SUMMARY
Trichomonas vaginalis is a parasitic protozoan that causes trichomonosis, a sexually-transmitted disease, with serious sequelae to women and men. As the host-parasite relationship is complex, it is important to investigate biochemical aspects of the parasite that contribute to our understanding of trichomonal biology and pathogenesis. Nucleoside triphosphate diphosphohydrolase 1 (NTPDase 1), which hydrolyses extracellular ATP and ADP, and ecto-5′-nucleotidase, which hyrolyses AMP, have been characterized in laboratory isolates of T. vaginalis. Here we show that the extracellular ATP:ADP hydrolysis ratio varies among fresh clinical isolates, which presented higher ATPase and ADPase activities than long-term-grown isolates. Growth of parasites in iron-replete and iron-depleted medium resulted in different, albeit minor, patterns in extracellular ATP and ADP hydrolysis among isolates. Importantly, some isolates had low or absent ecto-5′-nucleotidase activity, regardless of environmental conditions tested. For isolates with ecto-5′-nucleotidase activity, high- and low-iron trichomonads had increased and decreased levels of activity, respectively, compared to organisms grown in normal TYM-serum medium. This suggests a regulation in expression of either the enzyme amounts and/or activity under the control of iron. Finally, we found no correlation between the presence or absence of dsRNA virus infection among trichomonad isolates and NTPDase and ecto-5′-nucleotidase activities.
Keywords: Trichomonas vaginalis, nucleoside triphosphate diphosphohydrolase, ecto-5′-nucleotidase, fresh isolates, heterogeneity
INTRODUCTION
Trichomonas vaginalis is a parasitic protozoan that causes trichomonosis (Kassai et al. 1988), the number one, non-viral sexually transmitted disease (STD). There are 250 million new cases occurring each year worldwide (WHO, 2001; Weinstock, Berman & Cates, 2004). Trichomonosis has major health consequences for women, including adverse pregnancy outcomes (Cotch et al. 1997), predisposition to cervical cancer (Viikki et al. 2000), and increased susceptibility to HIV/AIDS (Sorvillo & Kerndt, 1998). Men infected with T. vaginalis may have a urethritis, and HIV-positive men with trichomonosis-urethritis have higher numbers of infectious HIV particles in semen, facilitating HIV transmission (Hobbs et al. 1999). Considering the serious impact of this STD on public health, it is important to study biochemical aspects of the parasite that may contribute to host infection and pathogenesis.
Several reports have shown that more than one half of clinical T. vaginalis isolates harbour double-stranded RNA (dsRNA) virus (Wang, Wang & Alderete, 1987; Wendel et al. 2002). The dsRNA virus infection affects the protein composition and growth kinetics of trichomonads and up-regulates the expression of the phenotypically varying P270 protein and cysteine proteinases (Khoshnan & Alderete, 1994). Further, iron-acquisition is preparatory to successful host parasitism and pathogenesis (Alderete, 1999). Iron modulates T. vaginalis growth rate, metabolic activities, and the expression of virulence genes, such as those involved in cytoadherence, binding to fibronectin, and resistance to complement lysis (Lehker, Arroyo & Alderete, 1991; Alderete, Provenzano & Lehker, 1995; Crouch, Benchimol & Alderete, 2001; Alderete et al. 2004).
Extracellular nucleotides such as ATP, ADP, UTP, and UDP may act as signalling compounds and can be inactivated by hydrolysis via ectonucleotidases. The ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family and the ecto-5′-nucleotidase are involved in the extracellular nucleotide hydrolysis pathway (Zimmermann, 2001). NTPDase1 (CD39, ecto-apyrase, ecto-ATP-diphosphohydrolase, EC 3.6.1.5) hydrolyses ATP and ADP at similar rates producing AMP and inorganic phosphate (Sarkis et al. 1995; Wang & Guidotti, 1996). The ecto-5′-nucleotidase (CD73, EC 3.1.3.5) hydrolyses AMP producing adenosine (Zimmermann, 2001). These enzymes are surface-located and require divalent cations (usually Ca++ or Mg++) and an alkaline pH. In this study, we tested the hypothesis that the activities of NTPDase1 (Matos et al. 2001) and ecto-5′-nucleotidase (Tasca et al. 2003a) would be elevated among fresh clinical isolates of T. vaginalis compared to long-term-grown isolates. Indeed, fresh isolates have elevated rates of ATP and ADP hydrolysis and, surprisingly, had different ATP:ADP hydrolysis ratios. Further, we show that some isolates had low or absent ecto-5′-nucleotidase activity regardless of environmental conditions examined. In addition, growth of parasites in high- or low-iron medium had no significant effect on NTPDase but increased and decreased, respectively, ecto-5′-nucleotidase activity in isolates that presented activity under normal conditions.
MATERIALS AND METHODS
Parasites and culture conditions
All fresh clinical isolates were axenized and used as reported before by us (Lehker et al. 1991; Khoshnan & Alderete, 1994; Alderete et al. 2004). Organisms were grown for no longer than 1 week and cultured at 37 °C by daily passage in Trypticase-yeast extract-maltose (TYM) medium supplemented with 5% heat-inactivated normal horse serum (Diamond, 1957). The dsRNA virus-infected (V+) laboratory isolate 347 V+ and virus-minus 347 V- progeny trichomonads have been described in detail before and have been grown for extended periods. The 347 V- progeny parasites were agar cloned from the parental 347 V+ isolate (Wang et al. 1987) after 3 weeks of batch culture, as described (Khoshnan & Alderete, 1994). Low-iron trichomonads were cultivated in TYM containing 50 μm of the iron chelator 2,2-dipyridil (2,2-DP; Sigma Chemical Co., St Louis, MO) at 37 °C for 24 h (Lehker et al. 1991) followed by resuspension of organisms in medium with 50 μm 2,2-DP for an additional 24 h. For high-iron organisms, low-iron parasites were washed once and suspended in TYM-serum medium containing 200 μm ferrous ammonium sulfate (Sigma) for incubation at 37 °C for 24 h. All experiments involving the isolates grown under different conditions were performed on at least 3 separate occasions.
Enzymes assays
All organisms from different T. vaginalis isolates were from the mid-logarthmic phase of growth, were viable based on trypan blue exclusion and were highly motile. Trichomonads remained viable and motile throughout the enzyme incubation time-period. Parasites were harvested and washed 3 times with 0·85% (w/v) NaCl solution. Intact organisms (1·5 × 106 trichomonads/ml) were added to the NTPDase reaction mixture (50 mm Tris buffer, pH 7·2, and 5 mm CaCl2) for measuring ATP and ADP hydrolysis. The same number of parasites were added to the ecto-5′-nucleotidase reaction mixture containing 50 mm Tris buffer, pH 7·5, and 3 mm MgCl2 (Tasca et al. 2003a). The samples were pre-incubated for 5 min at 37 °C in 200 μl of the reaction mixture. The final concentrations for ATP and ADP for NTPDase were 1 mm and for AMP for ecto-5′-nucleotidase was 3 mm. The reaction of hydrolysis of ATP, ADP and AMP was stopped by adding 200 μl of 10% (v/v) trichloroacetic acid (TCA). The samples were chilled on ice for 10 min before assaying the release of inorganic phosphate (Pi) (Chan, Delfert & Junger, 1986). The time of incubation and numbers of organisms were chosen for linearity of the reactions. Controls included intact organisms added to the reaction mixtures containing TCA in order to correct non-enzymatic hydrolysis of substrates, and the averages of control values were subtracted from the test samples. Specific activity is expressed as nmol Pi released/min/1·5 × 106 trichomonads, which corresponded to 0·3-0·8 mg/ml of protein. The same relative levels of enzyme activity and ATP:ADP hydrolysis ratios were obtained when specific activity was expressed as nmol Pi released/min/mg protein. All samples were run in triplicate with similar results achieved in at least 3 different parasite suspensions.
Protein determination
Protein was measured by the Coomassie Blue method (Bradford, 1976), using bovine serum albumin as standard.
RESULTS
Fresh clinical isolates display higher NTPDase activity and heterogeneity in ATP:ADP hydrolysis ratio
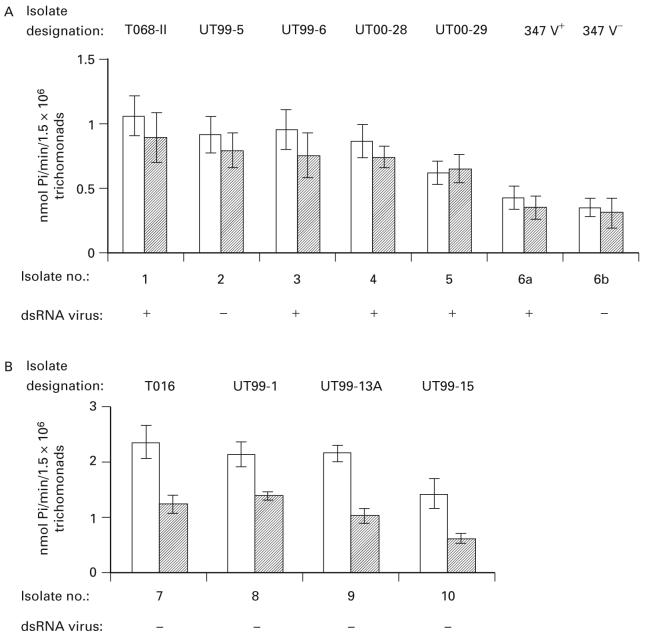
Fig. 1 shows the distinct levels of NTPDase activity for 9 representative fresh clinical isolates (numbered 1 through 5, part A and 7 through 10, part B) compared to a representative long-term-grown isolate (no. 6). Trichomonads of fresh isolates had NTPDase activity ranging from 1·4- to 2·4-fold higher when compared to parasites of isolate 347 V+ (no. 6a) and the corresponding virus-minus progeny 347 V- organisms (no. 6b). This was the case regardless of whether ATP or ADP served as substrate in the reaction. The lower level of NTPDase1 activity of isolate 347 is consistent with that of other long-term-grown isolates analysed previously by us (Tasca et al. 2003b). In part A, trichomonads of fresh isolates had NTPDase1 activity with a typical ATP:ADP hydrolysis ratio of 1 : 0·8 (Zimmerman, 1999). Further, there was no relationship between virus infection of clinical isolates and NTPDase1 activity. Interestingly, Fig. 1B illustrates that some fresh isolates had an ATP:ADP hydrolysis ratio of approximately 2 : 1. These data suggest that different isolates exhibit heterogeneity in the ATP:ADP hydrolysis ratio.
Fig. 1.
NTPDase activity among fresh Trichomonas vaginalis isolates (nos. 1-5 (A) and 7-10 (B) and a representative long-term-grown (no. 6) isolate. Isolate 347 V+ (no. 6a) and 347 V- (no. 6b) refer to presence or absence of dsRNA virus, respectively, as described in the Materials and Methods section. The enzyme activities are those using ATP (clear bars) and ADP (hatched bars) as substrates. Isolates (B) were separated from (A) because of the ATP:ADP hydrolysis ratio of ∼2 : 1, that is distinct from that of NTPDase1 (isolates nos. 1-5). Data are expressed as the mean±S.D. for at least 3 experiments.
Heterogeneity and lack of ecto-5′-nucleotidase activity among some isolates
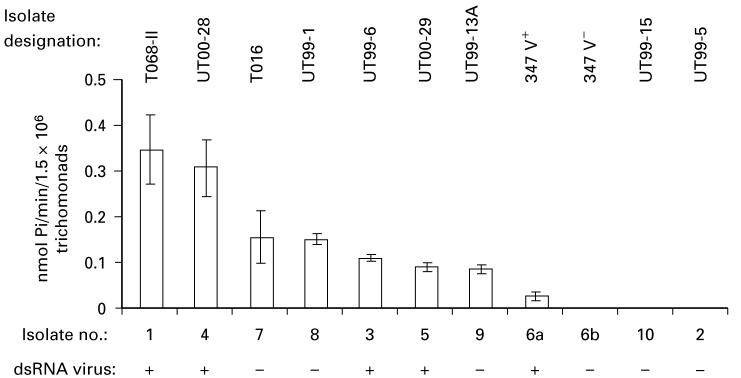
Fig. 2 presents, in decreasing amounts, the extent of heterogeneity of ecto-5′-nucleotidase activity among isolates. The level of enzyme activity for isolate 347 V+ (no. 6a) was very low at 0·024±0·008 nmol Pi released/min/1·5 × 106 trichomonads (mean±s.d., n=9). The progeny 347 V- (6b) and fresh isolates UT99-5 (no. 2) and UT99-15 (no. 10) had no detectable ecto-5′-nucleotidase activity. Because of the unexpected dramatic differences in ecto-5′-nucleotidase activity, we grew isolates number 2, 6a, 6b and 10 under varying environmental conditions to determine if this enzymatic activity could be increased. For example, in data not shown, growth of these isolates under nutrient limitation (1% serum) did not enhance ecto-5′-nucleotidase activity. Lastly and not unexpectedly, based on findings of Fig. 1, there was no correlation between ecto-5′-nucleotidase activity (AMP hydrolysis) and presence or absence of dsRNA virus in the isolates.
Fig. 2.
The extent of variation in the ecto-5′-nucleotidase activity among fresh Trichomonas vaginalis isolates and the long-term-grown 347 isolate. Data are expressed as the mean±S.D. for at least 3 experiments.
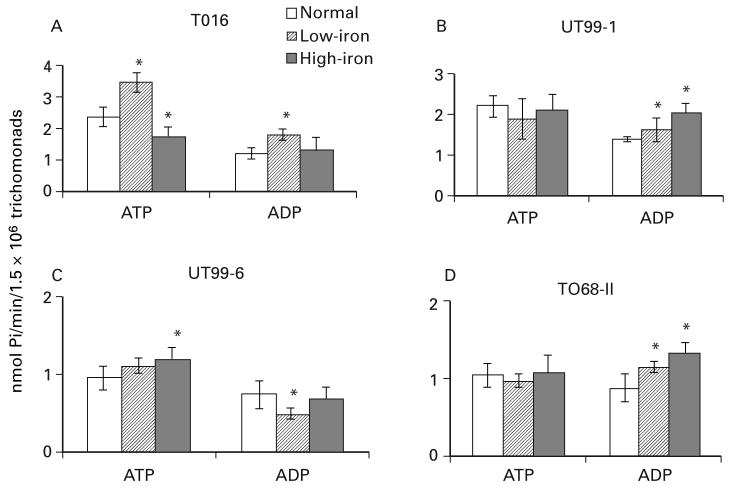
Effect of iron on NTPDase activity
Fig. 3 shows that, for representative virus-minus (A and B) and virus-harbouring (C and D) fresh isolates, there were statistically significant, albeit minor, varied and different NTPDase activities among the isolates grown in either iron-replete or iron-limited medium. Also, there were no clear-cut trends with either iron condition and the activity of NTPDase regardless of substrate. For example, only low-iron parasites of isolate T016 had significant elevated levels of ATP hydrolysis. Likewise, high-iron decreased ATP hydrolysis in only the T016 isolate, while slightly but significantly (P<0·05) increased ATPase activity in only UT99-6. Low-iron T016, UT99-1 and T068-II trichomonads had slightly increased ADP hydrolysis activity compared to normal parasites, while high-iron organisms of UT99-1 and T068-II isolates had increased ADP hydrolysis.
Fig. 3.
Representative experiments on the effect of growth of trichomonads in low- (centre, hatched bars) versus high-iron (solid black bars) medium on NTPDase activity among 2 virus-minus (T016 and UT99-1) and 2 virus-harbouring (UT99-6 and T068-II) fresh Trichomonas vaginalis isolates. Different iron conditions were compared with normal TYM-serum medium. Bars represent the mean±S.D. for at least 3 experiments. Results were analysed statistically by the Student’s t-test (P<0·05). Statistically significant enzyme activity differences when compared to control are indicated by an asterisk.
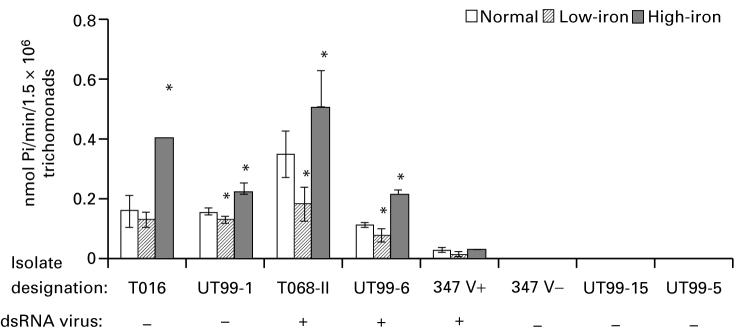
Iron modulates ecto-5′-nucleotidase activity
Finally, based on the results of Fig. 2, we tested the effect of iron among representative fresh isolates with detectable high, moderate and low ecto-5′-nucleotidase activities. High- versus low-iron trichomonads had significantly increased and decreased enzyme activity, respectively, compared to organisms grown in normal medium (Fig. 4). On the other hand, growth in high-iron medium did not enhance AMP hydrolysis in the 347 V+ isolate that had very low ecto-5′-nucleotidase activity. High-iron parasites of progeny 347 V- and isolates UT99-5 and UT99-15 remained without activity. These results show that, for fresh isolates with ecto-5′-nucleotidase activity under normal conditions, iron may be a modulator for increasing either the amount of enzyme or the level of enzymatic activity.
Fig. 4.
Effect of growth of trichomonads in low- (centre, hatched bars) versus high-iron (solid black bars) medium on ecto-5′-nucleotidase activity among representative fresh Trichomonas vaginalis isolates and the long-term-grown 347 isolate. Different iron conditions were compared with normal TYM-serum medium. Bars represent the mean±S.D. for at least 3 experiments. Results were analysed statistically by Student’s t-test (P<0·05). Statistically significant enzyme activity differences when compared to control are indicated by an asterisk.
DISCUSSION
Nucleoside 5′-triphosphates and 5′-diphosphates may be hydrolysed by NTPDase1 to NTPDase6, members of the E-NTPDase family (Zimmermann, 2001). We previously characterized NTPDase1 activity in T. vaginalis (Matos et al. 2001; Tasca et al. 2003b). In this study, we tested the hypothesis that the extracellular ATP, ADP and AMP hydrolysis in T. vaginalis is higher in fresh clinical isolates, and indeed, higher enzymatic activities were seen in fresh compared to representative long-term-grown isolates used here and in our earlier study (Tasca et al. 2003b). It is noteworthy that heterogeneity exists among isolates regarding the ratio of ATP:ADP hydrolysis. The kinetic data for the NTPDase permit two possibilities. While some isolates have NTPDase1 activity (ATP:ADP ratio was 1 : 0·8), other isolates may have a different NTPDase with an ATP:ADP ratio approximating 2 : 1 Alternatively, the different ATP:ADP hydrolysis ratio may be due to structural alterations in the enzyme, which then leads to distinct susbtrate preferences, as has been suggested (Zimmermann, 2001). There are reports of ATP:ADP hydrolysis ratios of 2 : 1 for rat brain synaptosomes (Battastini et al. 1991), and the NTPDase8 was recently identified in mouse as responsible for this hydrolysis ratio (Bigonnesse et al. 2004). Recently, different ATP:ADP hydrolysis ratios in NTPDase were found in Trypanosoma cruzi epimastigotes (1 : 1 ratio) and trypomastigotes (2 : 1 ratio) (Fietto et al. 2004). Whether T. vaginalis trophozoites have NTPDase1 with a distinct hydrolysis ratio or a different enzyme awaits experimental verification.
Ecto-5′-nucleotidase is the last step in the enzymatic chain producing adenosine. T. vaginalis isolates had dramatic differences in levels of ecto-5′-nucleotidase activity, and surprisingly, some isolates had little or no enzymatic activity requiring further studies to clarify the absence of activity in these isolates. This is noteworthy because T. vaginalis is dependent on salvage pathways to generate de novo nucleotides (Heyworth, Gutteridge & Ginger, 1982, 1984). Also, adenosine was shown to be the primary precursor of the entire purine nucleotide pool in T. vaginalis, and adenine is converted to GMP via adenosine (Munagala & Wang, 2003). It would seem that in T. vaginalis, the NTPDase and ecto-5′-nucleotidase are absolute requirements to produce adenosine and for the parasite to acquire the nucleoside through the uptake pathway. Therefore, this essential nucleoside adenosine produced by the parasite may be lacking in some isolates. Different growth conditions were without effect in elevating levels of ecto-5′-nucleotidase activity, especially for isolates with little or no enzyme and/or activity. It is possible that unique environmental conditions not used here may up-regulate expression of the enzyme activity. Alternatively, the trichomonads may have a transcriptional and/or translational defect, or the expressed protein may be functionally silent due to a mutation. This lack of ecto-5′-nucleotidase activity by T. vaginalis may have important consequences for both host and parasite during infection. For example, adenosine is an anti-inflammatory agent that can bind specific receptors to regulate the consequences of inflammation (Cronstein et al. 1992; Bouma et al. 1997). Thus, decreased amounts or lack of adenosine as an anti-inflammatory agent could result in acute symptoms due to leukocytic infiltration among patients infected with ecto-5′-nucleotidase-deficient organisms.
It is conceivable that T. vaginalis organisms might modulate the nucleotide levels in response to external factors. One such factor, iron, is known to modulate expression of virulence factors of T. vaginalis, such as adhesins (Alderete et al. 2004) and proteinases (Alderete et al. 1995). Interestingly, growth of trichomonads in iron-replete and iron-depleted medium had overall little dramatic effect in distinct patterns in NTPDase activity among fresh isolates, regardless of dsRNA virus infection. In contrast, the fact that high- and low-iron trichomonads had increased and decreased ecto-5′-nucleotidase activity, respectively, among fresh isolates with activity detectable under normal medium conditions may be significant during infection. The higher rates of growth and multiplication of T. vaginalis in an iron-rich environment may promote an inflammatory host response that would be tempered through increased production of adenosine.
While ectonucleotidases have been described in parasites (Vasconcelos et al. 1993; Meyer-Fernandes et al. 1997; Barros et al. 2000; Coimbra et al. 2002), the consequences of the enzymatic activities in relation to host responses or parasitism are unknown. For example, of interest is whether these proteins have functional diversity. For example, NTPDase1 (CD39) and ecto-5′-nucleotidase (CD73) are involved in the cellular signalling that regulates adhesion in lymphocytes (Kansas, Wood & Tedder, 1991; Airas et al. 1995). It would be important to determine whether ecto-5′-nucleotidase mediates the known T. vaginalis binding to fibronectin and laminin (Costa e Silva Filho, de Souza & Lopes, 1988; Crouch & Alderete, 1999; Crouch et al. 2001) as has been found for mammalian cells (Stochaj et al. 1989; Méhul et al. 1993). This requires the availability of reagents such as pure enzyme and/or antibody. At this time, analyses of gene banks using known sequences of these enzymes (Resta et al. 1993; Maliszewski et al. 1994; Asai et al. 1995; Vasconcelos et al. 1996; Fietto et al. 2004) did not reveal any putative T. vaginalis homologues for NTPDase and ecto-5′-nucleotidase.
Acknowledgments
This work was supported by Public Health Service grants AI 43940 and AI 45429 from the National Institutes of Health. T.T. is recipient of a fellowship from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq), Brazil. We would like to express our appreciation to all members of the J.F.A. laboratory for their support to T.T. during this project, and to the laboratories of G.A.D.C. and J.J.F.S. for their invaluable support and discussions throughout this work.
REFERENCES
- AIRAS L, HELLMAN J, SALMI M, BONO P, PUURUNEN T, SMITH DJ, JALKANEN S. CD73 is involved in lymphocyte binding to the endothelium: characterization of lymphocyte-vascular adhesion protein 2 identifies it as CD73. Journal of Experimental Medicine. 1995;182:1603–1608. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDERETE JF. Trichomonas vaginalis, a model mucosal parasite. Reviews in Medical Microbiology. 1999;10:165–173. [Google Scholar]
- ALDERETE JF, NGUYEN J, MUNDODI V, LEHKER MW. Heme-iron increases levels of AP65-mediated adherence by Trichomonas vaginalis. Microbial Pathogenesis. 2004;36:263–271. doi: 10.1016/j.micpath.2003.12.007. [DOI] [PubMed] [Google Scholar]
- ALDERETE JF, PROVENZANO D, LEHKER MW. Iron mediates Trichomonas vaginalis resistance to complement lysis. Microbial Pathogenesis. 1995;19:93–103. doi: 10.1006/mpat.1995.0049. [DOI] [PubMed] [Google Scholar]
- ASAI T, MIURA S, SIBLEY LD, OKABAYASHI H, TAKEUCHI T. Biochemical and molecular characterization of nucleoside triphosphate hydrolase isozymes from the parasitic protozoan Toxoplasma gondii. The Journal of Biological Chemistry. 1995;270:11391–11397. doi: 10.1074/jbc.270.19.11391. [DOI] [PubMed] [Google Scholar]
- BARROS FS, DE MENEZES LF, PINHEIRO AAS, SILVA EF, LOPES AHCS, DE SOUZA W, MEYER-FERNANDES JR. Ectonucleotide diphosphohydrolase activities in Entamoeba histolytica. Archives of Biochemistry and Biophysics. 2000;375:304–314. doi: 10.1006/abbi.1999.1592. [DOI] [PubMed] [Google Scholar]
- BATTASTINI AM, DA ROCHA JB, BARCELLOS CK, DIAS RD, SARKIS JJ. Characterization of an ATP diphosphohydrolase (EC 3.6.1.5) in synaptosomes from cerebral cortex of adult rats. Neurochemistry Research. 1991;16:1303–1310. doi: 10.1007/BF00966661. [DOI] [PubMed] [Google Scholar]
- BIGONNESSE F, LEVESQUE SA, KUKULSKI F, LECKA J, ROBSON SC, FERNANDES MJ, SÉVIGNY J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- BOUMA MG, JEUNHOMME TMMA, BOYLE DL, DENTENER MA, VOITENOK NN, VAN DEN WILDENBERG FAJM, BUURMAN WA. Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. Journal of Immunology. 1997;158:5400–5408. [PubMed] [Google Scholar]
- BRADFORD MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:218–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHAN K, DELFERT D, JUNGER KD. A direct colorimetric assay for Ca2+-ATPase activity. Analytical Biochemistry. 1986;157:375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- COIMBRA ES, GONÇALVES-DA-COSTA SC, CORTE-REAL S, DE FREITAS FGR, DURÃO AC, SOUZA CSF, SILVA-SANTOS MI, VASCONCELOS EG. Characterization and cytochemical localization of an ATP diphosphohydrolase from Leishmania amazonensis promastigotes. Parasitology. 2002;124:137–143. doi: 10.1017/s0031182001001056. [DOI] [PubMed] [Google Scholar]
- COSTA E SILVA FILHO F, DE SOUZA W, LOPES JD. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proceedings of the National Academy of Sciences, USA. 1988;85:8042–8046. doi: 10.1073/pnas.85.21.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTCH MF, PASTOREK JG, II, NUGENT RP, HILLIER SL, GIBBS RS, MARTIN DH, ESCHENBACH DA, EDELMAN R, CAREY JC, REGAN JA, KROHN MA, KLEBANOFF MA, RAO AV, RHOADS GG. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sexually Transmitted Diseases. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- CRONSTEIN BN, LEVIN RI, PHILIPS M, HIRSCHHORN R, ABRAMSON SB, WEISSMAN G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. Journal of Immunology. 1992;148:2201–2206. [PubMed] [Google Scholar]
- CROUCH M-LV, ALDERETE JF. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology. 1999;145:2835–2843. doi: 10.1099/00221287-145-10-2835. [DOI] [PubMed] [Google Scholar]
- CROUCH M-LV, BENCHIMOL M, ALDERETE JF. Binding of fibronectin by Trichomonas vaginalis is influenced by iron and calcium. Microbial Pathogenesis. 2001;31:131–144. doi: 10.1006/mpat.2001.0455. [DOI] [PubMed] [Google Scholar]
- DIAMOND LS. The establishment of various trichomonads of animals and man in axenic cultures. Journal of Parasitology. 1957;43:488–490. [PubMed] [Google Scholar]
- FIETTO JL, DE MARCO R, NASCIMENTO IP, CASTRO IM, CARVALHO TM, DE SOUZA W, BAHIA MT, ALVES MJ, VERJOVSKI-ALMEIDA S. Characterization and immunolocalization of an NTP diphosphohydrolase of Trypanosoma cruzi. Biochemical and Biophysical Research Communication. 2004;316:454–460. doi: 10.1016/j.bbrc.2004.02.071. [DOI] [PubMed] [Google Scholar]
- HEYWORTH PG, GUTTERIDGE WE, GINGER CD. Purine metabolism in Trichomonas vaginalis. FEBS Letters. 1982;141:106–110. doi: 10.1016/0014-5793(82)80026-4. [DOI] [PubMed] [Google Scholar]
- HEYWORTH PG, GUTTERIDGE WE, GINGER CD. Pyrimidine metabolism in Trichomonas vaginalis. FEBS Letters. 1984;176:55–60. doi: 10.1016/0014-5793(84)80910-2. [DOI] [PubMed] [Google Scholar]
- HOBBS MM, KZEMBE P, REED AW, MILLER WC, NKATA E, ZIMBA D, DALY CC, CHAKRABORTY H, COHEN MS, HOFFMAN I. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sexually Transmitted Diseases. 1999;26:381–387. doi: 10.1097/00007435-199908000-00003. [DOI] [PubMed] [Google Scholar]
- KANSAS GS, WOOD GS, TEDDER TF. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. Journal of Immunology. 1991;146:2235–2244. [PubMed] [Google Scholar]
- KASSAI T, CORDERO DEL CAMPILLO M, EUZEBY J, GAAFAR S, HIEPE T, HIMONAS CA. Standardized nomenclature of animal parasitic diseases (SNOAPAD) Veterinary Parasitology. 1988;29:299–326. doi: 10.1016/0304-4017(88)90148-3. [DOI] [PubMed] [Google Scholar]
- KHOSHNAN A, ALDERETE JF. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. Journal of Virology. 1994;68:4035–4038. doi: 10.1128/jvi.68.6.4035-4038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHKER MW, ARROYO R, ALDERETE JF. The regulation by iron of the synthesis of adhesins and cytoadherence levels in the protozoan Trichomonas vaginalis. Journal of Experimental Medicine. 1991;174:311–318. doi: 10.1084/jem.174.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALISZEWSKI CR, DELESPESSE GJ, SCHOENBORN MA, ARMITAGE RJ, FANSLOW WC, NAKAJIMA T, BAKER E, SUTHERLAND GR, POINDEXTER K, BIRKS C, ALPERT A, FRIEND D, GIMPEL SD, GAYLE RB., III The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. Journal of Immunology. 1994;153:3574–3583. [PubMed] [Google Scholar]
- MATOS JAA, BORGES FP, TASCA T, BOGO MR, DE CARLI GA, FAUTH MG, DIAS RD, BONAN CD. Characterisation of an ATP diphosphohydrolase (Apyrase, EC 3.6.1.5) activity in Trichomonas vaginalis. International Journal for Parasitology. 2001;31:770–775. doi: 10.1016/s0020-7519(01)00191-6. [DOI] [PubMed] [Google Scholar]
- MÉHUL B, AUBERY M, MANHERZ H-G, CODOGNO P. Dual mechanism of laminin modulation of ecto-5′-nucleotidase activity. Journal of Cellular Biochemistry. 1993;52:266–274. doi: 10.1002/jcb.240520303. [DOI] [PubMed] [Google Scholar]
- MEYER-FERNANDES JR, DUTRA PML, RODRIGUES CO, SAADNEHME J, LOPES AHCS. Mg-dependent ecto-ATPase activity in Leishmania tropica. Archives of Biochemistry and Biophysics. 1997;341:40–46. doi: 10.1006/abbi.1997.9933. [DOI] [PubMed] [Google Scholar]
- MUNAGALA NR, WANG CC. Adenosine is the primary precursor of all purine nucleotides in Trichomonas vaginalis. Molecular and Biochemical Parasitology. 2003;127:143–149. doi: 10.1016/s0166-6851(02)00330-4. [DOI] [PubMed] [Google Scholar]
- RESTA R, HOOKER SW, HANSEN KR, LAURENT AB, PARK JL, BLACKBURN MR, KNUDSEN TB, THOMPSON LF. Murine ecto-5′-nucleotidase (CD73): cDNA cloning and tissue distribution. Gene. 1993;133:171–177. doi: 10.1016/0378-1119(93)90635-g. [DOI] [PubMed] [Google Scholar]
- SARKIS JJF, BATTASTINI AMO, OLIVEIRA EM, FRASSETTO SS, DIAS RD. ATP diphosphohydrolases: an overview. Journal of Brazilian Association for the Advancement of Science. 1995;47:131–136. [Google Scholar]
- SORVILLO F, KERNDT P. Trichomonas vaginalis and amplification of HIV-1 transmission. Lancet. 1998;351:213–214. doi: 10.1016/S0140-6736(05)78181-2. [DOI] [PubMed] [Google Scholar]
- STOCHAJ U, DIECKHOFF JM, CRAMER M, MANNHERZ HG. Evidence for the direct interaction of chicken gizzard 5′-nucleotidase with laminin and fibronectin. Biochimica et Biophysica Acta. 1989;992:385–392. doi: 10.1016/0304-4165(89)90101-3. [DOI] [PubMed] [Google Scholar]
- TASCA T, BONAN CD, DE CARLI GA, BATTASTINI AM, SARKIS JJ. Characterization of an ecto-5′-nucleotidase (EC 3.1.3.5) activity from intact cells of Trichomonas vaginalis. Experimental Parasitology. 2003a;105:167–173. doi: 10.1016/j.exppara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- TASCA T, BORGES FP, BONAN CD, DE CARLI GA, BATTASTINI AM, SARKIS JJ. Effects of metronidazole and tinidazole on NTPDase1 and ecto-5′-nucleotidase from intact cells of Trichomonas vaginalis. FEMS Microbiology Letters. 2003b;226:379–384. doi: 10.1016/S0378-1097(03)00637-2. [DOI] [PubMed] [Google Scholar]
- VASCONCELOS EG, NASCIMENTO PS, MEIRELLES MNL, VERJOVSKI-ALEMIDA S, FERREIRA ST. Characterization and localization of an ATP diphosphohydrolase on the external surface of tegument of Schistosoma mansoni. Molecular and Biochemical Parasitology. 1993;58:205–214. doi: 10.1016/0166-6851(93)90042-v. [DOI] [PubMed] [Google Scholar]
- VASCONCELOS EG, FERREIRA ST, CARVALHO TMU, DE SOUZA W, KETTLUN AM, MANCILLA M, VALENZUELA MA, VERJOVSKI-ALMEDA S. Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni. The Journal of Biological Chemistry. 1996;36:22139–22145. doi: 10.1074/jbc.271.36.22139. [DOI] [PubMed] [Google Scholar]
- VIIKKI M, PUKKALA E, NIEMINEN P, HAKAMA M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncologica. 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- WANG AL, WANG CC, ALDERETE JF. Trichomonas vaginalis phenotypic variation occurs only among trichomonads with double-stranded RNA virus. Journal of Experimental Medicine. 1987;166:142–150. doi: 10.1084/jem.166.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG TF, GUIDOTTI G. CD39 is an ecto-(Ca2+, Mg2+)-apyrase. The Journal of Biological Chemistry. 1996;271:9898–9901. [PubMed] [Google Scholar]
- WEINSTOCK H, BERMAN S, CATES W., Jr. Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspectives on Sexual and Reproductive Health. 2004;36:6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- WENDEL KA, ROMPALO AM, ERBELDING EJ, CHANG T-H, ALDERETE JF. Double-stranded RNA viral infection of Trichomonas vaginalis infecting patients attending a sexually transmitted diseases clinic. Journal of Infectious Diseases. 2002;186:558–561. doi: 10.1086/341832. [DOI] [PubMed] [Google Scholar]
- WORLD HEALTH ORGANIZATION . Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections. Overview and Estimates. WHO; Geneva: 2001. [Google Scholar]
- ZIMMERMANN H. Two novel families of ectonucleotidases: molecular structures, catalytic properties, and a search for function. Trends in Pharmacological Sciences. 1999;20:231–236. doi: 10.1016/s0165-6147(99)01293-6. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Development Research. 2001;52:44–56. [Google Scholar]