Abstract
Cadherin cell adhesion molecules play crucial roles in vertebrate development including the development of the visual system. Most studies have focused on examining functions of classical type I cadherins (e.g. cadherin-2) in visual system development. There is little information on the function of classical type II cadherins (e.g. cadherin-6) in the development of the vertebrate visual system. To gain insight into cadherin-6 role in the formation of the retina, we analyzed differentiation of retinal ganglion cells, amacrine cells and photoreceptors in zebrafish embryos injected with cadherin-6 specific antisense morpholino oligonucleotides. Differentiation of the retinal neurons in cadherin-6 knockdown embryos (cdh6 morphants) was analyzed using multiple markers. We found that expression of transcription factors important for retinal development was greatly reduced, and expression of Notch-Delta genes and proneural gene ath5 was altered in the cdh6 morphant retina. The retinal lamination was present in the morphants, although the morphant eyes were significantly smaller than control embryos due mainly to decreased cell proliferation. Differentiation of the retinal ganglion cells, amacrine cells and photoreceptors was severely disrupted in the cdh6 morphants due to a significant delay in neuronal differentiation. Our results suggest that cadherin-6 plays an important role in the normal formation of the zebrafish retina.
Keywords: cell adhesion molecules, retinal ganglion cells, amacrine cells, photoreceptors, optic nerve, development
Introduction
The vertebrate retina, including the zebrafish retina, is a well-laminated structure consisting of alternating cellular and synaptic layers. Retinal ganglion cells (RGCs), residing in the innermost cell layer near the basal surface of the retina, called the retinal ganglion cell layer (gcl), are the first retinal cells to become postmitotic, beginning around 28 hours post fertilization (hpf). The earliest differentiating RGCs are located in the anteroventral retina. Differentiating RGCs produce several dendrites (toward the apical direction), and an axon (toward the basal direction). The earliest RGC axons arrive at the optic chiasm at about 35 hpf, reach the anterior optic tectum at about 45 hpf, and cover the entire lobe of the optic tectum by 72 hpf (Stuermer 1988; Burrill and Easter, 1994). Differentiating zebrafish RGCs can be labeled using antibodies for various markers including anti-HuC/HuD (labeling the soma), anti-acetylated tubulin (labeling mainly the processes), anti-Pax6 (labeling the soma), and zn5 (labeling both the soma and processes) (Malicki et al., 2003; Masai et al., 2003; Avanesov et al., 2005).
Photoreceptors (rods and cones), found in the outer nuclear layer (onl) near the apical surface of the retina, become postmitotic much later than RGCs. Differentiating photoreceptors can be distinguished by their expression of photoreceptor-specific markers (e.g. zpr-1, Larison and BreMiller, 1990). Like the RGCs, the earliest differentiating photoreceptors, beginning around 45 hpf, are located in the anteroventral region of the retina, and by 72 hpf, expression of the photoreceptor-specific markers has expanded to the entire retina (Malicki, 1999).
Amacrine cells, located in the innermost portion of the inner nuclear layer (inl), develop later than RGCs, but earlier than photoreceptors. Differentiating zebrafish amacrine cells can be first detected at 41 hpf (Godinho et al., 2005). As development proceeds, amacrine cells generate numerous processes, and by 50 hpf, a plexus of neurites is formed projecting toward the gcl (Godinho et al., 2005). Developing zebrafish amacrine cells are well-labeled with various markers including the anti-HuC/HuD, anti-Pax6 and anti-parvalbumin antibodies (Malicki et al., 2003; Masai et al., 2003; Avanesov et al., 2005).
Molecular mechanisms underlying RGC, amacrine cell and photoreceptor development have been under intense investigation. Regulator molecules such as atonal homologues 3 and 5 (ath3 and ath5), BarH, NeuroD, Pax6, sonic hedgehog (shh), bone morphogenetic proteins (BMPs), members of retinal homeobox (e.g. Rx1) and orthodenticle homeobox families (e.g. otx2), and cone-rod homeobox (Crx), play crucial roles in differentiation of the vertebrate retinal cells (Chow, 2001; Marquardt and Gruss, 2002; Harada et al., 2007). Cadherin cell adhesion molecules have also been implicated in the development of these retinal cells (see below).
Cadherins are transmembrane molecules that mediate cell-cell adhesion mainly through homophilic interactions (Takeichi, 1991; Gumbiner, 1996). Functional studies of two classical type I cadherins (e.g. cadherin-2 and cadherin-4, also known as N-cadherin and R-cadherin, respectively) in various vertebrate species including zebrafish showed that these molecules regulate the formation of the vertebrate visual system (Matsunaga et al., 1988; Riehl et al., 1996; Stone and Sakaguchi, 1996; Inoue and Sanes, 1997; Treubert-Zimmermann et al., 2002; Malicki et al, 2003; Masai et al., 2003; Babb et al., 2005, Liu et al., 2007). There is little information on the function of other cadherins (e.g. cadherin-6, -7, and -10, members of the type II cadherins, Nollet et al., 2000) in the development of the visual system. In this study we showed that cadherin-6 was expressed by specific zebrafish retinal cells (e.g. RGCs and amacrine) during critical stages of their development, and interfering with cadherin-6 function using translation blocking morpholino antisense oligonucleotides severely disrupted differentiation of zebrafish retinal cells, indicating that cadherin-6 plays a crucial role in the development of the vertebrate visual system.
Methods
Zebrafish
Zebrafish (Danio rerio) were maintained as described in the Zebrafish Book (Westerfield, 2000). Zebrafish embryos were obtained from breeding of wild-type adult zebrafish. Embryos for whole-mount immunocytochemistry or in situ hybridization were raised in PTU (1-phenyl-2-thiourea, 0.003%) to prevent melanization. All animal-related procedures were approved by the Care and Use of Animals in Research Committee at the University of Akron.
MO injections and mRNA synthesis
Two translation blocking morpholino antisense oligonucleotides (MOs; cdh6MO1: 5’-AAG AAG TAC AAT CCA AGT CCT CAT C-3’ (Kubota et al., 2007), cdh6MO2: 5’-TCC GCT CTT AGG GTG TCT TAC AGG G-3’), and a MO with five-mismatched nucleotides (5-mis cdh6MO1: 5’-AAc AAG TAg AAT gCA AcT CCT gAT C-3’) were used in the study. These MOs, designed by and purchased from Gene Tools (Philomath, OR), were used as described (Nasevicius and Ekker, 2000). Compared with data bases using BLAST, the cdh6 MOs sequences showed no significant similarities to any sequences other than zebrafish cdh6 (GenBank accession number: AB193290). MOs were microinjected into one- to four-cell stage embryos at 2 nl (6-12 ng for cdh6MO1, 12 ng for 5-mis cdh6MO1, 3.4-6.8 ng for cdh6MO2) in Daneau buffer (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES pH 7.6).
Injected embryos were allowed to develop at 28.5°C until the embryos reached desired stages (e.g. 50 hpf), anesthetized in 0.02% MS-222 and fixed in 4% paraformaldehyde and processed as described below. To label individual retinal cells, some embryos were injected with a solution containing a cdh6 MO or a control (5-mis) MO (the same amount as above), together with eGFP DNA (30 ng). This eGFP expressing vector is under the control of a zebrafish heat shock promoter 70 (HSP70/4 eGFP, Shoji et al., 1998). Injected embryos were heat shocked (37°C for one hour at 36 hpf) to activate the heat shock promoter, followed by returning to 28.5°C until the embryos reached desired stages (e.g. 53 hpf), anesthetized and fixed as described above.
Tissue processing
To prepare tissue for whole mount in situ hybridization or immunohistochemistry, the fixed embryos were rinsed in 0.1 M phosphate buffered saline (PBS, pH 7.4), followed by placing the embryos in increasing concentrations of methanol, and stored in 100% methanol at -20°C. To prepare tissue for immunohistochemistry on tissue sections, the fixed embryos were washed in PBS, processed through a graded series of increasing sucrose concentrations, and placed in 20% sucrose in PBS overnight at 4°C. The embryos were then embedded and frozen in a mixture of OCT embedding compound and 20% sucrose (1:1, v/v). A cryostat was used to obtain 10 μm (30 μm for embryos co-injected with the eGFP plasmid DNA) sections collected on pretreated glass slides (Fisher Scientific, Pittsburgh, PA), dried at room temperature and stored at -80°C.
In situ hybridization
cDNAs used to generate the cRNA probes were kindly provided by Pamela Raymond at the University of Michigan (for crx, deltaC, notch1a, otx5 and rx1 genes), and Deborah Stenkamp at the University of Idaho (for ath5 and neuroD). A cDNA fragment corresponding to the nucleotides 228-1359 of the zebrafish cdh6 (GenBank accession no. AB193290) was obtained using reverse transcriptase-polymerase chain reaction with total RNA from 50 hpf zebrafish embryos (Liu et al., 2006). Procedures for the synthesis of digoxigenin-labeled cRNA probes, and whole mount in situ hybridization were described previously (Liu et al., 1999; Babb et al., 2005; Liu et al., 2006). For each cRNA probe, control embryos (uninjected or embryos injected with the 5-misMO) and embryos injected with one of the cdh6 MOs (cdh6 morphants) were processed at the same time, side by side. For immunocytochemical detection of the digoxigenin-labeled cRNA probes, anti-digoxigenin Fab fragment antibodies conjugated to alkaline phosphatase were used, followed by an NBT/BCIP color reaction step (Roche Molecular Biochemicals, Indianapolis, IN).
Cadherin-6 antibody production
A synthetic peptide 5’-RMHKSTHLVAVVISDGHFPMQS-3’, corresponding to zebrafish cadherin-6 amino acid residues 557-578, was conjugated to keyhole limpet hemocyanin and used to immunize two rabbits (Covance Research Products, Inc., Denver, PA). The resulting crude rabbit polyclonal antiserum was affinity purified by covalently linking the synthetic peptide to Affi-Gel 10 resin (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. The cadherin-6 antiserum was diluted with an equal volume of 5X PBS and applied to a chromatography column packed with the resin. The flow-through was reapplied twice, and the column was rinsed with 10 column volumes of 5X PBS to eliminate non-specific binding. The cadherin-6 antibody was eluted with 10 column volumes of 0.1 M sodium citrate supplemented with 10% ethylene glycol (pH 2.5), neutralized with 1 M Tris buffer (pH 8) and then concentrated with Centriprep concentrators (Bio-Rad).
Immunoblotting, Immunohistochemistry, and TUNEL labeling
Detailed procedures for immunoblotting, whole mount immunohistochemistry and immunostaining on tissue sections were described previously (Liu et al., 1999; Liu et al., 2002; Babb et al., 2005). Primary antibodies used were anti-acetylated tubulin (1:1,000; Sigma, St. Louis, MO), anti-β-catenin (1:500, Sigma), anti-eGFP (1:1,000; Medical & Biological Laboratories, Woburn, MA), anti-HuC/HuD (1:2000; Molecular Probes/Invitrogen, Carlsbad, CA), anti-Pax6 (1:500; Chemicon International, Inc., Temecula, CA), anti-parvalbumin (1:500; Chemicon International Inc.), anti-histone H3 (1:500; Chemicon International, Inc.), cadherin-6 (1:500 and 1:300 for immunoblotting and immunofluorescent methods, respectively), zn-5 (1:1,500, Zebrafish International Resource Center, University of Oregon, Eugene, OR), znp-1 (1:250; The Developmental Studies Hybridoma Bank, the University of Iowa, Iowa City, IA) and zpr-1 antibodies (1:1,000; Zebrafish International Resource Center). For immunofluorescent microscopy, an anti-rabbit or anti-mouse secondary antibody conjugated with Cy3 or FITC (Jackson ImmunoResearch Laboratories, West Grove, PA) was used. A biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) was used for immunoperoxidase methods, and visualization of the reaction was achieved by using a DAB kit (Vector Laboratories).
Terminal dUTP nick-end labeling (TUNEL) was performed on whole-mount embryos using the Roche in situ cell death detection kit (Roche Molecular Biochemicals), according to the manufacturer’s instructions.
Microscopy and Photography
Stained whole mount embryos or tissue sections were analyzed with an Olympus BX51 compound microscope equipped with a SPOT digital camera. Quantitative data from each embryo processed for the histone H3 immunostaining was obtained from two alternate sections (to avoid counting the same positive cells twice) through the central retina, using the size and presence of the lens as reference points. To analyze cadherin-6 function on differentiation of individual retinal cells, morphology (soma shape and size, presence of axon and dendrites and the number of the dendrites) of well-labeled eGFP expressing cells in the ganglion cell layer (RGCs) and inner portion of the inner nuclear layer (amacrine cells) was recorded digitally and/or using Camera lucida drawings under 100X objective lens with Nomarski optics. For amacrine cells, only processes projected toward the ganglion cell layer were recorded. Digitized images were processed, with the same values of contrast, sharpening, and hue/saturation, with Adobe Photoshop (Mountain View, CA). Unpaired Student t-test was used to determined statistical significance.
Results
Cadherin-6 expression in the developing retina
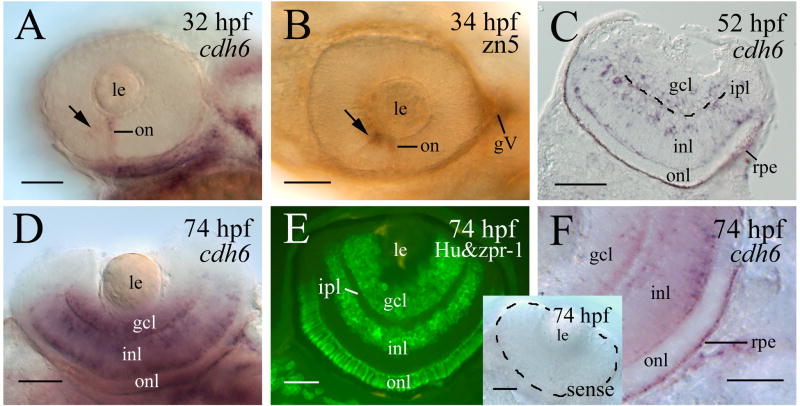
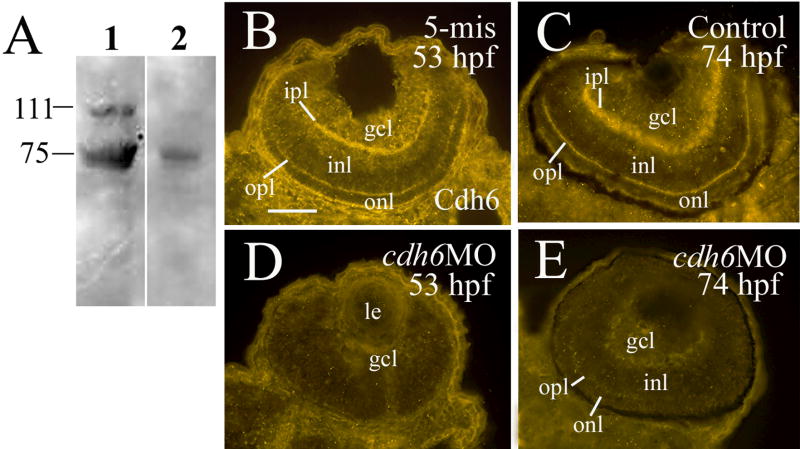
Cadherin-6 message (cdh6) expression was first detected in the developing zebrafish retina at 32 hpf. cdh6 was detected in the optic nerve and the anteroventral region of the retina, where the earliest differentiating retinal cells, the retinal ganglion cells (RGCs), are located (Fig. 1A and B). As development proceeded, cdh6 expression increased greatly in the retina, mainly found in the retinal ganglion cell layer (gcl), the inner portion of the inner nuclear layer (inl) where presumptive amacrine cells reside, and the outer most region of the inl where presumptive horizontal cells are localized (Fig. 1C-E). The retinal pigmented epithelium also contained cdh6 (Fig. 1C and F). Similar expression pattern was observed using the affinity purified polyclonal cadherin-6 anti-peptide antibody (Fig. 2A-C). Moreover, both the inner plexiform layer, where retinal ganglion cell dendrites contact amacrine and bipolar cell processes, and the outer plexiform layer, where horizontal, bipolar and photoreptor processes contact, were strongly labeled by the antibody (Fig. 2B and C). Specificity of this antibody was demonstrated by immunoblotting using zebrafish embryonic tissues (lysates of whole embryos at 50 to 74 hpf). The antibody detected a band at ~ 110 kDa (Fig. 2A), a molecular mass similar to other zebrafish cadherin proteins (Cdh6) (e.g. cadherin-1, -2, -4, Bitzur et al., 1994; Liu et al., 2001a; Liu et al., 2001b; Babb and Marrs, 2004), and Cdh6 from other vertebrates (Cho et al., 1998; Inoue et al., 1998; Nakagawa and Takeichi, 1998; Ruan et al., 2006). A couple of lower molecular mass bands were also present in the immunoblot. These bands likely represented degradation products of the Cdh6 (Inoue et al., 1998; Nakagawa and Takeichi, 1998), because their staining was greatly reduced when the antibody had been preincubated with the synthetic cadherin-6 peptide used for the generation of the cadherin-6 antibody. The Cdh6 is likely target for proteolysis, since only the high molecular mass, the 110 kDa band could be detected in fresh tissue in the presence of strong protease inhibitors (phenylmethylsulfonyl fluoride and protease inhibitor cocktail).
Figure 1.
cdh6 expression in the developing zebrafish retina. Panels A, B and D show whole mount eyes (anterior to the left, dorsal is up for panels A and B, and dorsal is down for panel D). Panels A, D and F are whole mount eyes processed for cdh6 in situ hybridization. Panel B is a whole mount eye processed for zn5 immunostaining (labeling early differentiating retinal ganglion cells). Panel C is a retinal cross section (dorsal to the left) of an embryo processed for cdh6 whole mount in situ hybridization, while panel E is immunostaining of a retinal cross section (dorsal to the left) using anti-HuC/HuD (labeling the retinal ganglion cells and amacrine cells) and zpr-1 antibodies (labeling the photoreceptor layer). Arrows in panels A and B indicate labeling in the anteroventral region of the retina. The inner plexiform layer (ipl) in panel C is indicated by the dashed line. Panel F is a higher magnification of the posteroventral quadrant of a whole mount eye (anterior to the left and dorsal up) showing labeling in the retinal pigmented epithelium (rpe). The insert in panel F shows a whole mount eye (outlined by the dashed line) processed for in situ hybridization using a cdh6 sense probe. Other abbreviations: gcl, retinal ganglion cell layer; gV, trigeminal ganglion; inl, inner nuclear layer; le, lens; on, optic nerve; onl, outer nuclear layer. Scale bars = 50 μm.
Figure 2.
Cadherin-6 antibody and cadherin-6 protein expression in the developing zebrafish retina. Panel A shows immunoblot using 55 hpf whole zebrafish embryo lysate demonstrating specificity of an affinity purified zebrafish cadherin-6 antibody. Staining of the 110 kDa molecular mass band was greatly reduced when the cadherin-6 antibody (lane 1) had been preincubated with excess cadherin-6 synthetic peptide used to generate the antibody (lane 2). The same amount of protein (40 μg total protein/lane) was loaded. Panels B-E are retinal cross sections (dorsal to the left) showing cadherin-6 protein (Cdh6) expression in an embryo injected with the control 5-mis MO (panel B), a control embryo (panel C) and cdh6 morphants (panels D and E). Abbreviation: opl, outer plexiform layer. Other abbreviations are the same as in Figure 1. Scale bar = 50 μm.
MO injections
Morpholino antisense oligonucleotide (MO) techniques effectively and selectively block gene function, and these reagents have mainly been employed for developmental studies in vertebrates such as Xenopus and zebrafish (Ekker, 2000; Nasevicius and Ekker, 2000; Andermann et al., 2002; Ando et al., 2005; Knaut et al., 2005; Bellipanni et al., 2006; Eaton and Glasgow, 2006). Injection of translation blocking cdh6 MOs into one- to four-cell stage zebrafish embryos resulted in embryos that had similar phenotype such as smaller eye and head (cdh6 is expressed in the forebrain and retina, Liu et al., 2006), edema in the thorax, short yolk extension, and/or curved body (due to disrupted kidney function) (Fig. 3; Table 1), as described by Kubota and colleagues (2007). Injection of lower dosages of the cdh6 MOs (cdh6MO1, 6.0 ng/embryo, or cdh6MO2, 3.4 ng/embryo) resulted in most embryos with moderate defects (e.g. small eyes and head, short yolk extension, slight or no edema in the thorax, with straight body; the second embryo in Fig. 3A; Table 1). Injection of higher dosages of cdh6 MOs (cdh6MO1, 12.0 ng/embryo, or cdh6MO2, 6.8 ng/embryo) resulted in embryos with more severe phenotypes (smaller eyes and head, apparent edema in the thorax, and/or curved body; the third embryo in Fig. 3A; Table 1) that were similar to those obtained by Kubota and colleagues (2007). The gross ` were not obvious at 24 hpf, but became apparent at 40-50 hpf. Measurements of the eye size (circumference in microns) of live embryos at 50 hpf revealed that cdh6 morphant eyes (576.8 ± 21.3, n = 20 eyes) were significantly smaller (p<0.001) than uninjected control embryo eyes (751.4 ± 35.7, n = 20 eyes). In contrast, injection of the control MO with five changes or mismatches in the cdh6MO1 sequence (making it unable to hybridize with the cdh6 sequence; 5-mis) resulted in embryos that were indistinguishable in morphology and neural marker staining from uninjected control embryos (Table 1; also see below). The specificity of the cdh6MO1 was demonstrated by rescuing the phenotype with synthetic cdh6 mRNA injections (Kubota et al., 2007). Moreover, Cdh6 expression was greatly reduced in cdh6 morphants (Fig. 2D and E) compared to control MO injected embryos (Fig. 2B) or uninjected embryos (Fig. 2C).
Figure 3.
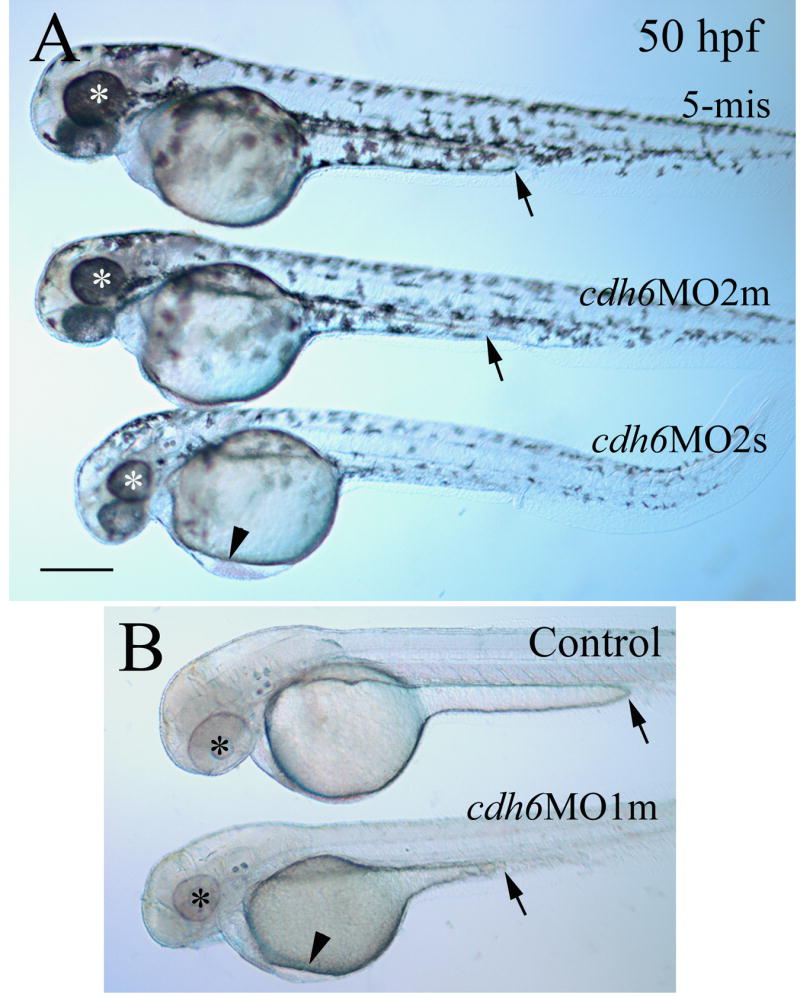
Gross morphological defects in cdh6 morphants. Lateral views of live embryos (anterior to the left and dorsal up) with embryos in panel A developed in normal tank water, while embryos in panel B developed in PTU treated tank water. Eyes in embryos are indicated by asterisks. Edema in the thorax is indicated by an arrowhead, while an arrow points to the end of the yolk extension, which is seen better in the PTU treated embryos in panel B. Abbreviations: cdh6MO2m, moderately affected embryos injected with cdh6MO2; cdh6MO2s, severely affected embryos injected with cdh6MO2. cdh6MO1m, moderately affected embryos injected with cdh6MO1. Scale bar = 250 μm.
Table 1.
Effects of cdh6MOs injection on zebrafish development
| Number of embryos with moderate gross defects (%) | Number of embryos with severe gross defects (%) | Number of embryos examined at 48-50 hpf | |
|---|---|---|---|
| Uninjected control | 0 | 0 | 346 |
| cdh6MO1 (6 ng) | 104 (67.1%) | 6 (3.9%) | 155 |
| (12 ng) | 31 (21.3%) | 99 (68.3%) | 145 |
| cdh6MO2 (3.4 ng) | 131 (76.6%) | 11 (6.4%) | 171 |
| (6.8 ng) | 19 (8.1%) | 176 (75.2%) | 234 |
| 5-misMO (12 ng) | 7 (3.4%)* | 204 |
Only one of the seven embryos showed thorax edema and curved body, and the gross defects in the remaining six embryos (much smaller and truncated body) were different from the cdh6 morphants.
Analysis of apoptosis and cell proliferation in cdh6 morphants
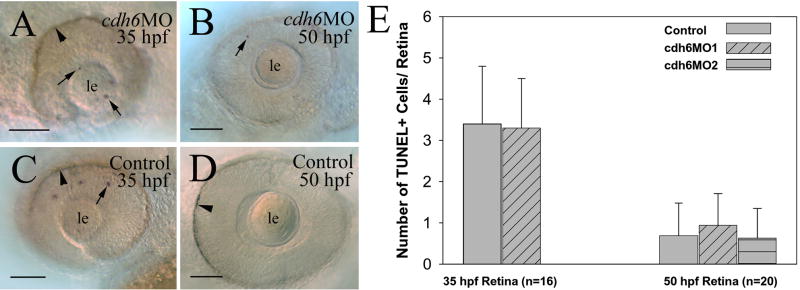
To determine whether the small eye phenotype in cdh6 morphants was mainly due to increased cell death or reduced cell proliferation, we performed TUNEL staining (Fig. 4), and histone H3 immunostaining experiments (Fig. 5). There were very few apoptotic cells in the retina of both young (35 hpf) and older (50 hpf) cdh6 morphants (Fig. 4A, B and E), which was similar to uninjected control embryos (Fig. 4C, D and E).
Figure 4.
Apoptosis analysis using TUNEL staining. Panels A-D are lateral views of whole mount eyes from embryos processed for TUNEL staining. Anterior is to the left and dorsal is up for these images. Arrows point to some TUNEL positive cells, while arrowheads indicate retinal pigmented epithelium. The number (n) in panel E represents the number of retinas examined for each group of embryos (e.g. control embryos vs. cdh6MO1 injected embryos). Abbreviations are the same as in Figure 1. Scale bars = 50 μm.
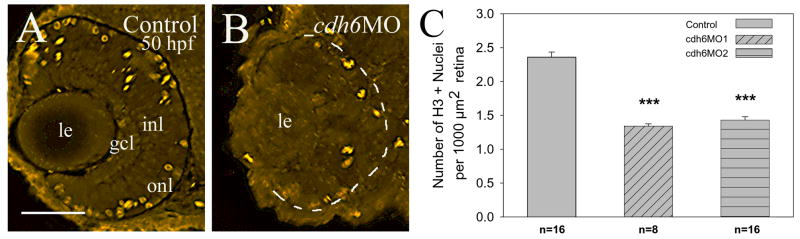
Figure. 5.
Histone-H3 immunostaining. Panels A and B are cross sections (8 μm) from central retina (dorsal up) processed for histone H3 immunostaining, The periphery of the morphant eye in panel B is outlined by the dashed line. The number (n) in panels C represents the number of retinas examined for each group. Asterisks indicate highly significant differences (p<0.001) between the control and each morpholino treatment (e.g. cdh6MO1). Abbreviations are the same as in Figure 1. Scale bars = 50 μm.
Mitotic nuclei in the control and cdh6 morphant retinas were revealed using the histone H3 immunostaining (Fig. 5; Adams et al., 2001). Due to differences in their eye sizes, we compared the number of histone H3 positive nuclei per unit area of the retina, instead of the number of histone H3 positive cells per retina in control and morphant retinas. Although most labeled cells in both the control and morphant retinas were located in the peripheral region at 50 hpf, the control retina (Fig. 5A) contained significantly more proliferative cells than cdh6 morphant retina (Fig. 5A-C).
Expression of transcription factors and notch-delta genes altered in the cdh6 morphant retina
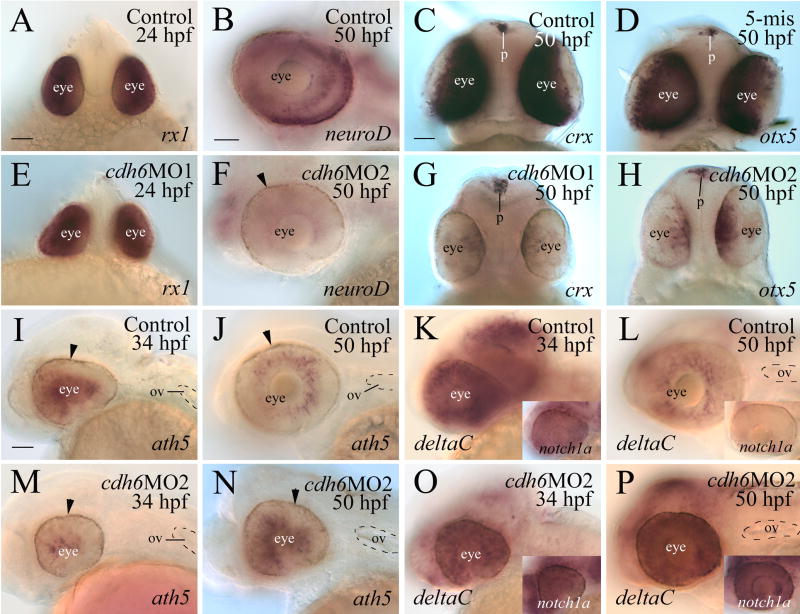
To determine whether cadherin-6 regulates retinal differentiation, five transcription factors known markers for retinal differentiation and/or involved in the formation of the vertebrate retina (Table 2; rx1 (Chuang et al., 1999; Chuang and Raymond, 2001), NeuroD (Morrow et al., 1999; Inoue et al., 2002; Yan et al., 2005), crx (Furukawa et al., 1997; Blackshaw et al., 2001; Liu et al., 2001; Shen and Raymond, 2004), otx5 (Gamse et al., 2002), and ath5 (Masai et al., 2000; Kay et al., 2005)), were examined in control and morphant retinas. rx1 was expressed by the entire retina at 18 and 24 hpf in both the control and cdh6 morphants (Fig. 6A and E; Table 2), suggesting that disruption of cadherin-6 function did not affect formation of the eye primordium and differentiation of the early retina. neuroD was expressed by many retinal cells, particularly in the outer portion of the retina in control embryos at 50 hpf (Fig. 6B; Korzh et al., 1998), while there was only weak neuroD expression in the cdh6 morphant retina at this stage (Fig. 6F; Table 2). crx and otx5 transcripts were strongly expressed by the entire retina in control embryos (Fig. 6C and D, but their expression was confined to a ventral patch in the morphant retina (Fig. 6G and H; Table 2). In contrast, crx and otx5 expression in the pineal gland was similar between the control and morphant retinae (Fig. 6C, D, G and H).
Table 2.
Effects of cdh6MOs injection on expression of transcription factors and notch-delta genes in the retina
| rx1 | neuroD | crx | otx5 | ath5 | deltaC | notch1a | |
|---|---|---|---|---|---|---|---|
| 18 hpf (n=20 for each probe) | ND | ND | ND | ND | ND | ND | |
| Uninjected control | 0% | ||||||
| cdh6MO1 | 0% | ||||||
| cdh6MO2 | 0% | ||||||
| 24 hpf | ND | ND | ND | ND | ND | ND | |
| Uninjected control (n=40) | 0% | ||||||
| cdh6MO2 (n=24) | 0% | ||||||
| 34 hpf | ND | ND | ND | ND | |||
| Uninjected control (n=25 for each probe) | 0% | 0% | 0% | ||||
| cdh6MO2 (n=20 for each probe) | 100% | 0% | 0% | ||||
| 50 hpf | ND | ||||||
| Uninjected control (n=20 for each probe) | 0% | 0% | 0% | 10% | 0% | 0% | |
| cdh6MO1 | 100% | 100% | 100% | ND | ND | ND | |
| cdh6MO2 | 100% | 100% | 100% | 85% | 90% | 100% | |
| 5-misMO | ND | 0% | 0% | ND | ND | ND |
n, number of eyes examined; %, percentages of greatly reduced or altered staining (staining area and/or staining intensity), compared to the majority of control embryos. For cdh6 morphants, only those with obvious gross morphological defects were used. Abbreviation: ND, analysis not done.
Figure 6.
Expression of transcription factors and Notch-Delta genes in the control and cdh6 morphant retinae. Panels A, C, D, E, G and H are in-face views (dorsal up) of embryo heads from embryos processed for whole mount in situ hybridization. The remaining panels are lateral views (anterior to the left and dorsal up) of whole mount eyes and/or heads. The arrowhead in panels F, I, J, M and N points to retinal pigmented epithelium. Abbreviations: p, pineal gland; ov, otic vesicle. Panels A and E, B and F, C, D, G and H are of the same magnifications, respectively. Panels I-P are of the same magnifications. Scale bars = 50 μm.
To determine whether neurogenesis was affected in the cdh6 morphant retina, we compared expression of the proneural gene ath5 between the control embryos and cdh6 morphants. Strong ath5 expression was detected in the retina of 34-50 hpf control embryos (Fig. 6I and J; Masai et al., 2000; Kay et al., 2005). ath5 expression was reduced in the morphant eyes at 34 hpf (Fig. 6M; Table 2), while its expression in the morphant eyes at 50 hpf (Fig. 6N) resembled expression in the control eyes of the younger stage embryos (Fig. 6I).
As with other classical cadherins, cadherin-6 is known to mediate cell-cell adhesion mainly through homophilic binding (Nakagawa and Takeichi, 1995; Shimoyama et al., 2000). Blocking cadherin-6 function may affect cell-cell contacts, which in turn may affect Notch-Delta signaling in the cdh6 morphant. Notch-Delta signaling is involved in lateral inhibitory signals that prevent differentiation of retinal neurons, maintenance of proliferating retinal progenitors, and promotion of Müller glial differentiation (Perron and Harris, 2000; Livesey and Cepko, 2001; Ahmad et al., 2004). To determine whether Notch-Delta signaling was affected in cdh6 morphants, we examined expression of notch1a and deltaC, zebrafish Notch and Delta gene family members, respectively, in the morphant retina. Both genes are strongly and widely expressed in the retina of young embryos (24-36 hpf; Smithers et al., 2000; Fig. 6K and insert), and their expression domains became more confined as development proceeds (Fig. 6L and insert). In the retina of 2.5-4 day larvae, notch1a and deltaC expression is restricted to the proliferating marginal zone (Smithers et el., 2000). Their expression in the retina of 34 hpf cdh6 morphants (Fig. 6O and insert) was similar to the levels seen in control embryos of the same stage (Fig. 6K and insert), but their expression remained high in the 50 hpf morphant retina (Fig. 6P and insert).
Differentiation of retinal ganglion and amacrine cells was severely disrupted in the cdh6 morphant retina
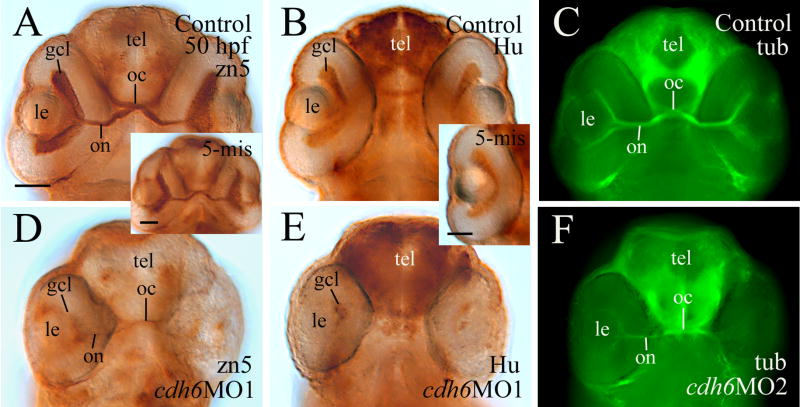
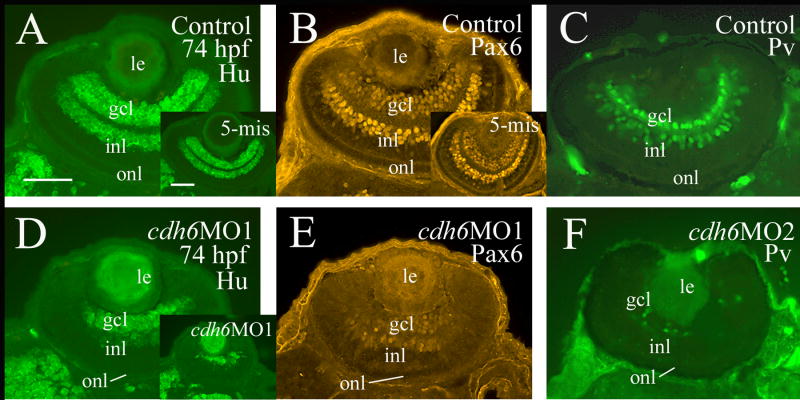
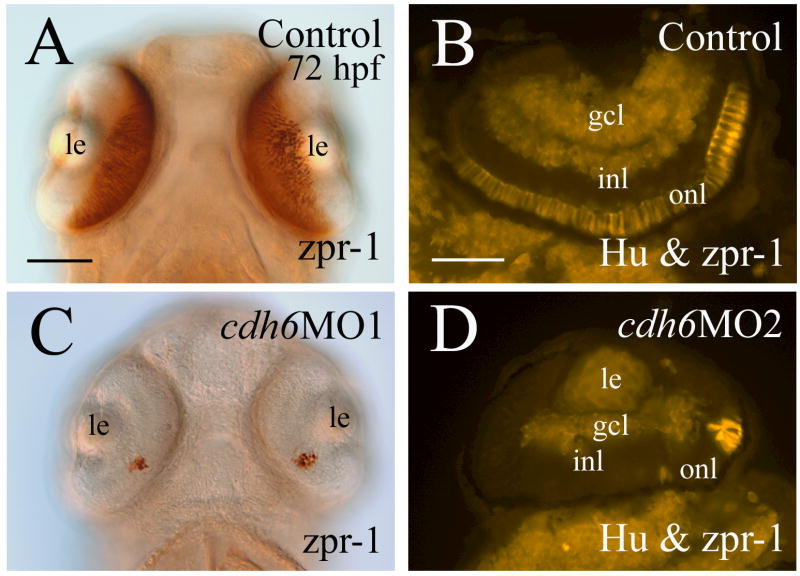
β-catenin immunostaining (labeling cell membrane of retinal cells and retinal synaptic layers) was performed to examine the general organization of the cdh6 morphant retina (Fig. 7). Although retinal lamination was barely detectable at 50 hpf in the morphant retina (Fig. 7C and insert), it became obvious by 74 hpf (Fig. 7D). This result was confirmed by znp-1 immunostaining (labeling synaptic layers, Fig. 7D insert). Since cdh6/Cdh6 was detected in the gcl and inner portion of the inl during critical stages of zebrafish RGC and amacrine cell development, perturbing cadherin-6 function in the embryonic zebrafish may disrupt formation of these retinal cell types. RGC differentiation was analyzed using several neural markers known to label differentiating zebrafish RGCs (Malicki et al., 2003; Masai et al., 2003; Table 3). A distinct gcl, shown by zn5 (labeling both RGC body and axons) and anti-HuC/HuD immunostaining (labeling RGC soma), was observed in 2-day old control embryos or embryos injected with the control MO (Fig. 8A and insert, B and insert). Expression of these markers, although still confined mainly to the inner portion of the retina, was greatly reduced in cdh6 morphants (Fig. 8D and E; Table 3). A thick zn5-positive optic nerve was found exiting each retina, crossing at the base of the diencephalon (optic chiasm) with the optic nerve from the other retina, and projecting toward the brain in the control embryos (Fig. 8A and insert), while in cdh6 morphants there was only a very thin retinal axonal bundle labeled by zn-5 immunostaining (Fig. 8D). Similar results were obtained using anti-acetylated tubulin (labeling mainly the RGC axons) immunostaining (Fig. 8C and F; Table 3). The RGC defects persisted in 3-day old larvae as revealed by anti-HuC/HuD and anti-Pax6 immunostaining (Fig. 9; Table 3). These two markers and anti-parvalbumin antibody also strongly labeled amacrine cells (Malicki et al., 2003; Masai et al., 2003; Avanesov et al., 2005) in control or 5-misMO injected 3-day old larvae (Fig. 9A-C and their inserts), while expression of these three markers was greatly diminished in the morphant retinae (Fig. 9D-F; Table 3), suggesting that differentiation of amacrine cells was disrupted in the cdh6 morphant retina.
Figure 7.
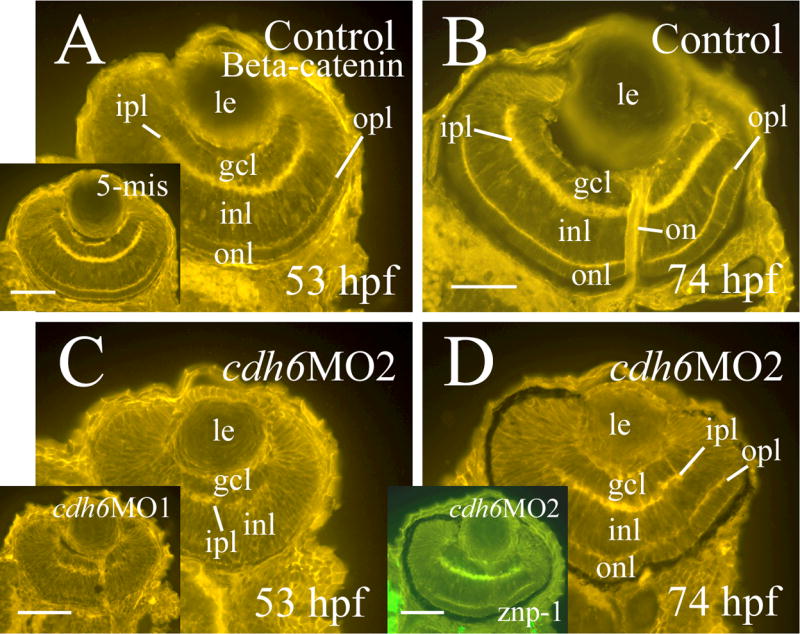
Retinal organization of control embryos (panels A and B) and cdh6 morphants (panels C and D) revealed by β-catenin immunostaining. All panels show retinal cross sections (dorsal to the left) labeled with a β-catenin antibody (panels A-C and inserts) or a znp-1 antibody (panel D insert). Images on the left column (panels A, C and their inserts) are from embryos raised in PTU treated fish tank water, while images from the right column (panels B, D and panel D insert) are from embryos raised in regular fish tank water. Abbreviations are the same as in Figure 1. Scale bars = 50 μm.
Table 3.
Effects of cdh6MOs injection on zebrafish retinal development revealed by immunostaining
| Hu | Hu&zpr-1 | Parv | Pax6 | tub | zn5 | |
|---|---|---|---|---|---|---|
| 50 hpf | ||||||
| Uninjected control | 0% (n=12) | ND | ND | 0% (n=10) | 13.3% (n=15)* | 0% (n=30) |
| cdh6 morphants with obvious gross morphological defects | 100% (n1=20)
100% (n2=20) |
ND
ND |
ND
ND |
100% (n1=16)
100% (n2=16) |
100% (n1=14)
100% (n2=12) |
100% (n1=32)
100% (n2=40) |
| cdh6 morphants with little or no gross morphological defects | 25.0% (n1=20)*
35.7% (n2=14)* |
ND | ND | 20.0% (n2=10)* | 33.3% (n1=12)* | 30.0% (n2=10)* |
| 5-misMO injected | 10% (n=10)* | ND | ND | 0% (n=10) | 0% (n=10) | 21.4% (n=14)* |
| 72-74 hpf | ||||||
| cdh6 morphants with obvious gross morphological defects | 100% (n1=10)
100% (n2=10) |
100% (n1=20)
100% (n2=20) |
100% (n1=8)
100% (n2=10) |
100% (n1=8)
100% (n2=8) |
ND | ND |
| cdh6 morphants with little or no gross morphological defects | 28.6% (n1=14)* | 41.7% (n1=12)*
35.0% (n2=20)* |
30.0% (n1=10)*
33.3% (n2=12)* |
20.0% (n2=10)* | ND | ND |
| 5-misMO injected | 0% (n=12) | 10.0% (n=20)* | 0% (n=10) | 8.3% (n=12)* | ND | ND |
n, number eyes examined; n1 and n2, the numbers of eyes from embryos injected with cdh6MO1 and cdh6MO2, respectively. %, percentages of greatly reduced staining (staining area and/or intensity) compared to the majority of control embryos.
The retinal defects in these embryos were milder than those from morphants with obvious gross morphological defects.
Abbreviations: Hu, anti-HuC/HuD immunostaining; Hu & zpr-1, anti-HuC/HuD and zpr-1 double immunostaining; ND, analysis not done; Parv, anti-parvalbumin immunostaining; Pax6, anti-Pax6 immunostaining; tub, anti-acetylated tubulin immunostaining; zn5, zn5 immunostaining.
Figure 8.
Analysis of RGC differentiation using zn-5 (panels A, D and panel A insert), anti-HuC/HuD (panels B, E and panel B insert) and anti-acetylated tubulin (panels C and F) immunostaining. All images are ventral views of whole mount heads (anterior up) or eye (panel B insert). Panels A, B, D and E are processed using immunoperoxidase methods, while panels C and F are processed using immunofluorescent methods. Abbreviation: oc, optic chiasm; tel, telencephalon. Other abbreviations are the same as in Figure 1. Scale bars = 50 μm.
Figure 9.
Analysis of RGC and amacrine cell differentiation using anti-HuC/HuD (Hu, panles A, D and their inserts), Pax6 (panels B, E and panel B insert), and parvalbumin (Pv, Panels C and F) immunostaining. All images are from cross sections with dorsal to the left. Panels A, B, D and E are from PTU treated embryos, while panels C and F are from embryos raised in regular fish tank water. The insert in panel D is from a severely affected embryo. Abbreviations are the same as in Figure 1. Scale bars = 50 μm.
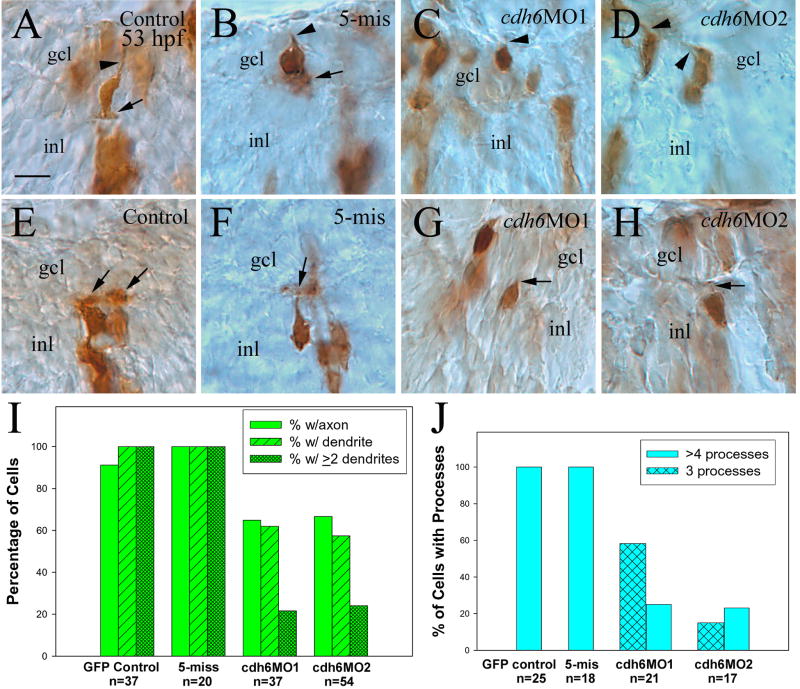
To determine cadherin-6 function on individual RGCs and amacrine cell differentiation, individual retinal cells were labeled with enhanced green fluorescent protein (eGFP) using an eGFP expressing vector under the control of a zebrafish heat shock promoter 70 (HSP70/4 eGFP, Shoji et al., 1998). Co-injection of a cdh6 MO with the HSP70/4 eGFP cDNA into one to four-cell stage zebrafish embryos, followed by activation of the heat shock promoter (37°C for one hour at 36 hpf), resulted in eGFP transfected cells throughout the embryo, including the retina. At 53 hpf, control eGFP expressing cells (from embryos injected with the eGFP containing vector only, or injected with the vector and the 5-misMO) exhibited differentiated morphology such as elongated cell body with an axon and several dendrites (Fig. 10A and B). Almost all control eGFP expressing RGCs contained an axon (>91%) and dendrites (100%), while an axon and dendrites were present in about two thirds of morphant eGFP expressing RGCs (Fig. 10C, D and I). Moreover, all control eGFP expressing RGCs had two or more dendrites, but less than one quarter of cdh6 morphant eGFP expressing RGCs contained two or more dendrites (Fig. 10I).
Figure 10.
Analysis of cadherin-6 function in differentiation of individual RGCs (panels A-D, and I) and amacrine cells (panels E-H, and J) labeled with an anti-eGFP antibody. All images are from cross sections (30 μm). Arrowheads point to axons, while arrows indicate dendrites. The number (n) in panels I and J represents the number of retinal cells examined for each group. Abbreviations are the same as in Figure 1. Scale bar = 10 μm.
All control eGFP expressing amacrine cells had numerous processes (>4) projecting toward the gcl (Fig. 10E, F and J), while only about one quarter of cdh6 morphant eGFP expressing amacrine cells had four or more processes (Fig. 10G, H and J).
Cadherin-6 function in differentiation of photoreceptors was examined using a photoreceptor-specific marker zpr-1 (labeling double cones, Fig. 11A and B). Zpr-1 expression was greatly reduced and confined to a ventral patch in the peripheral (outer) lamina of the cdh6 morphant retina (Fig. 11C and D).
Figure 11.
Expression of photoreceptor-specific marker zpr-1 is greatly reduced in cdh6 morphants. Panels A and C (ventral view, dorsal up) are the head region of whole mount embryos processed for in situ hybridization. Panels B and D are cross retinal sections (anterior to the left) processed for anti-HuC/HuD (labeling the gcl and inner portion of the inl) and zpr-1 (labeling the onl) double immunostaining. Abbreviations are the same as in Figure 1. Scale bars = 50 μm.
Discussion
Cadherin-6 promotes RGCs and amacrine cells differentiation
Cdh6/cdh6 is expressed in both RGCs and amacrine cells during critical periods of their development in zebrafish retina, suggesting that cadherin-6 participates in the differentiation of these retinal cells, as supported by the results of this study. Unlike cadherin-2 and cadherin-4, which are involved in both initiation and elongation of RGC axons and dendrites (Lele et al., 2002; Malicki et al., 2003; Masai et al., 2003; Babb et al., 2005), cadherin-6 appears to be more involved in the elongation of RGC axons and elongation and/or branching of RGC dendrites, rather than their initiation. A majority of cdh6 morphant RGCs had an axon and dendrites, but only a few axons exited the retina, suggesting disrupted elongation. Also, cdh6 morphant RGCs had fewer dendrites, which could be due to perturbations in elongation, branching or both.
Differentiation of amacrine cells was severely affected in cdh6 morphants, as shown by anti-HuC/HuD, anti-Pax6 and anti-parvalbumin immunostaining, as well as by examination of eGFP-labeled individual amacrine cells. Reduced Pax6 labeling in the inner nuclear layer was also observed in cadherin-4 morphant retina (Babb et al., 2005). In contrast to the greatly reduced amacrine cell processes in cdh6 morphants shown in this study, amacrine processes became markedly increased in branching and length in cadherin-2 mutants (Masai et al., 2003), suggesting differential cadherin function in the amacrine cell differentiation.
It was surprising to find greatly reduced photoreceptor differentiation in the cdh6 morphant retina, because cdh6 expression is not detected in the outer nuclear layer where photoreceptors reside (Fig. 1). However, Cdh6/cdh6 is strongly expressed by RGCs, amacrine cells, cells adjacent to the outer nuclear layer, and retinal pigmented epithelium (Fig. 1), and differentiation of the RGCs and amacrine cells are severely disrupted in the cdh6 morphants (see above). Therefore, cadherin-6 may regulate retinal photoreceptor differentiation indirectly through its control on the differentiation of other types of retinal cells, which is similar to our findings in cadherin-4 morphants (Babb et al., 2005; Liu et al., 2007).
Cadherin-6 function in vertebrate retina development was recently studied in Xenopus by Ruan and colleagues (Ruan et al., 2006). The study focused on effects of cadherin-6 knockdown on the formation of the eye primordium, retinal lamination, retinal pigmented epithelium and lens development, while differentiation of specific subtypes of retinal cells (e.g. retinal ganglion cells, amacrine cells and photoreceptors) was only briefly discussed (Ruan et al., 2006). Similar to our findings, cdh6 loss-of-function in Xenopus resulted in small eye phenotype (Ruan et al., 2006). Moreover, in both zebrafish and Xenopus morphants, the reduced eye size is mainly due to decreased cell proliferation in the retina. This is different from embryos with disrupted cadherin-2 or cadherin-4 function in which significantly higher rates of cell death contributed to the eye defects (Pujic and Malicki, 2001; Malicki et al., 2003; Babb et al., 2005). Unlike Xenopus in which the loss of cadherin-6 function resulted in disrupted retinal lamination (Ruan et al., 2006), the zebrafish cdh6 morphant retina showed no disruption in the retinal lamination. This species difference may partially due to different expression patterns of this cadherin in the Xenopus and zebrafish developing eye: cdh6 expression in Xenopus eye primordium (optic cup) is earlier (stage 26 before lens formation), and its expression domain in the eye at this stage is larger (David and Wedlich, 2000) than that in the zebrafish eye at 32 hpf (when cdh6 is first detected in the retina which has a distinct lens). Moreover, the developing zebrafish retina expresses multiple cadherins including cadherin-2 that is shown to confer much stronger cell-cell adhesion than type II cadherins (Chu et al., 2006). This is consistent with the finding that zebrafish cadherin-2 mutants have severe retinal lamination defects (Masai et al., 2003; Malicki et al., 2003).
Possible mechanisms of cadherin-6 function in retinal development
Classical cadherins control cell motility, growth and differentiation through β–catenin-T-cell factor transcription factor pathway (canonical Wnt pathway), p120 catenin pathway (Rac or Rho GTPases), cadherin extracellular domain 4/FGF receptor pathway (receptor tyrosine kinases), and/or proteolytically generated cytoplasmic fragment pathway (reviewed by Wheelock and Johnson, 2003; Junghans et al., 2005). For example, cadherin-2 regulates myoblasts differentiation by activating RhoA, leading to increased expression of MyoD (Charrasse et al., 2002). It is possible that cadherin-6 functions in retinal cell differentiation by controlling regulatory gene expression such as ath5, crx, and neuroD, and affecting expression Notch-Delta. Normal expression of rx1, notch1a and deltaC in younger morphant (18-34 hpf) retinae suggests that specification of retinal identity does not require cadherin-6 function. Reduced expression of transcription factors and reduced staining of other retinal differentiation markers (see above); continued strong expression of notch1a and deltaC in 50 hpf morphant retinae; and similar ath5 expression pattern in younger control embryos (34 hpf) and 50 hpf morphants indicates that retinal differentiation is greatly delayed in cdh6 morphants. This delay in the retinal development is unlikely the result of a general delay in zebrafish development, because morphants have a similar body size and yolk size as control embryos (Fig. 3), and the distance between the eye and ear (a good indicator of developmental stage) is reduced in both the morphants and control embryos at 50 hpf (Fig. 6I, J, M, N, L and P).
Cadherins (e.g. cadherin-2) are known to inhibit cell proliferation in the vertebrate central nervous system, possibly by regulating β-catenin levels (Lele et al., 2002; Noles and Chenn, 2007). Cadherins also promote neuroblast proliferation, possibly by mediating interactions between glial cells and neuroblasts (e.g. DE-cadherin, Dumstrei et al., 2003), and promote proliferation of rhabdomyosarcomas (highly malignant tumors of skeletal muscle origin) (e.g. cadherin-4, Charrasse et al., 2004). Molecular mechanisms underlying cadherin-6 function in promoting retinal cell proliferation remain to be determined.
Conclusion
Numerous studies have shown that type I classic cadherins (e.g. cadherin-2 and cadherin-4) play crucial roles in the vertebrate visual system development (see above), but there is little information on type II classic cadherins (e.g. cadherin-6 and cadherin-7) function in the formation of this system. In this study, we analyzed developmental consequences of cdh6 loss-of-function, particularly in the retina, and we found that cadherin-6 regulates differentiation of the retinal ganglion cells, amacrine cells and photoreceptors. Moreover, we found that cadherin-6 functions in the retinal cell development (i.e., promoting retinal cell proliferation; little effect on the initiation of the retinal ganglion cell axon) are different from functions of type I classic cadherins (i.e., promoting retinal cell survival; great effect on the initiation of the retinal ganglion cell axon). Our findings also provide additional support for the idea that normal development of the vertebrate central nervous system requires coordinated cadherin activities.
Acknowledgments
We thank Drs. Pamela Raymond (University of Michigan) and Deborah Stenkamp (University of Idaho) for providing the cDNAs of the retinal genes, Dr. Babb-Clendenon (Indiana University) for technical assistance. This study was supported by NIH EY13879 (Q. Liu) and NIH DC006436 (J.A. Marrs).
Grant sponsor: NIH; grant numbers: R15 EY13879, R01 DC006436.
Footnotes
This is a preprint of an article published in Developmental Neurobiology (2008, 68:1107-1122, http://www.interscience.Wiley.com/)
Contributor Information
Qin Liu, Email: qliu@uakron.edu.
Richard Londraville, Email: londraville@uakron.edu.
James A. Marrs, Email: jmarrs@iupui.edu.
Amy L. Wilson, Email: amy45@iwon.com.
Thomas Mbimba, Email: iasse_th@yahoo.com.
Tohru Murakami, Email: tohru.murakami@gunma-u.ac.jp.
Fumitaka Kubota, Email: fkubota@med.gunma-u.ac.jp.
Weiping Zheng, Email: wzheng@uakron.edu.
David G. Fatkins, Email: dgf3@uakron.edu.
References
- Adams RR, Maiato H, Earnshaw WC, Carmena M. Essential roles of Drosophila inner centromere protein (IN-CENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J Cell Biol. 2001;153:865–880. doi: 10.1083/jcb.153.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I, Das AV, James J, Bhattacharya S, Zhao X. Neural stem cells in the mammalian eye: types and regulation. Semin Cell Dev Biol. 2004;15:53–62. doi: 10.1016/j.semcdb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- Ando H, Kobayashi M, Tsubokawa T, Uyemura K, Furtuta T, Okamoto H. Lhx2 mediates the activity of Six3 in zebrafish forebrain growth. Dev Biol. 2005;287:456–468. doi: 10.1016/j.ydbio.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Avanesov A, Dahm R, The Tuebingen 2000 Screen Consortium. Sewell WF, Malicki JJ. Mutations that affect the survival of selected amacrine cell subpopulations definea new class of genetic defects in the vertebrate retina. Dev Biol. 2005;285:138–155. doi: 10.1016/j.ydbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Babb SB, Marrs JA. E-cadherin regulates cell movements and tissue formation in early zebrafish embryos. Dev Dyn. 2004;230:263–277. doi: 10.1002/dvdy.20057. [DOI] [PubMed] [Google Scholar]
- Babb SB, Kotradi SM, Shah B, Chiappini-Williamson C, Bell LN, Schmeiser G, Chen E, Liu Q, Marrs JA. Zebrafish R-cadherin (Cdh4) controls visual system development and differentiation. Dev Dyn. 2005;233:930–945. doi: 10.1002/dvdy.20431. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, Chu F, Talbot WS, Weinberg ES. Essential and opposing roles of zebrafish beta-catenins in the formation of dorsal axial structures and neurectoderm. Development. 2006;133:1299–1309. doi: 10.1242/dev.02295. [DOI] [PubMed] [Google Scholar]
- Bitzur S, Kam Z, Geiger B. Structure and distribution of N-cadherin indeveloping zebrafish embryos: morphogenetic effects of ectopic over expression. Dev Dyn. 1994;201:121–136. doi: 10.1002/aja.1002010204. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Fraioli RE, Furukawa T, Cepko CL. Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell. 2001;107:579–589. doi: 10.1016/s0092-8674(01)00574-8. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Easter SS., Jr Development of the retinofugal projections in the embryonic and larval zebrafish (Brachydanio rerio) J Comp Neurol. 1994;346:583–600. doi: 10.1002/cne.903460410. [DOI] [PubMed] [Google Scholar]
- Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrasse S, Comunale F, Gilbert E, Delattre O, Gauthier-Rouviere C. Variation in cadherins and catenins expression is linked to both proliferation and transformation of Rhabdomyosarcoma. Oncogene. 2004;23:2420–2430. doi: 10.1038/sj.onc.1207382. [DOI] [PubMed] [Google Scholar]
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998;125:803–812. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]
- Chow R. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Chu YS, Eder O, Thomas WA, Simcha I, Pincet F, Ben-Ze’ev A, Perez E, Thiery JP, Dufour S. Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J Biol Chem. 2006;281:2901–2910. doi: 10.1074/jbc.M506185200. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Mathers PH, Raymond PA. Expression of three Rx homeobox genes in embryonic and adult zebrafish. Mech Dev. 1999;84:195–8. doi: 10.1016/s0925-4773(99)00077-5. [DOI] [PubMed] [Google Scholar]
- Chuang JC, Raymond PA. Zebrafish genes rx1 and rx2 help define the region of forebrain that gives rise to retina. Dev Biol. 2001;231:13–30. doi: 10.1006/dbio.2000.0125. [DOI] [PubMed] [Google Scholar]
- David R, Wedlich D. Xenopus cadherin-6 is expressed in the central and peripheral nervous system and in neurogenic placodes. Mech Dev. 2000;97:187–190. doi: 10.1016/s0925-4773(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Dumstrei K, Wang F, Hartenstein V. Role of DE-cadherin in neuroblast proliferation, neural morphogenesis, and axon tract formation in Drosophila larval brain development. J Neurosci. 2003;23:3325–3335. doi: 10.1523/JNEUROSCI.23-08-03325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JL, Glasgow E. The zebrafish bHLH PAS transcriptional regulator, single minded 1 (sim1), is required for isotocin cell development. Dev Dyn. 2006;235:2071–82. doi: 10.1002/dvdy.20848. [DOI] [PubMed] [Google Scholar]
- Ekker SC. Morphants: a new systematic vertebrate functional genomics approach. Yeast. 2000;17:302–306. doi: 10.1002/1097-0061(200012)17:4<302::AID-YEA53>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-sepecific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Shen YC, Thisse C, Thisse B, Raymond PA, Halpern ME, Liang JO. Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nat Genet. 2002;30:117–121. [Google Scholar]
- Godinho L, Mumm JS, Williams PR, Schroeter EH, Koerber A, Park SW, Leach SD, Wang RO. Targeting of amacrine cell neuritis to appropriate synaptic laminae in the developing zebrafish retina. Development. 2005;132:5069–5079. doi: 10.1242/dev.02075. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Parada L. Molecular regulation of visual system development: more than meets the eye. Genes & Dev. 2007;21:367–378. doi: 10.1101/gad.1504307. [DOI] [PubMed] [Google Scholar]
- Inoue T, Sanes JR. Lamina-specific connectivity in the brain: regulation: by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tanaka T, Suzuki SC, Takeichi M. Cadherin-6 in the developing mouse brain: expression along restricted connection systems and synaptic localization suggest a potential role in neuronal circuitry. Dev Dyn. 1998;211:338–351. doi: 10.1002/(SICI)1097-0177(199804)211:4<338::AID-AJA5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Inoue T, Hojo M, Bessho Y, Tano Y, Lee JE, Kageyama R. Math3 and NeuroD regulate amacrine cell fate specification in the retina. Development. 2002;129:831–842. doi: 10.1242/dev.129.4.831. [DOI] [PubMed] [Google Scholar]
- Junghans D, Haas IG, Kemler R. Mammalian cadherins and protocadherins:about cell death, synapses and processing. Cur Opin Cell Biol. 2005;17:446–452. doi: 10.1016/j.ceb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kay JN, Link BA, Baier H. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132:2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- Knaut H, Blader P, Strahle U, Schier AF. Assembly of trigeminal sensory ganglia by chemokine signaling. Neuron. 2005;47:653–666. doi: 10.1016/j.neuron.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Korzh V, Sleptsova I, Liao J, He J, Gong Z. Expression of zebrafish bHLH genes and ngn1 and nrd defines distinct stages of neural differentiation. Dev Dyn. 1998;213:92–104. doi: 10.1002/(SICI)1097-0177(199809)213:1<92::AID-AJA9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kubota F, Murakami T, Mogi K, Yorifuji H. Cadherin-6 is required for zebrafish nephrogenesis during early development. Int J Dev Biol. 2007;51:123–129. doi: 10.1387/ijdb.062200fk. [DOI] [PubMed] [Google Scholar]
- Larison KD, BreMiller R. Early onset of phenotype and cell patterning in the embryonic retina. Development. 1990;109:567–576. doi: 10.1242/dev.109.3.567. [DOI] [PubMed] [Google Scholar]
- Lele Z, Folchert A, Concha M, Rauch G-J, Geisler R, Rosa F, Wilson SW, Hammerschmidt M, Bally-Cuif L. parachute/n-cadherin is required formorphogenesis and maintained integrity of the zebrafish neural tube. Development. 2002;129:3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- Liu Q, Sanborn KL, Cobb N, Raymond PA, Marrs JA. R-cadherin expression in the developing and adult zebrafish visual system. J Comp Neurol. 1999;410:303–319. doi: 10.1002/(sici)1096-9861(19990726)410:2<303::aid-cne11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shen Y, Rest JS, Raymond PA, Zack DJ. Isolation and characterization of a zebrafish homologue of the cone rod homeobox gene. Invest Ophthalmol Vis Sci. 2001;42:481–487. [PubMed] [Google Scholar]
- Liu Q, Babb SG, Novince ZM, Doedens AL, Marrs JA, Raymond PA. Differential Expression of cadherin-2 and cadherin-4 in the developing and adult zebrafish visual System. Vis Neurosci. 2001a;18:923–933. [PubMed] [Google Scholar]
- Liu Q, Marrs JA, Chuang JC, Raymond PA. Cadherin-4 expression in the zebrafish central nervous system and regulation by ventral midline signaling. Dev Brain Res. 2001b;131:17–29. doi: 10.1016/s0165-3806(01)00241-3. [DOI] [PubMed] [Google Scholar]
- Liu Q, Londraville RL, Azodi E, Babb SG, Chiappini-Williamson C, Marrs JA, Raymond PA. Up-regulation of cadherin-2 and cadherin-4 in regenerating visual structures of adult zebrafish. Exp Neurol. 2002;177:396–406. doi: 10.1006/exnr.2002.8008. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu B, Wilson AL, Rostedt J. cadherin-6 message expression in the nervous system of developing zebrafish. Dev Dyn. 2006;235:272–278. doi: 10.1002/dvdy.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Frey RA, Babb-Clendenon SG, Liu B, Francl J, Wilson AL, Marrs JA, Stenkamp DL. Differential expression of photoreceptor-specific genes in the retina of a zebrafish cadherin-2 mutant glass onion and zebrafish cadherin-4 morphants. Exp Eye Res. 2007;84:163–175. doi: 10.1016/j.exer.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Malicki J. Development of the retina. In: Detrich HW III, Westerfield M, Zon LI, editors. The Zebrafish Biology. San Diego: Academic Press; 1999. pp. 273–299. [Google Scholar]
- Malicki J, Jo H, Pujic Z. Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev Biol. 2003;259:95–108. doi: 10.1016/s0012-1606(03)00181-7. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Gruss P. Generating neuronal diversity in the retina: one for nearly all. Trends in Neurosci. 2002;25:32–38. doi: 10.1016/s0166-2236(00)02028-2. [DOI] [PubMed] [Google Scholar]
- Masai I, Stemple DL, Okamoto H, Wilson SW. Midline signals regulate retinal neurogenesis in zebrafish. Neuron. 2000;27:251–263. doi: 10.1016/s0896-6273(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, Wilson SW, Okamoto H. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Hatta K, Takeichi M. Role of N-cadherin cell adhesion molecules in the histogenesis of neural retina. Neuron. 1988;1:289–95. doi: 10.1016/0896-6273(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Noles SR, Chenn A. Cadherin inhibition of –catenin signaling regulates the proliferation and differentiation of neural precursor cells. Mol Cell Neurosci. 2007;35:549–558. doi: 10.1016/j.mcn.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- Perron M, Harris WA. Determination of vertebrate reinal progenitor cell fate by the Notch pathway and basic helix-loop-helix transcription factors. Cell Mol Life Sci. 2000;57:215–223. doi: 10.1007/PL00000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujic Z, Malicki J. Mutation of the zebrafish glass onion locus causes early cell nonautonomous loss of neuroepithelial integrity followed by severe neuronal patterning defects in the retina. Dev Biol. 2001;234:454–469. doi: 10.1006/dbio.2001.0251. [DOI] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Ruan G, Wedlich D, Koehler A. Xenopus cadherin-6 regulates growth and epithelial development of the retina. Mech Dev. 2006;123:881–892. doi: 10.1016/j.mod.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Shen YC, Raymond PA. Zebrafish cone-rod (crx) homeobox gene promotes retinogenesis. Dev Biol. 2004;269:237–251. doi: 10.1016/j.ydbio.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Tsujimoto G, Kitajima N, Natori M. Identification of three type-II classic cadherins and frequent hterophilic interactions between different subclasses of type-II classic cadherins. Biochem J. 2000;349:159–167. doi: 10.1042/0264-6021:3490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers L, Haddon C, Juang YJ, Lewis J. Sequence and embryonic expression of deltaC in the zebrafish. Mech Dev. 2000;90:119–123. doi: 10.1016/s0925-4773(99)00231-2. [DOI] [PubMed] [Google Scholar]
- Shoji W, Yee CS, Kuwada JY. Zebrafish semaphorin Z1a collapses specific growth cones and alters their pathway in vivo. Development. 1998;125:1275–1283. doi: 10.1242/dev.125.7.1275. [DOI] [PubMed] [Google Scholar]
- Stone KE, Sakaguchi DS. Perturbation of the developing Xenopus retinotectal projection following injections of antibodies against ß1 integrin receptors and N-cadherin. Dev Biol. 1996;180:297–310. doi: 10.1006/dbio.1996.0302. [DOI] [PubMed] [Google Scholar]
- Stuermer CAO. Retinotopic organization of the developing retinotectal projection inthe zebrafish embryo. J Neurosci. 1988;8:4513–4530. doi: 10.1523/JNEUROSCI.08-12-04513.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:451–455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Treubert-Zimmermann U, Heyers D, Redies C. Targeting axons to specific fiber tracts in vivo by altering cadherin expression. J Neurosci. 2002;22:7617–7626. doi: 10.1523/JNEUROSCI.22-17-07617.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR. Cadherin-mediated cellular signaling. Cur Opin Cell Biol. 2003;15:509–514. doi: 10.1016/s0955-0674(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish Book. Eugene, OR: University of Oregon Press; 2000. p. 363. [Google Scholar]
- Yan RT, Ma W, Liang L, Wang SZ. bHLH genes and retinal cell fate specification. Mol Neurobiol. 2005;32:157–171. doi: 10.1385/MN:32:2:157. [DOI] [PMC free article] [PubMed] [Google Scholar]