuterick C, Jordanova A, Nelis E, De Vriendt E, Van Hul M, Seeman P, Mazanec R, Saifi GM, Szigeti K, Mancias P, Butler IJ, Kochanski A, Ryniewicz B, De Bleecker J, Van den Bergh P, Verellen C, Van Coster R, Goemans N, Auer-Grumbach M, Robberecht W, Milic Rasic V, Nevo Y, Tournev I, Guergueltcheva V, Roelens F, Vieregge P, Vinci P, Moreno MT, Christen HJ, Shy ME, Lupski JR, Vance JM, De Jonghe P, Timmerman V. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129(Pt 8):2093–102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- Wadley GD, McConell GK. Effect of nitric oxide synthase inhibition on mitochondrial biogenesis in rat skeletal muscle. J Appl Physiol. 2007;102(1):314–20. doi: 10.1152/japplphysiol.00549.2006. [DOI] [PubMed] [Google Scholar]
- Watson K, Haslam JM, Linnane AW. Biogenesis of mitochondria. 13. The isolation of mitochondrial structures from anaerobically grown Saccharomyces cerevisiae. J Cell Biol. 1970;46(1):88–96. doi: 10.1083/jcb.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel JM, Iwen KA, Seitz HJ. Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol. 2003;88(1):121–8. doi: 10.1113/eph8802506. [DOI] [PubMed] [Google Scholar]
- White R, Morganstein D, Christian M, Seth A, Herzog B, Parker MG. Role of RIP140 in metabolic tissues: connections to disease. FEBS Lett. 2008;582(1):39–45. doi: 10.1016/j.febslet.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Winder WW, Taylor EB, Thomson DM. Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc. 2006;38(11):1945–9. doi: 10.1249/01.mss.0000233798.62153.50. [DOI] [PubMed] [Google Scholar]
- Wooten WL, Cascarano J. The effect of thyroid hormone on mitochondrial biogenesis and cellular hyperplasia. J Bioenerg Biomembr. 1980;12(1−2):1–12. doi: 10.1007/BF00745009. [DOI] [PubMed] [Google Scholar]
- Wu CW, Ping YH, Yen JC, Chang CY, Wang SF, Yeh CL, Chi CW, Lee HC. Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol. 2007;220(3):243–51. doi: 10.1016/j.taap.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of Mitochondrial Biogenesis in Skeletal Muscle by CaMK. Science. 2002;296(5566):349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103(39):14379–84. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ma K, Song S, Elam MB, Cook GA, Park EA. Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha). J Biol Chem. 2004;279(52):53963–71. doi: 10.1074/jbc.M406028200. [DOI] [PubMed] [Google Scholar]


