Summary
The proteins AP65, AP51, AP33 and AP23 synthesized by Trichomonas vaginalis organisms in high iron play a role in adherence. Multigene families encode enzymes of the hydrogenosome organelles, which have identity to adhesins. This fact raises questions regarding the compartmentalization of the proteins outside the organelle and about the interactions of adhesins with host cells. Data here demonstrate the presence of the proteins outside the organelle under high-iron conditions. Fluorescence and immunocytochemical experiments show that high-iron-grown organisms coexpressed adhesins on the surface and intracellularly in contrast with low-iron parasites. Furthermore, the AP65 epitopes seen by rabbit anti-AP65 serum that blocks adherence and detects surface proteins were identified, and a mAb reacting to those epitopes recognized the trichomonal surface. Two-dimensional electrophoresis and immunoblot of adhesins from surface-labelled parasites provided evidence that all members of the multigene family were co-ordinately expressed and placed on the trichomonal surface. Similar two-dimensional analysis of proteins from purified hydrogenosomes obtained from iodinated trichomonads confirmed the specific surface labelling of proteins. Contact of trichomonads with vaginal epithelial cells increased the amount of surface-expressed adhesins. Moreover, we found a direct relationship between the levels of adherence and amount of adhesins bound to immortalized vaginal and ureter epithelial cells, further reinforcing specific associations. Finally, trichomonads of MR100, a drug-resistant isolate absent in hydrogenosome proteins and adhesins, were non-adherent. Overall, the results confirm an important role for iron and contact in the surface expression of adhesins of T. vaginalis organisms.
Introduction
Trichomonas vaginalis is responsible for trichomonosis (Kassai et al., 1988), the number one, non-viral sexually transmitted disease (STD). Among women, there will be up to 5 million new cases of trichomonal vaginitis in the USA (Cates, 1999) and 250 to 350 million world-wide (World Health Organization, 1995). There are health consequences for women with trichomonosis, including adverse pregnancy outcomes, predisposition to cervical cancer, and increased susceptibility to HIV seroconversion (Cotch et al., 1991; Wasserheit, 1992; Laga et al., 1993; Zhang and Begg, 1994; Yap et al., 1995; Zhang et al., 1995; Cotch et al., 1997). Among African-American women, this STD contributes significantly to health disparities through the higher rate of HIV (Sorvillo and Kerndt, 1998; Sorvillo et al., 2001). Men with T. vaginalis infection may present a non-chlamydial, non-gonococcal urethritis (Krieger et al., 1993). Importantly, symptomatic, HIV-positive men have higher concentrations of HIV in semen, facilitating HIV transmission (Hobbs et al., 1999).
The mechanisms of pathogenesis are multifactorial and virulence is greatly influenced by iron (Ryu et al., 2001). Adherence by this parasite to vaginal epithelial cells (VECs) is complex. Surface proteins (AP65, AP51, AP33 and AP23) appear to interact with host cells via ligand-receptor-type interactions and fulfil criteria of adhesins (Beachey et al., 1988). Multigene families encode these proteins (Alderete et al., 1995; 1998; Engbring et al., 1996; Engbring and Alderete, 1998a). Recombinant proteins of AP65, AP51 and AP33 competed with binding of the natural trichomonad adhesin to HeLa cells (Arroyo et al., 1992; Alderete et al., 1995; Engbring and Alderete, 1996a; Alderete, 1999). Furthermore, the amounts of adhesins were increased when organisms were grown in iron-replete medium (Lehker et al., 1991) and also upon binding to VECs (Arroyo et al., 1993). Characterization of a receptor-interactive domain for AP33 was performed, and a peptide of this domain of AP33 inhibited adherence to host cells (Engbring and Alderete, 1998b). Unexpectedly, AP65 had identity with decarboxylating malic enzyme and AP51 and AP33 with the β- and α-subunits of succinyl coenzyme A synthetase (SCS) (Alderete et al., 1995; 1998; O’Brien et al., 1996; Engbring and Alderete, 1998a; b), enzymes that reside within hydrogenosome organelles involved in fermentative oxidation of pyruvate (Müller, 1993; 1997; Kulda, 1999).
This discovery of molecular mimicry and functional diversity resulted in the questioning of the role of AP65 and the other proteins as adhesins versus a sole role as metabolic enzymes in hydrogenosomes. Alternatively, it raised the question whether only some members of the gene families are adhesins and others are enzymes within the organelles or whether these proteins are multifunctional. In addition, it was reported that surface AP65 of T. vaginalis is an erythrocyte-binding protein (Rappelli et al., 1995) and that AP65 also is released into cultures during growth (Addis et al., 1997). Extracellular AP65 appears to retain decarboxylating malic enzyme activity. It was also reported that AP65 (and the other adhesins) bound to epithelial cells, erythrocytes and Mycoplasma hominus in a non-specific fashion (Addis et al., 2000), albeit biochemical data to make this claim were unavailable. Additionally, antibodies to decarboxylating malic enzyme (AP65) and to the α-subunit of succinyl coenzyme synthetase (AP33) detected only proteins in hydrogenosomes (Brugerolle et al., 2000).
It was important therefore to continue characterization of the role of these proteins as adhesins. We hypothesized that surface expression and colocalization of the proteins on the surface of trichomonads occurred only when parasites are grown in an iron-replete medium and/or upon contact with the host cells. Furthermore, we tested whether epitope accessibility was responsible for the different results obtained with monospecific antiserum versus mAb recognition of proteins in different compartments. This report provides additional evidence on the surface placement of the proteins of T. vaginalis involved in adherence. Data suggest that all members of the multigene families are on the surface capable of binding to host cells, and surface expression is under the control of iron. Importantly, parasites in contact with host cells increased amounts of the surface adhesins. We further show quantitative relationships in the amounts of the adhesins bound to immortalized VECs and ureter epithelial cells that give different levels of adherence. Importantly, parasites of MR100 (Kulda, 1999), a laboratory-created, drug-resistant isolate lacking adhesins, were non-adherent to VECs. Overall, data reinforce the validity of past work that functional diversity as occurs for other microbial pathogens is central to the biology of this important STD agent.
Results
Detection of the surface adhesins
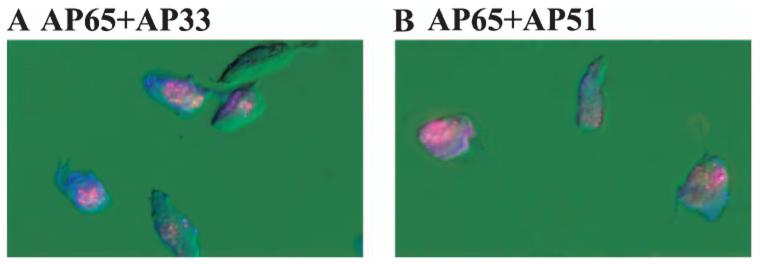
Previously we have shown that T. vaginalis organisms were examined for surface immunoreactivity with each immune rabbit anti-adhesin serum (IRS) to the four individual adhesins and, not unexpectedly, non-permeabilized trichomonads grown in high-iron medium were fluorescent. Weak fluorescence was observed using non-permeabilized parasites grown in a low-iron medium (Lehker et al., 1991; Arroyo et al., 1992). Therefore, it was now important to show whether these adhesins were colocalized. Double-labelling experiments were then performed with organisms treated with rhodamine-conjugated anti-AP65 antibody followed by incubation with fluorescein-conjugated anti-AP51 or anti-AP33 antibody. Figure 1A and B show coexpression of proteins on the parasite surface, evidenced by the blending of the combined colour on the surface. Similar results were obtained using anti-AP65 and anti-AP23 sera (not shown). No fluorescence was detected with pre-bleed normal rabbit serum (NRS). These results illustrate for the first time that the adhesins are colocalized on the trichomonad membranes and are consistent with the co-ordinated synthesis of the four proteins under high-iron conditions (Lehker et al., 1991).
Fig. 1.
Demonstrations of surface colocalization of AP65 with either AP33 (A) or AP51 (B) by fluorescence using non-permeabilized trichomonads of isolate T016 grown in a high-iron medium as described in Experimental procedures. Organisms were treated with antiserum IgG to adhesins conjugated individually to fluorescein (anti-AP65) or rhodamine (both anti-AP51 and AP33). Use of the individual antisera gave only the respective colour, and blending was observed indicative of coexpression. Little or no fluorescence was observed with prebleed NRS or with trichomonads grown in a low-iron medium (Lehker et al., 1991; Arroyo et al., 1992). In this experiment, fluorescence was examined with an Axiophot II Zeiss epifluorescent microscope.
Comparative immunocytochemistry with the IRS was carried out on trichomonads grown in high- versus low-iron medium. Figure 2 presents a representative experiment showing immunogold labelling with anti-AP65 serum. Similar results were obtained with the other IRS for AP51, AP33 and AP23. Figure 2A(1) shows the high numbers of gold particles detecting AP65 on the trichomonal surface, cytoplasm, vacuoles and flagella as well as in hydrogenosomes (A2) for high-iron-grown organisms. Only minimal labelling for low-iron-grown parasites was found on surface and in hydrogenosomes, respectively (B1 and B2). Neither omission of the primary anti-AP65 antibody nor pre-bleed NRS gave gold particles. Finally, consistent with Fig. 1, colocalization of different-sized gold particles conjugated to the distinct anti-adhesin IgG was observed by IEM. For example, both 5-nm-gold anti-AP65 IgG and 15-nm-gold anti-AP51 IgG were seen on the surface and throughout the different compartments as in part A1. Similar results were obtained both with the other combination of antisera to adhesins and with other fresh clinical isolates of T. vaginalis. These data suggest a role for iron in modulating the location of adhesins within trichomonads.
Fig. 2.
Immuno-electron microscopic detection of AP65 on cryosections on the surface and different sites of high-iron (A1) vs. low-iron grown (B1) trichomonads of Trichomonas vaginalis T068-II. Anti-AP65 serum IgG followed by gold-conjugated goat anti-rabbit IgG was used in this experiment. Large numbers of gold particles were evident within hydrogenosomes of high-iron (A2) parasites. Few particles (arrow) were detectable in the hydrogenosome (H) of low-iron (B2) organisms. Other antisera to adhesins gave similar results. The mAbs F11, F28-1 and DM116 (Fig. 3) to AP65 gave results like those in a2 and b2 in high- and low-iron respectively.
Differences in reactivity between rabbit antiserum and mAbs and generation of a new mAb reactive with the trichomonal surface
It was reported earlier (Engbring and Alderete, 1998b) that the mAb used originally to obtain AP33 (F5) (Arroyo et al., 1995) was unreactive with the surface of high-iron parasites by fluorescence. Furthermore, AP33 was found previously to have two receptor-interactive domains, which were toward the amino terminus relative to the mAb epitope. Similarly, the AP65 mAb F11 was unreactive with the surface of high-iron trichomonads (data not shown). We therefore evaluated by IEM the mAbs F5 and F11 and found immunogold localized only in hydrogenosomes as in Fig. 2A(2). Decreased numbers of gold particles were seen in hydrogenosomes for low-iron organisms as in Fig. 2B(2). At this stage, we hypothesized that the proteins outside the hydrogenosome had the mAb epitopes inaccessible for mAb recognition.
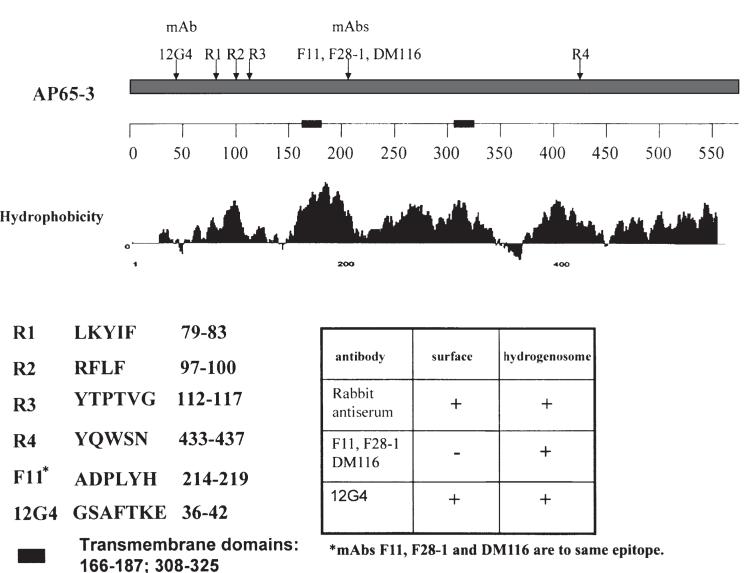
Therefore, as was done for AP33 (Engbring and Alderete, 1998b), we characterized the IRS and mAb epitopes for AP65. Figure 3 shows the results from using overlapping decapeptides obtained for AP65 that revealed three strongly reactive IRS epitopes at the amino terminus (R1, 79-LKYIF; R2, 97-RFLF; and R3, 112-YTPTVG) and a weakly reactive epitope at the carboxy terminus (R4, 433-YQWSN). The IgG1 mAb F11 and two new IgG1 mAbs, F28-1, and DM116 generated from distinct mAb libraries recognized the same epitope (214-ADPLYH). Based on this information and that obtained for AP33 that subclones of the amino terminus had receptor-binding activity (Arroyo et al., 1995), we hypothesized that a mAb reacting near the rabbit antiserum epitopes would lead to detection of the parasite surface. Because we were unsuccessful in three attempts to get mAbs to the NH4-terminal region, we resorted to a LamB-AP65 fusion using a subclone comprised of the first one-third of ap65-3 (O’Brien et al., 1996). Selection of a new mAb was based on both immunoblot detection of the LamB-AP65 recombinant and enzyme-linked immunoabsorbance assay (ELISA) reactivity to whole organisms (see Experimental procedures). The new IgG1 mAb, 12G4, was obtained, which recognized the 36-GSAFTKE epitope. Importantly, the AP65 mAb epitopes were in all ap65 family members. The blast database revealed some identity to other malic enzyme sequences. Further, the amino acid sequences 166-187 and 308-325 were identified as two strong transmembrane helices by expasy proteomics tools™ and suggested the N- and C-termini were extracellular domains. It is notable that this model is consistent with the presence of the rabbit anti-AP65 and mAb 12G4-reactive epitopes.
Fig. 3.
Identification of epitopes using overlapping decapeptides obtained for AP65-3 as described in the Experimental procedures section. The IgG1 mAb F11 and two new IgG1 mAbs, F28-1, and DM116 generated from distinct mAb libraries recognized the same epitope. A LamB-AP65 fusion protein used for immunization of mice resulted in generation of mAb 12G4 toward an epitope at the amino terminus of the clustered rabbit epitopes. The sequences of the epitopes are included on the left of the insert table, which presents the combined fluorescence and immunogold labelling results of separate experiments. The new mAb reacted with the parasite surface as shown in Fig. 4. Two transmembrane domains are represented on the numbered scale by bold, solid bars, and the amino acid numbers are indicated.
Finally, we performed fluorescence with the new mAb 12G4. Figure 4A(1) shows the surface detection with the new mAb 12G4 of AP65 in high-iron, non-permeabilized parasites. The mAb F11 reacted with the protein only in permeabilized organisms (B3). As another control, mAb L64 toward a cytoplasmic protein (Alderete et al., 1987) also was reactive only in permeabilized parasites (B5).
Fig. 4.
Fluorescence experiments showing detection of AP65 on the surface of non-permeabilized (A) and intracellularly of permeabilized (B) trichomonads of isolate T016 with mAb 12G4 characterized in Fig. 3. The mAbs F11 and L64 to AP65 and to an intracellular protein (Alderete et al., 1987), respectively, were immunoreactive only with permeabilized organisms (B3 and B5). No signal was evident with non-permeabilized trichomonads (A3 and A5). Brightfield photomicrographs of the same fields accompany the fluorescence pictures.
All adhesin family members reside on the parasite surface
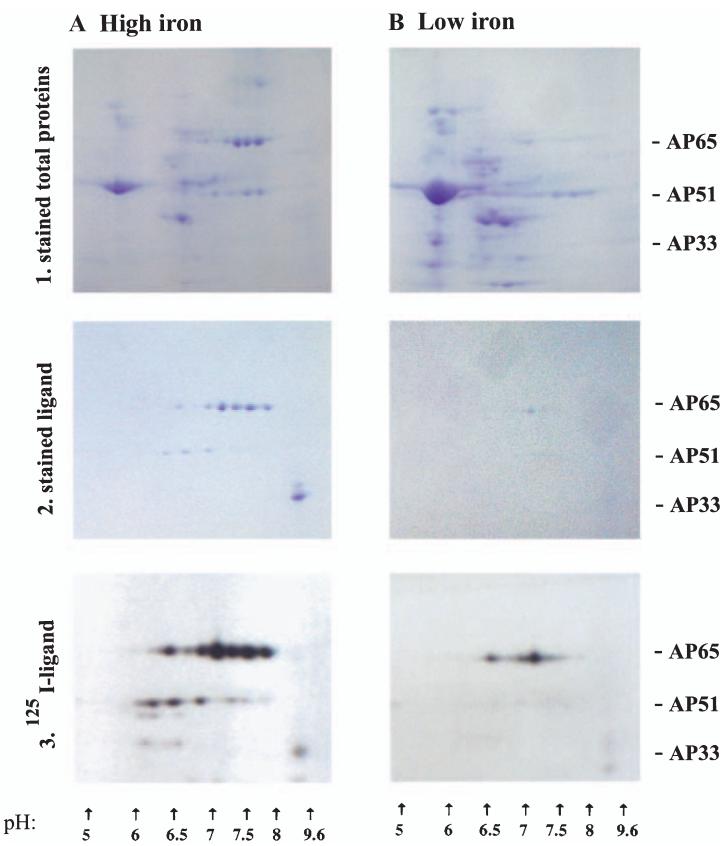
We next wanted to determine whether all members of the gene families were on the surface. Extracts of total proteins of surface-iodinated isolate T016 trichomonads, used originally to characterize the adhesins (Arroyo et al., 1992), were analysed by two-dimensional SDS-PAGE and autoradiography. Duplicate samples were subjected to a ligand assay to identify adhesins followed by two-dimensional analysis (two-dimensional ligand). Figure 5 shows the stained and autoradiography protein patterns of HeLa cell-binding proteins using extracts of high-iron trichomonads, and as can be seen, stained (A2) and iodinated (A3) patterns of proteins were similar. Six iodinated spots were readily detectable for AP65, and three spots were seen for AP51, consistent with the respective gene copy numbers (Lahti et al., 1992; Hrdyý and Müller, 1995; O’Brien et al., 1996; Alderete et al., 1998). The pIs of the stained and iodinated spots were consistent with those predicted based on the complete amino acid sequences of the proteins (Alderete et al., 1995; Hrdyý and Müller, 1995; Drmota et al., 1996; O’Brien et al., 1996; Alderete et al., 1998). Only one spot was evident for AP33 because the three AP33 proteins have almost identical pIs (Engbring and Alderete, 1998b) and due to the poor resolution of basic proteins in two-dimensional gels (Hochstrasser et al., 1988). Low-iron trichomonads had decreased amounts of the iodinated HeLa cell-binding proteins in stained gels (B2) and gave only one prominent AP65 spot in autoradiograms (B3). The different two-dimensional stained total protein patterns confirmed the high (A1) versus low-iron (B1) status of trichomonads (Lehker et al., 1991). Lastly, it should be noted that AP65 in stained gels and autoradiograms was quantitatively greater than the other proteins. AP23 was not readily visualized by two-dimensional analysis, possibly due to the low copy number of this protein (Alderete and Garza, 1985; Lehker et al., 1991). These data suggest that all members of the multigene families are on the surface and bind to cells.
Fig. 5.
Combined ligand assay with two-dimensional SDS-PAGE confirms the surface residence of the adhesin family members. Isoelectric focusing separated the total proteins or proteins from the ligand assay in a pH 3-10 ampholine gradient followed by electrophoreses and staining or autoradiography. Part 1. Coomassie brilliant blue stained total protein patterns of the duplicate samples of surface-iodinated organisms used in Parts 2 and 3 below. Trichomonads were grown in high- (A) and low-iron medium (B). The complex patterns of proteins are representative of equivalent cell numbers treated identically, and the different patterns are indicative of the iron status of trichomonads. Part 2. Stained patterns of proteins following the ligand assay, which enriches for the host cell-binding proteins of extracts of parasites grown in high (A) and low-iron medium (B). The same number of cell equivalents was used in the ligand assays. Part 3. Autoradiogram patterns of the stained gels in Part 2 showing iodinated protein spots of the two-dimensional gels. The X-ray film was exposed 8 h for both samples handled identically. It is noteworthy that immunoblots of duplicate samples of the stained gels of part 2A were performed comparing the rabbit antisera to the adhesins with mAbs F11 (AP65) and F5 (AP33). Not unexpectedly based on results recently published for AP33 (Engbring and Alderete, 1998a), the patterns of antibody reactivities were identical with those seen here for AP65 and AP33.
Furthermore, hydrogenosome organelles were purified from samples of surface-iodinated trichomonads used above in Fig. 5A. Identical cell equivalents of iodinated extracts of total proteins and purified hydrogenosomes were then used in the ligand assay. Figure 6 presents results of a representative experiment showing the complete absence of iodinated spots using hydrogenosomes (B2) compared with total proteins (A2) in autoradiograms exposed to X-ray film for the same time period. No spots in autoradiograms of B2 were visible despite exposure for longer periods. Parts A1 and B1 present immunoblots using as probes the mAbs 12G4 to AP65 and F5 to AP33. The corresponding proteins were apparent, albeit quantitatively less amounts of proteins were detected in the ligand assay using hydrogenosomes. The inserts in A1 and B1 illustrate the stained two-dimensional protein patterns of the duplicate gels showing again the presence of proteins with the same mobilities and pIs. As a control to confirm purification of organelles, the activity of hydrogenosome pyruvate/ferredoxin oxidoreductase was determined (Peterson and Alderete, 1984). In separate experiments, the hydrogenosome fractions had three- to fivefold higher specific activity compared with total extract, showing the enrichment for hydrogenosomes in the procedure (data not shown). These results confirm the specificity in our surface labelling procedure.
Fig. 6.
Comparison of two-dimensional patterns of total proteins (A) versus hydrogenosome proteins (B) of surface-iodinated T. vaginalis used in the ligand assay. Immunoblots of proteins after the ligand assay probed with mAbs F11 (AP65) and F5 (AP33) show quantitatively greater amounts of proteins in the total protein extracts (A1) compared with hydrogenosome extracts (B1). Inserts on bottom left of A1 and B1 are the Coomassie brilliant blue stained two-dimensional gels. The multiple bands for AP65 and AP51 are readily visible. The spot with a high pI at the lower right side corresponds to AP33, as seen in Fig. 5. Parts A2 and B2 present autoradiograms of gels of iodinated proteins of total trichomonad and hydrogenosome (B2) extracts, respectively, used in the ligand assay. No labelled proteins were evident for the hydrogenosome two-dimensional patterns even with prolonged exposure to X-ray film.
Lastly, as we already reported on the monospecific nature of anti-AP33 serum (Engbring and Alderete, 1998a), we took advantage of the two-dimensional-ligand assay to examine the polyclonal, monospecific nature of anti-AP65 and anti-AP51 sera. We obtained identical immunoblot reactions using rabbit anti-AP65 serum and the mAbs analysed above (Fig. 3), and both IRS for AP65 and AP51 reacted only with the spots in the two-dimensional gels in Fig. 5A(2), as no additional prominent spots were detected anywhere in the stained gels and blots. As another control to show specificity of the interaction of adhesins with HeLa cells, the mAb L64 to a cytoplasmic protein used above was unreactive in the two-dimensional ligand assay blots. These data show that the rabbit antisera are most likely monospecific.
Contact with VECs increases surface expression of adhesins
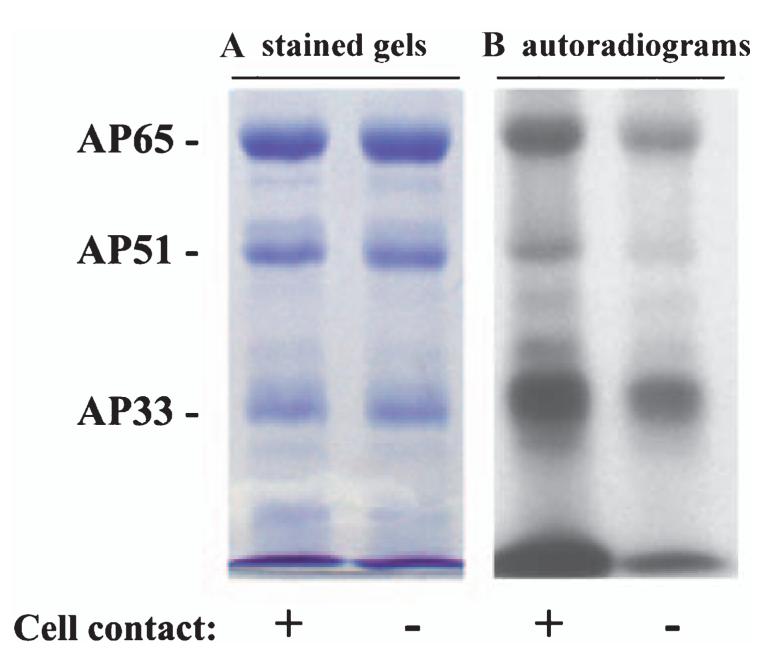
During the course of our studies, we noticed that contact of trichomonads with VECs increased amounts of surface adhesins. Trichomonads were either placed in contact with fixed immortalized cultures of AO or MS74 VECs for 30 min or suspended in the interaction medium without host cells for the same period. Parasites were removed and immediately iodinated for use in a ligand assay. Figure 7 shows that although the intensities of bands of cell-binding proteins were similar, greater amounts of AP65, AP51 and AP33 bands on autoradiograms resided on organisms after contact with host cells.
Fig. 7.
Contact with host cells shows increased amounts of surface-expressed adhesins. In this experiment, high iron trichomonads were first incubated with monolayers of immortalized AO VECs before removing and iodinating (see Experimental procedures). Part A shows the same amount of proteins enriched by the ligand assay, as evidenced by the equal intensity of Coomassie brilliant blue stained gels. Autoradiograms in part B illustrate a greater intensity of signal of the surface-labelled proteins of trichomonads after contact with the host cells. Equivalent parasite numbers were used in both samples, which were handled identically. Identical results were obtained with MS74 VECs.
Relationship between levels of adherence and amounts of adhesins binding to immortalized vaginal and ureter epithelial cells
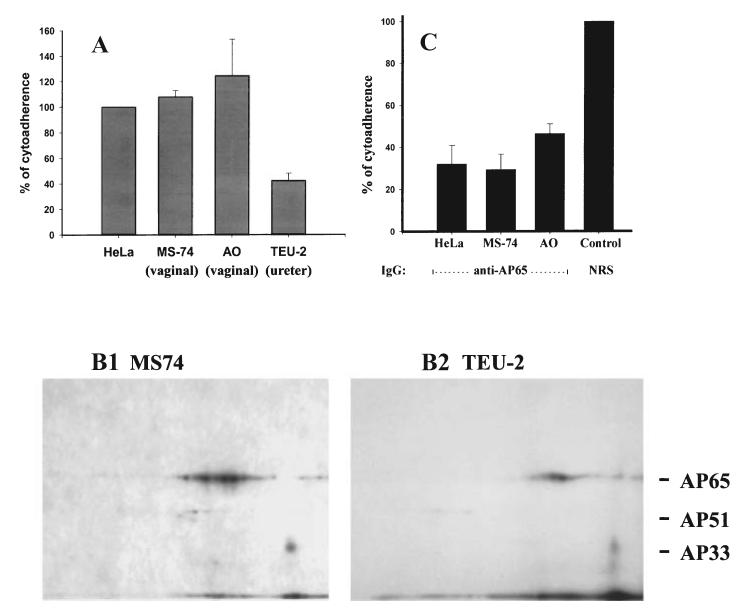
A recent report suggested non-specific binding of the adhesins to different host cells and bacteria (Addis et al., 2000). We therefore took advantage of the availability of immortalized VEC lines (MS74 and AO) and an immortalized ureter epithelial cell line (TEU-2) to examine the association of levels of adherence of T. vaginalis T016 organisms in relation to the amounts of adhesins bound to the cell surfaces in the two-dimensional ligand assay. Figure 8A shows results from five separate experiments and normalized for adherence to HeLa cells. Trichomonads reproducibly adhered to slightly higher levels to immortalized VECs compared with HeLa cells. Interestingly, TEU-2 ureter cells accommodated T. vaginalis organisms to ≈ 40% of that seen for HeLa cells and the VECs. Further, the two-dimensional ligand assay using extracts of surface-labelled parasites showed that immortalized MS74 VECs, HeLa and AO (data not shown) cells had increased amounts of bound adhesins as seen in Fig. 8B(1) compared to the TEU-2 ureter cells (B2). The two-dimensional ligand assay patterns strongly support the notion of a direct relationship between the levels of adherence and amounts of adhesins bound by cells. Furthermore, it has been demonstrated before that AP65 is a major adhesin for HeLa cells (Arroyo et al., 1993), and as AP65 is a major binding protein for these immortalized VECs (B1), we wanted to confirm a role for AP65 with these cells. Importantly, anti-AP65 IgG eluted from preparative blots of AP65 inhibited by up to 70% the adherence of trichomonads to the MS74 (part c), affirming a role in adherence for this protein as shown previously (Arroyo et al., 1993).
Fig. 8.
Associations between levels of adherence of T. vaginalis T016 with amounts of adhesins bound to host cell surfaces. Part A, a cytoadherence assay was performed with quadruplicate samples comparing HeLa cells with immortalized VECs (MS74 and AO) and a immortalized ureter epithelial cell line (TEU-2). Overnight cultures of the confluent monolayers of cells were incubated with equal numbers of highly motile and radiolabelled mid-logarithmic phase trichomonads (see Experimental procedures). All samples were handled identically. The adherence experiment was performed on five different occasions and with similar results. The mean of the results for each cell type was compared with that for HeLa cells, which were normalized to be 100%. Approximately 40% of parasites in the suspension were adherent for HeLa cells. Part B. Autoradiogram two-dimensional patterns showing the amounts of iodinated adhesins from an extract of surface-labelled parasites bound to the cell surfaces by the ligand assay. MS74 (B1), HeLa cells and AO VECs had increased amounts of bound adhesins compared with the TEU-2 ureter cells (B2). In this experiment, the two-dimensional ligand assay was performed identically using duplicate samples of the radioiodinated trichomonad proteins and fixed host cells. X-ray film was exposed for an identical period of time to the acrylamide gels. Part C. Inhibition of adherence was demonstrated with purified anti-AP65 IgG eluted from preparative blots of AP65 (Arroyo et al., 1993). Washed radiolabelled organisms were first pretreated with antibody before addition to the cell monolayers. The level of adherence achieved for control prebleed normal rabbit serum (NRS) IgG was similar to that obtained in a normal adherence assay using HeLa cells, and this was normalized to 100%.
The drug-resistant MR100 trichomonads lacking adhesins do not adhere
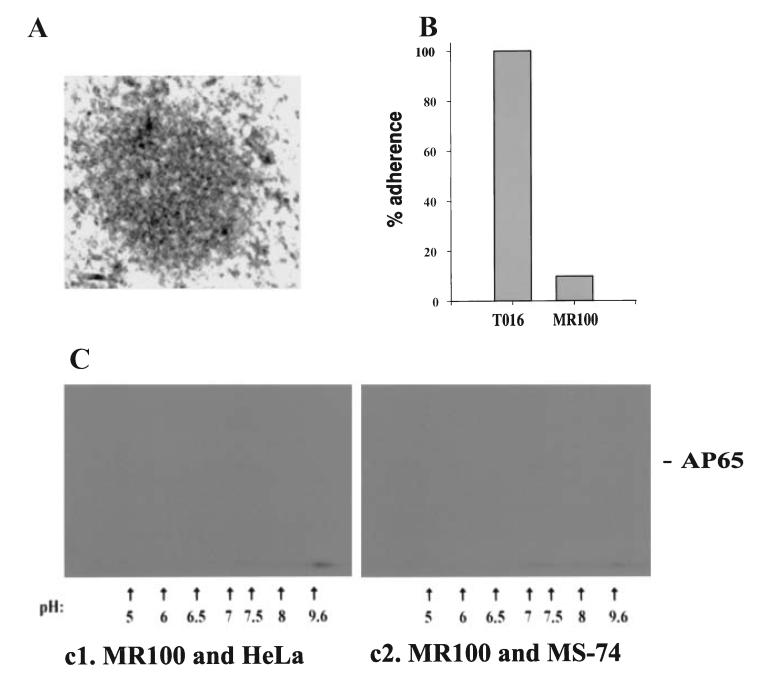
MR100 is a drug-resistant isolate lacking adhesins detectable by immunoblot of ligand assay proteins. IEM using IRS to the adhesins showed background levels of gold particles compartmentalized outside the hydrogenosome. Indeed, the organelles lacked the well defined double membrane and had few gold particles within hydrogenosomes (Fig. 9A) even when parasites were grown in high-iron medium (not shown). Furthermore, 9B shows results from a representative adherence experiment with AO VECs using quadruplicate samples that gave less than 2% standard deviation. The association of MR100 trichomonads to VECs was 5% that seen when compared with wild-type T016 trichomonads; similar results of only 1% to 5% levels were obtained in five separate experiments. Finally, autoradiograms of a two-dimensional-ligand assay had no VEC-binding proteins with both HeLa and MS74 cells (C). In this experiment, the X-ray film was exposed for the same period of time as that needed above (Fig. 8B) These data provide strong supporting evidence for a role of the trichomonad adhesin enzymes in adherence.
Fig. 9.
Absence of detectable adhesins and non-adherence of T. vaginalis MR100. Part A. A representative picture showing minimal labelling with gold-conjugated anti-rabbit IgG following treatment of the preparation with IRS to anti-AP65 IgG, as seen in Fig. 2A(2). The absence of the well defined double membrane for hydrogenosomes was apparent. Similar results were seen regardless of the iron status of trichomonads. Part B. A representative adherence assay using AO VECs comparing MR100 with wild-type trichomonads of T. vaginalis isolate T016 (Arroyo et al., 1993). All experiments were in quadruplicate and performed at least five different times. Part C. Autoradiograms showing the lack of any radioiodinated proteins by the two-dimensional ligand assay. This two-dimensional -ligand assay was performed at the same time as the adherence experiment in Fig. 8C. Identical duplicate samples of the radioiodinated MR100 trichomonad proteins were mixed with fixed host cells. Also as above, the X-ray film was exposed for a period of time identical to the positive ligand assay of Fig. 8B(1). Only did prolonged exposure of the X-ray films over several days were some protein spots visualized that migrated in the same area of the adhesins.
Discussion
The surface expression of each protein and colocalization of the proteins on the surface of parasites (Fig. 1) and at sites outside the hydrogenosome (Fig. 2) occurred only in trichomonads grown in a high-iron medium. Specific surface labelling revealed that all members of the multigene families were capable of residing on the parasite surface (Fig. 5). These findings are in accordance with original surface labelling experiments showing the predominance of iodinated bands corresponding with the mobilities of these multigene proteins (Alderete, 1983; Alderete et al., 1986b). Importantly, identical proteins were immunoreactive with both the rabbit antisera and mAbs (to AP65 and AP33), thus eliminating the possibility that the IRS is not monospecific and confirming results from an earlier study (Engbring and Alderete, 1998a).
What is clear is that the detection of the proteins on the surface depends on the experimental conditions. Among important considerations for reproducibility of results are: (i) that adhesins are released into the extracellular environment (Addis et al., 1997); (ii) that surface localization is a function of the high-iron status of the parasite (Fig. 2) (Lehker et al., 1991; Arroyo et al., 1992); and (iii) that regulation by iron is lost among long-term laboratory isolates (Lehker et al., 1991). Indeed, these same variables probably influence the amounts of these surface proteins detected by assays like fluorescence, immunocytochemistry, and surface labelling and autoradiography. These facts may help clarify conflicting findings by others and us, like the inability to visualize the AP51 and AP33 adhesin enzymes outside hydrogenosomes (Brugerolle et al., 2000). Equally noteworthy was a report with T. vaginalis and antisera to decarboxylating malic enzyme (Drmota et al., 1996), which detected the protein outside the hydrogenosome using immunogold labelling. In an unrelated report examining polyamine metabolism of Tritrichomonas foetus, the cattle trichomonad responsible for fetal wastage, immuno-electron microscopy with antibody to β-SCS showed gold particles outside the hydrogenosome, including the cytoplasm and vacuoles, suggesting the ability of this protein to be compartmentalized outside hydrogenosomes by the bovine trichomonad (Reis et al., 1999), possibly only under certain conditions. Furthermore, a recent report described iron-induced expression of hydrogenosomal proteins in Tt. foetus (Vanacova et al., 2001). The decarboxylating malic enzyme (AP65) and isoforms of SCS (AP51 and AP33) were among the proteins upregulated in Tt. foetus by high iron. Given that for T. vaginalis the adhesins are also hydrogenosome enzymes then, to our knowledge, our laboratory was the first to show that iron mediated regulation of these proteins of metabolism in trichomonads (Alderete et al., 1995; 1998; O’Brien et al., 1996; Engbring and Alderete, 1998a, b). These findings show the important role that iron plays in signalling for both transcription and protein compartmentalization within trichomonads.
Original cDNA clones and subclones expressing recombinant adhesin proteins localized the receptor-interactive domains of the adhesins at the amino terminus (Arroyo et al., 1995). Characterization of AP33 confirmed a receptor-binding epitope within the NH3-terminal region (Engbring and Alderete, 1998b). Interestingly, the F5 mAb to AP33 detected proteins only within hydrogenosomes, and the epitope was toward the carboxy terminus of the protein. We identified the epitopes reactive with the IRS and mAbs to AP65. It is remarkable that different fusions resulted in three mAbs to the identical epitope (214-ADPLYH) within AP65, which was distant from the three clustered epitopes toward the amino terminus seen by IRS (Fig. 3). Because of our lack of success at generating new mAbs at the amino terminus, we resorted to creating a LamB-AP65 chimeric protein with the first one-third of AP65-3 (O’Brien et al., 1996). The mAb 12G4 recognized the parasite surface but did not inhibit adherence, suggesting that the 12G4 epitope is not in juxtaposition with the receptor-binding domain possibly defined by the clustered rabbit epitopes, a situation similar to that seen for AP33 (Engbring and Alderete, 1998b). It is highly likely that the environment outside the hydrogenosome is very different from that found inside the organelle. This could result in an altered conformation of the protein with the epitope of mAb F11 inaccessible for antibody binding. Alternatively, the protein outside the hydrogenosome may be associated with other proteins, such as chaperones, thus masking the epitope. It is equally conceivable that the epitope of mAb F11 is modified, such as through phosphorylation of the tyrosine residue, something that awaits experimental verification.
Hydrogenosomal proteins possess a short N-terminal leader sequence for targeting and translocation into the organelle (Bradley et al., 1997). Whether the same or different sequences are required for partitioning to other sites must be considered in light of these results. If additional signals within the proteins are involved in targeting of the adhesin enzymes to different places, then the identification of other essential sequence-specific regions will help our understanding of protein segregation in this and possibly other protists. It is equally reasonable to hypothesize that iron induces synthesis of factors that act as chaperones for the adhesins to the different compartments. Alternatively, mRNAs might contain zipcodes, different from signal sequences, which determine localization within the organism that the transcript is translated (Kislauskis and Singer, 1992; Smalheiser, 1996). This phenomenon has been documented for the mRNAs of actin, vimentin and tubulin.
This report also shows a direct relationship between levels of adherence to immortalized VECs and ureter epithelial cells and amounts of adhesins bound to the cells (Fig. 8). We have shown previously that the binding of adhesins to the surface of host cells is specific. The criteria include saturation binding kinetics, competition experiments with the natural adhesins and a peptide for AP33, and demonstration of proteins on host cells accommodating the adhesins (Alderete and Garza, 1985; Alderete et al., 1988; Lehker et al., 1991; Arroyo et al., 1992; Engbring and Alderete, 1998a, b; O’Brien et al., 1996). Specific antibody inhibition of adherence was established, and, again, that anti-AP65 IgG inhibited adherence to immortalized VECs (Fig. 8C) reaffirms a role for adhesins in adherence. Further, adherence levels of isolate trichomonads correlated directly with the amounts of adhesins on the surface (Alderete and Garza, 1985; Arroyo et al., 1992). Finally, the absence of other cytoplasmic proteins binding to host cells provides evidence for the specific interaction of AP65, AP51 AP33, and AP23 with host cells. We believe that the results obtained by Addis et al. (2000) are in part compatible with our observations. For example, T. vaginalis organisms are capable of associating with different host cells to varying degrees, and one hypothesis to explain this may be the precedence regarding proteins that bind to different ligands through conserved receptor-interactive domains, which may bind to different receptors (Chen et al., 2000). Such common structures may reside on the surfaces of distinct target cells and therefore adhesins associating with different host cells may still occur through specific mechanisms, as discussed above. We also recognize that the hydrophobic nature of the trichomonad adhesins (Fig. 3) (Engbring and Alderete, 1998b) requires that the criteria of binding specificity, as discussed above, must be demonstrated with the different cells, bacteria and erythrocytes, and this was not evident in the report of Addis et al. (2000). Finally, the use of identical methods must be emphasized. It is noteworthy that we identified trichomonad surface proteins, which are distinct from adhesins, associating with erythrocytes (Lehker et al., 1990). Therefore, as reported by us (Millsap and Alderete, 2001), we believe that our results continue to provide evidence to reinforce the idea that the adhesins are involved in mediating cytoadherence.
AP65 is found in the supernatants of viable organisms (Addis et al., 1997), along with numerous other trichomonad proteins (Alderete and Garza, 1984; Addis et al., 1997). It is not inconceivable that the extracellular AP65 protein (Addis et al., 1997) associates with the parasite surface thereby acquiring adhesive function. However, it is noteworthy that a recent report using the same antibodies toward adhesins as used here found that the proteins were linked with the secretory pathway involving the Golgi apparatus (Benchimol et al., 2001). Clearly, many factors need to be taken into account in the study of adhesin-cell surface interactions.
Although we reported on the increased synthesis of adhesins upon trichomonal binding to host cells, data presented here show that contact with immortalized VECs by high-iron trichomonads optimally synthesizing the adhesins resulted in greater amounts of the adhesins on the parasite surface (Fig. 7). This finding may be significant in that existing intracellular adhesins may not be available for function until an adherence signal is received. This strategy would avoid accumulation of extracellular protein binding to cell surfaces, which would negate adherence through competition with available host cell receptors.
It was not surprising that MR100 was non-adherent (Fig. 9). MR100 is absent several hydrogenosome enzymes, including pyruvate:ferredoxin oxidoreductase and decarboxylating malic enzyme (AP65), both essential for optimal drug activation (Kulda, 1999). In our hands by immunocytochemical, immunoblot and ligand assays, T. vaginalis MR100 had little, if any, detectable decarboxylating malic enzyme and SCS. Based on what is known for Tt. foetus MR100, the parasites are unable to efficiently transcribe the genes for these and other hydrogenosome proteins (Land et al., 2001), and T. vaginalis may possess similar properties. The use of MR100 trichomonads therefore provides strong evidence for functional diversity of the hydrogenosome proteins.
In conclusion, trichomonads are part of a growing list of microbial pathogens that possess surface-associated enzymes with alternative, non-enzymatic functions (Alderete et al., 2001). With respect to the property adherence, what is clear is that for T. vaginalis this is multifaceted. Early reports showed the interaction of trichomonads with laminin of basement membranes (Costa e Silva-Filho et al., 1988) and serum components (Gold, 1992). More recently, the parasite was shown to specifically bind to mucin (Lehker and Sweeney, 1999), and the complex high affinity to associations with fibronectin and laminin were demonstrated (Crouch and Alderete, 1999; Crouch et al., 2001). What is clear is that this ancient protist has evolved to take advantage of a limited genome to be able to successfully parasitize the constantly changing and nutrient-limiting urogenital region of women and, in part, this is done through functional diversity of proteins.
Experimental procedures
Parasites and surface labelling
Batch cultivation of fresh clinical Trichomonas vaginalis isolates T016, 347 and T068-II (Arroyo et al., 1992; 1993; Provenzano et al., 1997; Crouch and Alderete, 1999; Crouch et al., 2001) was in trypticase-yeast extract-maltose (TYM) medium with 5% serum (Diamond, 1957). High- and low-iron-grown parasites were obtained by growing organisms in iron-replete medium and in medium depleted of iron with 2,2-dipyridyl (Sigma), as before (Lehker et al., 1991; Crouch et al., 2001). T. vaginalis MR100 was kindly provided by Dr Jaroslav Kulda of the Charles University of the Czech Republic and was cultivated in medium in the presence of 100 mg ml-1 of freshly prepared metronidazole (Kulda, 1999). Extrinsic radiolabelling of parasites was performed by the chloramine T procedure, as before (Garvey et al., 1977; Alderete, 1983) and with solid phase Iodogen (Pierce) (Greenwood et al., 1963), as recommended by the manufacturer. Iodogen was used to expose the organisms to milder reaction conditions and to show the specific surface labelling of parasites. Chloramine T obtained a higher resulting specific radioactivity, but both procedures gave identical results in radiolabelling of proteins as evaluated by assays described below.
Construction of the recombinant plasmid and expression of recombinant LamB-AP65
Escherichia coli pop6510 (thr leu thi metA lacA dex5 tonA supE recA) (Bouges-Bocquet et al., 1984; Charbit et al., 1988) served as the recipient for the recombinant derived from plasmid pAJC264.5. Oligonucleotide primers (Gibco) were prepared to the first third of AP65-3 (Fig. 3). The sense primer AP65-F-13 5′-GGGGAGATCTGATGCTCGCATCT TCAGTCG-3′ and the antisense primer AP65-R-399 5′-GGGGAGATCTGACTGGCGGTGTGTAGCCCA-3′ were used to create recombinants that span the protein with a BglII site or a BamHI site for insertion into position 153/154 of the amino acid sequence of the LamB protein via a unique BamHI site in the plasmid (Boulain et al., 1986; Wang et al., 1989). Plasmid was electroporated into E. coli pop6510 (charging voltage 1.8 kV, 186 ohm, 2.5 kV), and transformants were selected for ampicillin resistance. Correct orientation of the inserts was verified by polymerase chain reaction (PCR) using primers external and internal to the insert. Restriction fragments were purified with QiaQuick PCR Purification (Qiagen). Plasmid pAJC264.5 and recombinant plasmids were isolated using the Spin Miniprep Kit (Qiagen). The restriction enzymes and T4 ligase were used as per manufacturer protocols. The LamB gene controlled by the tac promoter was induced by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). A stationary preculture or recombinant E. coli in Luria-Bertani (LB) broth supplemented with ampicillin was diluted 1:100 in fresh LB medium with ampicillin and grown in a shaking incubator for 3 h at 37°C. IPTG was added to the bacteria in logarithmic phase, and the culture was incubated for another 3 h.
Production of LamB and LamB-AP65 antisera and AP65 mAb 12G4
Anti-LamB serum needed to monitor the construction and synthesis of recombinant LamB-AP65 protein was generated. Briefly, 50 ml of E. coli was cultured as above for expression of the recombinant LamB and LamB-AP65 proteins. The bacteria were harvested by centrifugation at 5900 g for 5 min at 4°C and washed once on 50 mM Tris-HCl, pH 8.0. The bacteria were resuspended in 5 ml resuspending buffer (20% sucrose and 100 mM Tris-HCl, pH 8.0) to which 0.1 ml 500 mM EDTA and 0.2 ml of 10 mg ml-1 of lysozyme was added. After incubating for 15 min at 37°C, 5 ml of extraction buffer (2% Triton X-100, 10 mM MgCl2, 50 mM Tris-HCl, pH 8.0) was added to the suspension and incubated for a further 30 min. The membrane fraction was collected by centrifugation at 2500 g for 10 min at 4°C, resuspended in 0.3 ml of electrophoresis sample buffer (Laemmli, 1970) and boiled for 5 min. The solubilized protein was separated on a preparative SDS-PAGE using 7% acrylamide gels. A strip of the gel was stained with Coomassie blue to visualize the LamB or LamB-AP65 protein, and the corresponding region of the acrylamide gel was cut out and eluted in 6 ml of water at 4°C for 18 h on a rocker platform. The eluted protein was concentrated, and the protein concentration estimated by SDS-PAGE using bovine serum albumin (BSA) as a standard. Finally, mice previously bled for preimmune sera that had no detectable LamB or AP65 antibody were immunized at bimonthly intervals with antigen in Freund’s complete adjuvant once followed by antigen in Freund’s incomplete adjuvant. Sera from mice were tested for antibody by immunoblotting the LamB or chimeric protein onto nitrocellulose. Positive controls to monitor production of the fusion protein included using both the anti-LamB and the rabbit anti-AP65 serum (IRS). Spleen cells from the LamB-AP65 immunized mice that had demonstrable anti-AP65 antibodies were used for production of hybridoma cells.
Hybridomas producing mAbs to the LamB:AP65-3 chimeric protein were produced using standard protocols, as described before for T. vaginalis (Alderete et al., 1986a). The mAb 12G4 was obtained by screening supernatants of antibody-producing hybridomas with different antigen preparations and using anti-mouse Ig followed by alkaline phosphatase conjugated goat anti-mouse IgG. The supernatants were tested by a whole-cell ELISA in which trichomonads were fixed to microtitre well plates to insure identification of surface-reactive mAb as before (Alderete, 1984). In addition, supernatants with antibody were tested on the LamB:AP65-3 nitrocellulose blots. A positive hybridoma by both assays resulted in limiting dilution and isolation of a new IgG1 mAb 12G4. Negative controls included using supernatant from the myeloma cells used for producing hybridomas or using only secondary antibody.
Immunofluorescence of parasites with mAb 12G4
First, 50 ml of highly motile T. vaginalis isolate T016 trichomonads grown in high iron-medium were washed once in phosphate-buffered saline (PBS) and fixed in 4% paraform-aldehyde and 0.25% glutaraldehyde for 1 h at RT. Then, cells were washed once in PBS and treated twice each for 5 min in 1 mg ml-1 of NaBH4 in PBS, pH 8.0, to reduce autofluorescence. Finally, fixed organisms were washed in PBS and resuspended in 10 ml of blocking solution (PBS containing 1% BSA and 50 mM glycine) for 1 h at RT on a rocker or stored at 4°C. Then, 1 × 106 parasites washed in PBS containing 1% horse serum were permeabilized by adding 250 μl of cold 70% ethanol and incubated for 5 min at 20°C. The cells were then resuspended in 200 μl of the mAb 12G4 diluted 1:100, F11 diluted 1:1, and L64 diluted 1:1 (all dilutions in PBS-1% horse serum) for shaking overnight at 4°C. Cells were washed in PBS-1% horse serum and resuspended in 200 μl of FITC-conjugated anti-mouse IgG (Sigma) diluted 1:100 and incubated for 3 h at RT. Finally, cells were washed in PBS and resuspended in 100 μl of PBS to be observed under the microscope at 1000× magnification. The mAbs F11 and L64 have been used before (Alderete et al., 1987; Arroyo et al., 1995; O’Brien et al., 1996; Engbring and Alderete, 1998b). As positive controls, trichomonads of isolates T068-II and 347 (Alderete et al., 1986b) that express the protein P270 on the surface were incubated for 2 h with a 1:5 dilution of hybridoma supernatant containing mAb C20A3 (IgG2a). Parasites treated identically with control hybridoma supernatant or in the absence of primary antibody were negative controls. The mAb L64 (IgG3) is to an intracellular protein of T. vaginalis (Alderete et al., 1987). In these experiments, and below, fluorescence experiments were repeated no less than four times and with duplicate samples.
Fluorescence and colocalization of adhesins with antiserum
Parasites were processed as detailed recently (Crouch and Alderete, 2001). Briefly, late logarithmic phase organisms grown o/n were pelleted and washed in prewarmed PHEM (50 mM MgCl2, 70 mM KCl, 10 mM EGTA, 20 mM Hepes, and 60 mM Pipes) buffer, pH 6.8, and fixed for 2 h with 4% paraformaldehyde freshly prepared in 100 mM sodium cacodylate buffer, pH 7.2. Then, parasites were bound to poly l-lysine-coated cover slips for treatment with IRS generated to the adhesins (Arroyo et al., 1992). After washing, trichomonads were treated for 1 h with either rhodamineconjugated goat anti-rabbit antibody. For double-labelling experiments, individual IRS IgG with either rhodamine- or fluorescein-conjugated was used for labelling. The cells were then washed in PBS and examined with an Axiophot II Zeiss microscope equipped with UV epifluorescence. Images were acquired with a Hamamatsu chilled CCD camera C5985. Colour pictures show a computer-generated green background with blue (fluorescein) and red (rhodamine) antibody reactivities (Fig. 1). Under no circumstances was non-specific fluorescence observed with prebleed control rabbit serum as controls.
SPOTs analysis
The antibody binding epitopes of AP65 for both mAb and IRS were determined by SPOTs™ membrane prepared with decapeptides offset by one amino acid by Sigma-Genosys. The detection system was changed from a colorimetric to a chemiluminescence assay using the ECL + Plus™ method (Amersham Pharmacia Biotech). Primary and secondary antibody dilutions were optimized as follows: 1:3000 for IRS, 1:100 for mAb 12G4, 1:1 for F11 and 1:10 000 for the corresponding secondary antibodies. Prebleed serum and either control myeloma supernatant or irrelevant mAbs were negative controls used in identical conditions. The membrane was blocked with 5% milk with 0.1% Tween-20 in TBS (20 mM Tris base and 137 mM NaCl, pH 7.6) for 1 h at RT. Then, the membrane was quick-washed twice for 2 min, followed by incubation in primary antibody for 1 h at RT. The membrane was then washed for 15 min followed by three each 5 min fresh rinses with high salt TBS with 0.1% Tween-20 (T-TBS). Peroxidase-conjugated anti-goat or anti-rabbit IgG was added and the membrane was incubated for an additional 1 h at RT. The membrane was washed as before in high salt T-TBS followed by developing the membrane by the ECL + Plus™ protocol and Hyperfilm ECL (Amersham). Intensely reacting decapeptides were overlapped to obtain the epitope sequence.
Immuno-electron microscopy (IEM) detection of surface proteins
Immuno-electron microscopy experiments involving immunogold labelling were performed no less than three times on numerous samples, and immuno-cytochemical protocols were recently detailed (Alderete et al., 2001; Crouch et al., 2001). Briefly, parasites washed three times in PHEM buffer (50 mM MgCl2, 70 mM KCl, 10 mM EGTA, 20 mM Hepes, 60 mM Pipes, pH 6.8) at 37°C were fixed o/n at RT in 100 mM sodium cacodylate buffer containing 0.4% glutaraldehyde, 4% paraformaldehyde, 0.5% sucrose, 1% picric acid, and 2 mM CaCl2. Fixed organisms were then washed three times over a 15 min period and dehydrated in ethanol before embedding in Unicryl (TedPella). Ultrathin sections of trichomonads processed as above were then collected in nickel grids and quenched using 50 mM ammonium chloride with 3% BSA. The organisms were then washed in a PBS-2% BSA buffer, pH 8.0, for 30 min. Grids were then incubated with IRS (1:100 dilution) or, as a positive control for some experiments, with mAb C20A3 to P270, as above. Other mAbs to adhesins described above for fluorescence were also used (Fig. 3). After incubation with antibody for 3 h at RT, the grids were washed before addition of 5 or 10 nm gold-conjugated, goat anti-rabbit or anti-mouse antibody (Sigma). For labelling cells with two distinct adhesin IRS, differentsized (5 nm to 15 nm) gold-conjugated, goat anti-rabbit IgG (Sigma) was added to grids. The grids were finally stained in the dark for 20 min at RT with 5% uranyl acetate followed by lead citrate. Sections were then visualized by transmission electron microscopy (TEM) using a JEOL 1210 model microscope.
Samples were pre-embedded to avoid loss of protein antigen detected by antibody. Parasites washed in warm PBS were fixed for 2 h at RT in 0.1% glutaraldehyde, 4% paraformaldehyde, 0.5% sucrose, 1% picric acid and 2 mM CaCl2 prepared in 100 mM sodium cacodylate buffer. After three washes in PBS, trichomonads were quenched for 30 min using 50 mM ammonium chloride containing 3% BSA followed by incubation with antibody for 3 h at RT under constant stirring conditions. After several washes in PBS over a 30 min period, the organisms were incubated with gold-conjugated secondary goat anti-mouse antibody for 1 h at RT with constant stirring. The cells were then washed three times in PBS followed by re-fixation in 2.5% glutaraldehyde in 100 mM sodium cacodylate buffer and routinely processed for TEM. Post-fixation of parasites was in 1% osmium tetroxide and potassium ferricyanide, after which samples were dehydrated in acetone and embedded in Epon.
Host cells, the adherence assay, and antibody inhibition of adherence
Immortalized human vaginal epithelial cells (MS74 and AO) and ureter epithelial cells (TEU-2) have been used to study adherence by microbial pathogens (Klumpp et al., 2002). Cells were grown in 45% DMEM and 45% keratinocyte-SFM (Life Technologies,) supplemented with 10% fetal bovine serum. Briefly, 2 × 106 cells were inoculated into T75 culture flasks with 25 ml medium. At 90% confluence, cells were harvested by trypsinization and washed with sterile PBS before passage for new o/n cultures. Cells used for adherence and ligand assays were never passaged more than eight times. HeLa cells monolayers were established and passaged for the adherence assay using standard protocols, as before (Arroyo et al., 1992).
For the adherence assay, 200 μl of suspension of 2 × 104 cells were seeded into individual wells of 96-well Stripwell plates (Costar Corning). After o/n incubation at 37°C, the monolayers were washed gently three times with prewarmed PBS followed by fixation with 3% glutaraldehyde (Arroyo et al., 1992) in PBS for 2 h at RT. Cells were then washed five times in PBS and blocked o/n with 500 mM glycine in PBS at 4°C. Before use, the wells were washed five times with PBS.
Trichomonads inoculated for o/n growth in T25 flasks with 10 ml medium were labelled with [3H]-thymidine. After washing three times with cold combined DMEM-TYM medium without serum (2:1, v/v), the parasites were adjusted to a density of 2 × 106 per ml in the same medium mixture. Then, 200 μl of the suspension was added to individual wells. All experiments were conducted using quadruplicate samples and repeated no less than five different times. After 45 min at 37°C, the wells were washed three times with at least an equal volume (200 μl) of medium mixture. The adherent trichomonads were measured by placing individual wells into mini-scintillation vials. The radioactivity was counted in a Beckman LS6500 instrument.
For adherence inhibition experiments performed using quadruplicate samples repeated no less than three separate times, affinity-purified IgG from prebleed NRS and rabbit anti-AP65 serum was prepared in the medium mixture as above at 100 μg ml-1 concentration. An equal volume of antibody was mixed with tritium-labelled trichomonads to give the same number of parasites (4 × 105) in 200 μl added to cell monolayers in microtitre wells as above. After a 10 min period of interaction at 37°C, the 200 μl was added to cells for an additional 45 min at 37°C. The wells were then washed three times in prewarmed medium mixture before measurement of radioactivity.
Ligand assay
The ligand assay for isolation of host cell-binding adhesin proteins was performed as before (Arroyo et al., 1992). Briefly, 106 glutaraldehyde-fixed HeLa cells, immortalized VECs, or immortalized ureter epithelial cells were incubated with a detergent extract derived from 2 × 107 solubilized trichomonads and, after incubation, cells were washed well to remove loosely associated trichomonad proteins. Boiling cells for 3 min in electrophoresis dissolving buffer (Laemmli, 1970) eluted the epithelial cell-binding proteins. These proteins were then analysed by SDS-PAGE and autoradiography. Alternatively, electrophoresed proteins were blotted onto nitrocellulose for immunodetection with antibody (Arroyo et al., 1992). After electrophoresis, duplicate gels were stained with Coomassie brilliant blue with standard protocols.
Isoelectric focusing (IEF), SDS-PAGE and immunoblotting
Isoelectric focusing gels and total protein samples were prepared using standard protocols (Hochstrasser et al., 1988), and attempts were made to diminish carbamylation for artificial results (Bjellqvist et al., 1993; Rabilloud, 1999). Adhesins from the ligand assay were prepared for IEF by eluting the adhesins from HeLa cells by addition of 250 μl of sample buffer I (350 mM SDS, 200 mM Tris-HCl and 150 mM DTT), followed by removal of cells by high-speed centrifugation. The eluted proteins were transferred to new tubes followed by addition of 230 μl of ice-cold acetone and incubation at 0°C for 20 min. After centrifugation at 4°C for 30 min, precipitated proteins were dissolved with 125 μl of sample buffer II (100 mM DTT, 4% CHAPS, 9.8 M urea, and 0.2% Bio-lytes pI 3-10 in 0.001% Bromophenol blue) (Hochstrasser et al., 1988). Sample loading and electrofocusing were as recommended by the Protean IEF Cell Instruction Manual (Bio-Rad Laboratories), and the focused gels were used immediately for SDS-PAGE using 10% acrylamide and 4% stacking gels. Proteins were visualized by Coomassie brilliant blue. Duplicate gels were transferred to nitrocellulose for immunoblotting. The mAbs DM116 (anti-AP65) and F5 (anti-AP33) and rabbit antisera to AP65, AP51 and AP33 detected the T. vaginalis proteins, as before (Arroyo et al., 1992).
Hydrogenosome isolation
The hydrogenosomes were isolated using slight modifications of published protocols (Drmota et al., 1996; Morgado Diaz and De Souza, 1997). Trichomonads (1 × 108) were washed twice in PBS followed by a final wash in homogenizing buffer (250 mM sucrose, 20 mM KCl, 10 mM KH2PO4, 5 mM MgCl2, and 20 mM Tris-HCl, pH 7.0). Cells were homogenized at 4°C with 50 strokes in a Thomas size B glass vessel with the Teflon pestle speed at 500 r.p.m. The volume was then adjusted to 10 ml with the same buffer and centrifuged at 1000 g for 10 min. The supernatant was separated and kept on ice while unbroken cells were resuspended and re-homogenized under identical conditions. The combined homogenates were kept on ice until 95% or greater cell lysis was achieved. The homogenate was transferred to a COREX glass centrifuge tube and centrifuged to sediment the nuclei and costae at 1500 g for 10 min at 4 °C. The supernatant was then transferred to a new tube for centrifugation at 12 100 g for 10 min. The sediment was then washed once more with homogenization buffer and was highly enriched for hydrogenosomes, which was used immediately for experiments or stored at -70°C.
As a measure of hydrogenosome purification, extracts of T. vaginalis and an equivalent cell number of hydrogenosomes in the preparation were used for measurement of pyruvate-ferredoxin oxidoreductase activity. The assay was a slight modification of that described previously (Peterson and Alderete, 1984). Briefly, the assay solution was 180 mM Na2HPO4 and 120 mM NaH2PO4, pH 7.0, 16 mM methylviologen, 0.04 mM coenzyme A, 570 mM b-mercaptoethanol, and 0.1% Triton X-100. The solution (100 ml) was placed in individual wells of microtitre plates and flushed with deoxygenated nitrogen gas in an anaerobic chamber. Parasites or hydrogenosomes were washed twice with anaerobic phosphate buffer (180 mM Na2HPO4 and 120 mM NaH2PO4, pH 7.0) and introduced into the assay mixture at a concentration equivalent to 5 × 105 parasites per 100 ml. After additional flushing with nitrogen, 50 μl of mineral oil was applied to each well. Finally, 50 μl of 20 mM pyruvate was added. The absorbance at 700 nm wavelength was monitored on a microtitre plate reader.
Nucleotide sequence accession number and data not shown
The nucleotide sequence of ap65-3 presented in Fig. 3 for epitope analysis has been assigned GenBank accession no. U35243. The other two ap65-1 and ap65-2 genes characterized by us (Alderete et al., 1995) have sequence accession nos. of U18346 and U18347. Given the length of the paper, some results were omitted and presented as ’data not shown.’ We will make available such data upon request.
Acknowledgements
This work was supported by Public Health Service grants AI 43940 and AI 45429 from the National Institutes of Health (J.F.A.) and grants from Financiadora de Estudos e Projetos, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, Programa de Núcleos de Excelencia, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior, and Associação Universitária Santa Úrsula (M.B.). The technical assistance of Rívea Cristina Custódio Rodrigues to the MB laboratory was greatly appreciated. The assistance of Anna Lazzell of the Institutional Hybridoma Facility of the UTHSCSA and the preliminary epitope characterizations by Kevin Millsap are acknowledged.
References
- Addis MF, Rappelli P, Cappuccinelli P, Fiori PL. Extracellular release by Trichomonas vaginalis of a NADP-dependent malic enzyme involved in pathogenicity. Microbial Pathogen. 1997;23:55–61. doi: 10.1006/mpat.1996.0128. [DOI] [PubMed] [Google Scholar]
- Addis MF, Rappelli P, Fiori PL. Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins. Infect Immun. 2000;68:4358–4360. doi: 10.1128/iai.68.7.4358-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF. Identification of immunogenic and antibody-binding proteins on the membrane of pathogenic Trichomonas vaginalis. Infect Immun. 1983;40:284–291. doi: 10.1128/iai.40.1.284-291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF. Enzyme-linked immunosorbent assay for detection of antibody to Trichomonas vaginalis: use of whole cells and aqueous extract as antigen. Br J Vener Dis. 1984;60:164–170. doi: 10.1136/sti.60.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF. Iron modulates phenotypic variation and phosphorylation of P270 in double-stranded RNA virus-infected Trichomonas vaginalis. Infect Immun. 1999;67:4298–4302. doi: 10.1128/iai.67.8.4298-4302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Garza GE. Soluble Trichomonas vaginalis antigens in cell-free culture supernatant. Molec Biochem Parasitol. 1984;13:147–158. doi: 10.1016/0166-6851(84)90109-9. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Garza GE. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985;50:701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Suprun-Brown L, Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986a;52:70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Garza GE, Smith J, Spence M. Trichomonas vaginalis: electrophoretic analysis reveals heterogeneity among isolates due to high molecular weight trichomonad proteins. Exp Parasitol. 1986b;61:244–251. doi: 10.1016/0014-4894(86)90158-x. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Demeš P, Gombošova A, Valent M, Janos śka A, Fabusśova M, Kasmala L, Metcalfe EC. Phenotype and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun. 1987;55:1037–1041. doi: 10.1128/iai.55.5.1037-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, Demeš P, Gombošova A, Valent M, Fabuśova M, Janosśka A, Stefanovic J, Arroyo R. Specific parasitism of purified vaginal epithelial cells by Trichomonas vaginalis. Infect Immun. 1988;56:2558–2562. doi: 10.1128/iai.56.10.2558-2562.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete JF, O’Brien JL, Arroyo R, Engbring JA, Musatovova O, Lopez O, et al. Cloning and molecular characterization of two genes encoding adhesion proteins involved in Trichomonas vaginalis cytoadherence. Mol Microbiol. 1995;17:69–83. doi: 10.1111/j.1365-2958.1995.mmi_17010069.x. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Engbring J, Lauriano CM, O’Brien JL. Only two of the Trichomonas vaginalis triplet AP51 adhesins are regulated by iron. Microb Pathogen. 1998;23:1–16. doi: 10.1006/mpat.1997.0167. [DOI] [PubMed] [Google Scholar]
- Alderete JF, Millsap KW, Lehker MW, Benchimol M. Enzymes on microbial pathogens and Trichomonas vaginalis: molecular mimicry and functional diversity. Cell Microbiol. 2001;3:1–13. doi: 10.1046/j.1462-5822.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- Arroyo R, Engbring J, Alderete JF. Molecular basis of host epithelial cell recognition by Trichomonas vaginalis. Mol Microbiol. 1992;6:853–862. doi: 10.1111/j.1365-2958.1992.tb01536.x. [DOI] [PubMed] [Google Scholar]
- Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete JF. Signalling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol Microbiol. 1993;7:299–309. doi: 10.1111/j.1365-2958.1993.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Arroyo R, Engbring J, Nguyen J, Musatovova O, Lopez O, Lauriano C, et al. Characterization of cDNAs encoding adhesin proteins involved in Trichomonas vaginalis cytoadherence. Arch Med Res. 1995;26:361–369. [PubMed] [Google Scholar]
- Beachey EH, Giampapa CS, Abraham SN. Bacterial adherence. Adhesin receptor-mediated attachment of pathogenic bacteria to mucosal surfaces. Am Rev Respir Dis. 1988;138:S45–S48. doi: 10.1164/ajrccm/138.6_Pt_2.S45. [DOI] [PubMed] [Google Scholar]
- Benchimol M, Ribeiro KC, Mariante RM, Alderete JF. Structure and division of the Golgi complex in Trichomonas vaginalis and Tritrichomonas foetus. Eur J Cell Biol. 2001;80:593–607. doi: 10.1078/0171-9335-00191. [DOI] [PubMed] [Google Scholar]
- Bjellqvist B, Sanchez JC, Pasquali C, Ravier F, Paquet N, Frutiger S, et al. Micropreparative two-dimensional electrophoresis allowing the separation of samples containing milligram amounts of proteins. Electrophoresis. 1993;14:1375–1378. doi: 10.1002/elps.11501401212. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B, Villatroyo H, Hofnung M. Linker mutagenesis in the gene of an outer membrane protein of Escherichia coli, LamB. J Cell Biochem. 1984;24:217–228. doi: 10.1002/jcb.240240304. [DOI] [PubMed] [Google Scholar]
- Boulain JC, Charbit A, Hofnung M. Mutagenesis by random linker insertion into the lamb gene of Escherichia coli K12. Mol Gen Genet. 1986;205:339–348. doi: 10.1007/BF00430448. [DOI] [PubMed] [Google Scholar]
- Bradley PJ, Lahti CJ, Plumper E, Johnson PJ. Targeting and translocation of proteins into the hydrogenosome of the protest Trichomonas: similarties with mitochondrial protein import. EMBO J. 1987;16:3484–3493. doi: 10.1093/emboj/16.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugerolle G, Bricheux G, Coffe G. Immunolocalization of two hydrogenosomal enzymes of Trichomonas vaginalis. Parasitol Res. 2000;86:30–35. doi: 10.1007/pl00008503. [DOI] [PubMed] [Google Scholar]
- Cates W. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Trans Dis. 1999;26:S2–S7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- Charbit A, Molla A, Saurin W, Hofnung Versatility of a vector for expressing foreign polypeptides at the surface of Gram-negative bacteria. Gene. 1988;70:181–189. doi: 10.1016/0378-1119(88)90116-3. [DOI] [PubMed] [Google Scholar]
- Chen Q, Heddini A, Barragan A, Fernandez V, Pearce SF, Wahlgren M. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J Exp Med. 2000;192:1–10. doi: 10.1084/jem.192.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa e Silva-Filho F, de Souza W, Lopes JD. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc Natl Acad Sci USA. 1988;85:8042–8046. doi: 10.1073/pnas.85.21.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotch MF, Pastorek JG, I.I., Nugent Yerg DE, Martin DH, Eschenbach DA. Demographic and behavioral predictors of Trichomonas vaginalis infection among pregnant women. Obstet Gynecol. 1991;78:1087–1092. [PubMed] [Google Scholar]
- Cotch MF, Pastorek JG, II, Nugent RP, Hillier SL, Gibbs RS, Martin DH, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Trans Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Alderete JF. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiol. 1999;145:2835–2843. doi: 10.1099/00221287-145-10-2835. [DOI] [PubMed] [Google Scholar]
- Crouch ML, Benchimol M, Alderete JF. Binding of fibronectin by Trichomonas vaginalis is influenced by iron and calcium. Microbial Pathogen. 2001;31:1–14. doi: 10.1006/mpat.2001.0455. [DOI] [PubMed] [Google Scholar]
- Diamond LS. The establishment of various Trichomonas of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- Drmota T, Proost P, Van Ranst M, Weyda F, Kulda J, Tachezy J. Iron-ascorbate cleavable malic enzyme from hydrogenosomes of Trichomonas vaginalis: purification and characterization. Molec Biochem Parasitol. 1996;83:221–234. doi: 10.1016/s0166-6851(96)02777-6. [DOI] [PubMed] [Google Scholar]
- Engbring JA, Alderete JF. Three genes encode distinct AP33 proteins involved in Trichomonas vaginalis cytoadherence. Mol Microbiol. 1998a;28:305–313. doi: 10.1046/j.1365-2958.1998.00784.x. [DOI] [PubMed] [Google Scholar]
- Engbring JA, Alderete JF. Characterization of Trichomonas vaginalis AP33 adhesin and cell surface interactive domains. Microbiol. 1998b;144:3011–3018. doi: 10.1099/00221287-144-11-3011. [DOI] [PubMed] [Google Scholar]
- Engbring JA, O’Brien JL, Alderete JF. Trichomonas vaginalis adhesin proteins display molecular mimicry to metabolic enzymes. Adv Exp Med Biol. 1996;408:207–223. doi: 10.1007/978-1-4613-0415-9_25. [DOI] [PubMed] [Google Scholar]
- Garvey JS, Cremer NE, Sussdorf DH. 125I or 131I-labelled proteins. In: Campbell DH, editor. Methods in Immunology. Benjamin, W.A. Inc; Reading, MA: 1977. pp. 171–182. [Google Scholar]
- Gold D. Adhesion of Trichomonas vaginalis to plastic surfaces: requirement for energy and serum constituents. Parasitol. 1992;105:55–62. doi: 10.1017/s0031182000073686. [DOI] [PubMed] [Google Scholar]
- Greenwood FC, Hunter WM, Glover JS. The preparation of 131I-labelled human growth hormone of high specific radioactivity. Biochem J. 1963;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs MM, Kazembe P, Reed AW, Miller WC, Nkata E, Zimba D, et al. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex Trans Dis. 1999;26:381–387. doi: 10.1097/00007435-199908000-00003. [DOI] [PubMed] [Google Scholar]
- Hochstrasser DF, Harrington MG, Hochstrasser AC, Miller MJ, Merril CR. Methods for increasing the resolution of two-dimensional protein electrophoresis. Anal Biochem. 1988;173:424–435. doi: 10.1016/0003-2697(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Hrydȳ I, Müller M. Primary structure of the hydrogenosomal malic enzyme of Trichomonas vaginalis and its relationship to homologous enzymes. J Euk Micro. 1995;42:593–603. doi: 10.1111/j.1550-7408.1995.tb05913.x. [DOI] [PubMed] [Google Scholar]
- Kassai T, Cordero del Campillo M, Euzeby J, Gaafar S, Hiepe T, Himonas CA. Standardized nomenclature of animal parasitic diseases (SNOAPAD) Vet Parasitol. 1988;29:299–326. doi: 10.1016/0304-4017(88)90148-3. [DOI] [PubMed] [Google Scholar]
- Kislauskis EH, Singer RH. Determinants of mRNA localization. Curr Opin Cell Biol. 1992;4:975–978. doi: 10.1016/0955-0674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Forrestal SG, Karr JE, Mudge CS, Anderson BE, Schaeffer AJ. Epithelial differentiation promotes the adherence of type 1-piliated Escherichia coli to human vaginal cells. J Infect Dis. 2002;186:1631–1638. doi: 10.1086/345557. [DOI] [PubMed] [Google Scholar]
- Krieger JN, Jenny C, Verdon M, Siegel N, Springwater R, Critchlow CW, Holmes KK. Clinical manifestations of trichomoniasis in men. Ann Intern Med. 1993;118:844–849. doi: 10.7326/0003-4819-118-11-199306010-00003. [DOI] [PubMed] [Google Scholar]
- Kulda J. Trichomonads, hydrogenosomes and drug resistance. Int J Parasitol. 1999;29:199–212. doi: 10.1016/s0020-7519(98)00155-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laga M, Manoka A, Kivuvu M, Malele B, Tulza M, Nzila N, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- Lahti CJ, d’Oliveira CE, Johnson PJ. Beta-succinyl-coenzyme A synthetase from Trichomonas vaginalis is a soluble hydrogenosomal protein with an amino-terminal sequence that resembles mitochondrial presequences. J Bacteriol. 1992;174:6822–6830. doi: 10.1128/jb.174.21.6822-6830.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land KM, Clemens DL, Johnson PJ. Loss of multiple hydrogenosomal proteins associated with organelle metabolism and high-level drug resistance in trichomonads. Exptl Parasitol. 2001;97:102–110. doi: 10.1006/expr.2001.4587. [DOI] [PubMed] [Google Scholar]
- Lehker MW, Chang TH, Dailey DC, Alderete JF. Specific erythrocyte binding is an additional nutrient acquisition system for Trichomonas vaginalis. J Exp Med. 1990;171:2165–2170. doi: 10.1084/jem.171.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehker MW, Arroyo R, Alderete JF. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. J Exp Med. 1991;174:311–318. doi: 10.1084/jem.174.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehker MW, Sweeney D. Trichomonad invasion of the mucous layer requires adhesins, mucinases, and motility. Sex Transm Infect. 1999;75:231–238. doi: 10.1136/sti.75.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millsap KW, Alderete JF. Issues relevant to specific molecules and mechanisms involved in colonization of host cells and tissues: Trichomonas vaginalis as a model mucosal pathogen. Recent Res Devel Microbiol. 2001;5:249–259. [Google Scholar]
- Morgado Diaz JA, De Souza W. Purification and biochemical characterization of the hydrogenosomes of the flagellate protozoan Trichomonas foetus. Eur J Cell Biol. 1997;74:85–91. [PubMed] [Google Scholar]
- Müller M. The hydrogenosome. J Gen Microbiol. 1993;139:2879–2889. doi: 10.1099/00221287-139-12-2879. [DOI] [PubMed] [Google Scholar]
- Müller M. Evolutionary origins of trichomonad hydrogenosomes. Parasitol Today. 1997;13:166–167. doi: 10.1016/s0169-4758(97)01036-3. [DOI] [PubMed] [Google Scholar]
- O’Brien JL, Lauriano CM, Alderete JF. Molecular characterization of a third malic enzyme-like ap65 adhesin gene of Trichomonas vaginalis. Microb Pathogen. 1996;20:335–349. doi: 10.1006/mpat.1996.0032. [DOI] [PubMed] [Google Scholar]
- Peterson KM, Alderete JF. Iron uptake and increased intracellular enzyme activity follow host lactoferrin binding by Trichomonas vaginalis receptors. J Exp Med. 1984;160:398–410. doi: 10.1084/jem.160.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano D, Khoshnan A, Alderete JF. Involvement of dsRNA virus in the protein composition and growth kinetics of host Trichomonas vaginalis. Arch Virol. 1997;142:939–952. doi: 10.1007/s007050050130. [DOI] [PubMed] [Google Scholar]
- Rabilloud T. Solubilization of proteins in 2D electrophoresis. In: Link AJ, editor. Methods in Molecular Biology. Humana Press; Totowa: 1999. pp. 9–19. [DOI] [PubMed] [Google Scholar]
- Rappelli P, Rocchigiani AM, Erre G, Colombo MM, Cappuccinelli P, Fiori PL. Sequence of cDNA coding for a 65 kD adhesive protein for the specific detection of Trichomonas vaginalis by PCR. FEMS Microbiol Lett. 1995;129:21–26. doi: 10.1016/0378-1097(95)00124-N. [DOI] [PubMed] [Google Scholar]
- Reis IA, Martinez MP, Yarlett N, Johnson PJ, Silva-Filho FC, Vannier-Santos MA. Inhibition of polyamine synthesis arrests trichomonad growth and induces destruction of hydrogenosomes. Antimicrob Agents Chemother. 1999;43:1919–1923. doi: 10.1128/aac.43.8.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JS, Choi HK, Min DY, Ha SE, Ahn MH. Effect of iron on the virulence of Trichomonas vaginalis. J Parasitol. 2001;87:457–460. doi: 10.1645/0022-3395(2001)087[0457:EOIOTV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR. Proteins in unexpected locations. Mol Biol Cell. 1996;7:1003–1014. doi: 10.1091/mbc.7.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvillo F, Kerndt P. Trichomonas vaginalis and amplification of HIV-1 transmission [letter] Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- Sorvillo F, Smith L, Kerndt P, Ash L. Trichomonas vaginalis, HIV, and Africans. Emerg Infect Dis. 2001;7:927–932. doi: 10.3201/eid0706.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacova S, Rasoloson D, Razga J, Hrdy I, Kulda J, Tachezy J. Iron-induced changes in pyruvate metabolism of Tritrichomonas foetus and involvement of iron in expression of hydrogenosomal proteins. Microbiol. 2001;147:53–62. doi: 10.1099/00221287-147-1-53. [DOI] [PubMed] [Google Scholar]
- Wang J, Michel V, Leclere C, Hofnung M, Charbit A. Immunogenicity of viral B-cell epitopes inserted into two surface loops of the Escherichia coli K12 LamB protein and expressed in an attenuated aroA strain of Salmonella typhimurium. Vaccine. 1999;17:1–12. doi: 10.1016/s0264-410x(98)00153-4. [DOI] [PubMed] [Google Scholar]
- Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Trans Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- World Health Organization . Global Program on AIDS. World Health Organization; Geneva, Switzerland: 1995. An overview of selected curable sexually transmitted diseases; pp. 2–27. [Google Scholar]
- Yap EH, Ho TH, Chan YC, Thong TW, Ng GC, Ho LC, Singh M. Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Genitourin Med. 1995;71:402–404. doi: 10.1136/sti.71.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Begg CB. Is Trichomonas vaginalis a cause of cervical neoplasia? Results from a combined analysis of 24 studies. Int J Epidemiol. 1994;23:682–690. doi: 10.1093/ije/23.4.682. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Graham S, Yu SZ, Marshall J, Zielezny M, Chen YX, et al. Trichomonas vaginalis and cervical cancer. A prospective study in China. Ann Epidemiol. 1995;5:325–332. doi: 10.1016/1047-2797(94)00101-x. [DOI] [PubMed] [Google Scholar]