Abstract
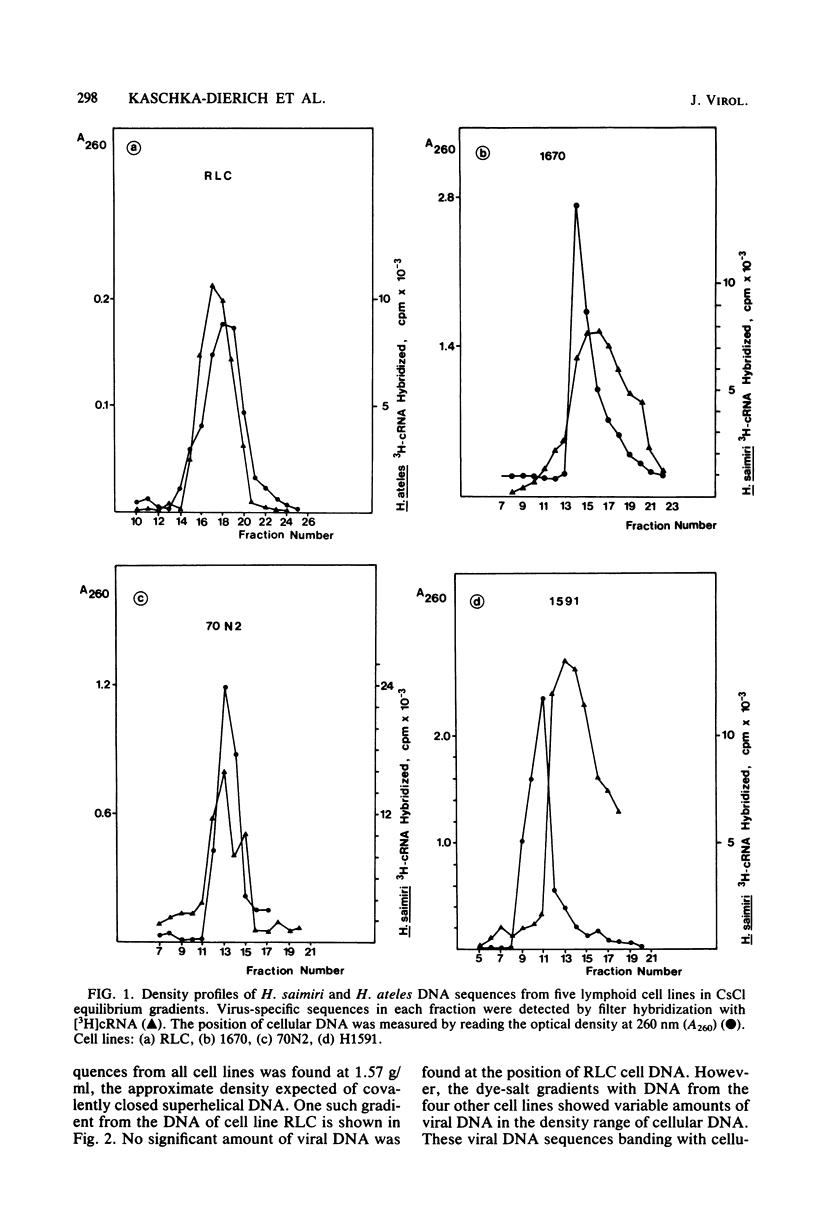
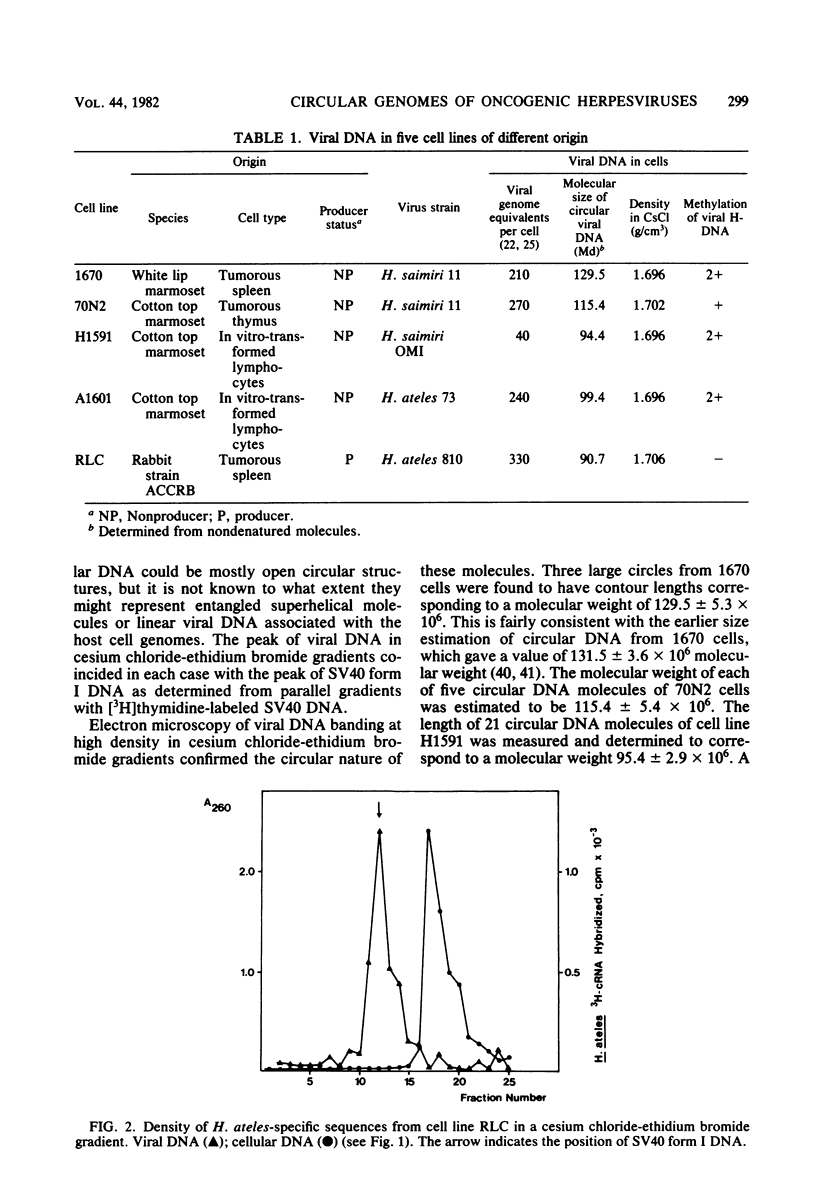
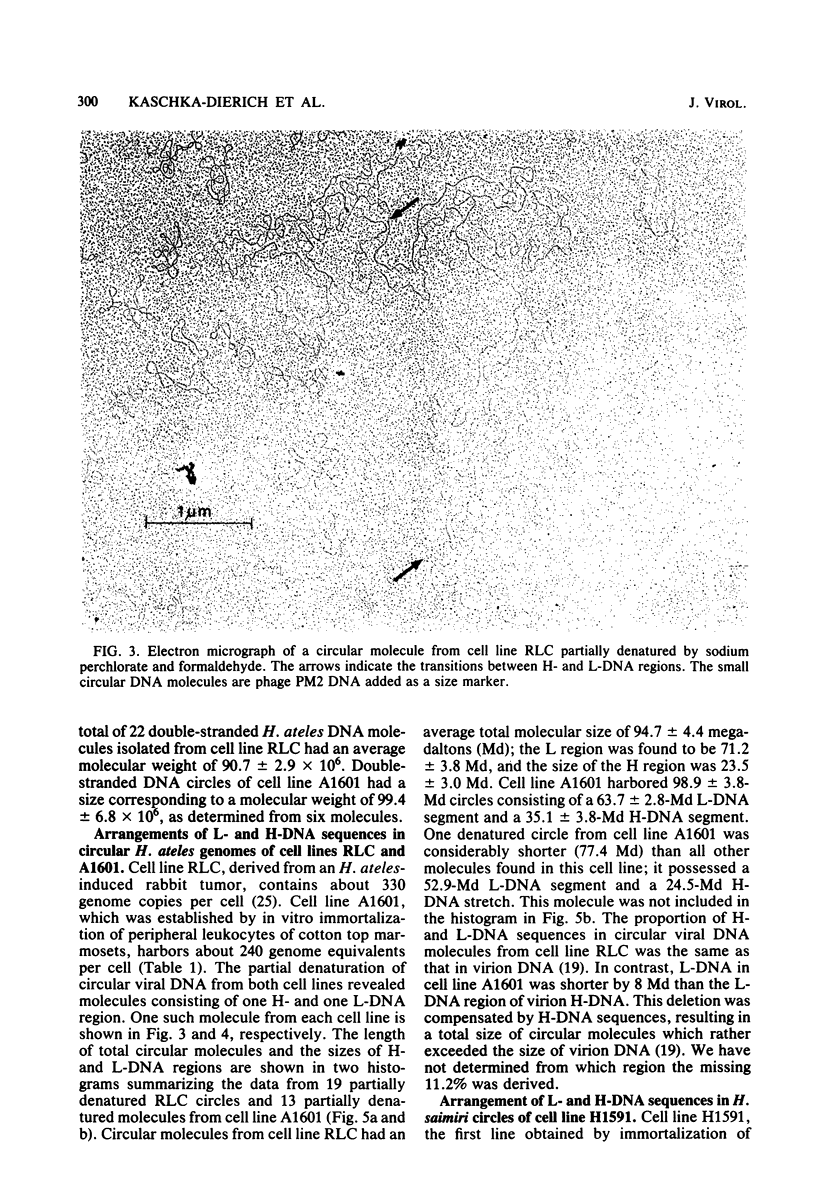
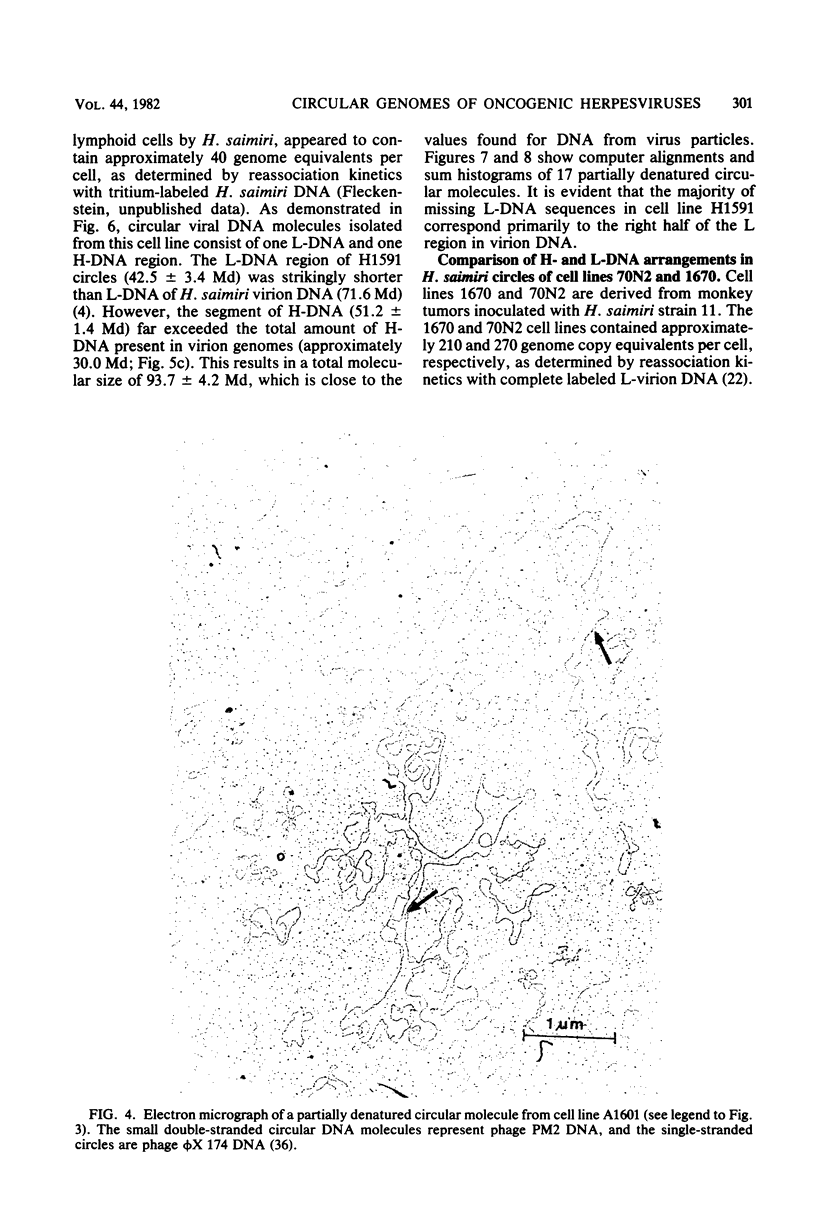
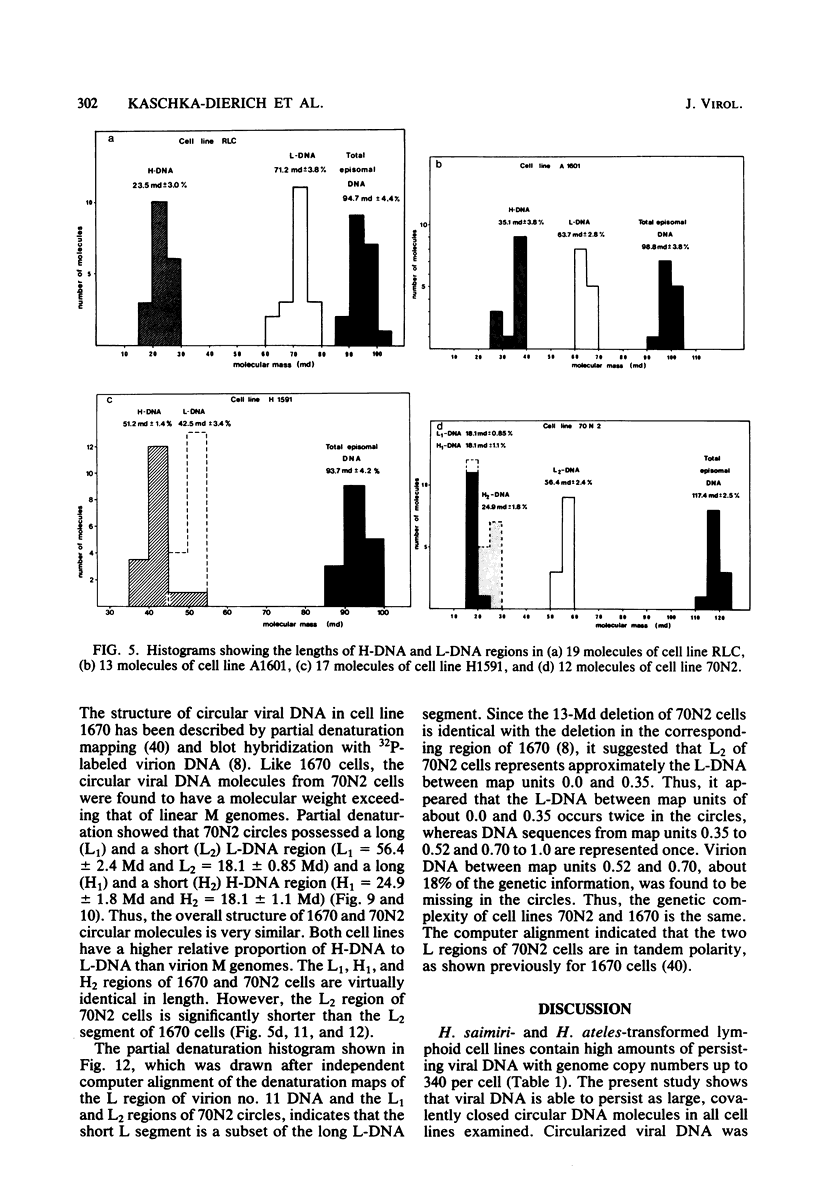
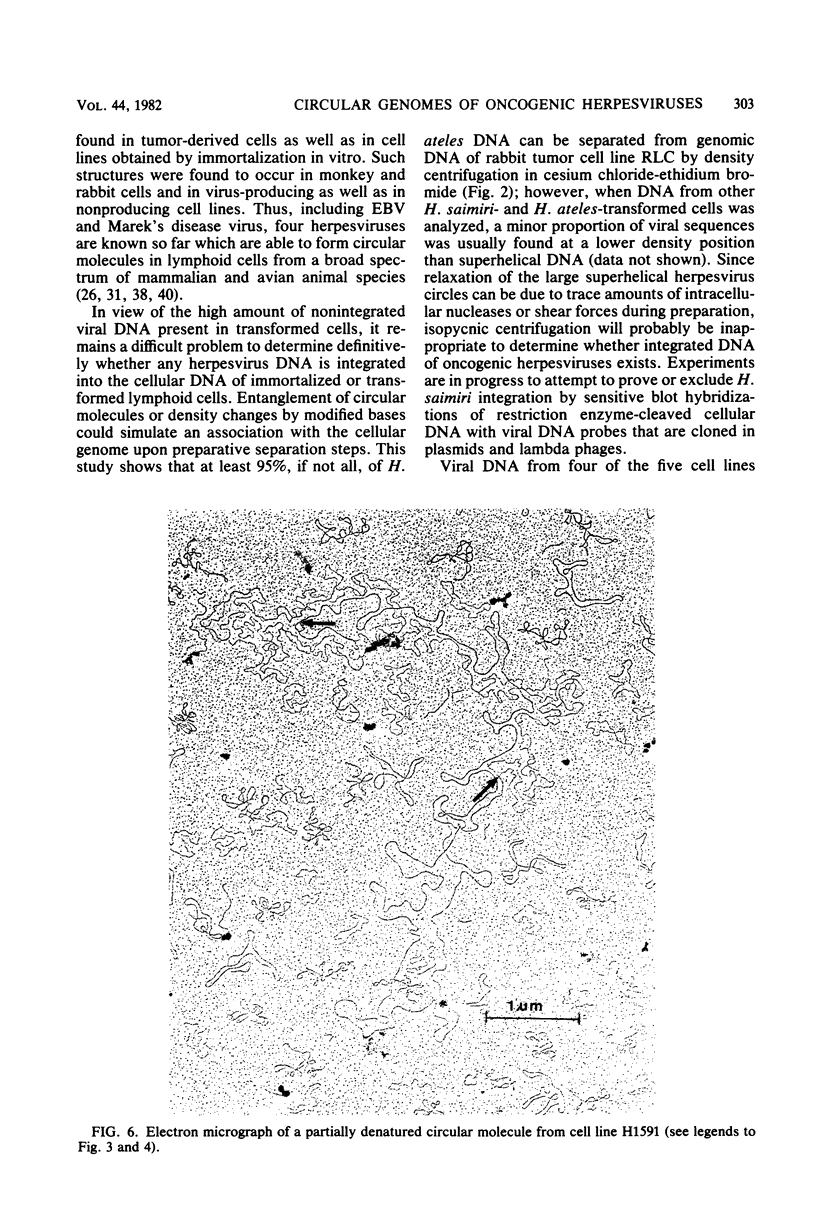
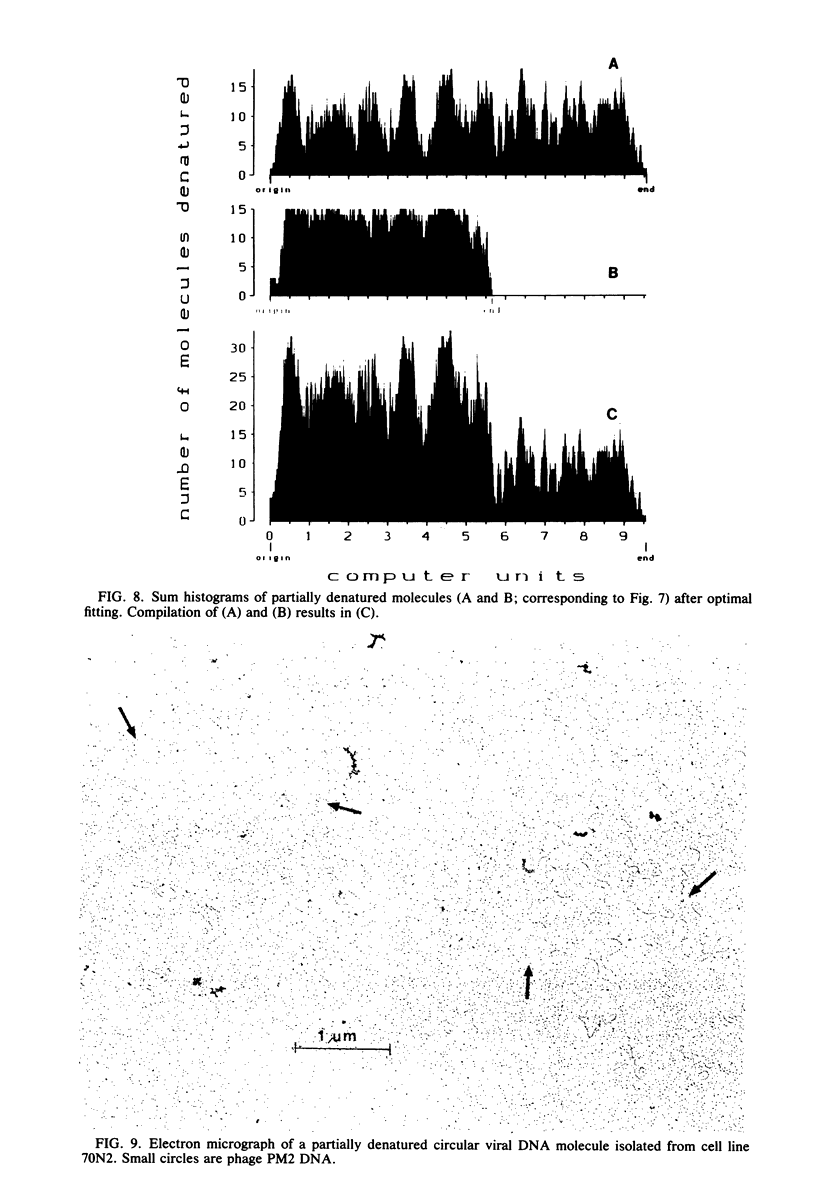
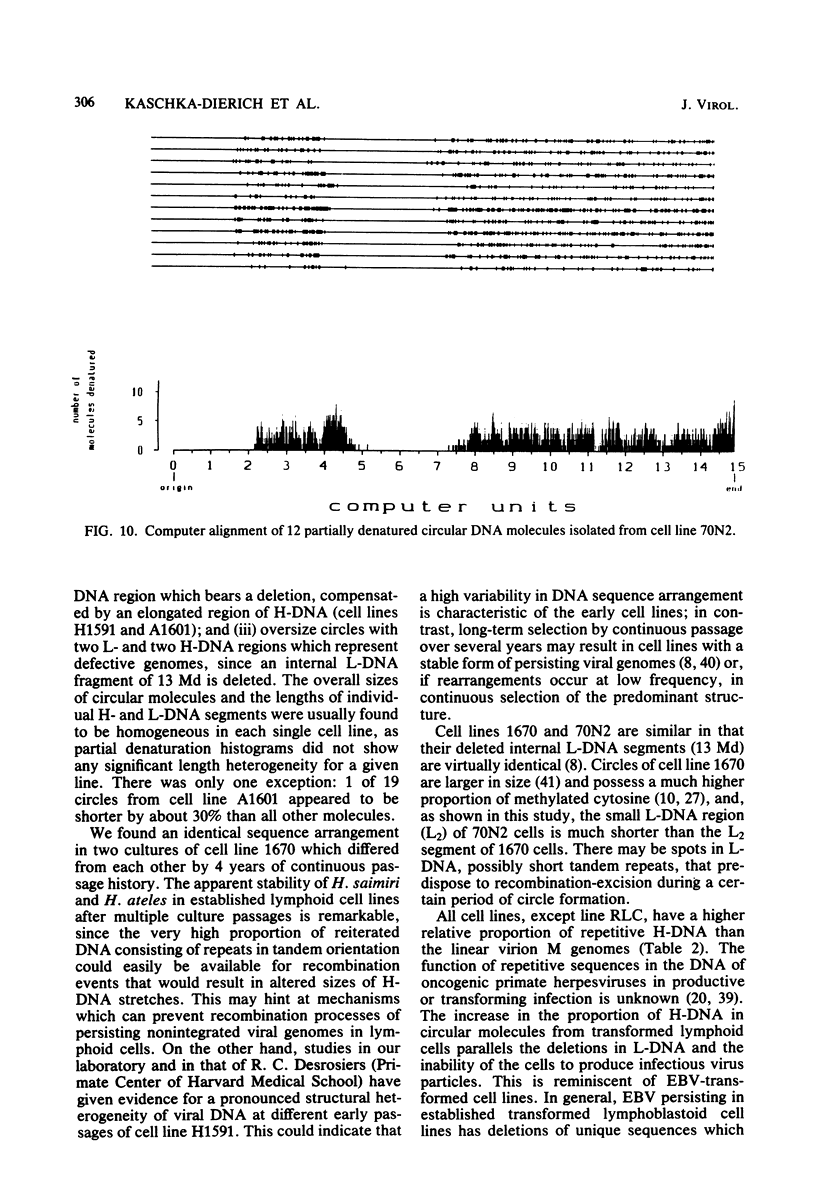
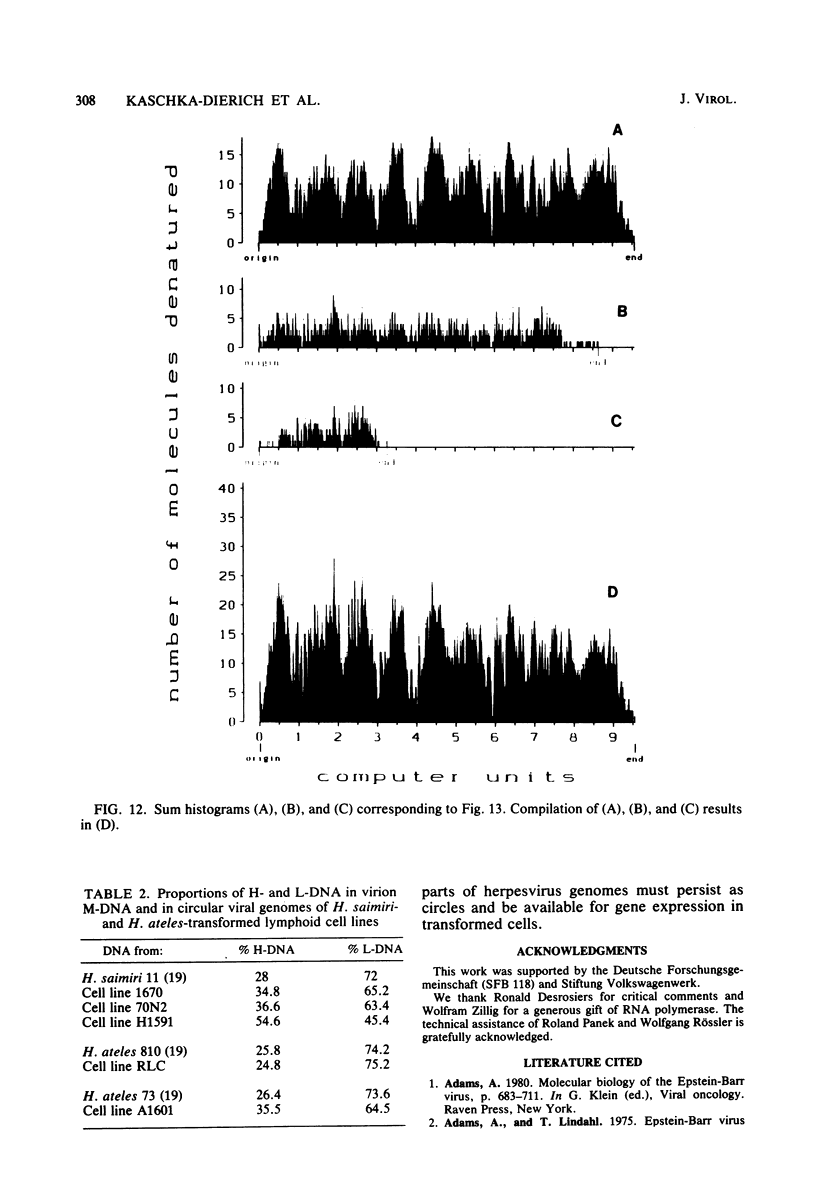
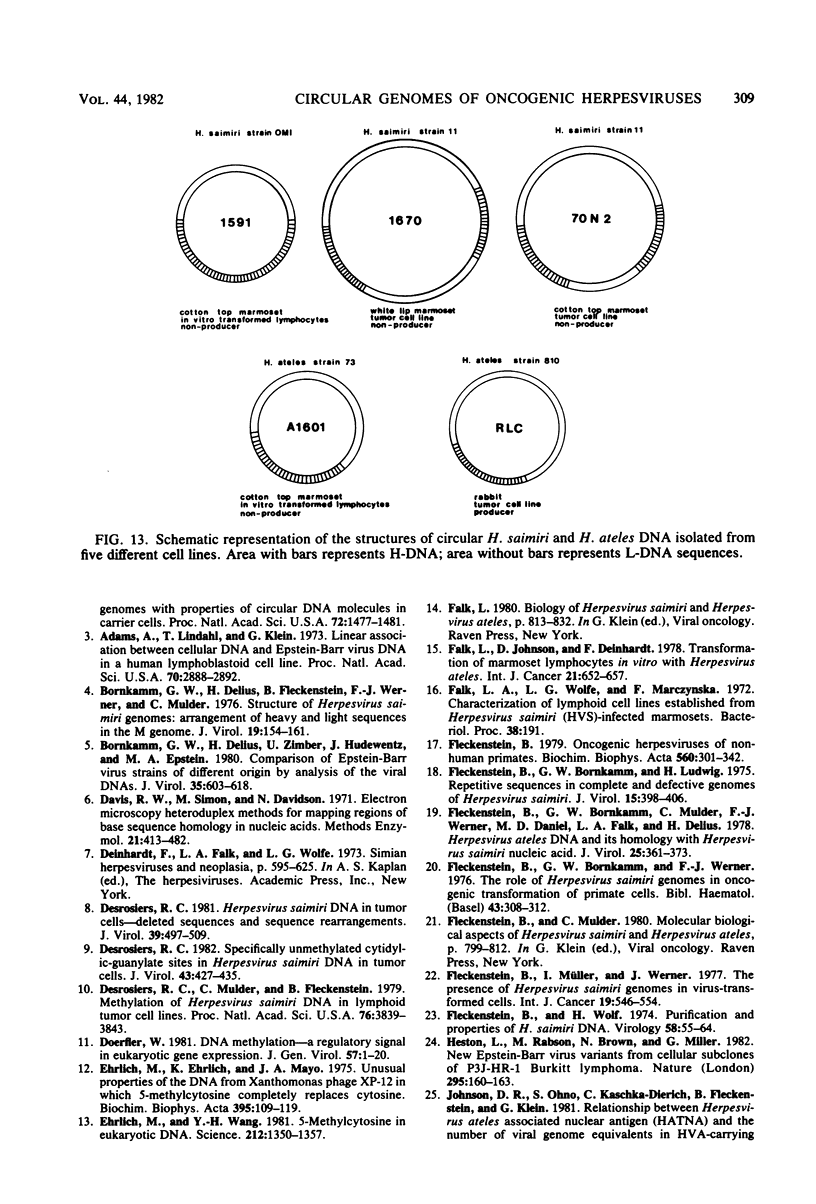
Nonintegrated, circular DNA molecules of Herpesvirus saimiri and Herpesvirus ateles were found in five lymphoid cell lines originating from tumor tissues or established by in vitro immortalization of T lymphocytes. The arrangement of unique (L) and repetitive (H) DNA sequences in circular viral genomes was analyzed by partial denaturation mapping followed by visualization with an electron microscope. Three types of circular viral DNA structures were found. (i) The virus-producing cell line RLC, which is derived from an H. ateles-induced rabbit lymphoma, contains circular viral genomes which consist of a single L-DNA and a single H-DNA region, both the same length as in virion DNA. (ii) The circular viral genomes of the nonproducer cell lines H1591 and A1601, in vitro transformed by H. saimiri and H. ateles, respectively, have deletions in the unique L-DNA region and larger H-DNA regions. Cell line A1601 lacks about 8% of virion L-DNA, and H1591 cells lack about 40% of viral L-DNA information. (iii) The nonproducing H. saimiri tumor cell lines 1670 and 70N2 harbor viral genomes with two L-DNA and two H-DNA regions, respectively. Both types of circular molecules have a long and a short L-segment. The sequence arrangements of circular DNA molecules from H. saimiri-transformed cell lines were compared with those of linear virion DNA by computer alignment of partial denaturation histograms. The L-DNA deletion in cell line H1591 was found to map in the right half of the virion DNA. Comparison of the denaturation patterns of both L regions of cell lines 1670 and 70N2 identified the short L regions as subsets of the long L regions. Thus, circular viral DNA molecules of all four nonproducer cell lines represent defective genomes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkamm G. W., Delius H., Zimber U., Hudewentz J., Epstein M. A. Comparison of Epstein-Barr virus strains of different origin by analysis of the viral DNAs. J Virol. 1980 Sep;35(3):603–618. doi: 10.1128/jvi.35.3.603-618.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. Herpesvirus saimiri DNA in tumor cells--deleted sequences and sequence rearrangements. J Virol. 1981 Aug;39(2):497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. Specifically unmethylated cytidylic-guanylate sites in Herpesvirus saimiri DNA in tumor cells. J Virol. 1982 Aug;43(2):427–435. doi: 10.1128/jvi.43.2.427-435.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation--a regulatory signal in eukaryotic gene expression. J Gen Virol. 1981 Nov;57(Pt 1):1–20. doi: 10.1099/0022-1317-57-1-1. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Ehrlich K., Mayo J. A. Unusual properties of the DNA from Xanthomonas phage XP-12 in which 5-methylcytosine completely replaces cytosine. Biochim Biophys Acta. 1975 Jun 16;395(2):109–119. doi: 10.1016/0005-2787(75)90149-5. [DOI] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Falk L., Johnson D., Deinhardt F. Transformation of marmoset lymphocytes in vitro with Herpesvirus ateles. Int J Cancer. 1978 May 15;21(5):652–657. doi: 10.1002/ijc.2910210517. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Ludwig H. Repetitive sequences in complete and defective genomes of Herpesvirus saimiri. J Virol. 1975 Feb;15(2):398–406. doi: 10.1128/jvi.15.2.398-406.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Mulder C., Werner F. J., Daniel M. D., Falk L. A., Delius H. Herpesvirus ateles DNA and its homology with Herpesvirus saimiri nucleic acid. J Virol. 1978 Jan;25(1):361–373. doi: 10.1128/jvi.25.1.361-373.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Werner F. J. The role of Herpesvirus saimiri genomes in oncogenic transformation of primate cells. Bibl Haematol. 1975 Oct;(43):308–312. doi: 10.1159/000399154. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Werner J. The presence of Herpesvirus Saimiri genomes in virus-transformed cells. Int J Cancer. 1977 Apr 15;19(4):546–554. doi: 10.1002/ijc.2910190416. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B. Oncogenic herpesviruses of non-human primates. Biochim Biophys Acta. 1979 Nov 30;560(3):301–342. doi: 10.1016/0304-419x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B., Wolf H. Purification and properties of Herpesvirus saimiri DNA. Virology. 1974 Mar;58(1):55–64. doi: 10.1016/0042-6822(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Adams A., Lindahl T., Bornkamm G. W., Bjursell G., Klein G., Giovanella B. C., Singh S. Intracellular forms of Epstein-Barr virus DNA in human tumour cells in vivo. Nature. 1976 Mar 25;260(5549):302–306. doi: 10.1038/260302a0. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Bauer I., Fleckenstein B., Desrosiers R. C. Episomal and nonepisomal herpesvirus DNA in lymphoid tumor cell lines. Haematol Blood Transfus. 1981;26:197–203. doi: 10.1007/978-3-642-67984-1_32. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Kemp J. D., Sutton D. W. A chemical and physical method for determining the complete base composition of plant DNA. Biochim Biophys Acta. 1976 Mar 4;425(2):148–156. doi: 10.1016/0005-2787(76)90020-4. [DOI] [PubMed] [Google Scholar]
- Kirk J. T. Effect of methylation of cytosine residues on the buoyant density of DNA in caesium chloride solution. J Mol Biol. 1967 Aug 28;28(1):171–172. doi: 10.1016/s0022-2836(67)80087-1. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Melendez L. V., Hunt R. D., King N. W., Barahona H. H., Daniel M. D., Fraser C. E., Garcia F. G. Herpesvirus ateles, a new lymphoma virus of monkeys. Nat New Biol. 1972 Feb 9;235(58):182–184. doi: 10.1038/newbio235182b0. [DOI] [PubMed] [Google Scholar]
- Meléndez L. V., Daniel M. D., Hunt R. D., Fraser C. E., Garciá F. G., King N. W., Williamson M. E. Herpesvirus saimiri. V. Further evidence to consider this virus as the etiological agent of a lethal disease in primates which resembles a malignant lymphoma. J Natl Cancer Inst. 1970 May;44(5):1175–1181. [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Nonoyama M. Latent DNA of Epstein-Barr virus: separation from high-molecular-weight cell DNA in a neutral glycerol gradient. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4658–4661. doi: 10.1073/pnas.71.12.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Silver S., Nonoyama M. Biochemical evidence of the nonintegrated status of Marek's disease virus DNA in virus-transformed lymphoblastoid cells of chicken. Virology. 1978 Jul 1;88(1):19–24. doi: 10.1016/0042-6822(78)90105-8. [DOI] [PubMed] [Google Scholar]
- Tracy S., Desrosiers R. C. RNA from unique and repetitive DNA sequences of Herpesvirus saimiri. Virology. 1980 Jan 15;100(1):204–207. doi: 10.1016/0042-6822(80)90569-3. [DOI] [PubMed] [Google Scholar]
- Werner F. J., Bornkamm G. W., Fleckenstein B. Episomal viral DNA in a Herpesvirus saimiri-transformed lymphoid cell line. J Virol. 1977 Jun;22(3):794–803. doi: 10.1128/jvi.22.3.794-803.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]







