Abstract
There are two distinct inhibitory GABAergic circuits in the neostriatum. The feedforward circuit consists of a relatively small population of GABAergic interneurons that receives excitatory input from the neocortex and exerts monosynaptic inhibition onto striatal spiny projection neurons. The feedback circuit comprises the numerous spiny projection neurons and their interconnections via local axon collaterals. This network has long been assumed to provide the majority of striatal GABAergic inhibition and to sharpen and shape striatal output through lateral inhibition, producing increased activity in the most strongly excited spiny cells at the expense of their less strongly excited neighbors.
Recent results, mostly from recording experiments of synaptically connected pairs of neurons, have revealed that the two GABAergic circuits differ markedly in terms of the total number of synapses made by each, the strength of the postsynaptic response detected at the soma, the extent of presynaptic convergence and divergence and the net effect of the activation of each circuit on the postsynaptic activity of the spiny neuron. These data have revealed that the feedforward inhibition is powerful and widespread, with spiking in a single interneuron being capable of significantly delaying or even blocking the generation of spikes in a large number of postsynaptic spiny neurons. In contrast, the postsynaptic effects of spiking in a single presynaptic spiny neuron on postsynaptic spiny neurons are weak when measured at the soma, and unable to significantly affect spike timing or generation. Further, reciprocity of synaptic connections between spiny neurons is only rarely observed.
These results suggest that the bulk of the fast inhibition that has the strongest effects on spiny neuron spike timing comes from the feedforward interneuronal system whereas the axon collateral feedback system acts principally at the dendrites to control local excitability as well as the overall level of activity of the spiny neuron.
Introduction
The basal ganglia comprise the largest subcortical system in the brain extending from the telencephalon through the midbrain. Among the many unique features of the basal ganglia is the fact that it is composed almost entirely (>98.8%; see Tepper et al., 2007) of GABAergic neurons. The neostriatum, the largest single nucleus in the basal ganglia, not surprisingly comprises almost entirely GABAergic neurons. The vast majority of these, at least 95%, in species ranging from rodent to primate (Kemp and Powell, 1971; Luk and Sadikot, 2001; Wilson, 2004 but see also Graveland et al., 1985) are medium sized spiny projection neurons that are also the only source of output from the nucleus. The remaining cell types comprise large aspiny cholinergic interneurons, and at least 3 distinct types of GABAergic interneurons (Kawaguchi, 1993; Kawaguchi et al., 1995).
These GABAergic interneurons were first characterized electrophysiologically, morphologically and neurochemically by Kawaguchi and colleagues (Kawaguchi, 1993, Kubota and Kawaguchi, 1994; Kawaguchi et al., 1995). These investigators described a medium sized GABAergic aspiny neuron with a fast-spiking (FS) phenotype that was immunoreactive for the calcium binding protein, parvalbumin (PV). A second aspiny interneuron, subsequently shown to be GABAergic (Kubota and Kawaguchi, 1994) was described that fired low threshold spikes and exhibited a persistent depolarizing plateau potential in response to depolarizing current injection that long outlasted the depolarizing stimuli which was termed the PLTS neuron (Kawaguchi et al., 1995), and in later papers just the LTS neuron (e.g., Kubota and Kawaguchi, 2000). The PLTS electrophysiological phenotype was shown to belong to a striatal interneuron previously shown to selectively express the neuropeptides somatostatin and NPY, and nitric oxide synthase (Emson et al., 1993). A third medium-sized aspiny GABAergic neuron was identified that was immunoreactive for calretinin but not for any of the other calcium binding proteins or neuropeptides found in other striatal interneurons (Kawaguchi et al., 1995). Its electrophysiological phenotype was not described at the time and still remains unknown.
Although most of the neurons in the striatum are GABAergic, most of the synapses are not, some 80% consisting of asymmetric glutamatergic synapses originating from the cortex and thalamus (Kemp and Powell, 1971; Ingham et al., 1998; for recent review, see Wilson, 2007). Nevertheless, GABAergic inhibition plays a most important role in the modulation of striatal output. One of the clearest demonstrations of this is the fact that blockade of striatal GABAA receptors by local application of bicuculline in vivo increases the spontaneous firing rate of striatal neurons by a factor of 3 or more (Nisenbaum and Berger, 1992).
There are two major potential sources of the fast GABAergic inhibition in striatum; feedforward inhibition from the GABAergic interneurons and feedback inhibition from the axon collaterals of the spiny neurons themselves. As the number of GABAergic synapses formed onto spiny neurons by the spiny cell axon collaterals is significantly greater than the number formed by the interneurons (e.g., Guzman et al., 2003; Koós et al., 2004), all other things being equal, one would expect the axon collateral inhibitory feedback system to be the predominant player in the control of spiny cell activity and spike timing, as proposed by many others in the past. Striatal organization was most often conceived of as a lateral inhibitory network (Groves et al., 1983; Wickens et al., 1991, 1995; Beiser and Houk, 1998; Bar-Gad and Bergman, 2001). Lateral inhibitory networks are typically considered to consist of each output neuron making symmetric reciprocal inhibitory synapses onto its neighbors. However, more recent data obtained from recording from synaptically connected interneuron-spiny cell and spiny cell-spiny cell pairs over the past 5 years are inconsistent with such a model of striatal function and suggest a significantly different type of functional organization.
Striatal Interneurons and Feedforward Inhibition
Fast-Spiking Interneurons
By far the best-characterized GABAergic interneurons are those that express parvalbumin (PV). Their somata average 16-18 μm in diameter and issue aspiny dendrites that branch modestly. There is some morphological heterogeneity, with one subtype exhibiting a smaller soma and a relatively restricted and varicose dendritic arborization in the region of 200-300 μm in diameter, and the other displaying a larger cell body and a more extended non-varicose dendritic field 500-600 μm in diameter (Kita et al., 1990; Kawaguchi, 1993; Bennett and Bolam, 1994a; Kawaguchi et al., 1995; Koós and Tepper, 1999). The neuron is characterized by an extremely dense local axonal plexus surrounding and extending beyond the dendritic field of the cell of origin that is heavily invested with presynaptic boutons. An example is shown in Figure 1.
Figure 1.
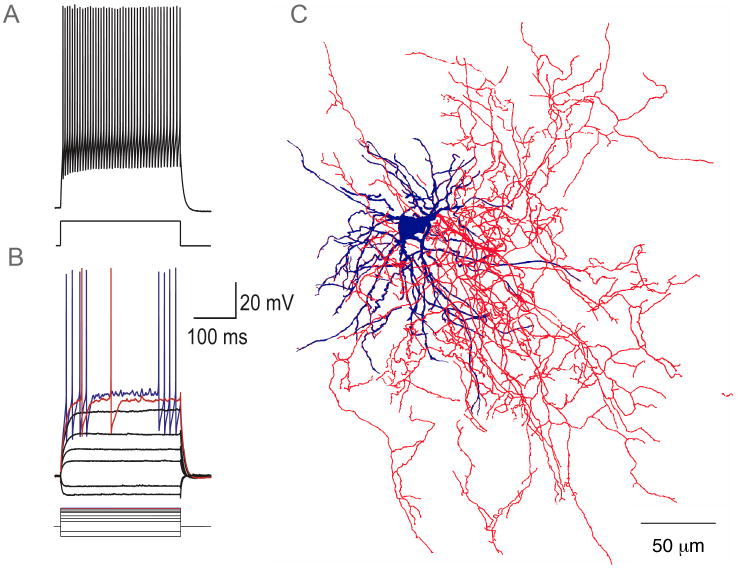
Whole cell recording and biocytin staining of a PV-immunoreactive striatal FS interneuron from a striatal slice from a 24 day old rat. A, B. The neuron is silent at rest and displays a relatively linear IV relation. Small increments in stimulus strength from a subthreshold value (most depolarized black trace in B) yield an irregular bursting pattern of firing (red and blue traces in B). Stronger stimuli evoke sustained firing that can exceed 200 Hz with relatively little spike frequency adaptation. C. Drawing tube reconstruction of a biocytin-stained FS interneuron from a striatal slice from a 21-day old rat. Note the dense, extensive local axon collateral arborization. Data redrawn from Koós and Tepper, 1999.
Unbiased stereological estimates put the numbers of PV interneuron number at about 0.7% of the total in rat neostriatum (Luk et al., 2001; Rymar et al., 2004; however, in our experience it is likely that there are other types of FS interneurons that are not PV-immunopositive so the actual proportion of FS interneurons is likely to be significantly greater than this; Koós, Ibanez-Sandoval and Tepper, unpublished). There is a strong medio-lateral gradient in the distribution of PV+ axons and terminals suggesting that these cells may be more integral to functioning in lateral striatum than medial striatum (Bolam and Bennett, 1995).
The neurons exhibit a very hyperpolarized membrane potential in vitro and do not fire spontaneously. When stimulated with a series of increasing amplitude depolarizing pulses, the neurons do not fire at all below a certain threshold. Over this threshold a tiny increment in stimulus amplitude results in a short burst of a few spikes. Further increases lead to episodes of high frequency firing interspersed with silent periods. Sufficiently strong stimuli evoke sustained firing at rates > 200Hz (Kawaguchi, 1993; Kawaguchi et al., 1995; Koós and Tepper, 1999, 2002, Bracci et al., 2002 Plotkin et al., 2005; Taverna et al., 2007). In the absence of a selective visible marker for these cells (e.g., Meyer et al., 2002; Freiman et al., 2006), they are usually targeted for recording in IR-DIC images by their larger cell body and are subsequently identified in whole cell recordings by this unusual firing pattern, illustrated in Figure 1.
PV+ interneurons receive a powerful excitatory input from cortex that differs from the cortical input to spiny cells in that the interneurons often receive multiple serial contacts from single corticostriatal axons within short distance (Ramanathan et al., 2002). This type of cortical innervation is consistent with observations that show that relatively weak cortical stimuli that are insufficient to elicit excitatory responses in spiny neurons evoke large scale immediate early gene expression in PV+ interneurons (Parthasarathy and Graybiel, 1997) and burst firing in presumed PV+ FS interneurons in vivo (Kita, 1993). PV+ interneurons also receive a powerful excitatory cholinergic input, presumably from striatal cholinergic interneurons (Chang and Kita, 1992; Kita, 1993; Koós and Tepper, 2002).
The predominant synaptic target of the FS interneuron identified by PV immunostaining, single cell filling or electrophysiological analysis, is the spiny projection neuron (Bennett and Bolam, 1994; Kubota and Kawaguchi, 2000). Over half of these boutons synapse proximally often forming pericellular baskets around spiny cell somata and making repeated contacts along proximal dendrites (Kita et al., 1990; Kita, 1993; Bennett and Bolam, 1994).
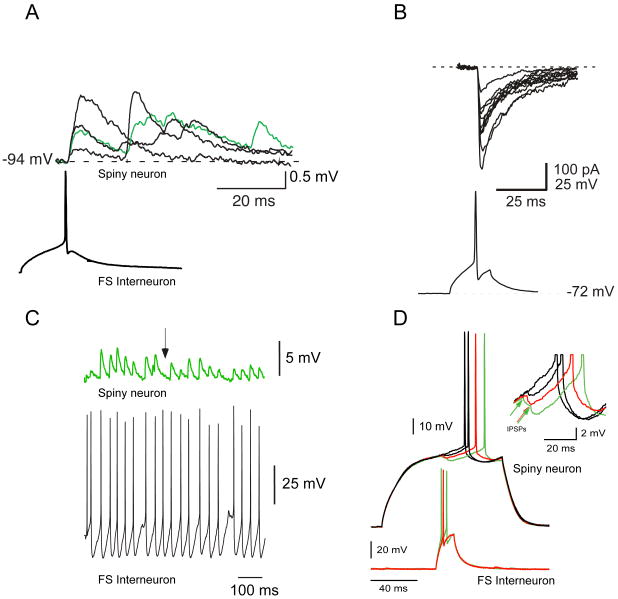
Paired whole cell recordings of FS interneurons and spiny cells revealed that at least 25% of the spiny cells within a 250 μm radius of a FS interneuron were synaptically connected to it. As most of these pairs were recorded with the intercellular distances averaging 100 μm, the connectivity ratio may actually be significantly higher, perhaps as high as 50%, as suggested by recent recordings in our laboratory (Tecuapetla, Koós and Tepper, unpublished observations). FS-Sp IPSPs were mediated predominantly or exclusively by GABAA receptors. Reciprocal connections, i.e., innervation of interneurons by innervated spiny cells were never observed (Koós and Tepper, 1999; Taverna et al., 2007). As in cortex (Galareta and Hestrin, 1999) and hippocampus (Meyer et al., 2002), striatal PV-immunoreactive interneurons themselves are connected by functional gap junctions that can help synchronize firing among active FS interneurons (Koós and Tepper, 1999). The FS-Spiny cell synaptic response was notable in that it was quite large. Single evoked spikes in FS interneurons produced unitary IPSPs in peri-threshold spiny neurons of about 1 mV or IPSCs over 100 pA, and short bursts of action potentials in FS interneurons led to compound IPSPs that could summate up to 7 mV or several hundred pA in spiny neurons (Koós and Tepper, 1999; Koós et al., 2004). The IPSP is functionally strong enough to delay or completely suppress firing in postsynaptic spiny neurons as illustrated in Figure 2 (Koós and Tepper, 1999, 2002).
Figure 2.
FS-spiny synaptic responses. A. Single action potentials evoked by current injection in FS interneurons elicited depolarizing IPSPs of up to several mV in amplitude in 25% of the spiny cells within a radius of 250 μm. B. Single FS interneuron presynaptic action potentials elicited large IPSCs in spiny cells recorded with a 140 mM CsCl internal. C. The FS-spiny synapse is very reliable, exhibiting a mean failure (arrow) rate of less than 1%. D. The FS-evoked IPSP is powerful enough to delay or even abolish postsynaptic spikes elicited by depolarizing pulses.
LTS Interneurons
During the course of the paired recording experiments, Koós and Tepper (1999) encountered another interneuron type not previously described. Although similar to a PLTS interneuron, this neuron was distinguished from the PLTS cell by a two-fold higher input resistance, a shorter duration action potential with a prominent biphasic afterhyperpolarization with a very fast initial component and the absence of the persistent depolarization that characterized the PLTS cell (see Figure 3). Similar to the FS interneurons, the LTS interneurons were also found to be synaptically connected to spiny cells and to exert similarly powerful inhibition capable of vetoing spiking in the postsynaptic neuron as shown in Figure 3B.
Figure 3.
LTS interneurons also exert strong inhibition on spike generation in spiny neurons. A. Responses of an LTS neuron to hyper- and depolarizing current pulses. Note the burst response at the start of the pulse (top trace) seen more clearly as riding on an LTS (arrow) evoked by lower amplitude stimuli in the bottom traces. Note relatively large slope (high input resistance) in the IV curve in the inset. B. Short burst of 3 spikes in a presynaptic LTS cell completely abolishes the evoked spike in a synaptically connected spiny neuron.
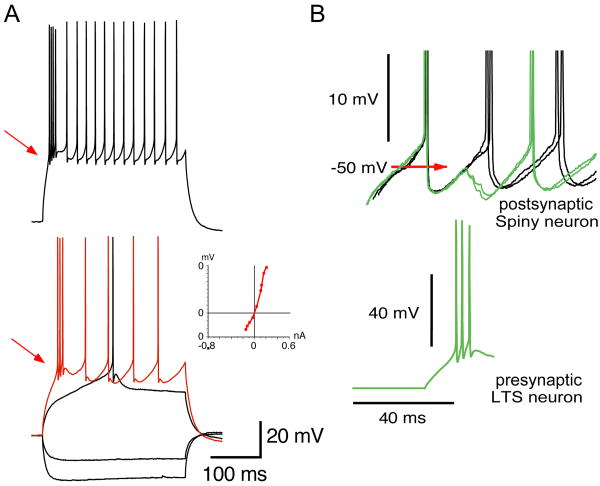
Finally, one additional type of presumed GABAergic interneuron was shown to elicit relatively strong inhibitory synaptic responses in spiny neurons as shown in Figure 4. This neuron exhibited an unusually deep and long-lasting spike afterhyperpolarization and fired rebound spikes following the offset of a hyperpolarizing current pulse delivered when the cell was depolarized (Figure 4B), characteristics different from those of either PV+ or LTS neurons. Depolarization from rest sometimes evoked a plateau-like potential that did not outlast the stimulus (Figure 4C).
Figure 4.
Second type of “novel” presumed striatal GABAergic interneuron. A. Spikes evoked by depolarizing stimuli exhibit deep, long afterhyperpolarizations. Note also the absence of a sag in response to hyperpolarizing pulses that is usually seen in PLTS neurons. B. Rebound spiking at the cessation of hyperpolarizing pulses delivered from a slightly depolarized membrane potential. C. Small depolarizing current injection elicits a plateau potential that does not outlast the stimulus. D. Single presynaptic spikes elicit IPSCs with low amplitude variance suggesting a very small number of release sites. E. Reconstruction of biocytin filled interneuron shows varicose dendrites (green) and a very sparse axonal arborization.
Single presynaptic spikes in this third type of GABAergic interneuron elicited IPSCs in postsynaptic spiny cells with low variance and high failure rate, suggesting that the number of synapses formed by the presynaptic neuron was very low, perhaps only 1. The cell was medium sized with modestly branching varicose dendrites and a very sparse local axonal arborization, a characteristic also very distinct from that of the FS or LTS interneurons, as shown in Figure 4.
Thus there are at least 3 different types of GABAergic interneurons that inhibit spiny cells in a feedforward manner. At least two of these, the FS and LTS interneurons, have been shown to exert powerful inhibitory control over spike timing in spiny neurons. Based on the size of the FS interneuronal axonal arborization, the density of spiny cells in the striatum and a 25% probability of synaptic connection between FS interneurons and surrounding spiny cells and the proportion of FS interneurons, it was estimated that spiny neurons receive inputs from between 4 and 27 FS interneurons, each of which is capable of vetoing spiking in the postsynaptic neuron (Koós and Tepper, 1999). With an estimated 135 spiny cells receiving input from each FS interneuron, it is clear that a very small number of striatal interneurons can exert very powerful control over both the general excitability as well as the precise spike timing in spiny neurons.
Spiny Cell Axon Collaterals and Feedback Inhibition
In addition to their extrastriatal projections, the spiny projection neurons give rise to a relatively dense local axon collateral arborization that is approximately coextensive with and usually extends beyond (sometimes far beyond) the dendritic arborization of the parent cell (Wilson and Groves, 1980; Kawaguchi et al., 1990). Electron microscopic analysis of intracellularly or immunocytochemically labeled spiny cell axons revealed that the principal targets of these local GABAergic collaterals were, nor surprisingly, other spiny cells (Wilson and Groves, 1980; Bolam et al., 1983). Most of these axons formed synapses with dendrites or spine shafts in the more distal regions of the cell, with only a small percentage forming axosomatic contacts. Given the overwhelming predominance of spiny cells in the neostriatum, each giving rise to a dense local dense axon collateral arborization innervating surrounding spiny cells, the prevailing view of neostriatal organization was one of a large lateral inhibitory network where neurons most strongly excited by cortical/thalamic inputs would most strongly inhibit their neighbors, leading to disinhibition of the strongly excited cell in a feedback loop that would result in a further increase in activity in the most strongly excited neuron with consequent increased inhibition exerted on its neighbors.
However, whereas some early electrophysiological and pharmacological studies provided evidence in favor of such a functional organization (e.g., Park et al., 1980; Katayama et al., 1981), other tests of the hypothesis using intracellular recordings of antidromic or orthodromic activation of spiny neurons or from pairs of spiny neurons shown to lie within each others' axon collateral fields, failed to reveal the expected monosynaptic inhibition (Pennartz and Kitai, 1991; Jaeger et al., 1994). Jaeger and colleagues concluded that lateral inhibition among spiny neurons was “weak or non-existent”, and suggested that the fast GABAergic inhibition seen in striatum under a number of conditions was likely mediated by interneurons.
Several years later spiny cell axon collateral inhibition was first reported by Tunstall, Oorschot, Keans and Wickens (2002) of the University of Otago. These investigators used paired sharp electrode recordings in acute striatal slices. Under these conditions, the probability of detecting synaptic transmission between spiny cell pairs was approximately 10%. Connections were always unidirectional - reciprocal innervation was not observed in this study. The unitary IPSPs were exceedingly small, some 277 μV in amplitude. Although it was sometimes possible to unequivocally identify these IPSPs in single sweeps, in most cases averages of 200 sweeps were necessary to separate the IPSP from the noise. In marked contrast to the very low FS-spiny synapse failure rate, the failure rate of spiny-spiny synapses was relatively high (>38%). Armed with the knowledge that the collateral IPSP was so small and prone to failure, studies from a number of different laboratories quickly replicated the results of Tunstall et al. (2002) in a variety of preparations. Subsequent studies in slices gave very similar results; connectivity probabilities of 12-16% (counting each pair of spiny cells as comprising 2 potential synaptic connections; see Tunstall et al., 2002, Koos et al., 2004), small amplitude IPSPs/IPSCs and an extremely low incidence of reciprocal connectivity (Czubayko and Plenz, 2002; Guzman et al., 2003; Plenz, 2003; Koós et al., 2004; Taverna et al., 2004; Venance et al., 2004). In addition spiny cell collateral synapses revealed a significantly greater short-term depression than that observed in the FS-spiny cell synapse, both in acute slices (Koós et al., 2004) and in organotypic cell cultures (Gustafson et al., 2005). Interestingly, studies of the spiny-spiny synapse in organotypic cell culture revealed a significantly larger collateral IPSP perhaps because of a significantly greater input resistance than reported for spiny cells in most studies in slices (Czubayko and Plenz, 2002; Gustafson et a., 20005; Tepper et al., 2004) although there was great variability with some synapses resembling those seen in acute slices. In addition spiny cell pairs in slice culture exhibited a considerably greater overall probability of connectivity (38%) and a significant degree of reciprocal connectivity (nearly one third of the connected pairs), suggesting that there is likely to be significantly greater connectivity in slice-co-cultures than in acute slices (Czubayko and Plenz, 2002; Gustafson et al., 2005). Despite this differences, the overall spiny-spiny synapse failure rate in observed in slice culture remained significantly higher than that observed for the FS-spiny synapses in slices (Koós and Tepper, 1999; Koós et al., 2004; Gustafson et al., 2005).
The IPSP/C amplitude differences between the feedforward and feedback circuits could be due to postsynaptic factors, differences in release probability and/or differences in the number of release sites. These parameters can be estimated by mean variance analysis (Clements and Silver, 2000) and nonstationary PSC fluctuation analysis (Scheuss et al., 2002) from IPSCs resulting from trains of presynaptic spikes. These methods have a significant advantage over several others in that they are technically simple, make fewer assumptions about the synaptic connection and perform better when the mean quantal content is small with respect to the noise, a feature of the spiny cell collateral synaptic response that was known from the original studies (Tunstall et al., 2002). In a study designed to directly compare feedforward and feedback inhibition in striatum, voltage clamp recordings of FS-spiny and spiny-spiny pairs with good voltage control were obtained from striatal slices under identical conditions (Koós et al., 2004), and the mean-variance and nonstationary PSC fluctuation analyses were applied. These analyses revealed that the two synapses were similar in quantal size and initial release probability, but differed in terms of peak synaptic current (51 vs. 269 pA) and number of release sites (2.9 vs. 6.7), which were both larger for FS-spiny synapses than for spiny-spiny synapses.
The larger amplitude of the FS-spiny IPSC was accounted for by two factors, the first of which is synaptic location. Electron microscopic data indicate that FS-spiny synapses are predominantly made proximally, on the soma and proximal dendrites of the spiny cell, an electrotonically favored location (Kita et al., 1990; Kita, 1993; Bennett and Bolam, 1994). In contrast, 88% of spiny cell collateral synapses are made in the more distal spiny regions of the dendrites (Wilson and Groves, 1980). Simulations have revealed that synapse location is a significant factor in somatic IPSP amplitude in spiny neurons as shown in Figure 6 (Wilson, 2007).
Figure 6.
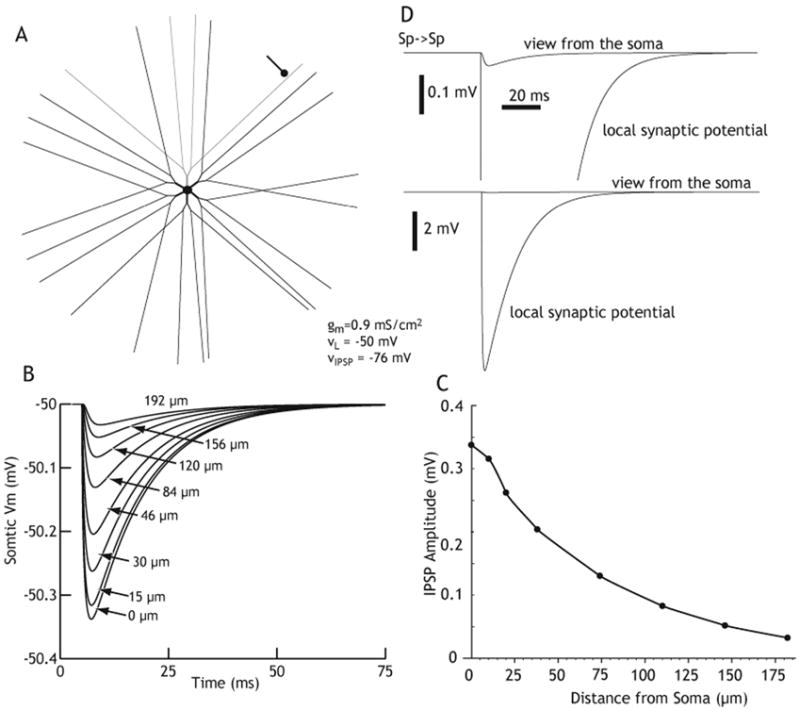
Computer simulation of the effects of distance of an inhibitory synaptic input from the amplitude recorded at the soma of a spiny neuron using parameters from in vitro recordings. Em= -50 mV (up state) and reversal potential = -76 mV. A. Model spiny neuron. B. IPSP recorded at soma for synapses located at varying distances. C. Peak IPSP at soma for synapses located at the indicated distances. D. Comparison of the amplitude of a typical Sp-Sp IPSP if viewed from the soma (upper) and at the dendritic site of origin (lower). Reprinted from Wilson (2007) with permission.
Consistent with these anatomical findings, spiny-spiny IPSCs recorded in whole cell mode with and without blocking potassium channels with intracellular cesium differed in amplitude by a factor of about 3 (18 vs. 51 pA), as did the estimated synaptic conductance (0.27 vs. 0.75 nS). The remainder of the difference in amplitude (and in the overall failure rate) was due to the greater number of release sites at FS-spiny vs. spiny-spiny synapses (Kóos et al., 2004). Qualitatively similar differences in the strength of feedforward and feedback inhibition have been reported by others (Guzman et al., 2003; Gustafson et al., 2005; Tecuapetla et al., 2005) and are apparent when reported amplitudes of FS-spiny and spiny-spiny synaptic responses are compared under equivalent conditions (Tepper et al., 2004).
It is important to emphasize that the spiny-spiny synapse only appears weak compared to the FS-spiny synapse at the level of the soma. At the dendritic site of origin, the spiny-spiny IPSP is likely to be about as powerful as that of the FS-spiny IPSP at the cell body (Figure 6C; Tepper et al., 2004; Wilson, 2007). Thus, although individual presynaptic spiny neurons are not very effective at affecting action potential generation in their postsynaptic spiny cell targets, a single spiny-spiny synapse can exert powerful effects on local dendritic processing. This could include strong influences on spike back-propagation, dendritic calcium entry and other events that could play a significant role in long-term corticostriatal and/or thalamostriatal plasticity (Kerr and Plenz, 2002, 2004; Plenz, 2003; Carter and Sabatini, 2004).
Nevertheless, recent findings that emphasize the sparse distribution of both the excitatory cortical input (Kincaid et al., 1998) and the recurrent collateral innervation, the very low incidence of symmetric and reciprocal connections and the small amplitude of individual feedback IPSPs at spiny cell somata (Tunstall et al., 2002; Koós et al., 2004; Taverna et al., 2004) make the original formulation of a lateral inhibitory network or even a modification based on lateral inhibition among functionally related groups of spiny cells untenable (Wilson, 2007). However a recent description of a biologically realistic simulation of a striatal network of 2500 spiny neurons in the up state (represented by 3000 synaptic inputs/sec) using reasonable assumptions of connectivity (0.2) and synaptic conductance (0.6 nS) based on mean data from several different laboratories, the operation of the axon collateral network was suggested to play a significant role in setting the overall level of activity of the network as well as amplifying the difference between weakly and strongly excited neurons (Wickens et al., 2007). Thus, while the type of strong lateral inhibitory dynamic originally proposed for the striatal axon collateral network is no longer valid, there may nonetheless be a significant effect of the feedback system on the overall level of activity.
Summary
In conclusion, there are two main types of GABAergic circuitry in the striatum, a feedforward inhibitory circuit mediated by several different types of GABAergic interneurons synapsing on spiny cells and an inhibitory feedback circuit mediated by the axon collaterals of the spiny neurons synapsing on other spiny neurons. There are far more inhibitory synapses in the feedback circuit than in the feedforward circuit, but each of the synapses is far less effective at controlling spiking in the neuron due to the much smaller amplitude of the synaptic response seen at the level of the cell body. The lower amplitude of the axon collateral IPSP/Cs is due to a combination of their distal synaptic location and lower number of release sites per cell compared to the GABAergic interneurons. Thus, the older view in which the main organization principle of striatal organization was seen as a classical lateral inhibitory network that acts to select and sharpen spiny cell output at the level of single spikes has given way to a more current view in which the feedforward interneuronal inhibitory circuitry functions to regulate the precision of spiny cell spike timing, whereas the feedback system functions more at the level of the dendrites to regulate local processes that could influence corticostriatal and/or thalamostriatal synaptic plasticity as well as overall levels of network activity.
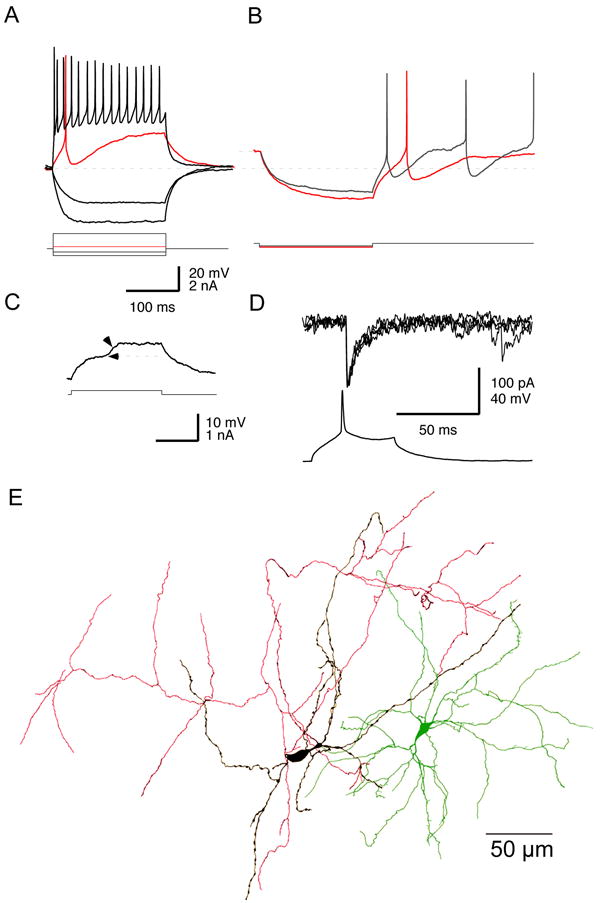
Figure 5.
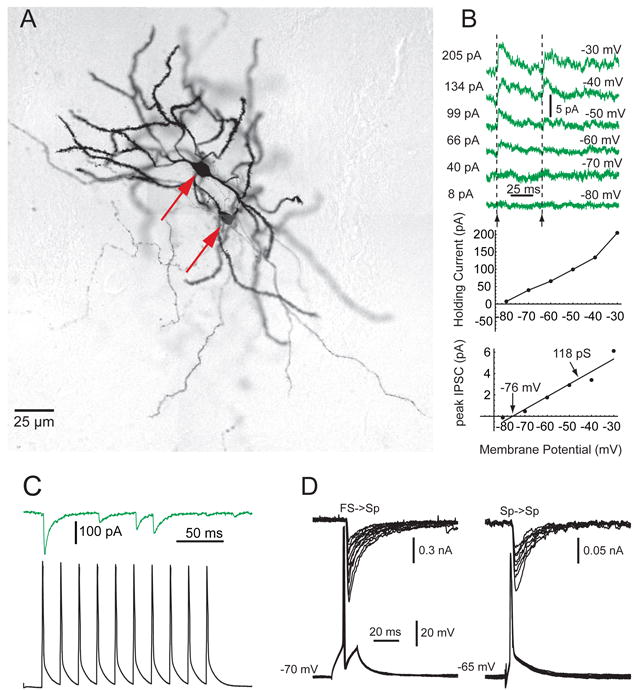
Feedback inhibition between spiny neurons. A. Synaptically connected pair of spiny neurons (arrows) in a striatal slice from an adult rat stained with biocytin after whole cell recording. B. Sp-Sp IPSCs recorded in perforated patch configuration in a slice from a 13 day old rat. Top. Presynaptic spikes were triggered at the arrows while holding the postsynaptic cell at the membrane potentials indicated on the right of the traces. Middle. Plot of the current required to hold the membrane at different potentials between -80 and -30 mV. Bottom. Slope of the VI plot from the data at top shows the conductance change due to the collateral synapse is only 118 pS. The amplitude goes to zero at the reversal potential of -76 mV. C. Response to a train of presynaptic spikes at 40 Hz shows frequent failures. D. Comparison of whole cell recordings of IPSCs in two different spiny cells, one elicited by single spikes in an FS interneuron (left) and another in a spiny cell (right). Note the difference in the voltage calibration and the approximately 6-fold difference in maximum IPSC amplitude. The internal contained 140 mM CsCl. Redrawn from Koós et al., (2004). Copyright 2004 by the Society for Neuroscience.
Acknowledgments
This work was supported by NS034865 (to JMT), NS052370 (to TK) and NS37760 (to CJW).
Footnotes
This manuscript is for the special issue “Basal Ganglia”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James M. Tepper, Center for Molecular and Behavioral Neuroscience, Rutgers-Newark, Newark, NJ, USA
Charles J. Wilson, University of Texas San Antonio, San Antonio, TX USA
Tibor Koós, Center for Molecular and Behavioral Neuroscience, Rutgers-Newark, Newark, NJ, USA.
References
- Bar-Gad I, Bergman H. Stepping out of the box: information processing in the neural networks of the basal ganglia. Curr Opin Neurobiol. 2001;11:689–95. doi: 10.1016/s0959-4388(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Beiser DG, Houk JC. Model of cortical-basal ganglionic processing: encoding the serial order of sensory events. J Neurophysiol. 1998;79:3168–88. doi: 10.1152/jn.1998.79.6.3168. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Localisation of parvalbumin-immunoreactive structures in primate caudate-putamen. J Comp Neurol. 1994;347:340–56. doi: 10.1002/cne.903470303. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–19. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Bennett BD. Microcircuitry of the neostriatum. In: Ariano MA, Surmeier DJ, editors. Molecular and Cellular Mechanisms of Neostriatal Function. RG Landes Company; Austin: 1995. pp. 1–20. [Google Scholar]
- Bolam JP, Somogyi P, Takagi H, Fodor I, Smith AD. Localization of substance P-like immunoreactivity in neurons and nerve terminals in the neostriatum of the rat: a correlated light and electron microscopic study. J Neurocytol. 1983;12:325–44. doi: 10.1007/BF01148468. [DOI] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Dopamine excites fast-spiking interneurons in the striatum. J Neurophysiol. 2002;87:2190–4. doi: 10.1152/jn.00754.2001. [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–93. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Chang HT, Kita H. Interneurons in the rat striatum: relationships between parvalbumin neurons and cholinergic neurons. Brain Res. 1992;574:307–11. doi: 10.1016/0006-8993(92)90830-3. [DOI] [PubMed] [Google Scholar]
- Clements JD, Silver RA. Unveiling synaptic plasticity: a new graphical and analytical approach. TINS. 2000;23:105–113. doi: 10.1016/s0166-2236(99)01520-9. [DOI] [PubMed] [Google Scholar]
- Czubayko U, Plenz D. Fast synaptic transmission between striatal spiny projection neurons. Proc Natl Acad Sci U S A. 2002;99:15764–9. doi: 10.1073/pnas.242428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emson PC, Augood SJ, Senaris R, Guerara Guzman R, Kishimoto J, Kadowaki K, Norris PJ, Kendrick KM. Chemical signalling and striatal interneurones. Prog Brain Res. 1993;99:155–65. doi: 10.1016/s0079-6123(08)61344-8. [DOI] [PubMed] [Google Scholar]
- Freiman I, Anton A, Monyer H, Urbanski MJ, Szabo B. Analysis of the effects of cannabinoids on identified synaptic connections in the caudate-putamen by paired recordings in transgenic mice. J Physiol. 2006;575:789–806. doi: 10.1113/jphysiol.2006.114272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–5. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–11. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Groves PM. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res. 1983;286:109–32. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Gustafson N, Gireesh-Dharmaraj E, Czubayko U, Blackwell KT, Plenz D. A comparative voltage and current-clamp analysis of feedback and feedforward synaptic transmission in the striatal microcircuit in vitro. J Neurophysiol. 2006;95:737–52. doi: 10.1152/jn.00802.2005. [DOI] [PubMed] [Google Scholar]
- Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci. 2003;23:8931–40. doi: 10.1523/JNEUROSCI.23-26-08931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Hernandez A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci. 2003;23:8931–40. doi: 10.1523/JNEUROSCI.23-26-08931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–43. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Miyazaki S, Tsubokawa T. Electrophysiological evidence favoring intracaudate axon collaterals of GABAergic caudate output neurons in the cat. Brain Res. 1981;216:180–6. doi: 10.1016/0006-8993(81)91286-5. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–23. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–35. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–38. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: light and electron microscopy. Philos Trans R Soc Lond B Biol Sci. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Dendritic calcium encodes striatal neuron output during up-states. J Neurosci. 2002;22:1499–512. doi: 10.1523/JNEUROSCI.22-05-01499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JN, Plenz D. Action potential timing determines dendritic calcium during striatal up-states. J Neurosci. 2004;24:877–85. doi: 10.1523/JNEUROSCI.4475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid AE, Zheng T, Wilson CJ. Connectivity and convergence of single corticostriatal axons. J Neurosci. 1998;18:4722–31. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H. GABAergic circuits of the striatum. Prog Brain Res. 1993;99:51–72. doi: 10.1016/s0079-6123(08)61338-2. [DOI] [PubMed] [Google Scholar]
- Kita H, Kosaka T, Heizmann CW. Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 1990;536:1–15. doi: 10.1016/0006-8993(90)90002-s. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–72. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. J Neurosci. 2002;22:529–35. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–22. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Three classes of GABAergic interneurons in neocortex and neostriatum. Jpn J Physiol. 1994;44 Suppl 2:S145–8. [PubMed] [Google Scholar]
- Kubota Y, Kawaguchi Y. Dependence of GABAergic synaptic areas on the interneuron type and target size. J Neurosci. 2000;20:375–86. doi: 10.1523/JNEUROSCI.20-01-00375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Mikawa S, Kawaguchi Y. Neostriatal GABAergic interneurones contain NOS, calretinin or parvalbumin. Neuroreport. 1993;5:205–8. doi: 10.1097/00001756-199312000-00004. [DOI] [PubMed] [Google Scholar]
- Luk KC, Sadikot AF. GABA promotes survival but not proliferation of parvalbumin-immunoreactive interneurons in rodent neostriatum: an in vivo study with stereology. Neuroscience. 2001;104:93–103. doi: 10.1016/s0306-4522(01)00038-0. [DOI] [PubMed] [Google Scholar]
- Meyer AH, Katona I, Blatow M, Rozov A, Monyer H. In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci. 2002;22:7055–64. doi: 10.1523/JNEUROSCI.22-16-07055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Berger TW. Functionally distinct subpopulations of striatal neurons are differentially regulated by GABAergic and dopaminergic inputs--I. In vivo analysis. Neuroscience. 1992;48:561–78. doi: 10.1016/0306-4522(92)90402-n. [DOI] [PubMed] [Google Scholar]
- Park MR, Lighthall JW, Kitai ST. Recurrent inhibition in the rat neostriatum. Brain Res. 1980;194:359–69. doi: 10.1016/0006-8993(80)91217-2. [DOI] [PubMed] [Google Scholar]
- Parthasarathy HB, Graybiel AM. Cortically driven immediate-early gene expression reflects modular influence of sensorimotor cortex on identified striatal neurons in the squirrel monkey. J Neurosci. 1997;17:2477–91. doi: 10.1523/JNEUROSCI.17-07-02477.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CM, Kitai ST. Hippocampal inputs to identified neurons in an in vitro slice preparation of the rat nucleus accumbens: evidence for feed-forward inhibition. J Neurosci. 1991;11:2838–47. doi: 10.1523/JNEUROSCI.11-09-02838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenz D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–43. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- Plotkin JL, Wu N, Chesselet MF, Levine MS. Functional and molecular development of striatal fast-spiking GABAergic interneurons and their cortical inputs. Eur J Neurosci. 2005;22:1097–108. doi: 10.1111/j.1460-9568.2005.04303.x. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Hanley JJ, Deniau JM, Bolam JP. Synaptic convergence of motor and somatosensory cortical afferents onto GABAergic interneurons in the rat striatum. J Neurosci. 2002;22:8158–69. doi: 10.1523/JNEUROSCI.22-18-08158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, Sadikot AF. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol. 2004;469:325–39. doi: 10.1002/cne.11008. [DOI] [PubMed] [Google Scholar]
- Scheuss V, Schneggenburger R, Neher E. Separation of presynaptic and postsynaptic contributions to depression by covariance analysis of successive EPSCs at the calyx of Held synapse. J Neurosci. 2002;22:728–739. doi: 10.1523/JNEUROSCI.22-03-00728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Canciani B, Pennartz CM. Membrane properties and synaptic connectivity of fast-spiking interneurons in rat ventral striatum. Brain Res. 2007;1152:49–56. doi: 10.1016/j.brainres.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Taverna S, van Dongen YC, Groenewegen HJ, Pennartz CM. Direct physiological evidence for synaptic connectivity between medium-sized spiny neurons in rat nucleus accumbens in situ. J Neurophysiol. 2004;91:1111–21. doi: 10.1152/jn.00892.2003. [DOI] [PubMed] [Google Scholar]
- Taverna S, van Dongen YC, Groenewegen HJ, Pennartz CM. Direct physiological evidence for synaptic connectivity between medium-sized spiny neurons in rat nucleus accumbens in situ. J Neurophysiol. 2004;91:1111–21. doi: 10.1152/jn.00892.2003. [DOI] [PubMed] [Google Scholar]
- Tecuapetla F, Carrillo-Reid L, Guzman JN, Galarraga E, Bargas J. Different inhibitory inputs onto neostriatal projection neurons as revealed by field stimulation. J Neurophysiol. 2005;93:1119–26. doi: 10.1152/jn.00657.2004. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Abercrombie ED, Bolam JP. Basal ganglia macrocircuits. Prog Brain Res. 2007;160:3–7. doi: 10.1016/S0079-6123(06)60001-0. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–9. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tunstall MJ, Oorschot DE, Kean A, Wickens JR. Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol. 2002;88:1263–9. doi: 10.1152/jn.2002.88.3.1263. [DOI] [PubMed] [Google Scholar]
- Venance L, Glowinski J, Giaume C. Electrical and chemical transmission between striatal GABAergic output neurones in rat brain slices. J Physiol. 2004;559:215–30. doi: 10.1113/jphysiol.2004.065672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Alexander ME, Miller R. Two dynamic modes of striatal function under dopaminergic-cholinergic control: simulation and analysis of a model. Synapse. 1991;8:1–12. doi: 10.1002/syn.890080102. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Arbuthnott GW, Shindou T. Simulation of GABA function in the basal ganglia: computational models of GABAergic mechanisms in basal ganglia function. Prog Brain Res. 2007;160:313–29. doi: 10.1016/S0079-6123(06)60018-6. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Kotter R, Alexander ME. Effects of local connectivity on striatal function: stimulation and analysis of a model. Synapse. 1995;20:281–98. doi: 10.1002/syn.890200402. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Basal Ganglia. In: Shepherd GM, editor. The Synaptic Organization of the Brain. 5th. Oxford University Press; Oxford: 2004. pp. 361–414. [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]