SUMMARY
Neutrophils are the predominant inflammatory cells found in the vaginal discharge of patients with Trichomonas vaginalis infection. However, it is not known whether neutrophil apoptosis is induced by live T. vaginalis. Therefore, we examined whether T. vaginalis can influence neutrophil apoptosis, and also whether caspase-3 and the Bcl-2 family members are involved in the apoptosis. Thus, human neutrophils were incubated with live T. vaginalis and neutrophil apoptosis was evaluated by Giemsa, annexin V-PI, and DiOC6 stainings. The neutrophil apoptosis was significantly higher in those incubated with T. vaginalis than in the control group. When trichomonads were pre-treated with mAb to AP65 (adhesin protein), or when trophozoites were separated from neutrophils using a Transwell chamber, neutrophil apoptosis was significantly reduced. The activation of caspase-3 was evident in neutrophils undergoing spontaneous apoptosis but was markedly enhanced during T. vaginalis-induced apoptosis. Moreover, the inhibition of caspase-3 effectively reduced T. vaginalis-induced apoptosis. Trichomonad-induced apoptosis was also associated with reduced expression of the neutrophil anti-apoptotic protein, Mcl-1. These results indicate that T. vaginalis alters Mcl-1 expression and caspase-3 activation, thereby inducing apoptosis of human neutrophils.
Keywords: Trichomonas vaginalis, neutrophil, apoptosis, caspase-3, Mcl-1
INTRODUCTION
Trichomonas vaginalis commonly causes vaginitis and perhaps cervicitis in women and urethritis in both sexes (1). In pregnant women, trichomonads are implicated in the premature rupture of membranes, premature delivery, and the delivery of low birth weight infants (2,3). In addition, trichomoniasis has been implicated as a risk factor for human immunodeficiency virus (HIV) transmission (4,5). More than 173 million people worldwide are infected annually by this parasite (6) and its prevalence rate in Kuri City, Korea, was reported to be 10·4% (7).
Although T. vaginalis is known to be a non-invasive microorganism, it elicits the vaginal mucosal infiltration of inflammatory cells, especially neutrophils. As proof of neutrophil infiltration, Rein et al. (8) reported a polymorphonuclear leucocytes (PMN)/epithelial cell ratio > 1 in 47 of 65 (72%) women with trichomoniasis by wet mount, and 15% of these women had PMN/epithelial cell ratios > 10. Also, PMN fused with trichomonads have been detected in vaginal smears of trichomoniasis by microscopic observations (9).
Only a few studies have been carried out on the response of neutrophils to T. vaginalis infection. T. vaginalis-induced recruitment of neutrophils is known to be mediated via the IL-8 expressed by neutrophils in response to activation by live T. vaginalis (10), although the role of infiltrated neutrophils in the pathogenesis of T. vaginalis infection has not yet been clearly characterized.
Neutrophils have a shorter life span than other leucocytes. After egress from the bone marrow, neutrophils leave the circulation within 6-10 h and migrate into tissues, where they undergo constitutive apoptosis in 1-2 days. However, the life span of neutrophils can be either prolonged or shortened by signals from the micro-environment (11). Various inflammatory mediators such as cytokines and bacterial products, and local conditions such as hypoxia and the expression of Fas/Fas ligand, are known to promote or suppress neutrophil apoptosis at the site of infection, thus regulating the progression of inflammatory responses (12). However, no report to date has described neutrophil apoptosis after T. vaginalis stimulation.
Recent studies implicate the involvement of caspases and members of the Bcl-2 protein family in the regulation and execution of neutrophil apoptosis (13). The caspases, a family of cysteine proteases, are activated by a set of pro-apoptotic signals (14). Caspase-3, also called executioner caspase, recognizes and cleaves the sequence Asp-Glu-Val-Asp (DEVD) in poly-ADP-ribose polymerase (PARP), resulting in irreversible nuclear alteration (15). In fact, a caspase-3 null cell line does not exhibit DNA fragmentation or some of the distinct morphological features typical of apoptotic cells such as shrinkage or blebbing (16). The Bcl-2 protein family constitutes an important intracellular checkpoint of apoptosis within a common cell death pathway. Recently, multiple Bcl-2 homologues have been identified as either pro-apoptotic or anti-apoptotic checkpoints upstream of the caspases (17-19). Moreover, the balance between pro-apoptotic and anti-apoptotic pathways determines the fate of cells in many systems.
In this study, to elucidate the role of neutrophils in vaginal inflammation caused by T. vaginalis infection, we examined neutrophil apoptosis induced by live T. vaginalis and evaluated the role of regulatory factors such as caspase-3 and Bcl-2 protein family, which are involved in neutrophil apoptosis. We demonstrate that co-incubation with T. vaginalis results in the apoptosis of neutrophils, and that this is associated with an increase of caspase-3 activity and Mcl-1 protein levels.
MATERIALS AND METHODS
Reagents
Cycloheximide, PIPES [piperazine-N,N’-bis(2-ethanesulphonic acid)], and Histopaque 1077 were purchased from Sigma (St Louis, MO, USA). 3,3′-Dihexyloxacarbocyanine iodide (DiOC6) was from Molecular Probes (Eugene, OR, USA). Caspase-3 inhibitor zDEVD-fmk and caspase-8 inhibitor zIETD-fmk were from Calbiochem (Nottingham, UK), Dextran T500 was from Pharmacia (Uppsala, Sweden), and fetal bovine serum and Trizol reagent were from Gibco BRL (Gaithersburg, MD, USA).
Culture of Trichomonas vaginalis
The Trichomonas vaginalis isolate KT-4, used in the present study, was isolated from a Korean female with acute vaginitis (20). Parasites were grown in a complex trypticase-yeast extract-maltose (TYM) medium supplemented with 10% heat-inactivated horse serum (21).
Isolation of human neutrophils
Fresh human blood was drawn from healthy donors and treated with heparin, and neutrophils were isolated from it using a method previously described, with minor modifications (22). Briefly, 10 volumes of blood were mixed with 2 volumes of dextran [4·5% dextran T500 suspended in PIPES buffer (25 mm PIPES, 50 mm NaCl, 5 mm KCl, 25 mm NaOH, 5·4 mm glucose, pH 7·4)]. After 30 min sedimentation at 37°C, neutrophils were obtained by layering on Ficoll-Hypaque (Histopaque 1077). After centrifuging at 385 g for 30 min at 4°C, the supernatant and mononuclear cells at the interface were carefully removed. The inside wall of the centrifuge tube was wiped twice with sterile gauze to remove adhering mononuclear cells. Erythrocytes in the sediment were lysed twice with sterile distilled water. Cell viability was determined using the trypan blue exclusion test (> 99%). Purity of neutrophils was confirmed morphologically (> 98%) and monocyte contamination was examined by phenotypic analysis using flow cytometry (Becton Dickinson, San Jose, CA, USA) after staining with fluorescein isothiocyanate (FITC)-conjugated anti-CD14 Ab (< 0·02%).
Culture conditions for neutrophil apoptosis
Freshly isolated neutrophils were cultured in suspension with RPMI-1640 medium supplemented with 10 mm HEPES and 10% fetal bovine serum. In a preliminary experiment to obtain an optimal neutrophil/trichomonad ratio for enhanced apoptosis, neutrophils (1 × 106) were co-cultured with live trophozoites at various neutrophil/trichomonad ratios (50: 1-10: 1) for 12 h, and the optimal ratio for increased apoptosis was found to be 10: 1; therefore 1 × 106 neutrophils were co-cultured with 1 × 105 live trophozoites in the subsequent experiments. For a positive control, neutrophils were treated with cycloheximide (20 μg/mL) to induce apoptosis.
To confirm whether close attachment of T. vaginalis to neutrophils was critical for induction of apoptosis, we used the 24-well Transwell insert system (Costar, Cambridge, MA, USA). These inserts, which have a porous (pore diameter 3 μm) membrane, serve as the upper chambers and ordinary tissue culture plate wells serve as the lower chambers. Medium containing trophozoites (2 × 105) was added to the upper chambers and neutrophil suspension (2 × 106) was added to the lower chamber. The plates were then incubated for 12 h, and apoptosis of neutrophils was determined.
To investigate whether adhesin protein of T. vaginalis affects neutrophil apoptosis, mAb to AP65 (a major adhesion protein of T. vaginalis) or a matched isotype control Ab (mouse IgG1, Sigma) was incubated with T. vaginalis at 37°C for 2 h. The mAb to AP65 was a mixture of 3 mAbs: anti-12G4 mAb, anti-DM116 mAb and anti-C55 mAb (23). After pre-incubation with the mAbs, the trichomonads were gently washed with culture medium prior to the 12 h co-culture with neutrophils.
Assays for neutrophil apoptosis
Morphologic assessment
Cellular morphology was ascertained by light microscopy following Giemsa staining of cytocentrifuged neutrophils. In addition, transmission electron microscopic observation was also carried out. A cytocentrifuge sample of 1 × 105 cells was prepared using a Shandon cytospin centrifuge (Shandon, UK) and was stained with Giemsa (12). Apoptosis was characterized by condensed and fragmented nuclei.
Transmission electron microscopy was conducted as follows: neutrophils (1 × 107) were incubated for 12 h, fixed with 3% glutaraldehyde in 0·1 m phosphate buffer (pH 7·3) for 3 h, washed three times, and postfixed with 1% osmium tetroxide in 0·1 m phosphate buffer for 2 h. The specimens were then dehydrated in an ethanol gradient, embedded in Epon, and polymerized at 60°C for 48 h. Ultrathin sections (50-70 nm) were stained with 1% uranyl acetate followed by lead citrate, and examined under a transmission electron microscope (H-7600S, Hitachi, Japan) (24).
Flow cytometric analysis
FITC-conjugated annexin V, which binds to phosphatidylserine, and propidium iodide (PI) were added to 1 × 105 cell suspension according to the manufacturer’s instructions (Apoptosis Detection Kit, R&D Systems, Minneapolis, MN, USA) and then incubated for 15 min at room temperature in the dark. Subsequently, the cells were analysed by flow cytometry. Early apoptotic cells were stained with annexin V alone, whereas necrotic cells and late apoptotic cells were stained with both annexin V and PI.
Reduced mitochondrial transmembrane potential is known to occur late in the apoptotic process. In the present study, mitochondrial transmembrane potential was assessed using DiOC6 staining and flow cytometry. Briefly, neutrophils (2 × 106) were co-cultured with live trophozoites (2 × 105/mL) for 1-24 h and washed with cold PBS, after which cells (5 × 105/mL) were incubated with DiOC6 (40 nm in PBS) for 15 min at 37°C (23). A total of 10 000 cells were analysed by a flow cytometry using a FACSCalibur with CellQuest pro software (BD Bioscience, Germany). To distinguish neutrophils from T. vaginalis, forward and side scatter were simultaneously measured at each incubation time.
Determination of caspase-3 activity
Activation of caspase-3 was determined by detection of the chromophore p-nitroanilide (pNA) formed by the cleavage of the labelled caspase-3 substrate Asp-Glu-Val-Asp-pNA (DEVD-pNA) (ApoAlert caspase colorimetric assay kits, Clontech, CA, USA). In brief, 2 × 106 cells were lysed and incubated with DEVD-pNA for 1 h at 37°C. Optical density was measured at 405 nm, and activity of caspase-3 was estimated by the rate of pNA release (pM/min/2 × 106 cells). Control reactions in each experiment included the caspase-3 inhibitor zDEVD-fmk added prior to the addition of DEVD-pNA. This inhibitor completely blocks caspase-3 activation (25).
Western blot analysis of Bcl-2 family proteins
After neutrophils (2 × 106) were co-cultured with live T. vaginalis (2 × 105) for 5 h, 10 h and 20 h, cell lysates were prepared in ice-cold lysis buffer [20 mm Tris-HCl (pH 7·5) 200 μL, 60 mm β-glycerophosphate 600 μL, 10 mm EDTA 200 μL, 10 mm MgCl2 444 μL, 10 mm NaF 200 μL, 2 mm DTT 20 μL, 1 mm Na3VO4 50 μL, 1 mm APMSF 100 μL, 1% NP-40 100 μL, leupeptin (5 μg/mL) 50 μL, distilled water 8·036 mL, total volume 10 mL]. Twenty μg of each sample was electrophoresed in 12% SDS-PAGE and electroblotted onto nitrocellulose membranes (Hybond ECL, Amersham, Sweden). After blocking overnight at 4°C with 5% skimmed milk, blots were incubated with primary antibodies [anti-Bax mAb, mouse anti-Bak polyclonal Ab (BD PharMingen, San Diego, CA, USA; 2 μg/mL), anti-Bcl-2 mAb, anti-Mcl-1 mAb (Oncogene, CA, USA; No. AM50, 1: 200), and anti-actin mAb (Santa Cruz Biotechnology, Santa Cruz, CA, USA; No. sc-8432, 1: 200) in 1% BSA Tris-buffered saline and 0·1% Tween-20] for 2 h at room temperature. Blots were then incubated with horseradish peroxidase-conjugated IgG (Amersham) for 1 h at room temperature and developed using an enhanced chemiluminescence detection system (ECL, Amersham Pharmacia Biotech, Uppsala, Sweden) (25).
The relative quantity of each protein was determined by densitometric assay. The bands were analysed by quantity one software (version 4·5·0; Bio-Rad, Hercules, CA, USA) and protein quantity was compared after density compensation with an actin control.
Statistical analysis
The results are expressed as mean ± SEM of three to five independent experiments. The Mann-Whitney U-test was used for statistical analysis, and a P-value of < 0·05 was considered statistically significant.
RESULTS
Trichomonas vaginalis-induced apoptosis in neutrophils
To determine whether T. vaginalis induces apoptosis of human neutrophils, neutrophils were incubated with T. vaginalis for 12 h and their morphologies were examined using Giemsa staining. As shown in Figure 1(a), uninfected neutrophils underwent spontaneous apoptosis after 12 h, showing typical apoptotic morphological changes such as chromatin condensation, accompanied with loss of multilobular nuclear structure. However, when incubated with T. vaginalis, the apoptotic process was markedly accelerated (Figure 1b). Transmission electron microscopy showed the fine ultrastructural apoptotic morphologies of neutrophils, including chromatin condensation, crescent-shape chromatin margination, the coalescence of nuclear lobes into a single body, and preservation of cytoplasmic granules and cell membrane integrity (Figure 1c,d).
Figure 1.
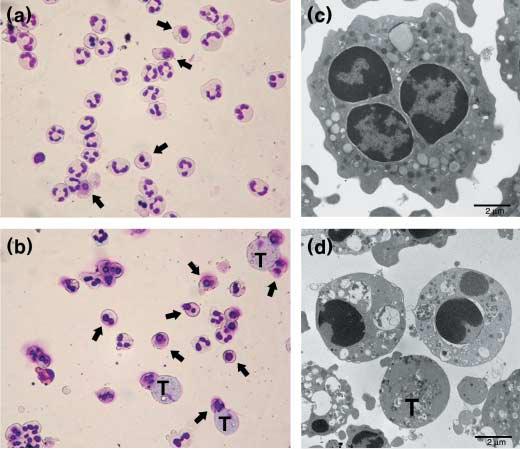
Trichomonas vaginalis induces apoptosis of human neutrophils. (a and b) Cytospin preparations of neutrophils were stained with Giemsa, and apoptotic cells (arrow) were seen morphologically (×400); (c and d) Transmission electron micrographs of neutrophils. The micrographs show neutrophils treated as follows: (a) neutrophils cultured with medium only for 12 h, (b) neutrophils co-cultured with live Trichomonas vaginalis (T) for 12 h, (c) freshly isolated normal neutrophils, (d) neutrophils and T. vaginalis were co-incubated for 12 h at 37°C. Several activated neutrophils surrounded one trichomonad (T). Two neutrophils show typical features of apoptosis (chromatin condensation).
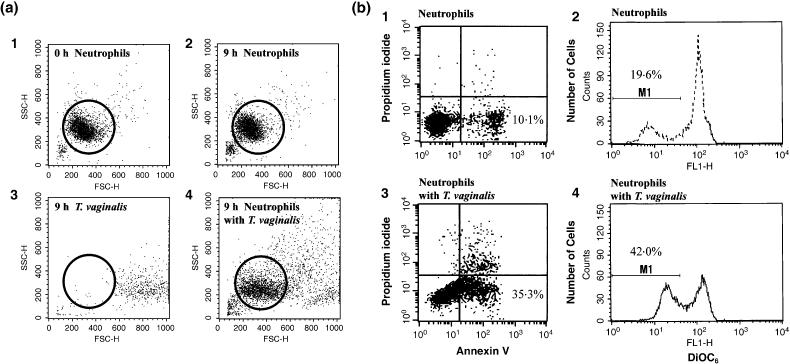
The morphological assessments of apoptosis were further confirmed by two flow cytometric methods. One was flow cytometry using FITC-annexin V stained cells to detect the externalization of phosphatidylserine to the outer leaflet of the cell membrane, and the other was flow cytometry after DiOC6 staining to detect reduced mitochondrial transmembrane potentials (Figure 2b). Neutrophils were distinguished from T. vaginalis by cell size and granularity in flow cytometric analysis (open circle; Figure 2a). The two staining methods (annexin V and DiOC6) showed similar levels of apoptosis. DiOC6 staining revealed that the spontaneous apoptosis of neutrophils without T. vaginalis increased with time (8·7% at 9 h and 19·6% at 12 h). When treated with T. vaginalis, apoptosis of neutrophils was accelerated showing significant increases of apoptosis at 12 h (26·1% at 9 h and 42·0% at 12 h) (Figure 3). Based on these results, subsequent experiments were performed by treating neutrophils with T. vaginalis for 12 h.
Figure 2.
Flow cytometric analysis of neutrophils co-incubated with live Trichomonas vaginalis. (a) Identification of neutrophils and T. vaginalis by forward/sideward scatter signals. Panels are as follows: (1) freshly isolated neutrophils, (2) neutrophils and (3) trichomonads cultured with medium only for 9 h, (4) neutrophils co-cultured with live trichomonads for 9 h. Open circles represent neutrophils selected for flow cytometric analysis. (b) Neutrophils were incubated with fluorescein isothiocyanate-conjugated annexin V, propidium iodide (1 and 3), and 40 nm DiOC6 (2 and 4) and analysed by flow cytometry. The area marked M1 contains the apoptotic neutrophil population. Neutrophils cultured with medium only for 12 h are shown in 1 and 2. Neutrophils co-cultured with T. vaginalis for 12 h are shown in 3 and 4.
Figure 3.
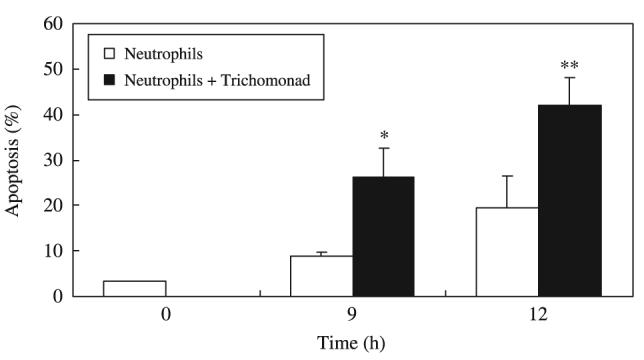
Time course of apoptosis of human neutrophils co-incubated with Trichomonas vaginalis. Neutrophils (1 × 106/mL) were co-cultured with live T. vaginalis (1 × 105/mL) for 0-12 h. The percentage of apoptotic cells was obtained by flow cytometry using DiOC6. The data represent mean ± SEM of four separate experiments. *P < 0·05; **P < 0·01, Neutrophils vs. Neutrophils + T. vaginalis.
The level of neutrophil apoptosis caused by live T. vaginalis was found to be dependent on the number of live trichomonads used. When 1 × 106 neutrophils were incubated with 2 × 104, 5 × 104, or 1 × 105 trichomonads (in neutrophil/trichomonads ratios of 50: 1, 20: 1, or 10: 1) for up to 12 h, the level of apoptosis was enhanced as the number of trophozoites increased (data not shown).
We reported previously that adherence between T. vaginalis and neutrophils is required for stimulation of IL-8 production by neutrophils (10). Adhesin proteins of T. vaginalis are known to be one out of multiple factors involved in adherence of T. vaginalis to vaginal epithelial cells (26-30). As shown in Figure 4, when T. vaginalis was pre-treated with an Ab against adhesin (anti-AP65 mAb), the apoptosis of neutrophils was reduced compared with neutrophils incubated with untreated trichomonads. Neutrophil apoptosis induced by trichomonads pre-treated with a matched isotype control Ab was similar to that induced by T. vaginalis without treatment.
Figure 4.
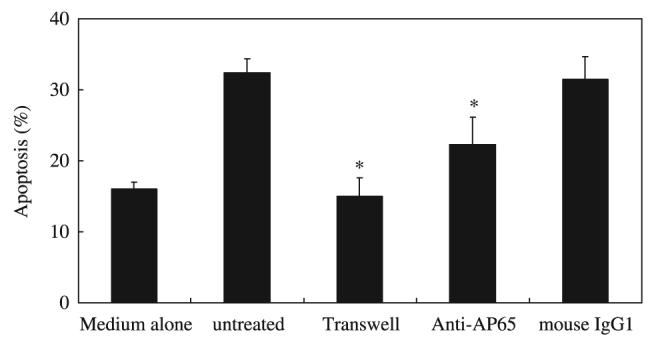
Effect of the adherence of Trichomonas vaginalis to neutrophils on the induction of neutrophil apoptosis. The Transwell chamber was used to prevent adhesion; trophozoites and neutrophils were placed in the upper and lower well, respectively, and incubated for 12 h. ‘Medium alone’ represents neutrophils incubated without T. vaginalis; ‘untreated’ represents neutrophils plus T. vaginalis in the absence of Ab. Apoptosis was also assessed for neutrophils incubated with T. vaginalis pretreated with mAb to adhesin protein AP65 or with an isotype-matched control Ab (mouse IgG1). The percentage of apoptotic cells was determined by flow cytometry using DiOC6. The data represent mean ± SEM of four separate experiments. *P < 0·05; control vs. treatment.
Next, the question of whether contact between T. vaginalis and neutrophils is critical for apoptotic induction was examined using the Transwell insert system. When suspensions containing trophozoites and neutrophils were placed in the upper and lower wells, respectively, and incubated for 12 h, the level of neutrophil apoptosis in the lower well was almost the same as that for neutrophils cultured without parasites (medium alone) (Figure 4).
Activation of caspase-3 in T. vaginalis-induced apoptosis
Caspase-3 is a key enzyme in the spontaneous apoptosis of neutrophils (31). The enzymatic activity of caspase-3 in lysates of neutrophils was measured using a synthetic substrate of caspase-3 (DEVD-pNA), which gives rise to a fluorescent product after cleavage by the activated enzyme. The caspase-3 activity of neutrophils alone incubated for 6 h and 12 h was 27·4 and 48·4 (pM/min/2 × 106 cells), respectively. Trichomonas vaginalis treatment doubled the level of caspase-3 activity compared to untreated neutrophils at 6 h and 12 h (54·8 and 95·9 pM/min/2 × 106 cells, respectively). At 18 h and 24 h, caspase-3 activity had decreased from the level at 12 h (Figure 5a).
Figure 5.

Analysis of caspase-3 activity in neutrophils and effect of caspase inhibitors. (a) Activation of caspase-3 was determined by detection of chromophore p-nitroanilide (pNA) after cleavage of the labelled substrate DEVD-pNA. After co-cultivation of neutrophils with Trichomonas vaginalis for 24 h, cell lysate supernatants were prepared at the designated times. The supernatants were incubated with DEVD-pNA for 1 h at 37°C. Optical density was measured at 405 nm and presented as the ratio of T. vaginalis-treated neutrophils/untreated neutrophils. *P < 0·05; significant difference from control group. (b) Effect of inhibitors of caspase-3 and caspase-8 on spontaneous and T. vaginalis-induced neutrophil apoptosis. Neutrophils (1 × 106/mL) were pre-treated for 10 min at 37°C with 25 mm zDEVD-fmk or zIETD-fmk to inhibit caspase-3 and 8, respectively. The cells were subsequently incubated with T. vaginalis (neutrophils/T. vaginalis, 10: 1) or in medium alone and then cultured for 12 h. Untreated cells (no inhibitors) were cultured with or without trichomonads for the same period of time. The percentage of apoptotic cells was obtained by flow cytometry using DiOC6. The data represent mean ± SEM of four separate experiments. (*, significantly different from cells not exposed to inhibitors; P < 0·05)
We then attempted to identify the caspases involved in T. vaginalis-induced apoptosis in neutrophils by using different caspase inhibitors. Thus, neutrophils were pre-treated with 25 μm zDEVD-fmk or zIETD-fmk, which inhibit caspase-3 and -8, respectively (25), and then the cells were exposed to T. vaginalis for 12 h. As shown in Figure 5(b), the inhibition of caspase-3 activity effectively reduced T. vaginalis-induced apoptosis from 48·2% (neutrophils alone) to 21·7% (neutrophils and zDEVD-fmk). Spontaneous apoptosis was also partially inhibited by zDEVD-fmk. In contrast, T. vaginalis-induced apoptosis was only slightly reduced by the caspase-8 inhibitor, zIETD-fmk, indicating the major involvement of caspase-3 in the neutrophil apoptosis.
Mcl-1 attenuation in neutrophils activated by T. vaginalis
To determine the changes of Bcl-2 family proteins, Western blotting was performed on protein extracts of neutrophils cultured in media alone or activated with T. vaginalis. An anti-apoptotic protein, Mcl-1 (13), expression was found to decrease in neutrophils incubated with T. vaginalis for 10 h and 20 h. The ratios of relative protein intensity (neutrophils with T. vaginalis/neutrophils only) were 1·10, 0·54 and 0·63 at 5 h, 10 h, and 20 h, respectively. By contrast, Bax and Bak protein levels were not different between T. vaginalis-treated and untreated neutrophils, although decreased intensity of bands was observed in all cell preparations after 10 h (Figure 6). Bcl-2 expression was undetectable in both infected and uninfected neutrophils.
Figure 6.
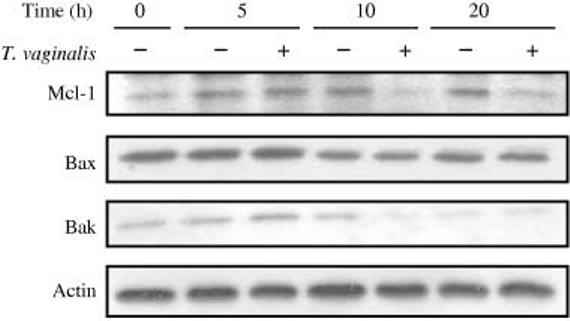
Western blot analysis of Bcl-2 family protein expression. After neutrophils were co-cultured with T. vaginalis for 5, 10 and 20 h, cell lysates were electrophoresed in 12% SDS-PAGE and electroblotted on nitrocellulose membranes. Blots were incubated with primary and horseradish peroxidase-conjugated secondary antibodies and then developed using an enhanced chemiluminescence detection kit. Representative Western blots of three separate experiments are shown.
DISCUSSION
In the present study employing light and electron microscopy and flow cytometry, live T. vaginalis was found to increase neutrophil apoptosis. Flow cytometric analysis using both annexin V-PI and DiOC6 staining showed similar rates of neutrophil apoptosis by both methods. Annexin V binds phosphatidylserine, which is exposed in the outer leaflet of the phospholipid bilayer during apoptosis or necrosis. Cells that retain membrane integrity, including viable and early apoptotic cells, do not take up PI. Therefore, the combined use of annexin and PI can be used to distinguish early-apoptotic from late-apoptotic or necrotic cells. DiOC6 staining was employed to follow changes of mitochondrial transmembrane potential in apoptotic neutrophils. Flow cytometric measurement of neutrophil apoptosis induced by interaction between neutrophil and live T. vaginalis has been thought to be difficult due to the similar sizes of T. vaginalis and neutrophils making it difficult to identify the two cell populations. However, we show that trichomonads can be differentiated from neutrophils on the basis of their higher forward scatter allowing each of the two populations to be gated separately.
An mAb against AP65, one of the four major adhesin proteins of T. vaginalis, reduced the level of neutrophil apoptosis induced by the parasite. The apoptosis-delaying effect of this anti-adhesin mAb is probably due to the inhibition of trichomonad adherence to neutrophils, since anti-AP65 mAb has been reported to reduce T. vaginalis adherence to vaginal epithelial cells (26). Using a Transwell chamber, we investigated the importance of contact between neutrophils and T. vaginalis for neutrophil apoptosis. The results of the Transwell experiments indicate that direct contact between neutrophils and T. vaginalis - most likely mediated by AP65 - is essential for neutrophil apoptosis to occur.
Human neutrophils are known to express the mRNAs of anti-apoptotic proteins, such as Mcl-1 and A1 (13), and levels of Mcl-1 protein have been reported to correlate with neutrophil survival (32,33). When neutrophils were pre-treated with GM-CSF or LPS (both of which are known to delay neutrophil apoptosis), Mcl-1 protein expression was increased (13). In our study, Mcl-1 protein levels in neutrophils were decreased after incubation with T. vaginalis. This result is in agreement with the finding of Moulding et al. (32) in which levels of Mcl-1 protein decreased prior to the onset of apoptosis.
The execution of the apoptotic pathway is mediated by caspases and human neutrophils express several different caspases; however, caspase-3 and -8 are known to be mainly activated during apoptosis (11). We found that the inhibition of caspase-3, but not caspase-8, effectively blocked T. vaginalis-induced apoptosis of neutrophils, and that stimulation with trichomonads markedly up-regulated caspase-3 activity. This suggests that T. vaginalis-induced apoptosis of neutrophil is mediated primarily through caspase-3 activation.
There are several studies that examine the role of increased neutrophil apoptosis in the pathogenesis of a variety of infectious diseases (34-37). Induction of apoptosis of cells associated with innate or adaptive immunity would appear to offer a means of countering a host antimicrobial defence measure. For example, induction of the apoptotic death of mononuclear and polymorphonuclear leucocytes by Fusobacterium nucleatum has been suggested to mediate immunosuppression in periodontal disease (38). It is also known that the apoptosis of neutrophils following phagocytosis by macrophages or fibroblasts provides a means of safely removing neutrophils from sites of inflammation, thus minimizing the risk of bystander tissue damage. This safe removal of neutrophils is essentially required for resolution of inflammation (39,40). Neutrophil infiltration is a ubiquitous feature of T. vaginalis-associated vaginitis (41,42) and is considered to be primarily responsible for the cytological damage (1,43); however, its role in the pathogenesis of trichomoniasis has not yet been clearly characterized.
In our previous study, we observed the production of IL-8 and Gro-α by neutrophils when stimulated with live T. vaginalis (10); these cytokines may subsequently induce more infiltration and recruitment of neutrophils by chemotaxis at the reaction site, and neutrophil accumulations are thought to cause continued inflammation and/or aggravated vaginal inflammation. In the present study, T. vaginalis was found to increase neutrophil apoptosis, which may contribute to the resolution of acute trichomoniasis. We are in the process of determining whether macrophages can phagocytose the apoptotic neutrophils induced by T. vaginalis. This would strongly suggest that increased neutrophil apoptosis by T. vaginalis contributes to the resolution of inflammation.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Research Foundation of the Korean Government (MOEHRD) R04-2003-000-10081-0 and by Hanyang University Korea, made in the program year of 2002. We thank Professor Woon Ki Paik at Hanyang University for his critical review of the manuscript, and Mr Han-Kyu Choi and Ms Ha-Young Park for expert technical assistance.
REFERENCES
- 1.Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation and laboratory diagnosis. J Infect Dis. 1993;141:137–143. doi: 10.1093/infdis/141.2.137. [DOI] [PubMed] [Google Scholar]
- 2.Cotch MF, Pastorek JG, 2nd, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Soper DE, Bump RC, Hurt WG. Bacterial vaginosis and trichomonas vaginitis are risk factors for cuff cellulitis after abdominal hysterectomy. Am J Obstet Gynecol. 1990;163:1016–1023. doi: 10.1016/0002-9378(90)91115-s. [DOI] [PubMed] [Google Scholar]
- 4.Laga M, Manoka A, Kivuvu M, et al. Non nucleated sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Sorvillo F, Smith L, Kerndt P, Ash L. Trichomonas vaginalis, HIV, and African-Americans. Emerg Infect Dis. 2001;7:927–932. doi: 10.3201/eid0706.010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections: Overview and Estimates. WHO; Geneva, Switzerland: 2001. [Google Scholar]
- 7.Ryu JS, Chung HI, Min DY, Cho YH, Ro YS, Kim SR. Diagnosis of trichomoniasis by polymerase chain reaction. Yonsei Med J. 1999;40:56–60. doi: 10.3349/ymj.1999.40.1.56. [DOI] [PubMed] [Google Scholar]
- 8.Rein MF, Sullivan JA, Mandell GL. Trichomonacidal activity of human polymorphonuclear neutrophils: Killing by disruption and fragmentation. J Infect Dis. 1980;142:575–585. doi: 10.1093/infdis/142.4.575. [DOI] [PubMed] [Google Scholar]
- 9.Demirezen S, Safi Z, Beksac S. The interaction of Trichomonas vaginalis with epithelial cells, polymorphonuclear leukocytes and erythrocytes on vaginal smears: light microscopic observation. Cytopathology. 2000;11:326–332. doi: 10.1046/j.1365-2303.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 10.Ryu JS, Kang JH, Jung SY, et al. Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun. 2004;72:1326–1332. doi: 10.1128/IAI.72.3.1326-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maianski NA, Maianski AN, Kuijpers TW, Roos D. Apoptosis of neutrophils. Acta Haematol. 2004;111:56–66. doi: 10.1159/000074486. [DOI] [PubMed] [Google Scholar]
- 12.Perskvist N, Long M, Stendahl O, Zheng L. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-XL via an oxygen-dependent pathway. J Immunol. 2002;168:6358–6365. doi: 10.4049/jimmunol.168.12.6358. [DOI] [PubMed] [Google Scholar]
- 13.Akgul C, Moulding DA, Edwards SW. Molecular control of neutrophil apoptosis. FEBS Lett. 2001;487:318–322. doi: 10.1016/s0014-5793(00)02324-3. [DOI] [PubMed] [Google Scholar]
- 14.Fadeel B, Orrenius S, Zhivotovsky B. Apoptosis in human disease: a new skin for the old ceremony? Biochem Biophys Res Commun. 1999;266:699–717. doi: 10.1006/bbrc.1999.1888. [DOI] [PubMed] [Google Scholar]
- 15.Depraetere V, Golstein P. Dismantling in cell death: molecular mechanism and relationship to caspase activation. Scand J Immunol. 1998;47:523–531. doi: 10.1046/j.1365-3083.1998.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- 17.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 18.Chao DT, Korsmeyer SJ. Bcl-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 19.Reed JC. Double identity for proteins of the Bcl-2 family. Nature. 1997;387:773–776. doi: 10.1038/42867. [DOI] [PubMed] [Google Scholar]
- 20.Ryu JS, Choi HK, Min DY, Ha SE, Ahn MH. Effect of iron on the virulence of Trichomonas vaginalis. J Parasitol. 2001;87:457–460. doi: 10.1645/0022-3395(2001)087[0457:EOIOTV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Diamond LS. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957;43:488–490. [PubMed] [Google Scholar]
- 22.Sim S, Yong TS, Park SJ, et al. NADPH oxidase-derived reactive oxygen species-mediated activation of ERK1/2 is required for apoptosis of human neutrophils induced by Entamoeba histolytica. J Immunol. 2005;174:4279–4288. doi: 10.4049/jimmunol.174.7.4279. [DOI] [PubMed] [Google Scholar]
- 23.Garcia AF, Chang TH, Benchimol M, Klumpp DJ, Lehker MW, Alderete JF. Iron and contact with host cells induce expression of adhesions on surface of Trichomonas vaginalis. Mol Microbiol. 2003;47:1207–1224. doi: 10.1046/j.1365-2958.2003.03366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sim S, Kim KA, Yong TS, Park SJ, Im KI, Shin MH. Ultrastructural observation of human neutrophils during apoptotic cell death triggered by Entamoeba histolytica. Korean J Parasitol. 2004;42:205–208. doi: 10.3347/kjp.2004.42.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JS, Kim JM, Jung HC, Song IS. Caspase-3 activity and expression of Bcl-2 family in human neutrophils by Helicobacter pylori water-soluble proteins. Helicobacter. 2001;6:207–215. doi: 10.1046/j.1523-5378.2001.00030.x. [DOI] [PubMed] [Google Scholar]
- 26.Alderete JF, Lehker MW, Arroyo R. The mechanism and molecules involved in cytoadherence and pathogenesis of Trichomonas vaginalis. Parasitol Today. 1995;11:70–74. [Google Scholar]
- 27.Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete JF. Signaling of Trichomonas vaginalis for amoeboid transformation and adhesin synthesis follows cytoadherence. Mol Microbiol. 1993;7:299–309. doi: 10.1111/j.1365-2958.1993.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert RO, Elia G, Beach DH, Suzanne K, Singh BN. Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect Immun. 2000;68:4200–4206. doi: 10.1128/iai.68.7.4200-4206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krieger JN, Ravdin JI, Rein MF. Contact-depent cytopathogenic mechanism of Trichomonas vaginalis. Infect Immun. 1985;50:778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendoza-Lopez MR, Becerril-Garcia C, Fattel-Facenda LV, et al. CP30, a cysteine proteinase involved in Trichomonas vaginalis cytoadherence. Infect Immun. 2000;68:4907–4912. doi: 10.1128/iai.68.9.4907-4912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pongracz J, Webb O, Wang K, Deacon E, Lunn OJ, Lord JM. Spontaneous neutrophil apoptosis involves caspase 3-mediated activation of protein kinase C-delta. J Biol Chem. 1999;274:37329–37334. doi: 10.1074/jbc.274.52.37329. [DOI] [PubMed] [Google Scholar]
- 32.Moulding DA, Quayle JA, Hart CA, Edwards SW. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. [PubMed] [Google Scholar]
- 33.Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. Bcl-2 family expression in human neutrophils during delayed and accelerated apoptosis. J Leukoc Biol. 2001;70:783–792. [PubMed] [Google Scholar]
- 34.Aga E, Katschinski DM, van Zandbergen G, et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 35.Rotstein D, Parodo J, Taneja R, Marshall JC. Phagocytosis of Candida albicans induces apoptosis of human neutrophils. Shock. 2000;14:278–283. doi: 10.1097/00024382-200014030-00006. [DOI] [PubMed] [Google Scholar]
- 36.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–3992. [PubMed] [Google Scholar]
- 37.Yoshiie K, Kim HY, Mott J, Rikihisa Y. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect Immun. 2000;68:1125–1133. doi: 10.1128/iai.68.3.1125-1133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jewett A, Hume WR, Le H, et al. Induction of apoptotic cell death in peripheral mononuclear and polymorphonuclear cells by an oral bacterium Fusobacterium nucleatum. Infect Immun. 2000;68:1893–1898. doi: 10.1128/iai.68.4.1893-1898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards SW, Moulding DA, Derouet M, Moots RJ. Regulation of neutrophil apoptosis. Chem Immunol Allergy. 2003;83:204–224. doi: 10.1159/000071562. [DOI] [PubMed] [Google Scholar]
- 40.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 41.Buchvald D, Demes P, Gombosova A, Mraz P, Valent M, Stefanovie J. Vaginal leukocyte characteristics in urogenital trichomoniasis. APMIS. 1992;100:393–400. doi: 10.1111/j.1699-0463.1992.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 42.Wolner-Hanssen P, Krieger JN, Stevens CE, et al. Clinical manifestations of vaginal trichomoniasis. JAMA. 1989;261:571–576. doi: 10.1001/jama.1989.03420040109029. [DOI] [PubMed] [Google Scholar]
- 43.Graves A, Gardner WA. Pathogenicity of Trichomonas vaginalis. Clin Obstet Gynecol. 1993;36:145–152. doi: 10.1097/00003081-199303000-00020. [DOI] [PubMed] [Google Scholar]