Abstract
The production of melanin in the hair and skin is tightly regulated by the melanocortin 1 receptor (MC1R) whose activation is controlled by 2 secreted ligands, α-melanocyte stimulating hormone (αMSH) and agouti signal protein (ASP). Since melanin is extremely stable, lasting years in biological tissues, the mechanism underlying the relatively rapid decrease in visible pigmentation elicited by ASP is of obvious interest. In this study, the effects of ASP and αMSH on the regulation of melanin synthesis and on visible pigmentation were assessed in normal murine melanocytes and were compared with the quick depigmenting effect of the tyrosinase inhibitor, phenylthiourea (PTU). αMSH increased pheomelanin levels prior to increasing eumelanin content over 4 days of treatment. Conversely, ASP switched off the pigment synthesis pathway, reducing eu- and pheo- melanin synthesis within 1 day of treatment that was proportional to the decrease in tyrosinase protein level and activity. These results demonstrate that the visible depigmentation of melanocytes induced by ASP does not require the degradation of existing melanin but rather is due to the dilution of existing melanin by melanocyte turnover, which emphasizes the importance of pigment distribution to visible color.
Keywords: eumelanin, pheomelanin, MC1R, ASP, αMSH, PTU, tyrosinase
Introduction
The melanocortin 1 receptor (MC1R) is expressed predominantly by melanocytes and is a key protein that regulates hair and skin color, which is critical to protecting the skin from UV-induced DNA damage. The activity of the MC1R is regulated positively by α-melanocyte stimulating hormone (αMSH) and negatively by agouti signal protein (ASP in mice, ASIP in humans). αMSH (encoded by the POMC locus) and ASP (encoded by the agouti locus) are secreted ligands that act through MC1R to regulate skin and hair color in mammals (Jackson, 1997) by controlling the amount and type of melanin synthesized by melanocytes in those tissues (Sakai et al., 1997; Siracusa, 1994; Suzuki et al., 1997; Wolff, 2003).
Melanin pigments in the hair and skin are of two basic types, black to brown eumelanin and red to yellow pheomelanin. The mechanism(s) involved in the switch between eumelanin and pheomelanin synthesis is not well understood but in the hair of mice, it is regulated by the time-specific expression of ASP in the dermal papilla. In mice, two important characteristics are controlled by agouti expression: a pale ventral pheomelanic coloration and the presence of “agouti” hairs, i.e. hairs containing a pheomelanic band against a dark eumelanic background. In humans, a polymorphism of the agouti gene which decreases its mRNA level has been associated with darker skin and hair and with brown eye color (Bonilla et al., 2005; Kanetsky et al., 2002; Meziani et al., 2005; Voisey et al., 2006). Variation in ASIP function may be one of several factors contributing to reduced pigmentation in some populations (Norton et al., 2007). Melanocytes treated with αMSH or ASP dramatically increase or decrease, respectively, their visible pigmentation over the course of 1 to 4 days (Sakai et al., 1997). In vitro studies have revealed that ASP inhibits total melanin content (Aberdam et al., 1998; Hunt & Thody, 1995; Siegrist et al., 1997). Since melanin is extremely stable, lasting years in biological tissues, the mechanism(s) underlying the relatively rapid decrease in visible pigmentation elicited by ASP is of obvious interest.
Phenylthiourea (PTU) is known to prevent melanin synthesis by directly inhibiting tyrosinase activity (Poma et al., 1999), without affecting levels of tyrosinase mRNA or mRNAs encoding other melanosomal proteins (TRP1, DCT, Pmel17, OA1) (Hall & Orlow, 2005). Therefore PTU can be used as a positive control that quickly and effectively inhibits melanin synthesis allowing one to compare its effects with those of ASP to determine if ASP leads to depigmentation by simply inhibiting pigment synthesis and/or by mediating the degradation of existing pigment.
In this study, we assessed the effects of 3 factors that affect melanin production (ASP, PTU and αMSH) in melan-a murine melanocytes. We evaluated the effects of those 3 factors on melanin contents of the cells as well as in the media of melanocytes treated for various times with those factors. The results provide interesting insights into the mechanism of action of MC1R regulation of pigment synthesis and visible pigmentation.
Results
Production and purification of ASP
αMSH and PTU are commercially available, but since ASP is not, we first needed to design an expression and purification protocol for that protein which retained its biological activity. We optimized conditions to purify murine ASP using several types of chromatography after its production by a recombinant virus transfected in insect cells. Briefly, fresh culture supernatants of insect cells 3 days after infection were filtered through 0.45 μm polyethersulfone membranes and were then purified by 2 successive ion-exchange chromatography steps. Proteins were eluted with a NaCl gradient and positive fractions (based on Coomassie blue staining and Western blotting) were pooled, concentrated and further purified by size-exclusion chromatography. The specificity and purity of the ASP was then analyzed using SDS-PAGE followed by immunoblotting with a specific ASP antibody and by silver staining (Figure 1). The band detected with the highest intensity by both methods was the expected size for ASP (∼16 kDa, 131 amino acids). Only minor contaminants were revealed by silver staining, confirming the high purity achieved by that purification process. The purified ASP retained its bioactivity as detailed in the following experiments.
Figure 1.
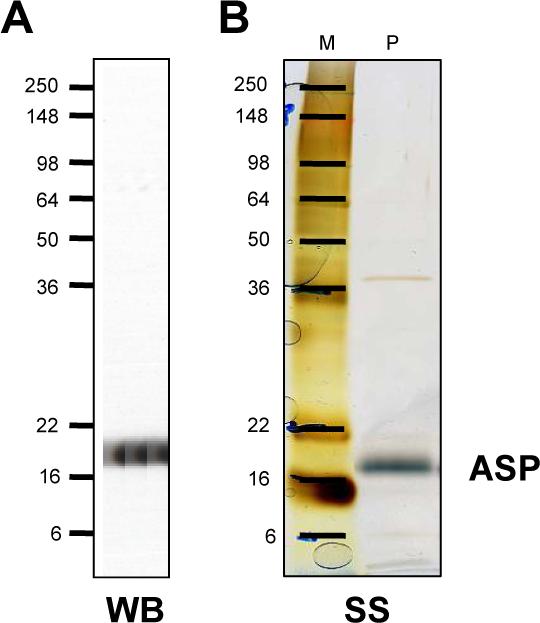
Production and purity of murine ASP used in these studies. ASP was expressed in a baculovirus system, purified by chromatography, and analyzed by SDS-PAGE on 14% Trisglycine gels visualized by Coomassie blue staining after each step of the process, as detailed in the Materials and methods. (A) Twenty μl of the purified ASP preparation (P) was analyzed by western blotting. (B) Twenty μl purified ASP analyzed by silver-staining; the position of molecular weight markers (M) is shown in kDa.
Effects of ASP, PTU and αMSH on eumelanin and pheomelanin synthesis
We then treated melanocytes with ASP, PTU or αMSH to determine their effects on the production of eumelanin and pheomelanin after 1, 2 or 4 days of treatment (Figure 2). The lightening effects of ASP and of PTU contrasted with the darkening effect of αMSH as evident by bright field microscopy of the cells (which allows only the pigmented cells to be seen), and particularly by the appearance of the melanocyte pellets (Figure 2 insets). The higher dendricity of cells treated with αMSH was evident, while ASP-treated cells were less dendritic than control- or PTU-treated cells. It should also be noted that all 3 factors had minor effects on melanocyte growth, αMSH decreasing cell number by 7% after 4 days while PTU and ASP decreased cells numbers by 14% and 25%, respectively, after 4 days of treatment days (averages of 3 independent experiments).
Figure 2.

Time course of treatment of melanocytes with ASP, PTU or αMSH. (top left) Experimental design: melanocytes were seeded 2 days prior (−2D) to the first treatment with 10 nM ASP, 200 μM PTU or 100 nM αMSH in fresh media (D0) to start the 4 days of treatment. At D2, media were collected and replaced with fresh media containing the drugs or vehicle as appropriate (4 and 2 days of treatment). At D3, appropriate drugs or vehicle were added to the remaining dishes for 1 day of treatment. Cells were collected at D4, frozen and lyophilized, while media were combined with those collected at D2, prior to lyophilization. Bright field microscopy of cells before harvesting of each treatment as noted (all photos at the same 10X original magnification and exposure settings); insets show representative cells at higher magnification (each at 40X) and the visual appearance of each cell pellet after harvesting.
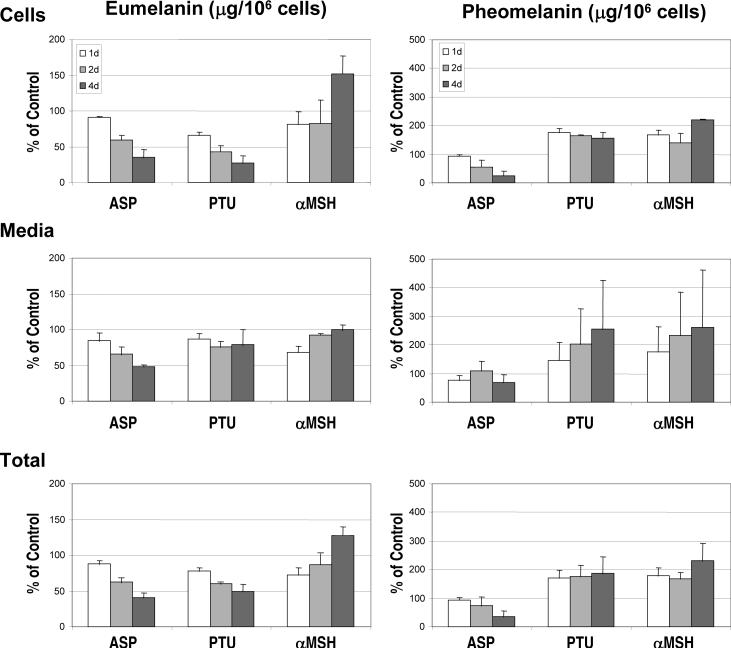
Eumelanin and pheomelanin contents in the treated melanocytes and in the media (which could potentially contain melanins secreted during the course of the experiment) were then analyzed by HPLC after chemical degradation. Melanin contents were normalized per cell number as each of those agents inhibited melanocyte proliferation after 4 days, as noted above. Figure 3 shows the contents per 106 cells of both types of melanins in melanocytes harvested after 1, 2 and 4 days of treatment, expressed as a percentage of the untreated controls. The data represent the means ± SD of 3 independent experiments. Consistent with the visible color of the cell pellets (shown above in Figure 2), ASP and PTU dramatically decreased levels of eumelanin in melanocytes within 2 days and within 1 day, respectively. Similarly, levels of eumelanin in the media were decreased by both drugs after 1 and 2 days of treatment, and after 4 days with ASP. In contrast, αMSH increased the level of eumelanin in melanocytes after 4 days of treatment, although eumelanin levels in the media of αMSH-treated cells were comparable to the untreated control (at 2 and 4 days), and were even lower after 1 day.
Figure 3.
Levels of eumelanin (left) and pheomelanin (right) in melanocytes and media after treatment with ASP, PTU or αMSH. Chemical measurements of PTCA (in duplicate) and 4-AHP as markers for eumelanin and pheomelanin, respectively, were performed on cells (top) harvested at D4 after the indicated time of treatment with or without ASP, PTU or αMSH, and on the medium (middle) from those 4 days of culture (collected at D2 and D4). Values are reported per 106 harvested cells (D4) and are expressed as a percentage of the control. Total melanin content (bottom) includes melanin in the cells and medium. Values represent the means ± SD of 3 independent assays.
When melanocytes were treated with ASP, there were significant decreases in levels of pheomelanin within 2 days in the cells and within 4 days in the media. In contrast, PTU and αMSH actually increased pheomelanin levels in melanocytes above the untreated control at all time points. Even though a high variability of pheomelanin content in media from PTU- or from αMSH-treated melanocytes was observed between the independent biological replicates, a time-dependent increase was seen. As a result, the total pheomelanin content (cell+media) seemed to be increased by PTU and slightly more by αMSH.
The depigmenting effect: inhibition and/or degradation of melanin synthesis?
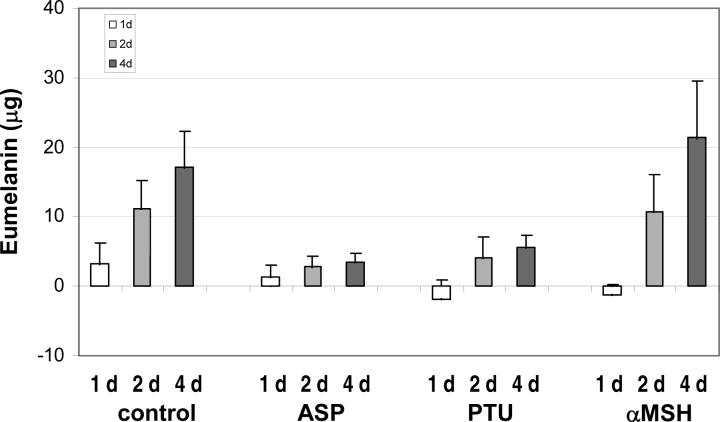
A key question is whether the depigmentation observed following treatment with ASP results from the degradation of existing melanin and/or the effective inhibition of new melanin production. Thus we further analyzed the results and compared the total eumelanin content after ASP, PTU or αMSH treatment with the amount of eumelanin present at the beginning of each treatment, which had started as indicated in the left upper panel of Figure 2. Figure 4 shows the amounts of eumelanin produced or degraded specifically during the various times of treatment with each drug. The eumelanin produced in the untreated controls increased progressively on days 1, 2 and 4. As expected, PTU inhibited further synthesis of melanin at all times of treatment which remained well below the level of the untreated control. Similar results were observed for ASP, although the level of eumelanin was reduced dramatically at 1 day and was quite similar to the amount produced during 2 and 4 days. Interestingly, the amount of eumelanin produced during αMSH treatment was greater than the control only after 4 days of treatment.
Figure 4.
Amounts of eumelanin generated specifically during treatment of melanocytes. The content of eumelanin prior to each treatment was evaluated in cells at D0 as well as in cells and media at D2 and D3, as indicated for Figure 2. The histograms show the calculated changes in eumelanin before and after with ASP, PTU or αMSH. Values represent the means ± SD in 3 independent assays.
Since it is possible that melanin byproducts might be produced due to oxidation of melanin (e.g. by endogenous peroxides), we determined whether there were any melanin degradation products secreted into the media of ASP-treated melanocytes by measuring pyrrole-2,3-dicarboxylic acid (PDCA) and pyrrole-2,3,5-tricarboxylic acid (PTCA) levels directly in the culture media without performing any oxidative degradation. In this regard, it has been recently reported that various forms of pyrrole acids are present in melanosomes (Ward et al., 2008) No such melanin degradation products were detected (data not shown). Therefore, the visible lightening of melanocytes treated with ASP or with PTU could simply correlate with a striking and effective inhibition of eumelanin synthesis without the degradation of any pre-existing melanin.
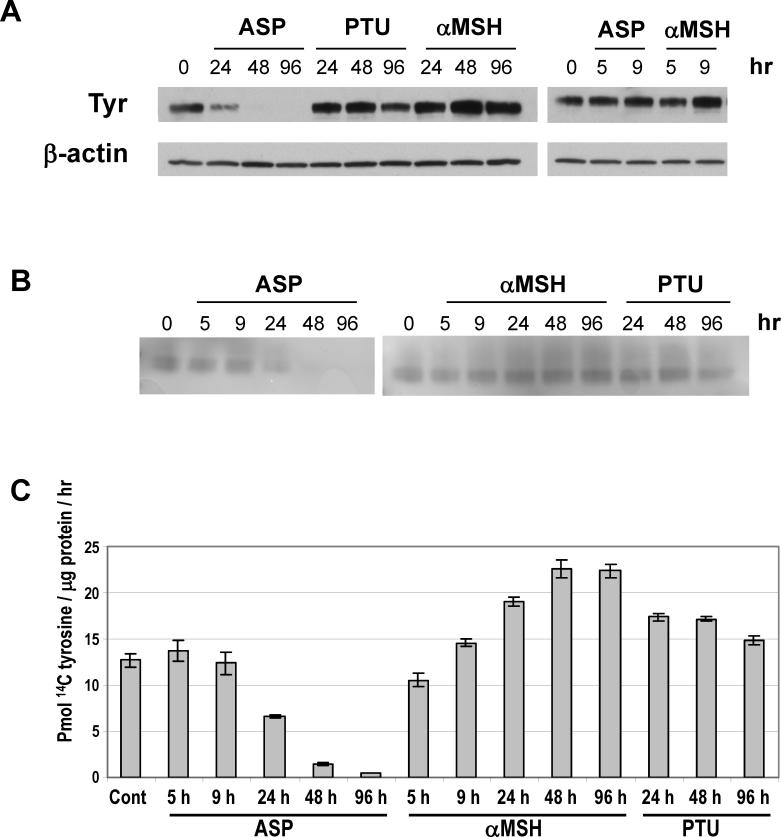
To determine whether ASP triggers a complete inhibition of melanogenesis, we characterized its effect on the regulation of tyrosinase, the key enzyme involved in melanogenesis. Protein levels of tyrosinase were assessed by immunoblotting after various times of treatment with ASP, and also with PTU or αMSH, as indicated in Figure 5. A significant decrease was observed in the level of tyrosinase in melanocytes treated for 24 hr with ASP, and that became maximal after 2 and 4 days. In contrast, tyrosinase levels were not altered by PTU, but were increased by αMSH after 2 and 4 days. In parallel, we determined the L-3,4-dihydroxyphenylalanine (DOPA) oxidation activity of tyrosinase (Figure 5B) and found a concomitant reduction of DOPA oxidase by ASP starting within 1 day. On the other hand, no change of DOPA oxidase activity was detectable after PTU treatment, whereas an increase was observed after 24 hr of treatment with αMSH. We confirmed those effects by analyzing the activity of tyrosinase using the 14C tyrosine assay and found consistent results (Figure 5C). The sum of these results clearly demonstrates that ASP inhibits melanogenesis by negatively regulating the expression of tyrosinase and, as a consequence, its enzymatic activity.
Figure 5.
Effects of ASP, PTU and αMSH on tyrosinase level and activity. (A) Melanocytes were incubated with ASP (10 nM), PTU (200 μM) or αMSH (100 nM) for the indicated times prior to preparing cell extracts for Western analysis. Proteins (7 μg/lane) were separated using 8−16% SDS-PAGE gels and were probed with the anti-tyrosinase antibody αPEP7 (1:10000). β-Actin was used as a control for loading. (B) Tyrosinase activity was assessed by the rate of DOPA oxidation on electrophoretically separated extracts (7 μg protein/lane). (C) Tyrosinase activity assay was performed as described under ‘Methods’ on 7 μg proteins incubated with 14C-tyrosine. Assays were performed in quadruplicate. Values represent means ± SD.
Dose-dependant inhibition of eumelanin and pheomelanin levels by ASP
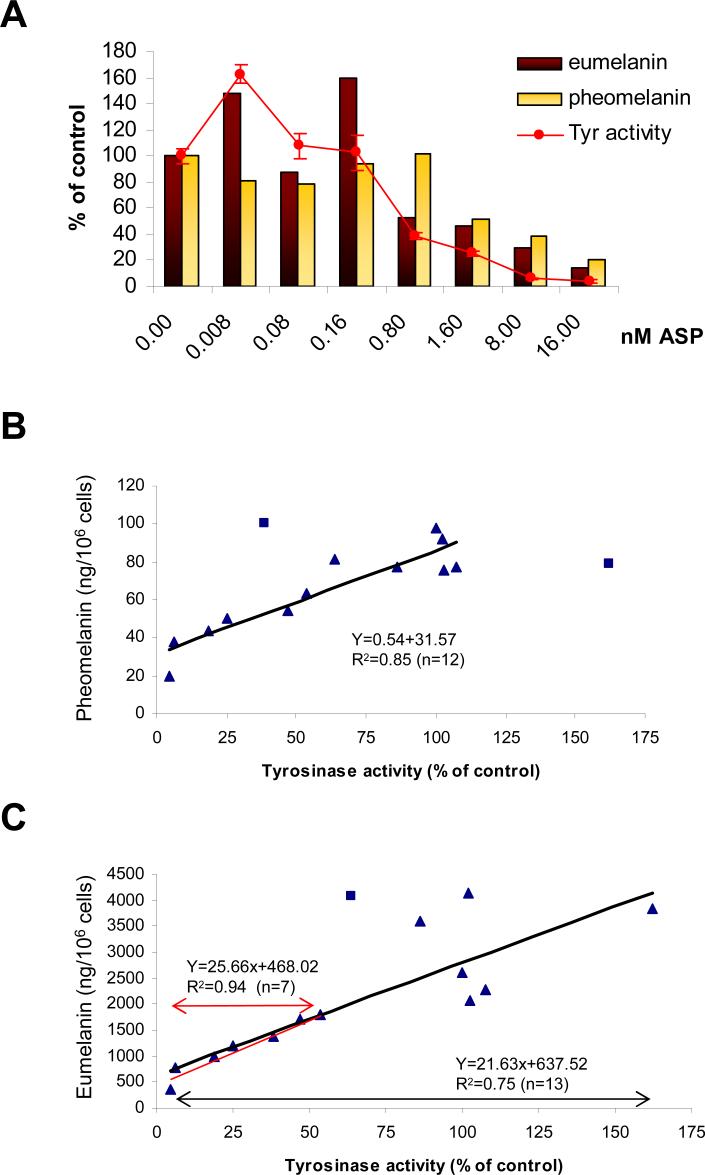
We also examined the dose-dependent inhibition of eumelanin and pheomelanin content by ASP after 3 days using a wide range of doses (from 8 pM to 16 nM) (Figure 6A). The total contents (cell and media) of eumelanin and pheomelanin are reported as percentages of the untreated melanocyte controls. We also measured tyrosinase activity using the 14C tyrosine assay in those same melanocyte populations. There was a striking dose-dependent decrease in enzyme activity and in eu- and pheo-melanin levels at ASP concentrations ≥0.8 nM (Figure 6A).
Figure 6.
ASP decreases eumelanin, pheomelanin and tyrosinase activity in a dose-dependant manner. (A) Melan-a melanocytes were treated with ASP at various concentrations as noted for 3 days. Measurements of eumelanin and pheomelanin were carried out on cells and media reported per 106 cells and were compared to controls. Tyrosinase activity was determined using a 14C-tyrosine assay as described in the Materials and methods. The results shown are representative of 2 different assays performed with an equivalent range of ASP concentrations. (B and C) 14 values for each parameter (eumelanin, pheomelanin and tyrosinase activity) from 2 different sets of experiments were analyzed and graphed to determine possible correlations between tyrosinase activity and the 2 types of melanins. (B) Linear correlation between tyrosinase activity and level of pheomelanin (r2=0.85, n=12). Two values (represented by squares) were not considered in calculating the equation because they were so far out of the range. (C) Correlation between tyrosinase activity and level of eumelanin: under 50% activity of tyrosinase, a linear correlation (r2=0.94, n=7) was observed. This correlation was not so dramatic for tyrosinase activities beyond 50% of the control. One value (represented by a square) among 14 was excluded in calculating the correlation coefficient (r2=0.75, n=13)
The relationships between tyrosinase activity and levels of eumelanin and pheomelanin were then plotted. The data show a positive linear correlation between tyrosinase activity and pheomelanin content (r2=0.85) (Figure 6B). A significant correlation (r2=0.94) was also found between eumelanin content and tyrosinase activity when the enzyme activity was below 50% of the control (Figure 6C), although at higher enzyme activity levels, the correlation coefficient dropped to 0.75. These data corroborate the strong correlation found in a previous study between the total melanin (the sum of eumelanin and pheomelanin) and the enzyme activity and protein levels of tyrosinase (Wakamatsu et al., 2006a).
Overall, we demonstrated the efficiency of 10 nM ASP to inhibit melanogenesis as rapidly as within 24 hr. Consequently, the lightening of cells cultured for several days in the presence of ASP or PTU is likely due to the inhibition of eumelanogenesis rather than to the degradation of existing pigment. We then tested whether visible lightening of cells would effectively result from the dilution of eumelanin (existing at the start of the treatment) with de novo daughter cells which remained unpigmented due to the inhibition of melanogenesis by ASP or PTU. To do that, we treated melanocytes seeded at low or high densities with PTU or ASP. At low density seeding, melanocytes were able to duplicate several times over the 4 day treatment period (Figure 7A, left panel) and the resulting cell pellets appeared much lighter than when cells were seeded at a higher density (right panel) and thus were unable to duplicate more than once. The inhibitory effects of PTU or ASP on melanin production was comparable in both conditions. Thus, the differences observed were based on the initial content of melanin (variable from one condition to the other) and on the rate of cell proliferation. The latter was not decreased by ASP when cells were already semi-confluent at the beginning of the experiment. To further confirm the effects of pigment dilution on visible color, we analyzed the effects of diluting pigmented melan-a (black) melanocytes with various ratios of unpigmented melan-c (albino) melanocytes. A clear lightening of the mixed population was proportional to the dilution of pigmented with unpigmented melanocytes. These experiments confirm that degradation of melanin is not required to depigment melanocytes cultured for several days with ASP or PTU, and support the contention that the dilution of existing pigment by cell proliferation is sufficient to explain the whitening effects on cells cultured in vitro.
Figure 7.
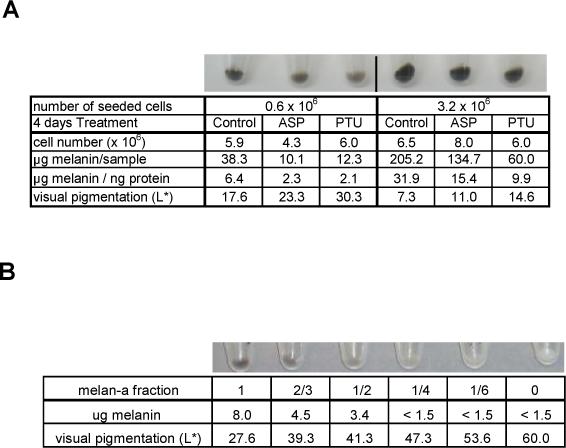
Visible depigmenting effects of ASP or PTU depends on the initial content of melanin, based on a dilution effect. (A) Six × 105 or 3.2 × 106 melanocytes were seeded in 10 cm dishes as noted and were treated 1 day later with ASP or PTU for 4 days where noted. Melanocytes seeded at 6 × 105 were able to grow exponentially, reaching a 10-fold increase in number, while melanocytes seeded at 3.2 × 106 could only double. Photos were taken after centrifugation of the harvested cells, and light intensity (L*) of each cell pellet was measured from the photo according to the CIE (ranging from 0/black to 100/white) (Coelho et al., 2006). Melanin contents were determined by photometric absorbance (405 nm) after solubilization of each pellet at 37°C in 1N NaOH, and are reported as μg melanin per ng protein. Cell number and total melanin contents are also reported. Results are representative of 2 independent experiments. (B) A mixed population of pigmented melan-a melanocytes and unpigmented albino melan-c melanocytes in various proportions in microcentrifuge tubes (106 cells per tube), emphasize the importance of the melanin dilution on the visible color.
Discussion
Mechanism of depigmentation following treatment with ASP or PTU
ASP, the physiological regulator of MC1R expressed in the dermal papilla of murine hair follicles (Slominski et al., 2005) and in human skin (Voisey et al., 2006), has been reported to reduce the total amount of melanin in vitro (Aberdam et al., 1998; Hunt & Thody, 1995; Siegrist et al., 1997). In this study, we characterized the dose-dependent reduction of intra- and extra-cellular levels of eumelanin by ASP in melanocytes (Figure 6). Furthermore, we demonstrated that ASP as well as PTU inhibits eumelanin synthesis as early as within 1 day and seems not to induce any degradation of pre-existing eumelanin (Figure 4). Ten nM ASP elicits a dramatic time-dependent down-regulation of tyrosinase expression which results in significant decreases in catalytic function (Figure 5). PTU is a potent pigment inhibitor commonly used to study biological mechanisms in the absence of pigment or to study the transdifferentiation of pigmented cells (Itoh & Eguchi, 1986). PTU is known to inhibit tyrosinase activity through its interaction with the 2 copper ions present in the active site and by increasing the rate of its degradation (Hall & Orlow, 2005), which may explain the stronger effect of PTU after 1 day compared with ASP (which inhibits tyrosinase only at the transcriptional level). Even though we did not see any inhibition of tyrosinase activity in proteins extracted from cells incubated with PTU (200 μM) for 1, 2 or 4 days, we confirmed the inhibitory effects of PTU on eumelanogenesis in several assays. These results suggest that the inhibition of tyrosinase by PTU is transient and reversible, and only occurs when PTU remains in contact with tyrosinase. We demonstrate for the first time that PTU inhibits eumelanin synthesis but does not significantly alter pheomelanin synthesis in melanocytes (Figure 3). These results are consistent with the proposal that the inhibition of tyrosinase activity leads preferentially to the suppression of eumelanin production during the synthesis of mixed-melanins (Ito, 2003; Wakamatsu et al., 2008).
As a consequence of the lack of eumelanin degradation by ASP or PTU based on our results, the in vitro depigmentation of melanocytes becomes visible only when pre-existing pigmented cells are diluted with unpigmented daughter melanocytes generated during the treatment (Figure 7). This observation explains why the decrease in pigmentation depends on cell turnover, and usually takes a couple of days in vitro and several days (or weeks) in vivo where the removal of melanin is accomplished by the normal regeneration of the skin (or hair). From a practical point of view, to obtain total depigmentation in vitro (e.g. in reconstructed skin) or in vivo (e.g. cosmetically), the removal of pigment by increasing cell turnover (e.g. by using an exfoliant), followed by or concomitant with treatment with melanogenic inhibitor(s) should be considered. It was surprising that a relatively small change (1:4 dilution) in cell number (and thus dilution of existing melanin) can have relatively profound effects on visible pigmentation (Figure 7). For example, in our experiments examining the effects of ASP, the melanin content was relatively stable over the 4 day course of the experiment, yet the cells and cell pellets were significantly depigmented within 2 days (cf Figure 2) at which time the number of melanocytes could have increased 4-fold at most. The dramatic effects of pigment redistribution on visible skin color have been reported over the years in many systems, e.g. in amphibians (Bagnara & Fernandez, 1993) and in human skin (Costin & Hearing, 2007).
Effect of αMSH on pigment synthesis
Physiologically, melanin production is positively regulated by the activation of MC1R by αMSH after UV radiation (Lin & Fisher, 2007; Rees, 2003). Our data show an increased production of eumelanin and of pheomelanin in melanocytes cultured with 100 nM αMSH. Interestingly, the increased production of pheomelanin occurs earlier (1, 2 and 4 days) than does the induction of eumelanin synthesis (4 days) (Figure 3). These data confirm a previous study which reported an increase of pheomelanin synthesis induced by αMSH in human melanocytes (Lassalle et al., 2003) and supports the hypothesis for mixed melanogenesis raised by Ito ( 2003) who proposed that melanogenesis proceeds in three distinctive steps: cysteinylDOPA formation, pheomelanin formation, followed by eumelanin formation. Furthermore, in humans, eumelanin and pheomelanin synthesis seem to be regulated proportionally to each other constitutively in the skin and after UV irradiation (Hennessy et al., 2005) and human melanocytes from darker skin produce more eumelanin and pheomelanin in culture than do melanocytes from lighter skin (Wakamatsu et al., 2006a). Those previous studies are consistent with the effects we observed in vitro by activating MC1R with αMSH or by inhibiting melanin synthesis with ASP. The fact that we observed an increased release of pheomelanin in the media of MSH- or PTU-treated cells compared with controls raises an interesting point. Does the secretion of melanins depend on regulatory events or could it be simply a consequence of their increased production? Further, pheomelanin has been shown to be released in significantly higher amounts than eumelanin from melanocytes to their environment, as attested by measurements in the serum and urine of healthy and of melanoma patients (Wakamatsu et al., 2006b). Therefore, it seems that the release of pheomelanin is rather a consequence of its higher production. In contrast, we did not observe such an increased release of eumelanin induced by αMSH at the different times studied (1, 2 and 4 days). Based on a previous study, αMSH increased eumelanin release in the media of melan-a melanocytes after 6 hr, but returned to the control level after 24 hr (Virador et al., 2002), which agrees with our data at 24 hr. Three hypothesis can be considered to understand the lack of increase in the release after 4 days when cellular eumelanin is increased by αMSH.
1) αMSH does not necessarily increase the transfer of melanin. Note that this transfer must occur even in the absence of αMSH in skin or hair to assure a constitutive level of pigmentation, and that POMC-deficient mice remain almost as dark as their littermates.
2) A mono-culture of melanocytes does not permit a possible positive regulation of eumelanin transfer by αMSH due to the lack of melanocyte-keratinocyte interactions and of subsequent secreted factors that would trigger such transfer.
3) The uptake of eumelanin released into the medium by melanocytes themselves can not be excluded, and consequently, an increase of eumelanin in the medium would become more difficult to detect. In contrast, the uptake of pheomelanin is less probable due to its higher solubility, explaining why increased levels of pheomelanin are measured in the medium. This third hypothesis is supported by the fact that αMSH enhances the dendricity of melanocytes rendering them more able to transfer melanin from one cell to another.
Is ASP a true positive regulator of pheomelanin synthesis?
ASP expression correlates closely with the presence of pheomelanin in mouse hairs (Cone et al., 1996; Furumura et al., 1996) and thus with the switch from eu- to pheo- melanin synthesis. However, we did not observe any increase in pheomelanin levels above the control at any concentration of ASP tested, nor has any other study to date shown that (e.g. on melanoma cells or melanocytes (Graham et al., 1997; Sakai et al., 1997)), even after the addition of cysteine (data not shown). ASP more likely switches on-off the entire pigment synthesis pathway.
Numerous observations support this hypothesis. First, the agouti banding pattern, commonly seen in hairs of various mammals, does not always consist of a pheomelanic band over a dark eumelanin background, but can also generate a non-pigmented white band. This is the case for the old world baboon P-cynocephaluhamadryas (Ito et al., 2001), among other examples. In mice, different alleles encoding tyrosinases with decreased activity (chinchilla > extreme dilution > albino) are responsible for reduced amounts of pheomelanin (chinchilla) in lethal yellow mice (Lamoreux et al., 2001) or for a total absence of pigments, including pheomelanin, in extreme dilution and albino mice (Galbraith, 1971; Silvers, 1958). The level of tyrosinase activity is a key parameter in pheomelanin synthesis as highlighted by the strong correlation found between levels of pheomelanin and tyrosinase activities ranging from 5 to 130% of the control (Figure 6B), tyrosinase levels being highly affected by ASP. Second, consider the observed ventral-specific expression of ASP (Millar et al., 1995) and the common white or cream rather than yellow ventral pigmentation of various mammals, including wild-type Aw/Aw mice. Finally, pheomelanin synthesis in mice does not necessarily require ASP, as in the case of non-agouti mice mutant for Mc1r (a/a, Mc1r e/e). It is also interesting to note that the color of lethal yellow mice which over-express ASP is paler than is the color of recessive yellow mice which lack Mc1r function (Ollmann et al., 1998).
When MC1R is inhibited by ASP or by a loss of function mutation in the receptor, eumelanin synthesis is inhibited due to the down-regulation of several melanogenic factors, and pheomelanin production relies on the chemical status of the cell environment, i.e. the content of available cysteine (del Marmol et al., 1996; Granholm et al., 1996), and of dopaquinone, which depends on the remaining levels of tyrosine and tyrosinase activity. The hypothesis that ASP can be considered as an inhibitor of melanocyte differentiation was raised 10 years ago (Aberdam et al., 1998). This is supported by the down-regulating effect of ASP towards many melanogenic components, such as Mitf, Tyr, Tyrp1 and Dct (Furumura et al., 2001; Sakai et al., 1997; Voisey et al., 2006) and by the presence of melanocytes in a dedifferentiated state in the white ventral skin of mice expressing ASP (as noted by Sviderskaya et al. ( 2001)).
Our data show that the activation of MC1R by αMSH enhances the pigmentary function of melanocytes to produce more eu- and pheo- melanin. The depigmentation induced by ASP results from the inhibited synthesis of both types of melanins concomitant with the dramatic decrease of tyrosinase activity, and does not result from the degradation of existing melanin. Because of the dramatic repercussions of a lighter pigmentation in human skin with respect to the risk of developing cutaneous cancers and in the light of the now well established involvement of MC1R in melanoma, it becomes of great interest to further characterize the mechanism underlying the effects of ASP via the MC1R and its possible role in reversing melanocyte differentiation.
Materials and methods
ASP synthesis and purification
Recombinant ASP was secreted from H5 insect cells infected with a baculovirus construct (Ollmann et al., 1998; Sakai et al., 1997) that contained the mouse cDNA and was cultured at 21°C. At 72 hr post-infection, fresh culture supernatants were filtered (0.45 μm polyethersulfone membrane) and were separated over two ion exchange columns (S Sepharose and Q Sepharose, 5 ml each) attached in series and equilibrated with 20 mM HEPES, pH 7.3, 75 mM NaCl. Columns were washed to baseline with equilibration buffer (20 mM HEPES, pH 7.3, 75 mM NaCl), then proteins were eluted with 0.1 to 1 M NaCl gradients over 20 column volumes. Fractions were analyzed by Coomassie blue staining of SDS-PAGE gels and by western immunoblotting to determine the presence and purity of ASP. Pools of fractions enriched in ASP were concentrated (5 kDa MWCO) to a volume of 2 ml, then were loaded onto a HiPrep 26/60 Sephacryl S-200 HR (GE Healthcare, Piscataway, NJ, USA) gel filtration column and eluted at 1.3 ml/min with equilibration buffer. Purified fractions of ASP were analyzed by Coomassie blue staining of SDS-PAGE gels and by Western immunoblotting as detailed below and were pooled appropriately. Expression of ASP and purification was performed by the Eukaryotic Expression Group NCI Frederick MD USA and the Protein Purification Group NCI Frederick MD. The degree of purification of the chosen pool was >90% as evaluated by silver staining performed according to the manufacturer's protocol (Invitrogen, Carlsbad, CA, USA).
SDS-PAGE and western blotting
ASP samples were prepared in reducing conditions using Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA, USA) before application to Novex Tris-glycine 14% pre-cast gels (Invitrogen). Proteins were visualized using 0.005% Coomassie Brilliant Blue R-250 in 0.3% v/v acetic acid during the different steps of purification. To analyze the presence of ASP after SDS-PAGE, blots were blocked in 5% nonfat milk in PBS-T for 1 h at room temperature, then were probed with an antibody against murine ASP (AB3400P, Millipore, Billerica, MA, USA). Melan-a melanocytes were harvested and solubilized in phosphate buffered saline (PBS) containing 1% Nonidet P40 (Calbiochem, San Diego, CA), and Protease Inhibitor cocktail (Roche, Mannheim, Germany) for 1 h on ice with occasional vortexing. Protein concentrations of extracts were measured using the BCA protein assay kit (Pierce, Rockford, IL). Cell extracts were separated by electrophoresis under reducing conditions on 8−16% gradient Tris-glycine gels (Invitrogen, Carlsbad, CA). After electrophoresis, proteins were transferred electrophoretically from the gels to Invitrolon polyvinylidene difluoride membranes (Invitrogen Corp). Blots were blocked in 5% nonfat milk in PBS-T for 1 hr at room temperature, then incubated with the anti-tyrosinase antibody αPEP7 or with anti-β-actin (Abcam, Cambridge, MA) at room temperature for 1 hr in 5% milk, and were then incubated with horseradish peroxidase-linked anti-rabbit or anti-mouse whole antibodies (Amersham Bioscience, Pittsburgh, PA) at room temperature for 1 hr. Antigens were detected using an ECL-plus Western Blotting Detection System (Amersham).
Cell culture and treatment conditions
Melan-a (black) and melan-c (albino) melanocytes (gifts from Prof. Dorothy C. Bennett, St. George's Hospital Medical School, London, UK) were cultured at 37°C with 5% CO2 in RPMI 1640 medium (Invitrogen) containing 5% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA), 0.084 % sodium bicarbonate (Invitrogen), 200 nM 2-otetradecanoylphorbol-13-acetate (Sigma, St Louis, MO, USA), 100 μM 2-mercaptoethanol (Sigma), 50 U/ml penicillin, 50 μg/ml streptomycin (Invitrogen) and 2 mM L-glutamine (Invitrogen) at pH 7.2.
Similar numbers of cells were plated in culture dishes at day 0 (D0), and culture media were replaced with fresh media containing (final concentrations reported) 10 nM ASP (in 20 μM HEPES, pH 7.3, 200 μM NaCl), 100 nM αMSH (in sterile water) (Sigma) or 200 μM PTU (in 0.4% ethanol or 0.1% DMSO) (Sigma) for the 4 days of treatment, or with medium containing the appropriate concentration of solvent used as a control. Two days later (D2), the resulting culture media were collected and stored in plastic centrifuge tubes (Becton Dickinson, Franklin Lakes, NJ, USA) at −80°C. Fresh media were added with the appropriate factors to continue the 4 day treatment samples and to begin the 2 day treatment samples. At D3, appropriate factors were added to begin the 1 day treatment samples. All culture media were collected and frozen at D4, while the cells in each dish were harvested, counted using a Cellometer Auto T4 (Nexcelom Bioscience, Lawrence MA) and frozen until used. Cells and culture media collected were then lyophilized and processed to analyze eumelanin and pheomelanin contents as detailed below. The results for melanin contents in the media reported take into account the total medium used during the 4 days of culture for each dish. In order to determine levels of melanins before 4, 2 and 1 days of treatment, cells of identical dishes were harvested and were frozen respectively at D0, D2 and D3. Three independent sets of experiments were performed according to this protocol. To determine the dose-effect of ASP on the synthesis of both pigments, cells were cultured 3 days in presence of various concentrations of ASP. Cells and media were harvested and processed as described earlier. Two set of experiments were performed with similar results.
Eumelanin and pheomelanin assays
The determination of eumelanin and pheomelanin contents were performed as described previously (Ito & Wakamatsu, 1994; Wakamatsu & Ito, 2002). The method involves the permanganate oxidation of eumelanin to form pyrrole-2,3,5-tricarboxylic acid (PTCA) and the hydriodic acid reductive hydrolysis of pheomelanin to form 4-amino-3-hydroxyphenylalanine (4-AHP). Contents of these specific degradation products were determined using HPLC assays, and were converted to eumelanin and pheomelanin contents by multiplying with factors of 50 and 9, respectively (Wakamatsu & Ito, 2002). Each measurement of eumelanin was performed in duplicate.
Tyrosinase activity determination
14C tyrosine assay
Proteins extracted from treated or from control melanocytes were harvested and solubilized in phosphate buffer saline (PBS) containing 1% Nonidet P40 (Calbiochem, San Diego, CA) and Protease Inhibitor cocktail (Roche, Mannheim, Germany) for 1 h on ice with occasional vortexing. Protein concentrations of extracts were determined using the BCA protein assay kit (Pierce, Rockford, IL). Seven μg of each sample were diluted into 30 μl solubilizing buffer as described earlier and were seeded into round-bottom 96-well microtiter plates. Ten μl of a solution containing chloramphenicol (1 mg/ml), cycloheximide (1 mg/ml), penicillin G (1000 units/ml) and bovine serum albumin (0.1 mg/ml) in 1 M potassium phosphate buffer, pH 7.2, was added along with 10 μl L-14C tyrosine (25 μCi/ml). The enzyme assays were run at 37°C for 1 h. After incubation, the samples were placed on ice and 40 μl of each sample was removed to Whatman 3MM filter-paper discs. Filters were washed as follows: one 15 min wash with 1 liter (per 50 filters) of 0.1 N HCl (containing unlabelled 0.1% tyrosine), two 15 min washes with 1 liter of 0.1 N HCl, two 5 min washes with 200 ml 95% ethanol, and one 5 min wash in 200 ml acetone. The filters were then allowed to dry in air, and were counted for radioactivity in 5 ml Hydrofluor in a Beckman LS 6500 liquid scintillation counter. Controls ordinarily used included blanks from which the substrate was omitted.
DOPA oxidation assay
Following extraction from treated or control cells, proteins were separated by electrophoreses in non-reducing conditions, and then transferred to PVDF membranes. Membranes were then incubated in 0.1 % DOPA solubilized in PBS for 3 hr at 37°C and were washed gently in PBS.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Cancer Institute at NIH. The authors wish to thank Ralph Hopkins, William Gillette, Dominic Esposito and Peter Frank of NCI for their expertise in purifying the ASP.
References
- Aberdam E, Bertolotto C, Sviderskaya EV, de Thillot V, Hemesath TJ, Fisher DE, Bennett DC, Ortonne JP, Ballotti R. Involvement of microphthalmia in the inhibition of melanocyte lineage differentiation and of melanogenesis by agouti signal protein. J. Biol. Chem. 1998;273:19560–19565. doi: 10.1074/jbc.273.31.19560. [DOI] [PubMed] [Google Scholar]
- Bagnara JT, Fernandez PJ. Hormonal influences on the development of amphibian pigmentation patterns. Zool. Sci. 1993;10:733–748. [Google Scholar]
- Bonilla C, Boxill LA, Donald SA, Williams T, Sylvester N, Parra EJ, Dios S, Norton HL, Shriver MD, Kittles RA. The 8818G allele of the agouti signaling protein (ASIP) gene is ancestral and is associated with darker skin color in African Americans. Hum. Genet. 2005;116:402–406. doi: 10.1007/s00439-004-1251-2. [DOI] [PubMed] [Google Scholar]
- Coelho SG, Miller SA, Zmudzka BZ, Beer JZ. Quantification of UV-induced erythema and pigmentation using computer assisted digital image evaluation. Photochem. Photobiol. 2006;82:651–656. doi: 10.1562/2005-08-02-TSN-635. [DOI] [PubMed] [Google Scholar]
- Cone RD, Lu D, Vage DI, Klungland H, Boston BA, Chen WB, Orth DN, Pouton C, Kesterson RA. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Rec. Prog. Hormone. Res. 1996;51:287–317. [PubMed] [Google Scholar]
- Costin GE, Hearing VJ. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- del Marmol V, Ito S, Bouchard B, Libert A, Wakamatsu K, Ghanem G, Solano F. Cysteine deprivation promotes eumelanogenesis in human melanoma cells. J. Invest. Dermatol. 1996;107:698–702. doi: 10.1111/1523-1747.ep12365591. [DOI] [PubMed] [Google Scholar]
- Furumura M, Potterf SB, Toyofuku K, Matsunaga J, Muller J, Hearing VJ. Involvement of ITF2 in the transcriptional regulation of melanogenic genes. J. Biol. Chem. 2001;276:28147–28154. doi: 10.1074/jbc.M101626200. [DOI] [PubMed] [Google Scholar]
- Furumura M, Sakai C, Abdel-Malek ZA, Barsh GS, Hearing VJ. The interaction of agouti signal protein and melanocyte stimulating hormone to regulate melanin formation in mammals. Pigment Cell Res. 1996;9:191–203. doi: 10.1111/j.1600-0749.1996.tb00109.x. [DOI] [PubMed] [Google Scholar]
- Galbraith DB. Expression of genes at the agouti locus and mitotic activity of the hair bulb of the mouse. Genetics. 1971;67:559–568. doi: 10.1093/genetics/67.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Wakamatsu K, Hunt G, Ito S, Thody AJ. Agouti protein inhibits the production of eumelanin and phaeomelanin in the presence and absence of α−melanocyte stimulating hormone. Pigment Cell Res. 1997;10:298–303. doi: 10.1111/j.1600-0749.1997.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Granholm DE, Reese RN, Granholm NH. Agouti alleles alter cysteine and glutathione concentrations in hair follicles and serum of mice (Ay/a, AwJ/AwJ, and a/a). J. Invest. Dermatol. 1996;106:559–563. doi: 10.1111/1523-1747.ep12344031. [DOI] [PubMed] [Google Scholar]
- Hall AM, Orlow SJ. Degradation of tyrosinase induced by phenylthiourea occurs following Golgi maturation. Pigment Cell Res. 2005;18:122–129. doi: 10.1111/j.1600-0749.2005.00213.x. [DOI] [PubMed] [Google Scholar]
- Hennessy A, Oh C, Diffey B, Wakamatsu K, Ito S, Rees JL. Eumelanin and pheomelanin concentrations in human epidermis before and after UVB irradiation. Pigment Cell Res. 2005;18:220–223. doi: 10.1111/j.1600-0749.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- Hunt G, Thody AJ. Agouti protein can act independently of melanocyte-stimulating hormone to inhibit melanogenesis. J. Endocrin. 1995;147:R1–R4. doi: 10.1677/joe.0.147r001. [DOI] [PubMed] [Google Scholar]
- Ito S. The IFPCS presidential lecture: a chemist's view of melanogenesis. Pigment Cell Res. 2003;16:230–236. doi: 10.1034/j.1600-0749.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K. An improved modification of permanganate oxidation of eumelanin that gives a constant yield of pyrrole-2,3,5-tricarboxylic acid. Pigment Cell Res. 1994;7:141–144. doi: 10.1111/j.1600-0749.1994.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Ito S, Wakamatsu K, Matsunaga N, Hearing VJ, Carey KD, Anderson S, Dooley TP. Cyclic oscillations in melanin composition within hairs of baboons. Pigment Cell Res. 2001;14:180–184. doi: 10.1034/j.1600-0749.2001.140307.x. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Eguchi G. In vitro analysis of cellular metaplasia from pigmented epithelial cells to lens phenotypes: a unique model system for studying cellular and molecular mechanisms of “transdifferentiation”. Dev. Biol. 1986;115:353–362. doi: 10.1016/0012-1606(86)90255-1. [DOI] [PubMed] [Google Scholar]
- Jackson IJ. Homologous pigmentation mutations in human, mouse and other model organisms. Hum. Mol. Gen. 1997;6:1613–1624. doi: 10.1093/hmg/6.10.1613. [DOI] [PubMed] [Google Scholar]
- Kanetsky PA, Swoyer J, Panossian S, Holmes R, Guerry D, Rebbeck TR. A polymorphism in the agouti signaling protein gene is associaed with human pigmentation. Amer. J. Hum. Gen. 2002;70:770–775. doi: 10.1086/339076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoreux ML, Wakamatsu K, Ito S. Interaction of major coat color gene functions in mice as studied by chemical analysis of eumelanin and pheomelanin. Pigment Cell Res. 2001;14:23–31. doi: 10.1034/j.1600-0749.2001.140105.x. [DOI] [PubMed] [Google Scholar]
- Lassalle MW, Igarashi S, Sasaki M, Wakamatsu K, Ito S, Horikoshi T. Effects of melanogenesis-inducing nitric oxide and histamine on the production of eumelanin and pheomelanin in cultured human melanocytes. Pigment Cell Res. 2003;16:81–84. doi: 10.1034/j.1600-0749.2003.00004.x. [DOI] [PubMed] [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Meziani R, Descamps V, Gerard B, Matichard E, Bertrand G, Archimbaud A, Ollivaud L, Saiag P, Lebbe C, Basset-Seguin N, Alberti C, Crickx B, Grandchamp B, Soufir N. Association study of the g.8818A>G polymorphism of the human agouti gene with melanoma risk and pigmentary characteristics in a French population. J. Dermatol. Sci. 2005;40:133–136. doi: 10.1016/j.jdermsci.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Millar SE, Miller MW, Stevens ME, Barsh GS. Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated. Development. 1995;121:3223–3232. doi: 10.1242/dev.121.10.3223. [DOI] [PubMed] [Google Scholar]
- Norton HL, Kittles RA, Parra E, McKeigue P, Mao X, Cheng K, Canfield VA, Bradley DG, McEvoy B, Shriver MD. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol. Biol. Evol. 2007;24:710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Lamoreux ML, Wilson BD, Barsh GS. Interaction of agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes & Devel. 1998;12:316–330. doi: 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poma A, Bianchini S, Miranda M. Inhibition of L-tyrosine-induced micronuclei production by phenylthiourea in human melanoma cells. Mutat. Res. 1999;446:143–148. doi: 10.1016/s1383-5718(99)00142-4. [DOI] [PubMed] [Google Scholar]
- Rees JL. Genetics of hair and skin color. Ann. Rev. Gen. 2003;37:67–90. doi: 10.1146/annurev.genet.37.110801.143233. [DOI] [PubMed] [Google Scholar]
- Sakai C, Ollmann M, Kobayashi T, Abdel-Malek ZA, Muller J, Vieira WD, Imokawa G, Barsh GS, Hearing VJ. Modulation of murine melanocyte function in vitro by agouti signal protein. EMBO J. 1997;16:3544–3552. doi: 10.1093/emboj/16.12.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist W, Drozdz R, Cotti R, Willard DH, Wilkison WO, Eberle AN. Interactions of α-melanotropin and agouti on B16 melanoma cells: evidence for inverse agonism of agouti. J. Recept. &Signal Trans. Res. 1997;17:75–98. doi: 10.3109/10799899709036595. [DOI] [PubMed] [Google Scholar]
- Silvers WK. An experimental approach to action of genes at the agouti locus in the mouse. III. Transplants of newborn Aw-, A-, and at- skin to Ay-, Aw-, A- and aa hosts. J. Exp. Zool. 1958;137:189–196. doi: 10.1002/jez.1401370110. [DOI] [PubMed] [Google Scholar]
- Siracusa LD. The agouti gene: turned on to yellow. Trends Genet. 1994;10:423–428. doi: 10.1016/0168-9525(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J. Invest. Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I, Tada A, Ollmann M, Barsh GS, Im S, Lamoreux ML, Hearing VJ, Nordlund JJ, Abdel-Malek ZA. Agouti signalling protein inhibits melanogenesis and the response of human melanocytes to α-melanotropin. J. Invest. Dermatol. 1997;108:838–842. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- Sviderskaya EV, Hill SP, Balachandar D, Barsh GS, Bennett DC. Agouti signaling protein and other factors modulating differentiation and proliferation of immortal melanoblasts. Devel. Dynam. 2001;221:373–379. doi: 10.1002/dvdy.1153. [DOI] [PubMed] [Google Scholar]
- Virador V, Muller J, Wu X, Abdel-Malek ZA, Yu Z-X, Ferrans VJ, Kobayashi N, Wakamatsu K, Ito S, Hammer JA, Hearing VJ. Influence of α-melanocyte stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. FASEB J. 2002;16:105–107. doi: 10.1096/fj.01-0518fje. [DOI] [PubMed] [Google Scholar]
- Voisey J, Gomez-Cabrera MC, Smit DJ, Leonard JH, Sturm RA, van Daal A. A polymorphism in the agouti signalling protein (ASIP) is associated with decreased levels of mRNA. Pigment Cell Res. 2006;19:226–231. doi: 10.1111/j.1600-0749.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Hu DN, McCormick SA, Ito S. Characterization of melanin in human iridal and choroidal melanocytes from eyes with various colored irides. Pigment Cell Melanoma Res. 2008;21:97–105. doi: 10.1111/j.1755-148X.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Ito S. Advanced chemical methods in melanin determination. Pigment Cell Res. 2002;15:174–183. doi: 10.1034/j.1600-0749.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Kavanagh R, Kadekaro AL, Terzieva S, Sturm RA, Leachman S, Abdel-Malek Z, Ito S. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006a;19:154–162. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Takasaki A, Kagedal B, Kageshita T, Ito S. Determination of eumelanin in human urine. Pigment Cell Res. 2006b;19:163–169. doi: 10.1111/j.1600-0749.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Ward WC, Lamb EC, Gooden D, Chen X, Burinsky DJ, Simon JD. Quantification of naturally occuring pyrrole acids in melanosomes. Photochem. Photobiol. 2008:84. doi: 10.1111/j.1751-1097.2008.00328.x. in press. [DOI] [PubMed] [Google Scholar]
- Wolff GL. Regulation of yellow pigment formation in mice: a historical perspective. Pigment Cell Res. 2003;16:2–15. doi: 10.1034/j.1600-0749.2003.00012.x. [DOI] [PubMed] [Google Scholar]