Abstract
Our previous studies showed that Fas expression correlates inversely with the metastatic potential of osteosarcoma (OS) cells and that the manipulation of Fas expression alters the lung metastatic phenotype. However, the role of VEGF in the growth and metastases of OS is unclear. The purpose of this study was to determine whether altering VEGF expression affects lung metastatic potential. LM7 metastatic OS cells were transfected with a small interfering RNA targeting human VEGF165 (LM7/siVEGF165) or a pcDNA4 plasmid expressing human VEGF165 (LM7/VEGF). We confirmed that VEGF165 expression was decreased in LM7/siVEGF165 cells and was increased in LM7/VEGF clones compared with control transfected clones. Fas expression was not altered in these transfected clones. We also transfected LM7 cells with Fas (LM7/Fas) or Fas together with VEGF165 (LM7/Fas/VEGF) to determine whether the overexpression of VEGF165 could enhance the metastatic potential of LM7 OS cells with high Fas expression (Fas+). LM7/siVEGF165 and LM7/Fas cells showed decreased lung metastatic potential. In addition, the overexpression of VEGF had no effect on the ability of LM7/Fas cells to form lung metastases. We therefore concluded that VEGF165 is critical to lung metastatic potential but is not sufficient to allow Fas+ OS cells to survive in the Fas ligand lung microenvironment.
Keywords: Fas, lung metastases, osteosarcoma, VEGF165, siVEGF165
Introduction
The lungs are the most common site for metastasis in patients with osteosarcoma (OS), with over 30% of patients developing lung metastases despite treatment with combination chemotherapy and surgery [1-4]. Patients who present with pulmonary metastases have a higher rate of treatment failure, with only 20% achieving long-term metastases-free survival. Salvage chemotherapy for OS lung metastases has been disappointing, showing questionable benefit in terms of improvement in overall survival. For these reasons, our laboratory has focused on understanding the biologic properties that contribute to metastasis to identify new therapeutic approaches for patients with OS lung metastases.
Angiogenesis has been specifically linked to increased growth and metastatic potential in human cancer [5]. Vascular endothelial growth factor (VEGF), particularly VEGF-α, has been demonstrated to play a pivotal role in tumor angiogenesis [6]. Increased VEGF expression has been demonstrated in colon, kidney, thyroid, bladder, ovary, and cervical cancers [7]. The overexpression of VEGF increased tumor growth and metastasis by stimulating neovascularization [8-10]. Therefore, antiangiogenic compounds are being investigated in patients who are unresponsive to chemotherapy as a means of inhibiting or controlling tumor growth. Inhibiting VEGF by using neutralizing antibodies to VEGF-α or VEGF receptor 2 has been one approach [11, 12].
Our previous studies showed that a small interfering RNA (siRNA) targeting the 165 isoform of vascular endothelial growth factor (siVEGF165) inhibited the growth of TC71 Ewing's sarcoma in a xenograft mouse model [13]. However, the role of VEGF in the growth and metastases of OS is unclear. We also demonstrated previously that Fas expression correlates inversely with the metastatic potential of OS cells [14-17]. Cells with high Fas expression (Fas+) failed to produce lung metastases when injected intravenously into nude mice, whereas a subclone with low Fas expression (Fas−) induced significant macroscopic pulmonary metastases [15]. In addition, we have shown that the manipulation of Fas expression alters the lung metastatic phenotype [15, 17]. The purpose of this study was to investigate whether decreasing or increasing VEGF expression in Fas− OS cells affects the metastatic phenotype. We showed that along with Fas, VEGF also contributes to the ability of OS cells to form lung metastases. Inhibiting VEGF165 reduced the metastatic potential of Fas− OS cells. However, increasing the expression of VEGF165 alone was not sufficient to allow Fas+ OS cells to form lung metastases. Fas+ OS cells transfected with VEGF and injected into mice did not induce pulmonary metastases. These data indicate that VEGF is a necessary but not sufficient component of the metastatic phenotype and that multiple proteins contribute to the ability of OS cells to grow in the lungs.
Materials and methods
Cell lines
The SAOS-2 human OS cell line was purchased from the American Type Culture Collection (Rockville, MD, USA). The LM7 cell line was derived from the SAOS-2 line by initial selection in 0.9% agarose followed by repeated intravenous recycling through the lungs of nude mice [14, 16]. The stable LM7/Fas and LM7/neo cells were obtained by transfection with a cytomegalovirus promoter-based pcDNA3 plasmid containing human Fas or neomycin control plasmid and selected with G418 antibiotic [15, 17]. These cells were maintained in Eagle's minimum essential medium (MEM) supplemented with 1x nonessential amino acids, 1 mM sodium pyruvate, 2x MEM vitamin solution, 2 mM L-glutamine, and 10% heat-inactivated fetal bovine serum (FBS) (see Reagents). The monolayer culture of cells was maintained in a 37°C humidified 5% CO2 incubator. The cells were tested periodically for mycoplasma contamination by reverse transcriptase-polymerase chain reaction (RT-PCR) (primer purchased from Sigma Genosys, The Woodlands, TX, USA) and were confirmed as being free of pathogenic murine viruses (National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, MD, USA).
Reagents
Eagle's MEM, Hanks' balanced salt solution without calcium or magnesium, nonessential amino acids, sodium pyruvate, MEM vitamin solution, L-glutamine, and 2.5% trypsin were purchased from Whittaker Bioproducts (Walkersville, MD, USA). The FBS was purchased from Atlanta Biologicals (Norcross, GA, USA). All reagents were free of endotoxin as determined by the Limulus amebocyte lysate assay (sensitivity limit, 0.025 ng/ml) purchased from Sigma (St. Louis, MO, USA).
Vector and cell transfection
The siRNA-expressing vector pSilencer2.1-U6 hygro was purchased from Ambion (Austin, TX). siRNA-expressing plasmids targeting human VEGF were constructed according to the manufacturer's instructions [13]. The targeted siVEGF-6 sequence was GTTCATGGATGTCTATCAG. The si-control vector sequence was CTACCGTTGTTATAGGTTCTCTTGAACACCTATAACAACGGTAGT [13]. These inserted sequences were verified by DNA sequencing. LM7 cells were transfected by using the nonliposomal transfection reagent FuGENE 6 (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer's instructions and then selected with 400 μg/ml of hygromycin B (Invitrogen Corporation, Carlsbad, CA). Decreased expression of VEGF in hygromycin B-resistant cells (LM7/siVEGF) was confirmed by using RT-PCR and enzyme-linked immunosorbent assay (ELISA).
To induce the overexpression of VEGF, the LM7 and LM7/Fas cells were transfected with cytomegalovirus promoter-based pcDNA4 plasmid containing human VEGF165 (provided by Dr. Raphael E. Pollock, The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA) or a neomycin control plasmid using FuGENE 6 as cited above. The cells transfected with VEGF165 were selected with 250 μg/ml of Zeocin selection reagent (Invitrogen). Increased expression of VEGF165 in Zeocin-resistant clones was confirmed by using RT-PCR and ELISA.
In vitro cell proliferation assay
LM7 and the various transfected LM7 clones were harvested in the mid-log growth phase, placed into 96-well plates, and incubated for 24, 48, 72, or 96 hours. The cells were labeled with 0.2 μCi/well of [3H] thymidine (ICN Biomedicals, Inc., Irvine, CA, USA) during the last 24 hours of incubation. They were then washed twice with Hanks' balanced salt solution and lysed with 0.1 ml of 0.1 N KOH. Radioactive incorporation was quantified with a plate harvester (Brandal Biomedical Research and Development Lab, Inc., Gaithersburg, MD, USA) and a Wallac 1450 Micro Beta counter (PerkinElmer Life Sciences, Turku, Finland). The doubling time was calculated by using the following formula: log2/log (n/no), where no is the counts per minute of cells incubated for 24 hours, and n is the counts per minute of cells incubated for 48, 72, or 96 hours.
ELISA
To quantify secreted VEGF165 protein from cloned cell lines, cells (5 × 103 cells/ml) were cultured into 96-well plates overnight at 37°C. The cultures then were washed twice with Hanks' balanced salt solution and incubated with fresh medium for an additional 24 hours. The supernatants were collected, and secreted VEGF165 protein was quantified by ELISA (R & D System Inc., Minneapolis, MN, USA) according to the manufacturer's recommendations. The viability of the cells in the plate was measured by MTT assay. The value of the VEGF165 protein was divided by the OD (ng/OD).
RT-PCR
RT-PCR was performed using a PTC-200 DNA Engine thermocycler (MJ Research, Incline Village, NV, USA). Total cellular RNA was extracted by using TRIzol reagent (Invitrogen). RNA (2 μg) was reverse transcribed with 15 units of AMV reverse transcriptase in 20 μl of reverse transcription reaction system volume (Promega, Madison, WI, USA) at 42°C for 15 min and then at 95°C for 5 min. The cDNA products were kept on ice, and 2 μl of cDNA sample were amplified by PCR for 30 cycles. Each cycle included denaturing at 94°C for 1 min, annealing at 55°C for 1 min, and extending at 72°C for 1 min with 0.5 units of TaqDNA polymerase (Roche Diagnostics), 1 μl of 10 μM dNTP mix, and 5 μl of 10 μM of human VEGF165 primer: sense (forward primer) 5'-TTCTGCTGTCTTGGGTGCATTGG, antisense (reverse primer) 5'-ATCTCTCCT-ATGTGCTGGCCTT. The VEGF165 primer was designed to produce a 323-bp fragment. Classic 18S (489 bp) or Classic II 18S primer:competimer (324 bp; Ambion) was used as an internal control. The total volume was 50 μl. The PCR products were isolated by electophoresis on 2% agarose gel stained with ethidium bromide and visualized under ultraviolet light. The results were quantified using the Quantity One machine (Bio-Rad Laboratories, Hercules, CA, USA).
Flow cytometric analysis
Flow cytometry was used to determine Fas expression. Cells were harvested, washed with fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline supplemented with 2% FBS and 10% NaAzide), and then incubated with PE anti-human CD95 antibody (Pharmingen, San Diego, CA, USA) diluted 1:50 in FACS buffer or 1:50 diluted PE-mouse antihuman IgG antibody (Pharmingen) on ice in the dark for 40 min. Cells were washed twice with FACSbuffer and then analyzed by a FACScan (Becton Dickinson, Mountain View, CA, USA).
Chemotactic migration and invasion assay
Cell migration and invasion were evaluated by using polycarbonate membrane transwell chambers (Corning, Acton, MA, USA) according to the manufacturer's recommendations. Forcell migration, the transwells were preincubated overnight with serum-free MEM in the upperchamber (insert) and 10% FBS MEM in the lower chamber. Cells (4 × 104 in 0.1 ml of serum-free MEM) were seeded into each insert, and the inserts were placed into the lower chamber, which contained 0.6 ml of mouse lung extract (25 mg of lung tissue per milliliter). To assess cellinvasion, 0.2 ml of 1:10 Matrigel (Pharmingen) in serum-free MEM was added to the transwellinsert and incubated for 2 h at 37°C, and then tumor cells were added. The cultures were then incubated for 24 h (for the migration assay) or 48 h (for the invasion assay) at 37°C. Cells that had not migrated or invaded remained on top of the membrane were wiped off with cottons wabs. The membranes were fixed and stained with a Diff Quick kit (Statlab, Lewisville, TX). The migrated or invaded cells on the bottom of the membranes were counted by using three high-power microscopic fields per membrane. The assays were performed in triplicate.
In vivo study
Four- to five-week-old pathogen-free athymic (T-cell deficient) nude mice were purchased from Charles River Laboratories (Wilmington, MA, USA). The mice were maintained and fed in a specific pathogen-free animal facility approved by the American Association of Laboratory Animal Care in accordance with current regulations and standards of the United States Department of Agriculture, The Department of Health and Human Services, and the National Institutes of Health. Mice were housed five to a cage and kept in a laminar-flow cabinet in specific pathogen-free conditions with a 12-h dark cycle for at least 1 week before initiation of the investigations. All animal protocols were approved by the Institutional Animal Care and Use Committee.
The LM7, control LM7/si, LM7/siVEGF165, control LM7/neo, LM7/VEGF165, LM7/Fas, LM7/Fas-control, or LM7/Fas/VEGF165 cells in the mid-log growth phase (106/0.2 ml) were injected into the lateral tail vein of nude mice. The mice were sacrificed 11 weeks later; the lungs were removed, weighed, and fixed in 10% formalin. The tumor nodules on the surface of the lungs were quantified and measured by using a dissection microscope.
Statistical analyses
Differences in cell doubling time, cell migration or invasion, and in vivo lung weights were evaluated by using the two-tailed Student's t test. A P value of <0.05 was considered statistically significant.
Results
Effects of altering VEGF165 on Fas expression
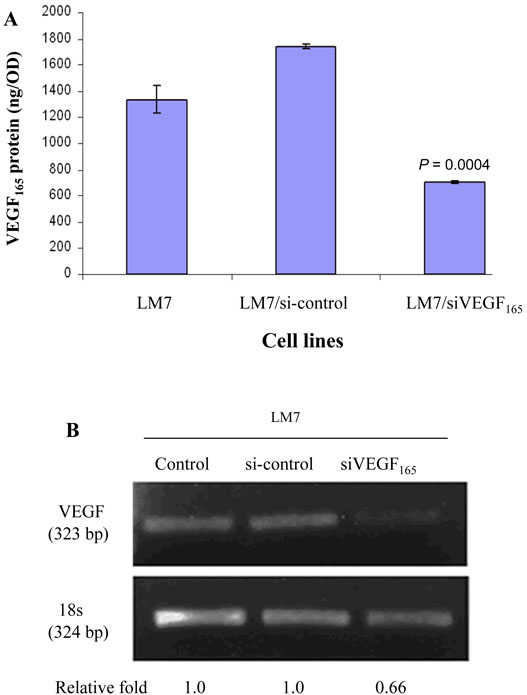
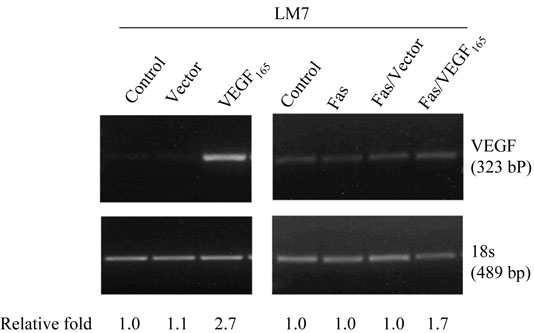
Transfection of LM7 cells with VEGF165 siRNA (LM7/siVEGF165) resulted in decreased VEGF165 expression and protein production compared with LM7/si-control cells (Fig. 1A, 1B; P < 0.001), with no change in Fas expression (data not shown). In contrast, transfection of LM7 cells with Fas resulted in increased Fas expression from 21.6% in LM7/vector cells to 81.1% in LM7/Fas cells but no effect on VEGF mRNA or protein production compared with control-vector transfected cells (Table 1, p > 0.8). Transfection of VEGF165 into LM7/Fas cells (LM7/Fas/VEGF165) also resulted in increased VEGF165 expression and protein production compared with LM7/Fas/vector cells (Fig. 2; Table 1, p <0.001). In all transfected cells including LM7/vector, LM7/Fas, and LM7/Fas/vector, the levels of VEGF165 were lower compared with LM7 parental cells (Table 1, p ≤0.05). However, the levels of VEGF165 were not significant different among these 3 transfected cells (Table 1, p > 0.8). It is possible that the vector transfection procedure resulted in decreased VEGF expression. Once again, compared with LM7/Fas or LM7/Fas/Vector cells, increased VEGF expression in the LM7/Fas cells did not change Fas expression (Table 1). Fas expression remained high in the LM7/Fas/VEGF165 cloned cells. There was no effect on cell doubling time after transfection with either siVEGF165, VEGF165, or Fas (data not shown). The doubling time for all transfected cells was between 25 and 32 h.
Fig. 1.
Transfection with siVEGF165 decreased expression of VEGF165 RNA and protein.
(A) Secreted VEGF protein in cultured supernatants was quantified by ELISA.
(B) Total RNA was extracted using TRIzol and analyzed by RT-PCR.
Table 1.
Expression of Fas and VEGF165 in LM7 cells after transfectiona
| Cell lines | Fas expressionb (%) |
VEGF (ng/OD)c |
|---|---|---|
| LM7 | 14.1 | 582.5 ± 69.59 |
| LM7/vector | 21.6 | 276.8 ± 22.0d |
| LM7/Fas | 81.1 | 256.6 ± 26.75d |
| LM7/Fas/vector | 82.5 | 283.3 ± 31.32d |
| LM7/VEGF165 | 21.0 | 2045.9 ± 48.9e |
| LM7/Fas/VEGF165 | 74.1 | 1297.1 ± 12.3f |
Abbreviations: OD = optic density
LM7 cells were transfected with empty vector alone, Fas, Fas + empty vector (Fas/vector), VEGF165, or Fas + VEGF165 (Fas/VEGF165).
Fas expression was quantified by flow cytometry analysis.
VEGF165 protein was measured by ELISA. The value of the VEGF165 protein was adjusted by the OD (MTT assay lived cells in the plate).
LM7/vector vs LM7 (level of VEGF), p = 0.0526, LM7/vector vs LM7/Fas (level of VEGF), p = 0.8059, LM7/Fas vs LM7/Fas/vector, p = 0.8130.
LM7/VEGF165 vs LM7/vector, P < 0.0001.
LM7/Fas/VEGF165 vs LM7/Fas/vector, P < 0.001.
Fig. 2.
Transfection of LM7 cells with Fas, VEGF165 or Fas + VEGF. After transfection with vector alone, VEGF165, Fas, Fas/Vector, or VEGF165 + Fas total RNA was extracted from the cloned cells and analyzed by RT-PCR.
Effect of VEGF165 suppression on metastatic potential
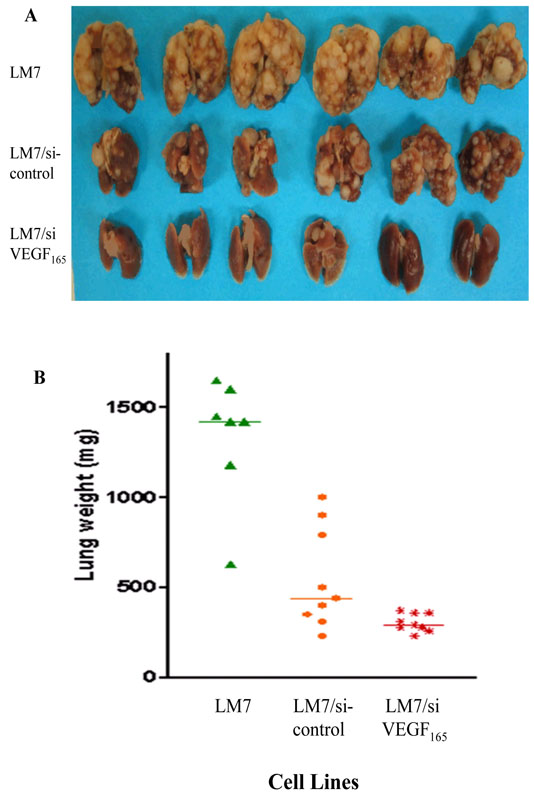
We next investigated whether altsering VEGF165 affected the ability of LM7 cells to form lung metastases. Mice were injected intravenously with LM7, LM7/si, or LM7/siVEGF165 cells and sacrificed 11 weeks later. Visible and microscopic lung metastases as well as lung weights were significantly reduced in mice injected with LM7/siVEGF165 cells compared to those injected with LM7/si cells (Fig. 3A, 3B); P < 0.02; Table 2).
Fig. 3.
Effect of altering VEGF165 on lung metastatic potential. Mice were injected intravenously with LM7, LM7/si, or LM7/siVEGF165 cells and were sacrificed 11 weeks later. Lung metastatic lesion, and lung weights were quantified.
Table 2.
Suppression of VEGF165 inhibits metastatic potentiala
| Visible lung metastasis |
Microscopic lung metastasis |
|||
|---|---|---|---|---|
| Cell lines | Incidenceb | Median no. of nodules (range) |
Incidenceb | Median no. of nodules (range) |
| LM7 | 7/7 | >200 (122 – >200) | 7/7 | >200 |
| LM7/si | 9/9 | 56 (12 – >200) | 9/9 | 43 (7 – >200) |
| LM7/siVEGF165 | 4/10 | 0 (0 – 15) | 6/10 | 0 (0 – 4) |
Mice were i.v. injected intravenously with the indicated cells (106). After 11 weeks, mice were sacrificed; lungs were removed, weighed, and fixed in 10% formalin. The number of visible and microscopic nodules were quantified.
Number of tumor-positive mice/per total number mice injected with cells.
We previously demonstrated that the expression of Fas reduces the metastatic potential of OS cells. We therefore next investigated whether the overexpression of VEGF165 could enhance the metastatic ability of Fas+ LM7 cells. For these investigations, we transfected LM7 cells with the Fas or VEGF gene alone or Fas together with VEGF165. The appropriate vector control cell lines were also cloned. Mice were injected intravenously with the various LM7 cell lines. Eleven weeks later, the mice were sacrificed, the lungs removed, and the number of metastases quantified. Transfection of LM7 cells with Fas resulted in increased Fas expression (Table 1) and decreased metastatic potential (Table 3). No visible lung metastases were detected, and only one of four mice had a single microscopic lesion in contrast to eight of eight mice injected with the control transfected cells. The double transfection of Fas and VEGF increased VEGF and Fas expression (Table 1) but did not increase the ability of the Fas+ LM7 cells to form lung metastases (Table 3). In additional, transfection with VEGF165 into LM7 cells increased VEGF165 protein in LM7/VEGF165 cells but not enhance lung metastases.
Table 3.
Overexpression of VEGF165 did not enhance metastatic potential of Fas+ cells
| Visible lung metastasis |
Microscopic lung metastasis |
|||
|---|---|---|---|---|
| Cell lines | Incidenceb | Median no. of nodules (range) |
Incidenceb | Median no. of nodules (range) |
| LM7 | 7/7 | 25 (0 – 77) | 9/9 | 22 (1 – 115) |
| LM7/vector | 6/8 | 15 (0 – 31) | 8/8 | 14 (4 – 44) |
| LM7/VEGF165 | 5/8 | 3 (0 – 24) | 5/8 | 5 (0 – 27) |
| LM7/Fas | 0/4 | 0 | 1/4 | 0 (0 – 1) |
| LM7/Fas/vector | 0/4 | 0 | 0/4 | 0 |
| LM7/Fas/VEGF165 | 0/4 | 0 | 0/4 | 0 |
Mice were i.v. injected intravenously with the indicated cells (106). After 11 weeks, mice were sacrificed; lungs were removed, weighed, and fixed in 10% formalin. The number of visible and microscopic nodules were quantified.
Number of tumor-positive mice/per total number mice injected with cells.
Effect of VEGF165 on cell migration and invasion
Cell migration and invasion are key to tumor progression and metastases. We therefore investigated whether altering VEGF165 affected these cellular functions. Because the lungs are the most commonly affected organ for osteosarcoma metastasis, we used lung extracts from mice as the chemoattractant. Increasing or decreasing VEGF165 had no effect on LM7 chemotaxis or invasion capacity (data not shown).
Discussion
Our findings demonstrated that VEGF165 is critical to the metastatic potential of OS cells and that suppression of VEGF165 inhibits the ability of human LM7 cells to form lung metastases. Both the incidence and the number of visible and microscopic metastatic lesions were significantly reduced after transfection with siVEGF165 (Table 2A; Fig. 3).
The importance of VEGF in the growth of primary tumors has been demonstrated by others. The transfection of antisense VEGF121 into human colorectal carcinoma cells for example, decreased VEGF production by a factor of 2 to 4 and inhibited the ability of these cells to form tumors in vivo [18]; in vitro growth was unaffected. Similarly, the transfection of antisense VEGF189 into human glioblastoma cells suppressed tumor growth after intracerebral injection [19]. Our studies are the first to show that the growth of OS cells in the lungs is severely compromised after suppression of VEGF165 production. Our data also confirmed that the Fas pathway plays a critical role in the ability of OS cells to form lung metastases.
LM7 cells are highly metastatic and express low levels of Fas compared with nonmetastatic parental SAOS cells [14, 15, 17]. The loss of Fas has been correlated with tumor progression in several other cancers as well [20, 21]. Up-regulation of Fas expression greatly compromised the metastatic potential of LM7 cells (Table 3). Of interest, the overexpression of VEGF165 in the Fas+ expressing LM7 cells was not able to restore the metastatic potentaial. Compared with control transfected LM7 cells, LM7/Fas/VEGF165 cells were unable to induce lung metastases following i.v. injection.
Taken together, these data support the hypothesis that multiple factors and pathways contribute to the metastatic signature of OS cells. Because Fas Ligand (FasL) is constitutively expressed in the lung microenvironment, we hypothesize that this is the first barrier or hurdle that OS cells confront in the lungs. Fas+ cells are eliminated by the FasL-expressing endothelial cells in the lung, whereas cells with low or no Fas expression are able to evade this host defense mechanism. Indeed, we have demonstrated that cells that express high levels of cell-surface Fas are rapidly cleared from the lung within 24 to 48 h [22, 23]. Blocking the Fas signaling pathway with Fas associated death domain dominant-negative allows the OS cells to survive and remain in the lung [22, 23]. Furthermore, mice that are deficient in FasL failed to clear Fas+ OS cells and developed Fas+ lung tumors after injection [22].
OS cells that evade FasL induced cell death, however, do not necessarily have the ability to form metastatic lesions in the lung, as demonstrated by our LM7/siVEGF165 cells. Once in the lung, tumor cells must be able to adhere to the local matrix and subsequently induce a vascular network to provide the necessary nutrients for cell expansion. As demonstrated by Khanna et al., [24], ezrin, a protein critical for cell invasion, adherence, and tethering, is required for OS metastases. Our data indicated that VEGF165 is another critical protein that is necessary for OS tumor growth in the lungs. We hypothesize that VEGF165 is involved in the expansion of the tumor vascular network after the OS cells enter the lung and evade FasL-induced cell death. Our data suggest that VEGF165 inhibition interfered with tumor vascular expansion resulting in the inability of the OS cells to form metastases in the lung. However, our data also suggest that the mere presence of VEGF165 is not enough to allow Fas+ OS cells to grow in the lung.
Metastatic spread of tumor cells from the primary area to distant organs involves multiple steps. The tumor cells must be able to degrade the basement membrane, gain access to the bloodstream, survive in the circulation, adapt to the new organ microenvironment, and induce the production of proteins that support tumor cell expansion. We propose that once OS cells reach the lungs, the first critical factor is Fas expression. If these cells are Fas+, the expression of ezrin, other adhesion proteins, or VEGF is irrelevant because the cells are rapidly cleared from the lungs having no time to become established. This hypothesis is supported by the finding that Fas+ human SAOS cells express ezrin [24] and VEGF [17] yet do not form lung metastases when injected intravenously [14]. By contrast Fas− LM7 cells (a subline of the SAOS parental cells) are not cleared from the lungs and readily form lung metastases. Individually both Fas and VEGF may be potential therapeutic targets for OS. Most likely they work independently with regard to lung metastasis. Understanding the factors that contribute to the metastatic phenotype may allow us to design specific and less toxic targeted therapies and provide an alternative for patients with OS lung metastases that are unresponsive to traditional chemotherapy. Indeed, we have demonstrated that inducing Fas expression in Fas− OS lung metastases results in tumor regression [22, 25, 26]. This suggests that altering tumor cell biology in established metastatic lesions can upset the balance in favor of tumor cell death. We propose that combining agents that alter Fas expression, block cell adhesion, and inhibit VEGF will be even more effective than targeting a single pathway.
Acknowledgments
The work was supported by National Institutes of Health grant CA-42992 (to E. S. Kleinerman) and National Cancer Institute grant CA-16672. We thank Ms. Joyce Furlough for manuscript preparation.
Abbreviations
- FACS
fluorescence-activated cell sorter
- OS
osteosarcoma
- RT-PCR
reverse transcriptase-polymerase chain reaction
- siRNA
small interfering RNA
- siVEGF165
small interfering RNA targeting VEGF165
- VEGF165
vascular endothelial growth factor isoform165
References
- 1.Eiber F, Giuliano A, Eckardt J, et al. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Onc. 1987;5:21–26. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe N. Chemotherapy in osteosarcoma. In: Muggia FM, editor. Experimental and Clinical Progress in Cancer Chemotherapy. Martinus Nijhoff Publishers; Boston: 1986. pp. 223–233. Advances and Controversies. [Google Scholar]
- 3.Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314:1600–1606. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 4.Goorin AM, Shuster JJ, Baker A, et al. Changing pattern of pulmonary metastases with adjuvant chemotherapy in patients with osteosarcoma: results from a multiinstitutional osteosarcoma study. J Clin Oncol. 1991;9:600–605. doi: 10.1200/JCO.1991.9.4.600. [DOI] [PubMed] [Google Scholar]
- 5.Fernando NH, Hurwitz HI. Inhibition of vascular endothelial growth factor in the treatment of colorectal cancer. Sem Oncol. 2003;30:39–50. doi: 10.1016/s0093-7754(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Sem Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SY, Nagane M, Huang HS, et al. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Science USA. 1997;94:12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oku T, Tjuvajev JG, Miyagawa Y, et al. Tumor growth modulation by sense and antisense vascular endothelial growth factor gene expression: effects on angiogenesis, vascular permeability, blood volume, blood flow, Fluorodeoxyglucose uptake, and proliferation of human melanoma intracerebral xenografts. Cancer Res. 1998;58:4185–4192. [PubMed] [Google Scholar]
- 10.Claffey KP, Brown LF, Del Angila LF, et al. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 11.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nature Med. 1999;5:1359–1364. doi: 10.1038/70928. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 13.Guan H, Zhou Z, Wang H, et al. A small interfering RNA targeting vascular endothelial growth factor inhibits Ewing's sarcoma growth in a xenograft mouse model. Clin Cancer Res. 2004;11:2662–2669. doi: 10.1158/1078-0432.CCR-04-1206. [DOI] [PubMed] [Google Scholar]
- 14.Jia S-F, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Met. 1999;17:501–506. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 15.Lafleur EA, Koshkina NV, Stewart J, et al. Increased Fas expression reduces the metastatic potential of human osteosarcoma cells. Clin Cancer Res. 2004;10:8114–8119. doi: 10.1158/1078-0432.CCR-04-0353. [DOI] [PubMed] [Google Scholar]
- 16.Jia S-F, Worth LL, Turan M, et al. Eradication of osteosarcoma lung metastasis using intranasal Gemcitabine. Anticancer Drugs. 2002;13:155–161. doi: 10.1097/00001813-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Worth LL, Lafleur EA, Jia S-F, et al. Fas expression inversely correlates with metastatic potential in osteosarcoma cells. Oncol Rep. 2002;9:823–827. [PubMed] [Google Scholar]
- 18.Okada F, Rak JW, Croix BS, et al. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Science USA. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng SY, Huang HJ, Nagane M, et al. Suppression of glioblastoma angiogenicity and tumorigenicity by inhibition of endogenous expression of vascular endothelial growth factor. Proc Natl Acad Science USA. 1996;93:8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owen-Schaub LB, van Golen KL, Hill LL, et al. Fas and Fas ligand interactions suppress melanoma lung metastasis. J Exp Med. 1998;188:1717–1723. doi: 10.1084/jem.188.9.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moller P, Koretz F, Leithauser F, et al. Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium. Int J Cancer. 1994;57:371–377. doi: 10.1002/ijc.2910570314. [DOI] [PubMed] [Google Scholar]
- 22.Gordon N, Koshkina NV, Jia S-F, et al. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential and reduces the effect of aerosol Gemcitabine. Clin Cancer Res. 2007;13:4503–4510. doi: 10.1158/1078-0432.CCR-07-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koshkina NV, Khanna C, Mendoza A, et al. Fas-negative osteosarcoma tumor cells are selected during metastasis to the lungs: the role the Fas pathway in metastatic process of osteosarcoma. Molecular Cancer Res. 2007;10:991–999. doi: 10.1158/1541-7786.MCR-07-0007. [DOI] [PubMed] [Google Scholar]
- 24.Khanna C, Khan J, Nguyen P, et al. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–3759. [PubMed] [Google Scholar]
- 25.Jia S-F, Worth LL, Densmore CL, et al. Aerosol Gene Therapy with PEI:IL-12 Eradicates Osteosarcoma Lung Metastases. Clinical Cancer Res. 2003;9:3462–3468. [PubMed] [Google Scholar]
- 26.Duan X, Jia S-F, Koshkina N, et al. Intranasal Interleukin-12 Gene Therapy Enhanced the Activity of Ifosfamide Against Osteosarcoma Lung Metastases. Cancer. 2006;106:1382–1388. doi: 10.1002/cncr.21744. [DOI] [PubMed] [Google Scholar]