Abstract
Background
This study tested the hypothesis that microstructural white matter abnormalities in frontostriatal-limbic tracts are associated with poor response inhibition on the Stroop task in depressed elders.
Method
Fifty-one elders with major depression participated in a 12-week escitalopram trial. Diffusion tensor imaging was used to determine fractional anisotropy (FA) in white matter regions. Executive function (response inhibition) was assessed with the Stroop task. Voxelwise correlational analysis was used to examine the relationship between Stroop performance and fractional anisotropy.
Results
Significant associations between FA and Stroop Color-Word Interference were evident in multiple frontostriatal-limbic regions, including white matter lateral to the anterior and posterior cingulate cortex, and white matter in prefrontal, insular and parahippocampal regions.
Conclusions
These findings suggest that microstructural white matter abnormalities of frontostriatal-limbic networks are associated with executive dysfunction of late-life depression. This observation provides the rationale for examination of specific frontostriatal-limbic pathways in the pathophysiology of geriatric depression.
Keywords: Stroop, MRI, depression, geriatric, DTI, executive function
INTRODUCTION
Executive dysfunction is present in a considerable number of older individuals with major depression (Alexopoulos et al 2002b; Elderkin-Thompson et al 2003; Lockwood et al 2002; Nebes et al 2001). Observations from acute treatment trials (Butters et al 2000; Nebes et al 2003) and from longer-term follow-up of depressed elders receiving uncontrolled treatment (Murphy and Alexopoulos 2003) suggest that impairment of executive functions remains present, albeit to a milder extent, even after depressive symptoms subside. Thus, in some depressed older patients executive dysfunction is a relatively stable trait only mildly exacerbated during depressed states.
Structural neuroimaging findings provide indirect support for the role of frontostriatal-limbic abnormalities in the executive dysfunction of late-life depression. White matter hyperintensities (WMH) are associated with executive dysfunction (Aizenstein et al 2002; Boone et al 1992; Lesser et al 1996), are more prevalent and severe in depressed older individuals than age-matched controls, and mainly occur in subcortical regions and their frontal white matter projections (Coffey et al 1990; Greenwald et al 1998; Krishnan 1993, Krishnan et al 1997; Lenze et al 1999; O’Brien et al 1996; Taylor et al 2003a, 2005; Tupler et al 2002). Gray matter volume reductions are present in multiple frontostriatal-limbic regions of older depressives, including the anterior cingulate, prefrontal cortices, the neostriatum, the hippocampus, and the amygdala (Ballmaier et al 2004; Krishnan et al 1992; Kumar et al 2000; Lai et al 2000; Steffens et al 2002; Taylor et al 2003b).
Diffusion tensor imaging (DTI) may reveal microstructural abnormalities in regions of cerebral networks critical to the pathophysiology of geriatric depression. We previously reported that compromised integrity of white matter lateral to the anterior cingulate gyrus [reduced fractional anisotropy (FA)] was associated with poor performance on the Stroop task in 13 older depressed patients (Alexopoulos et al 2002a). However, the focus on preselected regions did not reveal whether this association was limited to these areas. We report here an exploratory analysis employing voxelwise whole -brain methodology to identify brain regions in which Stroop Color-Word Interference performance is associated with FA in a new sample of 51 depressed older individuals. We hypothesized that poor Stroop Color-Word Interference performance, an index of response inhibition, is associated with reduced FA in frontostriatal-limbic regions.
METHODS AND MATERIALS
Participants
Participants were depressed patients aged 60 to 86 years recruited at a University-based Geriatric Psychiatry clinic. All participants signed informed consent. Participants met DSM-IV criteria (American Psychiatric Association 1994) for unipolar major depression and had a score > 18 on the 24-item Hamilton Depression Rating Scale (HDRS, Williams 1988). Exclusion criteria included: 1) history of psychiatric disorders (except personality disorders) prior to the onset of their depression; 2) severe or acute medical illness within 3 months preceding the study; 3) neurological disorders (i.e. dementia or delirium, history of head trauma, Parkinson’s disease); 4) use of drugs known to cause symptoms of depression (e.g. steroids, beta-blockers) and 5) Mini-Mental State Examination (MMSE, Folstein et al 1975) score < 24. These criteria resulted in a group of elderly patients with non-psychotic unipolar major depression without a diagnosable dementing disorder (Table 1).
Table 1.
Baseline demographic and clinical characteristics of 51 elderly patients with major depression
| Variable | Mean ± SD | Range |
|---|---|---|
| Age | 70.0 ± 5.9 | 60 – 86 yrs |
| Education | 15.8 ± 2.8 | 7 – 22 yrs |
| Gender (% male) | 43 % | |
| HDRS | 23.4 ± 4.4 | 18 – 34 |
| Age of first depression onset | 59.1 ± 17.8 | 10 – 80 yrs |
| MMSE | 28.2 ± 1.8 | 24 – 30 |
| Stroop Word Reading | 89.6 ± 14.4 | 67 – 126 |
| Stroop Color Naming | 60.8 ± 10.8 | 41 – 85 |
| Stroop Color-Word Interference | 32.1 ± 10.3 | 8 – 51 |
Note: Values are mean ± standard deviation unless otherwise noted.
HDRS = Hamilton Depression Rating Scale; MMSE = Mini Mental State Exam.
Clinical assessment
The Weill Cornell and NKI Institutional Review Boards approved all procedures. Trained raters blind to the study hypotheses conducted assessments. Diagnostic evaluation was conducted using the SCID (Spitzer and Williams 1995). Depression severity was quantified with the 24-item HDRS.
Subjects completed the Stroop test (Golden and Freshwater 2002) prior to starting study drug. This task consists of three parts; each part is scored independently and represents the number of correct responses in 45 seconds. First, subjects were presented with a list of the words “red”, “blue”, and “green” printed in black ink and were instructed to read each word aloud as quickly as possible. Next, participants were shown a similar page on which the words were replaced by “XXXX”s printed in red, blue, or green ink and were instructed to name the color of the ink (Color Naming; CN). Finally, subjects were presented with a list of the words “red”, “blue” and “green” printed in incongruent ink color (e.g. the word “red” written in blue ink) and instructed to name the ink color of each word (Color-Word Interference; CWI). This condition, which requires suppression of the automatic word-reading response, is a measure of response inhibition, an aspect of executive control (MacLeod 1991).
MRI Procedures
Scanning was performed with a 1.5T Siemens Vision Scanner. All but 5 scans took place during a single-blind placebo lead-in phase of the treatment trial. Patients received a 3D magnetization prepared rapidly acquired gradient echo (MPRAGE) scan (TR=11.6ms, TE=4.8ms, matrix=256×256, FOV=320 mm, NEX=1, slice thickness=1.25 mm, 172 slices, no gap, TI=1018 ms), as well as a turbo dual spin echo scan (TR=5s, TE=22/90 ms, rectangular matrix=190×256 interpolated to 256×256, FOV=240 mm, slice thickness=5 mm, 26 slices, no gap), and a diffusion tensor imaging scan (TR=6000 ms, TE=100 ms, matrix=128×128, FOV=300 mm, NEX=7, slice thickness=5 mm, 19 slices, no gap, b = 1000 s/mm2). Eight diffusion sensitization directions were used (Jones et al 1999). The latter two scans were acquired in an oblique axial plane parallel to the anterior commissure – posterior commissure axis.
Postprocessing
FA was computed in the original ‘patient space’ using software written in house (BAA). The FA images were corrected for susceptibility induced distortion and were transformed into Talairach space using methods described elsewhere (Ardekani et al 2003; Hoptman et al 2004). Intersubject registration was completed using ART (Ardekani et al 1995; Ardekani et al 2005). The average FA map (in standardized space) was segmented using Otsu’s (1979) algorithm and used to mask each image for white matter.
Data Analysis
We computed correlations between FA and Stroop performance in the Color-Word Interference condition using voxelwise correlational analyses, first using age as a covariate, and then with age and Stroop CN as covariates. To reduce Type I error, we first used a thresholding method (Baudewig et al 2003) that requires a significant correlation between FA and the performance data in a cluster of contiguous voxels. The approach identifies clusters of voxels signficantly (p<.01) associated with behavioral data and then specifies that at least one voxel be significant at a higher level (p<.001). We selected a cluster size of 100mm3. The resulting correlation maps were superimposed onto an MPRAGE image in Talairach space using AFNI (Cox 1996).
RESULTS
Significant positive correlations between CWI scores and FA after partialling out age were noted in multiple regions, including white matter lateral to the anterior and posterior cingulate cortex, left prefrontal white matter, and white matter in insular, posterior temporal, parahippocampal, and occipital regions (Figure 1, Panel A; Table 2). To examine the specificity of the CWI correlations, we examined frontal regions in which significant relationships remained between FA and Color-Word Interference, after partialling out the correlations between FA and Color-Naming condition performance. Significant positive correlations remained in frontal regions lateral to the left anterior cingulate, in the left insula, as well as in the left occipital cortex and the right cerebral peduncle (Figure 1, Panel B).
Figure 1.
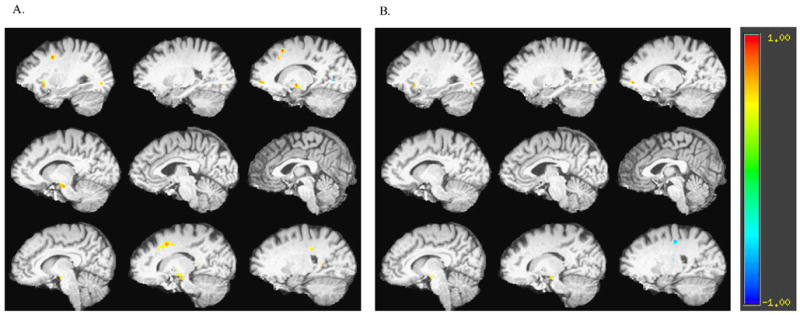
Correlation map of fractional anisotropy (FA) and Stroop Color Word Interference performance A) with age as a covariate and B) with age and Stroop Color Naming performance as covariates. Slices are presented from left to right hemisphere.
Table 2.
Areas of significant correlation between FA and Stroop Color Word Interference performance in older depressed patients after controlling for age
| Talairach Coordinates* | |||||
|---|---|---|---|---|---|
| x (+Right) | y (+Anterior) | z (Superior) | Cluster Size | R/L | Anatomical Region |
| −16 | 20 | 42 | 575 | Left | Superior frontal |
| −28 | 4 | 38 | 306 | Left | Middle frontal |
| 14 | 13 | 41 | 1198 | Right | Dorsal anterior cingulate |
| −15 | 50 | −2 | 291 | Left | Rostral anterior cingulate |
| −28 | 17 | −5 | 476 | Left | Insula |
| 18 | −31 | 34 | 261 | Right | Posterior cingulate |
| 41 | −44 | −3 | 1069 | Right | Parahippocampal |
| −31 | −52 | 9 | 504 | Left | Posterior temporal |
| 22 | −51 | 9 | 474 | Right | Posterior temporal |
| −13 | −10 | −11 | 979 | Left | Cerebral peduncles |
| 13 | −11 | −10 | 733 | Right | Cerebral peduncles |
| −26 | −77 | −4 | 280 | Left | Occipital |
| −16 | −67 | 2 | 267 | Left | Occipital |
Talairach coordinates represent the Centroid of the region.
DISCUSSION
The main finding of this study is that reduced FA in frontostriatal-limbic regions is associated with poor response inhibition on the Stroop in depressed older patients. These findings are consistent with imaging studies that implicate the ACC and dorsolateral prefrontal cortex in Stroop performance in healthy subjects (e.g. Leung et al 2000; MacDonald et al 2000; Pardo et al, 1990). To our knowledge, this is the first study to use a voxelwise analysis of FA to identify frontostriatal-limbic network microstructural abnormalities associated with aspects of executive dysfunction of geriatric depression.
Our observations are consistent with other findings of frontostriatal-limbic abnormalities in geriatric depression (e.g. Krishnan 1993; Kumar et al 2000; Steffens et al 2002; Taylor et al 2003a), and lend support to converging evidence that compromised white matter in frontostriatal-limbic pathways may lead to a “disconnection-syndrome” that interferes with the reciprocal regulation of dorsal cortical-ventral limbic structures in depression (for reviews see Alexopoulos 2002; Seminowicz et al 2004). We propose that these abnormalities, which may be caused by vascular, degenerative, or neurodevelopmental processes, not only contribute to executive dysfunction but also confer vulnerability to depression. There are two reasons for this assertion. First, these regions are thought to participate in mood regulation (for reviews see Davidson et al 2002, Phillips et al 2003), and second, some degree of executive dysfunction is the rule rather than the exception in geriatric depression.
This study is limited by its narrow assessment of executive functions and the lack of a non-depressed comparison group. Further, the sample size did not allow us to examine the contribution of education or other clinical variables to the association of FA to Stroop performance. Despite these limitations, identification of microstructural abnormalities associated with executive dysfunction can guide future studies of specific pathways associated with the pathophysiology of geriatric depression. DTI studies, for example, can use fiber tracking to identify with greater precision specific frontostriatal-limbic abnormalities present in geriatric depression. The identification of particular pathway abnormalities can generate investigations of specifically targeted novel therapeutic interventions.
Acknowledgments
Dr. Alexopoulos has received research grants by Forest Pharmaceuticals, Inc. and Cephalon and participated in scientific advisory board meetings of Forest Pharmaceuticals. He has given lectures supported by Forest, Cephalon, Bristol Meyers, Janssen, Pfizer, and Lilly and has received support by Comprehensive Neuroscience, Inc. for the development of treatment guidelines in late-life psychiatric disorders.
Footnotes
GRANT SUPPORT
This work was supported by NIMH grants RO1 MH65653 (GSA), K23 MH067702 (CFM), P30 MH68638 (GSA), K23 MH074818 (FGD) and by the Sanchez Foundation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Nebes RD, Meltzer CC, Fukui MB, Williams RL, Saxton J, et al. The relation of white matter hyperintensities to implicit learning in healthy older adults. Int J Geriatr Psychiatry. 2002;17:664–669. doi: 10.1002/gps.685. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002a;159:1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the “depression-executive dysfunction syndrome” of late life. Am J Geriatr Psychiatry. 2002b;10:98–106. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H. A fully automatic multimodality image registration algorithm. J Comput Assist Tomogr. 1995;19:615–623. doi: 10.1097/00004728-199507000-00022. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Guckemus S, Bachman A, Hoptman MJ, Wojtasek M, Nierenberg J. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. J Neurosci Methods. 2005;42:76. doi: 10.1016/j.jneumeth.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. NeuroReport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Ballmaier M, Toga AW, Blanton RE, Sowell ER, Lavretsky H, Peterson, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161:99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Dechent P, Merboldt KD, Frahm J. Thresholding in correlational analyses of magnetic resonance functional neuroimaging. Magn Reson Imaging. 2003;21:1121–1130. doi: 10.1016/j.mri.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill-Gutierrez E, Goldberg MA, et al. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG. Changes in cognitive functioning following treatment of late -life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187–189. doi: 10.1176/ajp.147.2.187. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Kumar A, Bilker WB, Dunkin JJ, Mintz J, Moberg PJ, et al. Neuropsychological deficits among patients with late-onset minor and major depression. Arch Clin Neuropsychol. 2003;18:529–549. doi: 10.1016/s0887-6177(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Stoelting; 2002. [Google Scholar]
- Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke. 1998;29:613–917. doi: 10.1161/01.str.29.3.613. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Ardekani BA, Butler PD, Nierenberg J, Javitt DC, Lim KO. DTI and impulsivity in schizophrenia: A first voxelwise correlational study. NeuroReport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Krishnan KR. Neuroanatomic substrates of depression in the elderly. J Geriatr Psychiatry Neurol. 1993;6:39–58. doi: 10.1177/002383099300600107. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–500. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, McDonald WM, Escalona PR, Doraiswamy PM, Na C, Husain MM, et al. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry. 1992;49:553–557. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bilker W, Jin Z, Udupa J. Atrophy and high intensity lesions: complementary neurobiological mechanisms in late-life depression. Neuropsychopharmacol. 2000;22:264–274. doi: 10.1016/S0893-133X(99)00124-4. [DOI] [PubMed] [Google Scholar]
- Lai T, Payne ME, Byrum CE, Steffens DC, Krishnan KR. Reduction of orbital frontal cortex volume in geriatric depression. Biol Psychiatry. 2000;48:971–975. doi: 10.1016/s0006-3223(00)01042-8. [DOI] [PubMed] [Google Scholar]
- Lenze E, Cross D, McKeel D, Neuman RJ, Sheline YI. White matter hyperintensities and gray matter lesions in physically healthy depressed subjects. Am J Psychiatry. 1999;156:1602–7. doi: 10.1176/ajp.156.10.1602. [DOI] [PubMed] [Google Scholar]
- Lesser IM, Boone KB, Mehringer CM, Wohl MA, Miller BL, Berman NG. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280–7. doi: 10.1176/ajp.153.10.1280. [DOI] [PubMed] [Google Scholar]
- Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cereb Cortex. 2000;10:552–60. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- Lockwood CA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119–1126. doi: 10.1176/appi.ajp.159.7.1119. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulated cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Murphy CF, Alexopoulos GS. Longitudinal association of initiation/perseveration and severity of geriatric depression. Am J Geriatr Psychiatry. 2003;11:1–7. [PubMed] [Google Scholar]
- Nebes RD, Butters MA, Houck PR, Zmuda MD, Aizenstein H, Pollock BG, et al. Dual-task performance in depressed geriatric patients. Psychiatry Res. 2001;102:139–151. doi: 10.1016/s0165-1781(01)00244-x. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37:99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Desmond P, Ames D, Schweitzer I, Harrigan S, Tress B. A magnetic resonance imaging study of white matter lesions in depression and Alzheimer’s disease. Br J Psychiatry. 1996;168:477–485. doi: 10.1192/bjp.168.4.477. [DOI] [PubMed] [Google Scholar]
- Otsu N. A threshold selection method from gray-level histograms. IEEE Trans Systems Man Cybernetics. 1979;9:63–66. [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci. 1990;87:256–9. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Patient Version. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Steffens DC, Payne ME, Greenberg DL, Byrum CE, Welsh-Bohmer KA, Wagner HR. Hippocampal volume and incident dementia in geriatric depression. Am J Geriatr Psychiatry. 2002;10:62–71. [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1–7. doi: 10.1016/j.pscychresns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Taylor WD, MacFall JR, Steffens DC, Payne ME, Provenzale JM, Krishnan KR. Localization of age-associated white matter hyperintensities in late-life depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003a;27:539–544. doi: 10.1016/S0278-5846(02)00358-5. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, McQuoid DR, Payne ME, Lee SH, Lai TJ. Smaller orbital frontal cortex volumes associated with functional disability in depressed elders. Biol Psychiatry. 2003b;3:144–149. doi: 10.1016/s0006-3223(02)01490-7. [DOI] [PubMed] [Google Scholar]
- Tupler LA, Krishnan KR, McDonald WM, Dombeck CB, D’Souza S, Steffens DC. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53:665–676. doi: 10.1016/s0022-3999(02)00425-7. [DOI] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]


