Abstract
Empathy is a complex social behaviour mediated by a network of brain structures. Recently, several functional imaging studies have investigated the neural basis of empathy, but few corroborative human lesion studies exist. Severe empathy loss is a common feature of frontotemporal lobar degeneration (FTLD), and is also seen in other neurodegenerative diseases. In this study, the neuroanatomic basis of empathy was investigated in 123 patients with FTLD, Alzheimer's disease, corticobasal degeneration and progressive supranuclear palsy using the Interpersonal Reactivity Index (IRI). IRI Empathic Concern and Perspective taking scores were correlated with structural MRI brain volume using voxel-based morphometry. Voxels in the right temporal pole, the right fusiform gyrus, the right caudate and right subcallosal gyrus correlated significantly with total empathy score (P < 0.05 after whole-brain correction for multiple comparisons). Empathy score correlated positively with the volume of right temporal structures in semantic dementia, and with subcallosal gyrus volume in frontotemporal dementia. These findings are consistent with previous research suggesting that a primarily right frontotemporal network of brain regions is involved in emotion processing, and highlights the roles of the right temporal pole and inferior frontal/striatal regions in regulating complex social interactions. This is the first large-scale lesion study to investigate the neural basis of empathy using correlational analytic methods. The results suggest that the right anterior temporal and medial frontal regions are essential for real-life empathic behaviour.
Keywords: dementia, empathy, frontotemporal lobar degeneration, temporal pole, VBM
Introduction
The recent emergence of social cognitive neuroscience has allowed psychological constructs such as empathy to be redefined based on neuroscientific evidence. In a recent review, Decety and Jackson (2004) examine converging lines of evidence from lesion and functional neuroimaging studies suggesting empathy derives from three main cognitive processes. In the first step, the other's emotion is ‘shared’, activating brain areas involved in subjective emotional experience such as the inferior frontal cortex, superior temporal cortex, amygdala, right somatosensory cortex, right temporal pole and right insula (Reiman et al., 1997; Carr et al., 2003). Second, one recognizes that the initiating agent of this subjective emotional experience is the other, not oneself. This ability to determine that the source of an internally represented emotion or intended goal is located outside of oneself requires perspective-taking (PT), which appears to be mediated by a brain circuit including the medial prefrontal cortex (Gallagher and Frith, 2003). This process also requires the capacity to assign agency, which is likely mediated by the heteromodal association area at the junction of the temporal, parietal and occipital lobes (Farrer et al., 2003; Ruby and Decety, 2003, 2004; Saxe and Wexler, 2005). Finally, the ability to accurately infer the other's perspective requires the intentional suppression of one's own viewpoint (Keysar et al., 2003; Royzman et al., 2003; Van Boven and Loewenstein, 2003; Bernstein et al., 2004). Both functional and developmental lesion studies in humans suggest that the frontal pole, approximately corresponding to Brodmann's area (BA) 10, may perform this regulatory function, actively inhibiting the self-perspective in order to allow the other's perspective to be considered (Anderson et al., 1999; Moll et al., 2001; Ruby and Decety, 2003, 2004; Shamay-Tsoory et al., 2003, 2005a).
While functional imaging of healthy controls is necessary to identify the functional circuits involved in empathy (Farrow et al., 2001; Decety and Chaminade, 2003; Shamay-Tsoory et al., 2005b; Vollm et al., 2005; Singer et al., 2006), only complementary data from human lesion studies can provide information about the relative importance of particular structures in those circuits, essentially demonstrating which structures are required for normal empathy in real-life situations and which are not. Many neuropsychiatric disorders are associated with deficits in empathy, including schizophrenia, Asperger's syndrome, sociopathy, post-traumatic brain injury and stroke. However, only a few studies have quantified brain–empathy correlations in patient groups (Shamay-Tsoory et al., 2003, 2005a), and many studies do not measure empathy by measuring the subjects' typical, real-life engagement in empathic behaviour.
A group of diseases that is of particular interest to the question of how brain damage leads to loss of empathy are neurodegenerative conditions that occur after normal social development has been established. Loss of empathy is an early and central symptom of frontotemporal lobar degeneration (FTLD), a focal neurodegenerative disorder involving primarily the frontal and temporal lobes. FTLD patients with predominantly temporal damage show dramatically increased interpersonal coldness (Rankin et al., 2003) and have pathologically low levels of cognitive and emotional empathy, while FTLD patients with primarily frontal atrophy seem to lose the capacity for empathic PT, but do not become significantly colder as a group. Alzheimer's patients, on the other hand, do not typically show significant changes in empathy (Rankin et al., 2005a). Empathy has yet to be directly studied in other neurodegenerative conditions involving behaviour changes, such as corticobasal degeneration (CBD) and progressive supra-nuclear palsy (PSP). Only a few descriptive studies have directly examined the brain structures mediating empathy loss in dementia patients. In a case study of four FTLD patients, Perry found that patients with damage to the temporal cortex of the non-language dominant hemisphere showed loss of empathy (Perry et al., 2001).
Thus, both patient and functional neuroimaging studies suggest that a network of brain regions in the temporal, parietal and frontal lobes is involved in empathy, but additional data from human lesion studies is needed. In this study, a psychometrically validated behaviour inventory was combined with quantitative analysis of structural MRI scans in order to investigate the neural basis of empathy in patients with neurodegenerative disease. The aim of the study was to determine the degree to which regional differences in brain volumes correspond to real-life empathic behaviour.
Material and methods
Subjects
A total of 123 patients diagnosed with one of six neurodegenerative diseases were recruited into the study from a dementia specialty clinic. These included 30 patients who met the Neary criteria for the frontotemporal dementia (FTD) variant of FTLD (typically characterized by bilateral frontal disease and a progressive behavioural syndrome; Rosen et al., 2002a), 26 with the semantic dementia (SeDe) variant of FTLD (typically characterized by left anterior temporal and orbitofrontal atrophy along with profound semantic loss; Boxer et al., 2003) and 8 with the progressive non-fluent aphasia (PNFA) variant of FTLD (typically characterized by focal dorsolateral atrophy of the left frontal lobe and non-fluent speech; Gorno-Tempini et al., 2004). In addition to the FTLD patients, 38 subjects had Alzheimer's disease [diagnosed by National Institute of Neurological Disorders and Stroke-Alzheimer Disease and Related Disorders Association (NINDS-ADRDA) criteria (McKhann et al., 1984)], 15 had CBD (Boxer et al., 2006) and 6 had PSP, diagnosed by the Litvan criteria (Litvan et al., 1996; Boxer et al., 2006). Patient diagnosis was derived by a multidisciplinary team consisting of neurologists, neuropsychologists, psychiatrists and nurses, who performed extensive behavioural, neuropsychological and neuroimaging assessments. Patients from diverse diagnostic groups with variable empathy scores and patterns of grey matter atrophy were included to provide variability in the sample and thus increase the power of the correlation analysis.
Twenty age-matched healthy normal subjects were included as a behavioural control group. Healthy control subjects were recruited through advertisements in local newspapers and recruitment talks at local senior community centres, followed by an extensive multidisciplinary clinical evaluation. For inclusion as healthy controls for this study, subjects had to have a normal neurological exam, a Clinical Dementia Rating (CDR) scale score = 0, Mini-Mental State Examination (MMSE) score ≥28/30 and delayed memory performance ≥25th percentile in both verbal and visuospatial domains. They also had to have had an informant to provide a corroborative report of their functioning.
All subjects and their informant/caregivers signed an institutional-review-board-approved research-consent form to participate in the study. Patients seen at the clinic represented a broad sample of the population in terms of ethnicity, sex, education level and socioeconomic status, and an attempt was made to recruit all available consecutive patients for this study. Subjects' demographic characteristics can be seen in Table 1. Patients' mean age was 63.4 (SD = 9.0), and they averaged 16 (SD = 2.6) years of education. There were 72 males and 51 females, patients' mean CDR score was 0.9 (SD = 0.6). Statistically significant differences were seen across groups in both age and sex, so these variables were included as covariates in all analyses.
Table 1.
Characteristics of patient sample classified by diagnostic group
| M (SD) | FTD (n = 30) | SeDe (n = 26) | PNFA (n = 8) | AD (n = 38) | CBD (n = 15) | PSP (n = 6) | All DX (n = 123) | NC (n = 20) | Overall F(df) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 59.5 (8.7) | 65.5 (7.1) | 58.1 (8.1) | 65.9 (10.4) | 62.9 (7.2) | 65.5 (6.5) | 63.4 (9.0) | 67.9 (7.9) | 3.28 (136, 6) | 0.0048 |
| Education | 16.0 (2.2) | 16.8 (3.0) | 16.4 (1.7) | 15.7 (3.6) | 14.9 (2.3) | 15.7 (2.3) | 16.0 (2.9) | 17.4 (2.7) | 1.44 (136,6) | n.s. |
| Sex (M/F) | 23/7 | 17/9 | 1/7 | 22/16 | 6/9 | 3/3 | 72/51 | 7/13 | χ2 = 17.46 | 0.0077 |
| CDR | 1.2 (0.7) | 0.9 (0.6) | 0.6 (0.6) | 0.8 (0.3) | 1.0 (0.5) | 0.8 (0.3) | 0.9 (0.6) | N/A | 1.56 (117, 5)* | n.s. |
| IRI-EC | 16.9 (5.6)† | 20.0 (9.5)† | 26.8 (3.9) | 26.4 (5.7) | 26.6 (5.1) | 25.5 (6.4) | 22.7 (7.7) | 28.6 (4.2) | 8.05 (134, 8) | <0.0001 |
| IRI-PT | 11.9 (4.8)† | 12.9 (6.3)† | 23.3 (5.6) | 18.2 (6.1)† | 21.1 (7.3) | 19.2 (4.8) | 16.0 (6.8) | 23.7 (5.3) | 9.62 (134, 8) | <0.0001 |
Values are listed as mean (SD).
Derived from Welch's ANOVA due to positive Levine's test suggesting inhomogeneity of variance
differs from NC at P < 0.05 based on a post hoc Dunnett—Hsu test controlling for age and sex.
Empathy testing
The Interpersonal Reactivity Index (IRI) is a questionnaire measure of empathy consisting of four 7-item subscales (Davis, 1983) that has previously been used with dementia patients (Rankin et al., 2005a) and patients with brain injuries (Shamay-Tsoory et al., 2003, 2005a). Two subscales were designed to measure the cognitive elements of empathy: Perspective Taking (PT: the tendency to spontaneously imagine the cognitive perspective of another person) and Fantasy (FS: the tendency to project oneself into the experiences of fictional characters). However, the FS scale has demonstrated problems with construct and criterion validity in that it positively correlates with at least three measures of social dysfunction in males, and correlates more highly with measures of emotionality than cognitive empathy (Davis, 1983). Thus, only the more psychometrically valid PT scale was used for this study to represent cognitive empathy. The second pair of IRI subscales was designed to measure emotional aspects of empathy: Empathic Concern (EC: the other-centred emotional response resulting from the perception of another's emotional state) and Personal Distress (PD: reflecting general anxiety and self-oriented emotional reactivity). The PD scale is negatively correlated with other measures of empathy and social competence (Davis, 1983), and has shown little predictive utility in the differential diagnosis of dementia (Rankin et al., 2005a). Thus only the better-validated EC scale was used in this study to represent the emotional elements of empathy. The PT and EC subscales were summed together to provide a total empathy score, and were also analysed separately for some analyses.
Loss of self-awareness and inaccurate assessment of one's own behaviour are primary symptoms of FTLD (Neary et al., 1998) and are common in other neurodegenerative diseases (Rankin et al., 2005b). Thus, for this study, it was assumed that patients were not necessarily capable of producing a valid assessment of their own capacity for empathy. Collecting data from caregivers has been established as an effective and reliable method for assessing personality change in patients with dementia (McCrae and Costa, 1989; Siegler et al., 1994; Williams et al., 1995), and this method has been used specifically with the IRI (Rankin et al., 2005a). Very high agreement has been found between primary and secondary caregivers on such measures, and caregiver relationship (spouse versus child) has not been found to change the report significantly (Strauss et al., 1993; Heinik et al., 1999). Informants were asked to fill out the IRI describing the subjects' current level of empathy. Raters were selected on a case-by-case basis with consideration given to the informant's frequency of contact with the patient, their described level of closeness, the rater's own cognitive capacity (e.g. in the case of an ageing spouse) and their willingness to participate. Spouses/partners were used whenever possible (79.7%), an adult child if no spouse was available (11.9%) and in some cases a sibling caregiver (6.0%) or parent caregiver (2.4%) was used as an informant. Questionnaires were completed within 3 months before or after the MRI scan, and the average span of time between questionnaire and scan was 4 days (SD = 37 days).
Structural MRI
MRI scans were obtained on a 1.5-T Magnetom VISION system (Siemens, Iselin, NJ) equipped with a standard quadrature head coil. A volumetric magnetization prepared rapid gradient echo MRI (MPRAGE, TR/TE/TI = 10/4/300 ms) was used to obtain T1-weighted images of the entire brain, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness.
Voxel-based morphometry
The voxel-based morphometry (VBM) technique utilizes an image pre-processing step (spatial normalization, segmentation, modulation and smoothing) followed by statistical analysis. Both stages were performed using the SPM2 software package (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm) running on Matlab 6.5.1 (MathWorks, Natick, MA). MRI images were pre-processed following standard procedures of the optimized method (Good et al., 2001). To optimize the spatial normalization of the subjects' images into a common anatomical space, custom a priori and template grey, white, CSF and whole-brain images were created from the T1-weighted scans of 100 subjects with the same diagnoses analysed in this study, including 25 FTLD, 22 SeDe, 8 PNFA, 27 AD, 12 CBD and 6 PSP patients. To improve image spatial pre-processing, a two-step segmentation procedure was applied. First, T1-weighted images were segmented into grey, white and CSF components in native space. Each grey matter image was then normalized to the custom grey matter template. The parameters obtained from the grey matter normalization were then applied to the original anatomical image. Finally, normalized images were segmented again into grey matter, white matter and CSF. A further modulation step was performed by multiplying grey matter voxel values by the Jacobian determinants derived from the spatial normalization step. Spatially normalized, segmented and modulated grey matter images were then spatially smoothed with a 12 mm FWHM isotropic Gaussian kernel.
Empathy analyses
Covariates-only statistical models were used to show the relationship between empathy scores and grey matter volume. Normal controls were not used for any VBM analysis, because by definition they were not expected to have significant variability in either brain volume or empathy score, thus could cause restriction of range in the VBM regressions. Age and sex were entered into the model as nuisance covariates, and total intracranial volume was used as a global covariate to correct for individual differences in head size. Regionally specific differences in grey matter volumes at each voxel were assessed using the general linear model, and the significance of each effect was determined using the theory of Gaussian fields. In all analyses of main effects, the statistical threshold was set at P < 0.05 after whole-brain correction (SPM family-wise error, or FWE) in order to correct for multiple comparisons across many voxels.
First, the main effect of empathy was tested using the total empathy score (sum of EC + PT) in a [1] t-contrast (with additional zeros for nuisance covariates), assuming that decreased empathy would be associated with decreased grey matter volumes in this patient population. In order to investigate whether each of the two empathy subscale scores (EC and PT) showed similar patterns of atrophy, we looked at the separate effects of EC and PT, using two different design matrices and performing a [1] t-contrast with additional zeros for nuisance covariates. Finally, the unique effects of PT and EC were analysed by entering both variables into the same design matrix, and performing [1 0] and [0 1] t-contrasts to determine which voxels were uniquely related to one subscale while controlling for the effects of the other.
Hypotheses
Based on the functional circuit suggested in Decety and Jackson's model of empathy (Decety and Jackson, 2004), the areas of atrophy predicted to correspond to empathy would include the dorsomedial prefrontal cortex (Gallagher and Frith, 2003), the right posterior superior temporal sulcus at the temporal–occipital junction (Allison et al., 2000; Castelli et al., 2000; Saxe and Wexler, 2005) and the right temporal pole (Castelli et al., 2000; Gallagher et al., 2000; Perry et al., 2001).
Results
Behavioural results
An omnibus analysis of variance using a general linear model, controlling for age and sex, showed significant differences in both empathy subscale scores (EC and PT) across subject groups (P < 0.0001) (Table 1). Levine's test for homogeneity of variance was significant for both EC and PT (P < 0.05), so a Welch's ANOVA was used to determine the omnibus F-statistic. FTD and SeDe patients showed significantly lower EC and PT scores than NCs (P < 0.05 based on a post hoc Dunnett–Hsu test controlling for age and sex). Unlike previously published findings using the IRI (Rankin et al., 2005a) with probable AD patients, this group of mixed possible/probable AD patients had significantly worse PT than controls. Examination of the scores across the sample suggests that this may have occurred because a few AD individuals had extremely poor scores that were statistical outliers. Boxplots of empathy scores across the seven subject groups show that scores were widely distributed across each group (Figs 1 and 2).
Fig. 1.
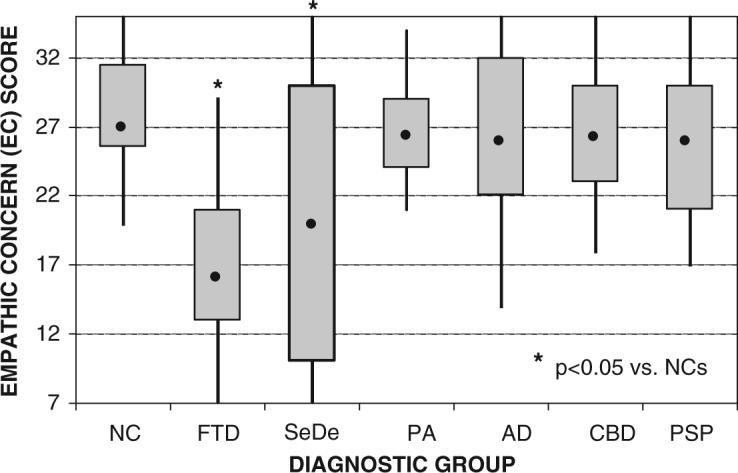
Boxplots of EC score classified by the six diagnostic groups compared with an age-matched healthy comparison group of 25 subjects (NC = normal controls; FTD = frontotemporal dementia variant of FTLD; SeDe = semantic dementia variant of FTLD; PNFA = progressive non-fluent aphasia variant of FTLD; AD = Alzheimer's disease; CBD = corticobasal degeneration; PSP = progressive supranuclear palsy). The dot inside of each plot represents the group's median score. The box represents the range of scores for the group's second and third quartiles. The whiskers represent the score range for the top and bottom quartiles. Group comparisons were performed using a post hoc Dunnett–Hsu test controlling for sex and age.
Fig. 2.

Boxplots of PT score classified by the six diagnostic groups compared with an age-matched healthy comparison group of 20 subjects (NC = normal controls; FTD = frontotemporal dementia variant of FTLD; SeDe = semantic dementia variant of FTLD; PNFA = progressive aphasia variant of FTLD; AD = Alzheimer's disease; CBD = corticobasal degeneration; PSP = progressive supranuclear palsy). The dot inside of each plot represents the group's median score. The box represents the range of scores for the group's second and third quartiles. The whiskers represent the score range for the top and bottom quartiles. Group comparisons were performed using a post hoc Dunnett–Hsu test controlling for sex and age.
Neuroimaging results
Total empathy score
Empathy score (the sum of EC + PT) significantly correlated with grey matter volume in the right temporal pole, the right fusiform gyrus and right medial inferior frontal region (P < 0.05, FWE whole-brain correction) (see Table 2 and Fig. 3). Because the VBM methodology used in this study requires that brains be smoothed to 12 mm in order to correct for variable inter-individual brain morphology, the structure corresponding to this finding cannot be unequivocally identified. Of the two peak voxels in the significant cluster in the main-effects analysis, one set of coordinates corresponds to the subcallosal gyrus, which would be consistent with orbitofrontal/subgenual cingulate damage. The second peak coordinates correspond to the right caudate, and the significant voxels in the cluster follow the curve of the caudate up along the wall of the lateral ventricle, strongly suggesting involvement beyond the cortical grey matter in BA 25. Additionally, the nucleus accumbens is located in the midst of this same region, and appears to be subsumed into this significant cluster. A plot of voxel intensity by total empathy score showed no outliers on the independent variable, and empathy scores were widely distributed throughout the range of voxel intensities, suggesting that there was no restriction of range (see Fig. 4).
Table 2.
Regions where empathy scores positively correlated with grey matter tissue density, corrected for FWE across the whole brain at a significance level of P < 0.05
| Anatomic region | BA | Cluster size | x | y | z | Z-score | FWE |
|---|---|---|---|---|---|---|---|
| Total empathy score (sum of EC + PT) | |||||||
| R temporal pole | 21 | 2421 | 58 | 10 | −33 | 5.52 | 0.001 |
| 20 | 2421 | 40 | 18 | −35 | 4.92 | 0.009 | |
| 38 | 2421 | 41 | 15 | −25 | 4.65 | 0.028 | |
| R anterior fusiform gyrus | 20 | 985 | 39 | −17 | −41 | 5.07 | 0.005 |
| R caudate/subcallosal gyrus | 25 | 1093 | 10 | 10 | −3 | 4.90 | 0.010 |
| 25 | 5 | 20 | −2 | 4.67 | 0.025 | ||
| Main effect of EC subscale | |||||||
| R temporal pole | 21 | 554 | 58 | 10 | −33 | 5.09 | 0.004 |
| 21 | 554 | 48 | 17 | −36 | 4.64 | 0.029 | |
| R caudate/subcallosal gyrus | 25 | 692 | 4 | 22 | −2 | 4.80 | 0.015 |
| R caudate/subcallosal gyrus | 25 | 692 | 11 | 12 | −5 | 4.77 | 0.017 |
| R inferior frontal gyrus | 47 | 3 | 34 | 24 | −11 | 4.53 | 0.046 |
| Main effect of PT subscale | |||||||
| R temporal pole | 21 | 372 | 59 | 9 | −33 | 5.00 | 0.006 |
| R temporal pole | 20 | 171 | 38 | 18 | −34 | 4.65 | 0.028 |
| R anterior fusiform gyrus | 20 | 892 | 40 | −16 | −40 | 4.98 | 0.007 |
| R posterior fusiform gyrus | 30 | 122 | 17 | −33 | −20 | 4.78 | 0.017 |
| R caudate/subcallosal gyrus | 25 | 36 | 7 | 4 | −1 | 4.61 | 0.033 |
Fig. 3.

Continuous, unthresholded map of t-scores for empathy total score analysis across all diagnostic groups, superimposed on a normal control template image (SPM2: single_subj_T1.mnc). The figure represents axial slices taken every 3 mm from z = −51 to z = +18. t-Scores range from 2.00 (blue–black) to 5.91 (red), and include slices from all three clusters containing significant voxels in the analysis.
Fig. 4.
Scatterplot of total empathy score (EC + PT) versus voxel intensity at the most significant voxel in the right anterior temporal lobe cluster (58, 10, −33). Voxel intensities were extracted from the SPM analysis after adjustment for age, sex and total intracranial volume.
Emotional and cognitive empathy subscale scores
The main effect of emotional empathy, as measured by the EC subscale, included voxels at the right temporal pole, the right caudate/subcallosal gyrus and the right inferior frontal gyrus (P < 0.05, FWE whole-brain correction) (Table 2 and Fig. 5). Cognitive empathy, measured with the PT subscale, also included voxels at the right temporal pole and the right caudate/subcallosal gyrus, as well as clusters in both the right anterior and posterior fusiform gyrus (P < 0.05, FWE). These results demonstrate that the anatomic correlates in the total empathy score analysis do not come from only one of the two subscales, but are reflected in both PT and EC.
Fig. 5.

(A) Main effect of EC score, showing rendered, sagittal (x = 7) and axial (z = 0) views of voxels significantly related to EC score at P < 0.001 uncorrected for multiple comparisons across the whole brain. Maps of significant correlation were superimposed on sections of a normal brain template image (SPM2: single_subj_T1.mnc). The design matrix for this analysis contained only EC score, with sex, age and TIV included as nuisance covariates, and a t-test was used. (B) Main effect of PT score, showing voxels significantly related to PT score at P < 0.001 uncorrected. The design matrix for this analysis contained only PT score, with sex, age and TIV included as nuisance covariates, and a t-test was used.
No unique effects of PT or EC were found at a corrected level of significance. Voxels in the right caudate/subcallosal gyrus (2, 25, 1; z = 3.87), the right gyrus rectus of the frontal lobe (13, 18, −13; z = 3.30) and the right cerebellum (26, −43, −39; z = 3.31) showed a trend in the EC analysis when the effects of PT were statistically removed (P < 0.001 uncorrected). Voxels in the left inferior frontal gyrus (−49, 27, −19; z = 3.74), the right cerebellum (16, −33, −19; z = 3.61), the left superior frontal gyrus (−13, 43, 39; z = 3.28) and the right fusiform gyrus (44, −21, −36; z = 3.24) showed a trend in the PT analysis when EC was removed. The significant anatomic overlap between PT and EC (Table 2 and Fig. 5) may have occurred partly because PT and EC scores showed an unusually high correlation in this sample (r = 0.71, P < 0.0001), while in published normative studies using the IRI with normal young adults (n = 770 and n = 460), much smaller correlations (ranging from r = 0.32 to r = 0.38) between EC and PT are typical (Davis, 1983). Future studies using measures of emotional and cognitive empathy that are less correlated with each other are necessary to adequately investigate the differential anatomy of these two aspects of empathy.
Post hoc analyses
Group analysis comparing atrophy patterns in patients with high versus low empathy scores
In order to explore whether these results were due to methodological artefact, a confirmatory group analysis was performed to examine whether patients with low empathy scores would show greater atrophy in the regions highlighted by the correlation analysis. The 31 patients with the lowest total empathy score (PT + EC), representing the bottom quartile of empathy, were compared with the 31 patients in the top quartile, again entering age, sex and TIV into the model as nuisance covariates. We accepted a level of significance of P < 0.001 uncorrected for multiple comparisons within the brain areas of interest previously identified in the correlation analyses (right temporal pole, anterior and posterior fusiform gyrus, caudate/subcallosal gyrus, inferior frontal gyrus), and accepted a P < 0.05 (FWE) level of significance for areas outside of these regions of interest. This group analysis confirmed the correlational results, showing that patients with the highest empathy scores had significantly more atrophy in the right temporal pole (46, 17, −38; z = 4.08), the right anterior fusiform gyrus (46, 17, −38; z = 4.38) and the right caudate/subcallosal gyrus (12, 8, −7; z = 4.06). No other area showed greater atrophy in the low empathy patients.
Diagnostic subgroup interactions
Because the different dementias in our sample have been associated with diverse patterns of brain atrophy, we explored whether different parts of the empathy circuit identified in the main effect results were contributed predominantly by a single diagnostic group. The three diagnostic subgroups showing some behavioural empathy deficits (FTD, SeDe and AD) were analysed separately from the rest of the sample in a series of group by covariate analyses. For these analyses, total empathy score (PT + EC) was used as the covariate of interest and diagnostic group was modelled as an interaction (30 FTD subjects versus all non-FTD subjects for the first design matrix; 26 SeDe subjects versus all non-SeDe subjects for the second design matrix; 38 AD subjects versus all non-AD subjects for the third design matrix) using [0 0 1 0] contrasts. The significance levels inside and outside a priori areas of interest were defined the same way as in the post hoc quartile analysis described above. Patients with FTD showed voxels in the right subcallosal gyrus (8, 27,−3; z = 3.90) that positively correlated with empathy, while SeDe patients showed voxels in the right temporal pole (44, 17, −35; z = 4.30) that positively correlated with empathy. No voxels in any of the a priori regions of interest were significantly related to empathy in the AD group. No voxels outside of these areas were significant in any of these diagnostic groups.
Discussion
VBM was used to correlate MRI-derived brain volumes with an observer-based measure of empathy in patients with neurodegenerative disease. The primary finding was that lower levels of empathy corresponded most significantly with atrophy of the right temporal pole, the right anterior fusiform gyrus and the right medial inferior frontal cortex. The finding that empathy corresponds with multiple areas in the frontal and temporal lobes is consistent with previous fMRI studies (Farrow et al., 2001; Decety and Chaminade, 2003; Shamay-Tsoory et al., 2005b; Vollm et al., 2005), but further elucidates which brain areas are likely to directly result in empathy loss when damaged.
Temporal lobe structures
Our finding that empathy loss correlates with damage to the right anterior temporal lobe complements functional imaging evidence that has suggested that this area is involved in emotion processing (Lane et al., 1997; Carr et al., 2003) and different aspects of person-perception (Adolphs, 1999; Allison et al., 2000). Both left and right temporal poles may be functionally recruited when normal controls perform empathic tasks (Reiman et al., 1997; Carr et al., 2003; Vollm et al., 2005). However, our data suggest that damage to the right temporal pole correlates more strongly with impaired empathy. Many patients in our study had significant left temporal pole damage as part of a typical SeDe atrophy pattern (Rosen et al., 2002a), so there was adequate variability in both right and left temporal lobe volume to have yielded a more bilateral result, yet this was not found. Mesulam (1998) has theorized that the temporal poles act as `transmodal epicentres' where information from multiple sensory modalities is combined to form complex, symbolic, personalized representations. While the left temporal pole is involved in word–meaning connections, he suggests that the right temporal pole creates symbolic socio-emotional precepts that aid in face recognition. Our evidence that right temporal pole damage is sufficient to produce empathy deficits suggests that these multimodal socio-emotional precepts may involve not only face recognition, but also the ability to recognize and symbolize others' emotional states in relation to personal emotions and autobiographical experiences.
Two areas of the fusiform gyrus were seen to correlate with empathy loss in our patients: the right fusiform face area and a more anterior portion of the fusiform gyrus. The right fusiform is involved in facial perception and recognition (Hadjikhani and De Gelder, 2003; Lewis et al., 2003), skills which are likely related to empathy.
The amygdala has shown activation in some fMRI studies of empathy (Carr et al., 2003; Vollm et al., 2005). Despite the fact that numerous patients had significant amygdala damage in this study, however, this structure did not show a direct relationship to real-life empathic behaviour. Because these data are based on structural damage rather than functional mapping, they do not contradict the evidence showing that the amygdala is often activated during social emotion processing. Instead, they suggest that there were patients in this study who had amygdala damage but were capable of empathic behaviour in daily life, or patients who had diminished empathy but did not have amygdala damage.
Frontal lobe structures
Our results suggest that both medial orbitofrontal cortex (OFC) and ventral striatal structures contribute to empathy. The role the OFC plays in empathic behaviour may be clarified by first examining what is known about the functional architecture of the OFC. Kringelbach and Rolls (2004) suggest that after analysing 87 functional imaging studies resulting in OFC activations, there appear to be two gradients of function in the OFC. A medial–lateral gradient appears in which the more medial areas are involved in processing the reward value of a particular stimulus, while the more lateral areas encode stimuli in terms of their potential for punishment. There is also an anterior–posterior gradient in which the reward value for more concrete, primary reinforcing factors, such as touch and taste, are encoded in the most posterior OFC, while the value of increasingly complex, abstract, or symbolic secondary reinforcing factors, such as money, are encoded in the anterior OFC. Based on the Kringelbach and Rolls analysis, the postero-medial area significantly related to emotional empathy in our study likely represents the reinforcement value of simple sensory stimuli, which probably include the visceral sensations that accompany an emotional experience.
Our study found that the right medial OFC, but not the left, was strongly related to empathy in these neuro-degenerative disease patients. Functional imaging studies have not been able to clearly delineate separate roles for the right and left OFC, though some lesion studies support the hypothesis that they do perform different functions. In a study correlating OFC damage with behavioural outcomes, Tranel et al. (2002) found that the right ventromedial prefrontal cortex is more directly involved in social conduct, decision-making and emotional processing than the left. They suggest that the syndrome of social deficits often cited as a result of orbitofrontal damage is actually specific to the right hemisphere. Other lesion studies also support the idea that this medial area of the OFC is involved in emotion processing. While emotion comprehension and expression are primarily mediated by structures outside of the frontal lobes, particularly the temporal, insular and somatosensory cortices (Adolphs et al., 2000), patients with OFC lesions show deficits in emotion recognition, both in facial and vocal modalities. A number of studies examining patients with unilateral or bilateral OFC lesions suggest a trend implicating the right medial portion of the OFC in social emotion processing across input modalities (Hornak et al., 1996; Blair and Cipolotti, 2000; Beer et al., 2003; Hornak et al., 2003; Mah et al., 2005).
There is considerable interconnection between the posterior medial OFC and underlying subcortical structures, including the head of the caudate and the nucleus accumbens. The function of these ventral striatal structures appears to overlap with that of the OFC. Reynolds and Berridge (2002) suggest that, much like the posterior medial OFC, the ventral striatum is involved in evaluation of stimuli that are concrete, primary reinforcers, particularly with respect to reward expectancy (Breiter et al., 2001; Knutson and Cooper, 2005). In a meta-analysis of the functional neuroanatomy of emotion, Phan et al. (2002) showed that the majority of studies found ventral striatal activations in response to both happiness and disgust. Also, Phan et al. (2005) found that nucleus accumbens activity increased with the emotional intensity and self-relatedness of stimuli. Carr et al. (2003) found that the right striatum was activated when normal subjects imitated emotional faces, but not when they merely observed them. An empathic response clearly relies in part on the emotional intensity of the empathy-inducing stimulus, as well as the degree to which that stimulus can be related to one's own situation or history. According to our data, damage to the subcortical structures that mediate this capacity appears sufficient to reduce empathic behaviour in real-life situations. Phan hypothesizes that, like the amygdala, the nucleus accumbens functions to provide a simple signal to the organism that a stimulus has significance and merits additional attention. Perhaps without this capacity, one cannot recognize that another's emotional state has personal significance and a potential reward or punishment value, thus one fails to engender an empathic response to it.
The recent literature examining the functional neuro-anatomy of empathy strongly suggests that dorsomedial frontal structures are involved in the PT aspects of empathy (Farrow et al., 2001; Gallagher and Frith, 2003; Shamay-Tsoory et al., 2003, 2005a; Decety and Jackson, 2004), and patients with damage to this area of the brain, particularly the right frontal pole corresponding to medial BA 10, have demonstrated poorer PT scores on the IRI (Shamay-Tsoory et al., 2003, 2005a). While this area did not appear in the main-effects analyses, which were performed with a rigorous whole-brain correction for FWE, clusters corresponding to the anterior cingulate and to BA 10 did appear at a trend level (P < 0.001 uncorrected) as part of the main effect of both empathy subscales (Figs 3 and 5). If the dorsomedial BA 10 cluster is directly related to empathy in our patients, but has a weaker correlation than the right temporal pole and the right inferior frontal areas, this would be consistent with the Decety and Jackson (2004) theory of the functional architecture of empathy. It suggests that the emotional elements of empathy are foundational, while downstream cognitive processes such as empathic PT may be dependent on the capacity to perform the initial emotion-sharing step. Diminished capacity to create shared emotional representations may be adequate to produce empathy impairment in real-life behaviour.
Clinical implications for neurodegenerative disease patients
Despite the fact that they do have some medial frontal atrophy (Rosen et al., 2002a), direct analysis of the SeDe group confirmed that empathy correlated with right temporal structures more than medial frontal structures in this group. However, SeDe patients showed behavioural impairment in the cognitive as well as emotional aspects of empathy, as has been previously reported (Perry et al., 2001; Rankin et al., 2005a). Potentially, right temporal damage primarily causes the SeDe patients to lose emotion-sharing and emotional responsiveness, which in turn may be adequate to cause the loss of downstream cognitive functions such as PT, whether or not frontal areas are preserved. Behaviourally, the very wide range of scores in this group likely corresponds to inter-individual variation in the degree of left-sided versus right-sided temporal atrophy.
Unlike in previous studies (Rankin et al., 2005a), FTD patients in this sample were behaviourally impaired in both the cognitive and emotional aspects of empathy. A direct anatomic analysis of empathy in the FTD group did not show a relationship to temporal lobe structures, but empathy was directly related to the volume of the subgenual cingulate/subcallosal gyrus in the inferior frontal cortex. The fact that FTD and SeDe patients obtained similar scores on both cognitive and emotional empathy subscales of the IRI despite different contributory anatomy, and that PT and EC scales were highly correlated in this group, may call into question the validity of using this measure with these patients. Particularly given the inconsistent evidence regarding FTD patients' capacity for emotional empathy, a valuable future analysis would be to compare FTD patients to a uniform group of predominantly right-sided SeDe patients in order to directly contrast the emotional empathy deficits resulting from right temporal versus isolated inferior frontal damage.
The AD, PNFA, CBD and PSP groups showed normal emotional empathy, but showed a wider variation of performance in PT. Direct anatomic analysis of empathy loss in AD patients did not yield any significant results, perhaps because this small subgroup of subjects with poor empathy scores provided inadequate power to detect a specific anatomic substrate in this group. Given their intact emotional empathy, these patients' poorer and more variable PT supports the theory that empathic PT is a more complex and multi-determinate downstream cognitive process (Decety and Jackson, 2004), and suggests it may be more susceptible than emotional empathy to subtle impairments, even in the context of non-FTLD neurodegenerative diseases.
This study also provides quantitative support for the frequently observed clinical observation that dementia patients with right-sided disease are more likely to show deficits in empathy and other aspects of social functioning. Even at a very low correlation threshold, right-sided structures predominated (Fig. 3). These results are consistent with numerous studies suggesting that right frontal damage can result in loss of social pragmatics and social reasoning (Stuss et al., 2001; Tranel et al., 2002), while right temporal pathology can result in a loss of emotional responsiveness and a failure to correctly interpret social and emotional cues (Perry et al., 2001; Rosen et al., 2002b).
Conclusions
Using VBM with structural MRI images, the anatomic substrate of empathy loss were delineated in a group of patients with diverse patterns of cortical atrophy due to neurodegenerative disease. This study provides lesion data suggesting that the right anterior temporal lobe and inferior frontal cortex are regions associated with real-life loss of empathy. These findings complement the existing functional imaging data which, because of image artefacts, often underestimate the role of these regions. These results also suggest that the use of psychometrically validated social– psychological measures with neurodegenerative patients may significantly facilitate the investigation of social behaviours in this clinical group. Ideally, further clarification of the neuroanatomic and neurocognitive mechanisms for diminished empathy in patients with neurodegenerative disease may give clinicians and families the power to better predict and compensate for the often distressing changes in patients' emotions and social behaviour.
Acknowledgements
This research was supported in part by the National Institute on Aging (NIA) grants 5-K23-AG021606-02 and AG19724-01A1, the State of California, Alzheimer's Disease Research Center of California (ARCC) grant 01-154-20, and the Larry L. Hillblom Foundation, Inc., grant 2002/2J.
Abbreviations
- CBD
corticobasal degeneration
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- IRI
Interpersonal Reactivity Index
- PSP
progressive supranuclear palsy
- SeDe
semantic dementia
- VBM
voxel-based morphometry
References
- Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3:469–79. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–90. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the sts region. Trends Cogn Sci. 2000;4:267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey E, Keltner D, Scabini D, Knight R. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. J Pers Soc Psychol. 2003;85:594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Attence C, Loftus G, Meltzoff AN. We saw it all along: visual hindsight bias in children and adults. Psychol Sci. 2004;15:264–7. doi: 10.1111/j.0963-7214.2004.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123:1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Rankin KP, Miller BL, Schuff N, Weiner MW, Gorno-Tempini ML, et al. Cinguloparietal atrophy distinguishes Alzheimer's disease from semantic dementia. Arch Neurol. 2003;60:949–56. doi: 10.1001/archneur.60.7.949. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller B, et al. Patterns of brain atrophy differentiate corticobasal degeneration from progressive supranuclear palsy. Arch Neurol. 2006;63:81–6. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith CD. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Per Soc Psychol. 1983:44. [Google Scholar]
- Decety J, Chaminade T. Neural correlates of feeling sympathy. Neuropsychologia. 2003;41:127–38. doi: 10.1016/s0028-3932(02)00143-4. [DOI] [PubMed] [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18:324–33. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Zheng Y, Wilkinson ID, Spence SA, Deakin JF, Tarrier N, et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–8. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Good CD, Ashburner J, Frackowiak RS. Computational neuroanatomy: new perspectives for neuroradiology. Rev in Neurol (Paris) 2001;157:797–806. [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognitive and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, De Gelder B. Seeing fearful body expressions activates the fusiform cortex and amygdala. Curr Biol. 2003;13:2201–5. doi: 10.1016/j.cub.2003.11.049. [DOI] [PubMed] [Google Scholar]
- Heinik J, Keren P, Vainer-Benaiah Z, Lahav D, Bleich A. Agreement between spouses and children in descriptions of personality change in Alzheimer's disease. Isr J Psychiatry Relat Sci. 1999;36:88–94. [PubMed] [Google Scholar]
- Hornak J, Rolls ET, Wade D. Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia. 1996;34:247–61. doi: 10.1016/0028-3932(95)00106-9. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O'Doherty J, Bullock PR, et al. Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain. 2003;126:1691–712. doi: 10.1093/brain/awg168. [DOI] [PubMed] [Google Scholar]
- Keysar B, Lin S, Barr DJ. Limits on theory of mind in adults. Cognition. 2003;89:25–41. doi: 10.1016/s0010-0277(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–7. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–33. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lewis S, Thoma RJ, Lanoue MD, Miller GA, Heller W, Edgar C, et al. Visual processing of facial affect. Neuroreport. 2003;14:1841–5. doi: 10.1097/00001756-200310060-00017. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Mah L, Arnold MC, Grafman J. Deficits in social knowledge following damage to ventromedial prefrontal cortex. J Neuropsychiatry Clin Neurosci. 2005;17:66–74. doi: 10.1176/jnp.17.1.66. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PTJ. Different points of view: self-reports and ratings in the assessment of personality. In: Forgas JP, Innes JM, editors. Recent advances in social psychology: an international perspective. Elsevier; Amsterdam: 1989. pp. 429–39. [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mesulam M. From sensation to cognition. Brain. 1998;121:1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Moll J, Eslinger PJ, Oliveira-Souza R. Frontopolar and anterior temporal cortex activation in a moral judgment task: preliminary functional MRI results in normal subjects. Arq Neuropsiquiatr. 2001;59:657–64. doi: 10.1590/s0004-282x2001000500001. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Rosen HR, Kramer JH, Beer JS, Levenson RL, Miller BL. Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7:145–60. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in pet and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan K, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Soc Biol Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Mychack P, Miller BL. Double dissociation of social functioning in frontotemporal dementia. Neurology. 2003;60:266–71. doi: 10.1212/01.wnl.0000041497.07694.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, Miller B. Patterns of cognitive and emotional empathy in frontotemporal dementia. Cogn Behav Neurol. 2005a;18:28–36. doi: 10.1097/01.wnn.0000152225.05377.ab. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL. Self-awareness and personality change in dementia. J Neurol Neurosurg Psychiatry. 2005b;75:632–9. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, et al. Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry. 1997;154:918–25. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste ‘liking’/‘disliking’ reactions, place preference/avoidance, and fear. J Neurosci. 2002;15:7308–20. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002a;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Perry RJ, Murphy J, Kramer JH, Mychack P, Schuff N, et al. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002b;125:2286–95. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Royzman EB, Cassidy KW, Baron J. ‘I know, you know’: Epistemic egocentricism in children and adults. Rev Gen Psychol. 2003;7:38–65. [Google Scholar]
- Ruby P, Decety J. What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur J Neurosci. 2003;17:2475–80. doi: 10.1046/j.1460-9568.2003.02673.x. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J Cogn Neurosci. 2004;16:988–99. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15:324–37. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired ‘affective theory of mind’ is associated with right ventromedial prefrontal damage. Cogn Behav Neurol. 2005a;18:55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Lester H, Chisin R, Israel O, Bar-Shalom R, Peretz A, et al. The neural correlates of understanding the other's distress: a positron emission tomography investigation of accurate empathy. Neuroimage. 2005b;27:468–72. doi: 10.1016/j.neuroimage.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Siegler IC, Dawson DV, Welsh KA. Caregiver ratings of personality change in Alzheimer's disease patients: a replication. Psychol Aging. 1994;9:464–6. doi: 10.1037//0882-7974.9.3.464. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss ME, Pasupathi M, Chatterjee A. Concordance between observers in descriptions of personality change in Alzheimer's disease. Psychol Aging. 1993;8:475–80. doi: 10.1037//0882-7974.8.4.475. [DOI] [PubMed] [Google Scholar]
- Stuss D, Gallup GG, Alexander MP. The frontal lobes are necessary for ‘theory of mind’. Brain. 2001;124:279–86. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Van Boven L, Loewenstein G. Social projection of transient drive states. Pers Soc Psychol Bull. 2003;29:1159–68. doi: 10.1177/0146167203254597. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor ANW, Richardson P, Corcoran R, Stirling J, McKie S, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Williams R, Briggs R, Coleman P. Carer-rated personality changes associated with senile dementia. Int J Geriatr Psychiatry. 1995;10:231–6. [Google Scholar]



