Abstract
Cytochrome P450scc (CYP11A1) can hydroxylate vitamin D3 to produce 20-hydroxyvitamin D3 and other poorly characterized hydroxylated products. The present study aimed to identify all the products of vitamin D3 metabolism by P450scc, as well as the pathways leading to their formation. Besides 20-hydroxyvitamin D3, other major metabolites of vitamin D3 were a dihydroxyvitamin D3 and a trihydroxyvitamin D3 product. The dihydroxyvitamin D3 was clearly identified as 20,23-dihydroxyvitamin D3 by NMR, in contrast to previous reports that postulated hydroxyl groups in positions 20 and 22. NMR of the trihydroxy product identified it as 17α,20,23-trihydroxyvitamin D3. This product could be directly produced by P450scc acting on 20,23-dihydroxyvitamin D3, confirming that hydroxyl groups are present at positions 20 and 23. Three minor products of D3 metabolism by P450scc were identified by MS and by examining their subsequent metabolism by P450scc. These products were 23-hydroxyvitamin D3, 17α-hydroxyvitamin D3 and 17α,20-dihydroxyvitamin D3 and arise from the three P450scc-catalysed hydroxylations occurring in a different order. We conclude that the major pathway of vitamin D3 metabolism by P450scc is: vitamin D3 → 20-hydroxyvitamin D3 → 20,23-dihydroxyvitamin D3 → 17α,20,23-trihydroxyvitamin D3. The major products dissociate from the P450scc active site and accumulate at a concentration well above the P450scc concentration. Our new identification of the major dihydroxyvitamin D3 product as 20,23-dihydroxyvitamin D3, rather than 20,22-dihydroxyvitamin D3, explains why there is no cleavage of the vitamin D3 side chain, unlike the metabolism of cholesterol by P450scc.
Keywords: CYP11A1, cytochrome P450scc, hydroxyvitamin D3, NMR, vitamin D3
Cytochrome P450scc (CYP11A1) catalyzes the first enzymatic step in steroid synthesis: the cleavage of the side chain of cholesterol to produce pregnenolone [1]. This three-step reaction occurs in mitochondria and involves sequential hydroxylation at C22 and C20, and subsequent oxidative cleavage of the bond between C20 and C22. It has now been established that cytochrome P450scc can also act on both vitamins D2 and D3, as well as their precursors ergosterol and 7-dehydrocholesterol [2–6]. For these substrates, cleavage of the side chain occurs for 7-dehydrocholesterol only, with the other substrates undergoing hydroxylations at substrate-specific sites without cleavage. For vitamin D3, initial hydroxylation is at C20 of the side chain, with subsequent hydroxylation reported to occur at C22 [2,4]. A small amount of trihydroxyvitamin D3 was also produced but the sites of hydroxylation in this product are unknown [2,4]. Metabolism of vitamin D3 by P450scc has not only been observed with purified enzyme, but also in intact rat adrenal mitochondria, where a number of hydroxylated vitamin D derivatives are produced [4].
In addition to its well characterized role in regulating calcium metabolism, the hormonally active form of vitamin D3, 1α,25-dihydroxyvitamin D3, displays anti-cancer, immunoregulatory and endocrine effects, and is also a potent antiproliferative and differentiation-inducing agent in different cell types, including epidermal keratinocytes [7–10]. To determine whether the major products of P450scc action on vitamin D3 have similar biological activity to 1α,25-dihydroxyvitamin D3, we produced them enzymatically using P450scc and subjected the products to phenotypic testing using skin cells cultured in vitro. Specifically, 20-hydroxyvitamin D3 has been identified as strong inducer of programmed keratinocyte differentiation, acting with a similar potency to that of 1α,25-dihydroxyvitamin D3 [10]. This finding indicates that P450scc-catalysed metabolism of vitamin D3 can result in production of biologically active derivatives with potential physiological and therapeutic implications.
The present study aimed to determine the pathways for the metabolism of vitamin D3 by P450scc and to identify both major and minor products. NMR was carried out on two of the major products, and identified them as 20,23-dihydroxyvitamin D3 and 17α,20,23-trihydroxyvitamin D3. Previously 20,23-dihydroxyvitamin D3 had been incorrectly identified as 20,22-dihydroxyvitamin D3 [4], but our new NMR data obtained from two independent samples shows that C23, and not C22, is hydroxylated. Minor products and/or intermediates were identified by purifying them and determining the substrates from which they arose by the action of P450scc, as well as the products that they gave rise to. This revealed both the major and minor pathways for the metabolism of vitamin D3 by P450scc.
Results
Metabolism of vitamin D3 by P450scc
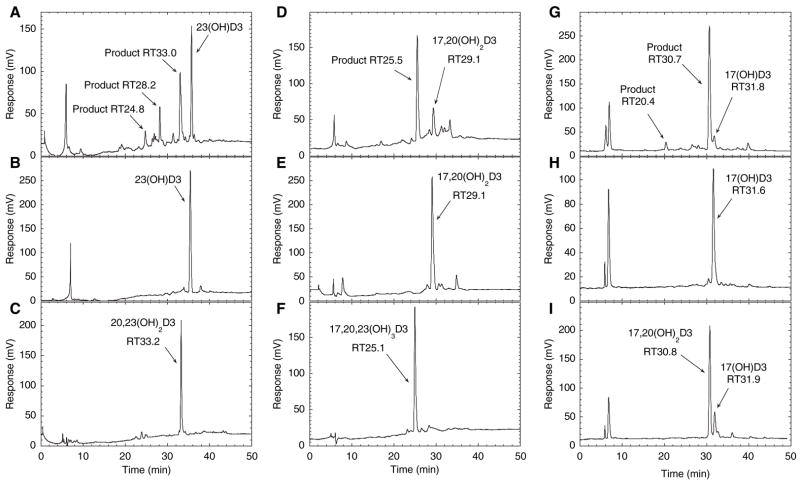
Six different products, in sufficient amounts to permit quantitation and subsequent characterization, were observed when vitamin D3 was incubated with P450scc in 0.45% 2-hydroxypropyl-β-cyclodextrin (cyclodextrin). A typical chromatogram of these products after 1 h of incubation is shown in Fig. 1A, together with a zero-time control (Fig. 1B). A time course for the metabolism of vitamin D3 by cytochrome P450scc in cyclodextrin is shown in Fig. 2. In agreement with previous studies [2,4], the major product was 20-hydroxyvitamin D3 [retention time (RT) = 33 min], identified by an authentic standard from our previous study [4]. Another major product, initially identified as 20,22-dihydroxyvitamin D3 (RT = 30 min), was subsequently shown to be 20,23-dihydroxyvitamin D3. This characterization is described subsequently, but its new identification will be used throughout to avoid confusion. In addition, four other products with retention times of 32, 26.7, 26 and 22 min (Fig. 1A) were observed in sufficient amounts throughout the time course to permit quantitation.
Fig. 1.
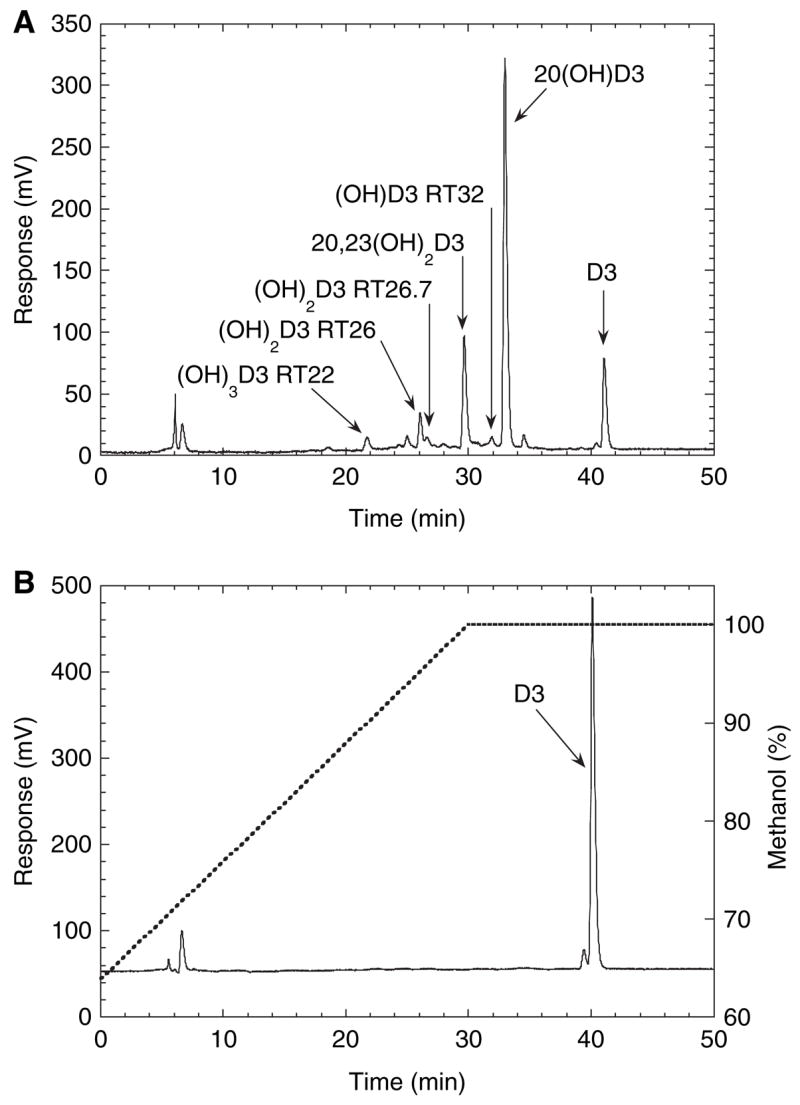
Chromatogram showing products of vitamin D3 metabolism by P450scc. Vitamin D3 (50 μM) dissolved in cyclodextrin to a final concentration of 0.45%, was incubated with 1.0 μM P450scc for 1 h in a reconstituted system containing adrenodoxin and adrenodoxin reductase. Samples were extracted and analyzed by reverse-phase HPLC. (A) Test reaction; (B) control incubation (zero time) showing the vitamin D3 substrate and the methanol gradient used for elution. (OH)D3, monohydroxyvitamin D3; (OH)2D3, dihydroxyvitamin D3; (OH)3D3, trihydroxyvitamin D3.
Fig. 2.
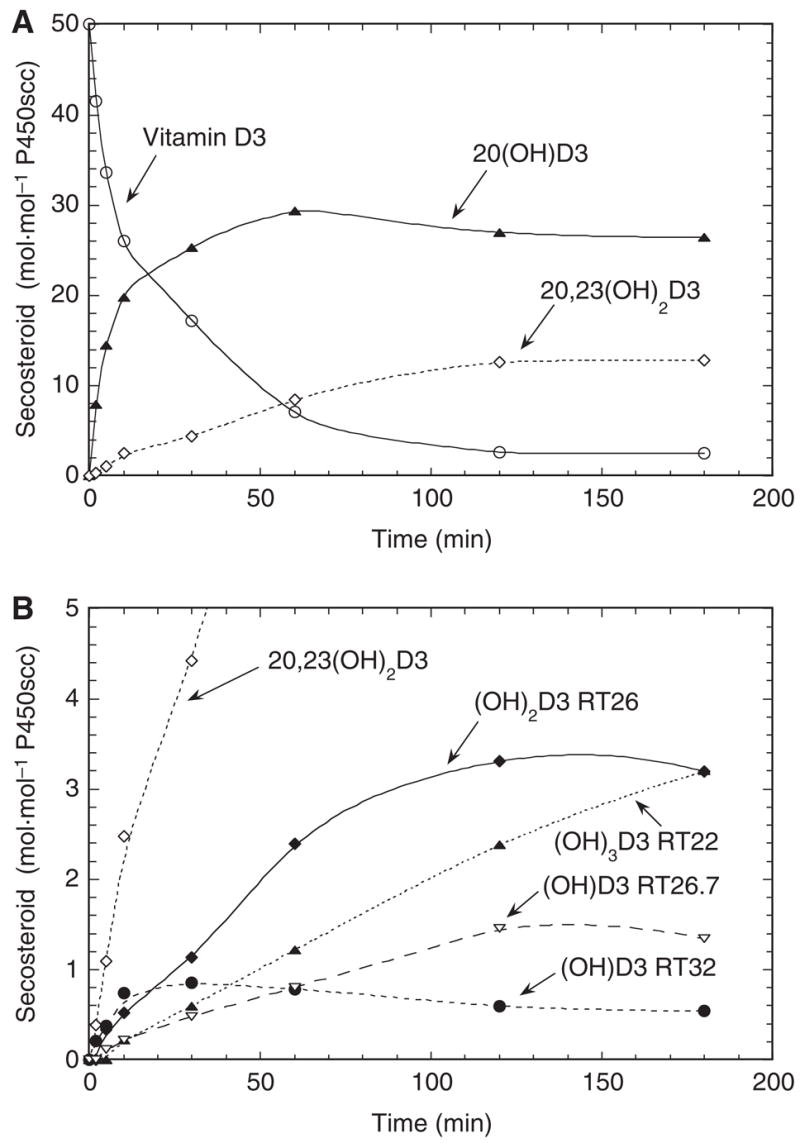
Time course for the metabolism of vitamin D3 in cyclodextrin. Vitamin D3 (50 μM) dissolved in cyclodextrin to a final concentration of 0.45% was incubated with 1.0 μM P450scc for the indicated times. (A) Consumption of vitamin D3 and major metabolites produced; (B) minor metabolites of vitamin D3.
The electrospray mass spectrum for the product with RT = 22 min (Fig. 1) showed the major ion at m/z 455.4 (432.4 + Na+), thus arising from trihydroxyvitamin D3. A major ion at m/z 887.6 corresponded to Na+ complexed to two trihydroxyvitamin D3 molecules. The electrospray mass spectrum for the product with RT = 26 min in Fig. 1 gave the major ion as m/z 439.4 (416.4 + Na+) from which the sample was identified as dihydroxyvitamin D3. A major ion was also observed at m/z 855.7, which corresponded to Na+ complexed to two dihydroxyvitamin D3 molecules. The electrospray mass spectrum for the product with RT = 32 min in Fig. 1 gave the major ion as m/z 423.4 (400.4 + Na+) from which the sample was identified as monohydroxyvitamin D3. An ion at m/z 439.4 corresponded to hydroxyvitamin D3 complexed to K+ (400.4 + 39), whereas an ion at m/z 823.5 corresponded to Na+ complexed to two hydroxyvitamin D3 molecules. The product with RT = 26.7 min in Fig. 1 was subjected to MS with electron impact ionization. This gave the molecular ion (m/z 400) with major fragment ions 398 (M – 2H) and 380 (398 –H2O). This product was identified as monohydroxyvitamin D3.
Because cyclodextrin provides an artificial means of holding vitamin D3 in solution, we also examined the metabolism of vitamin D3 incorporated into the membrane of phospholipid vesicles, more closely resembling the normal membrane environment of P450scc [11–13]. Figure 3 shows that the same products are made in vesicles and cyclodextrin, although there are some changes to their relative proportions. The rate of production of 20-hydroxyvitamin D3 in vesicles fell dramatically after 6 min of incubation, despite less than 20% of the vitamin D3 being consumed.
Fig. 3.
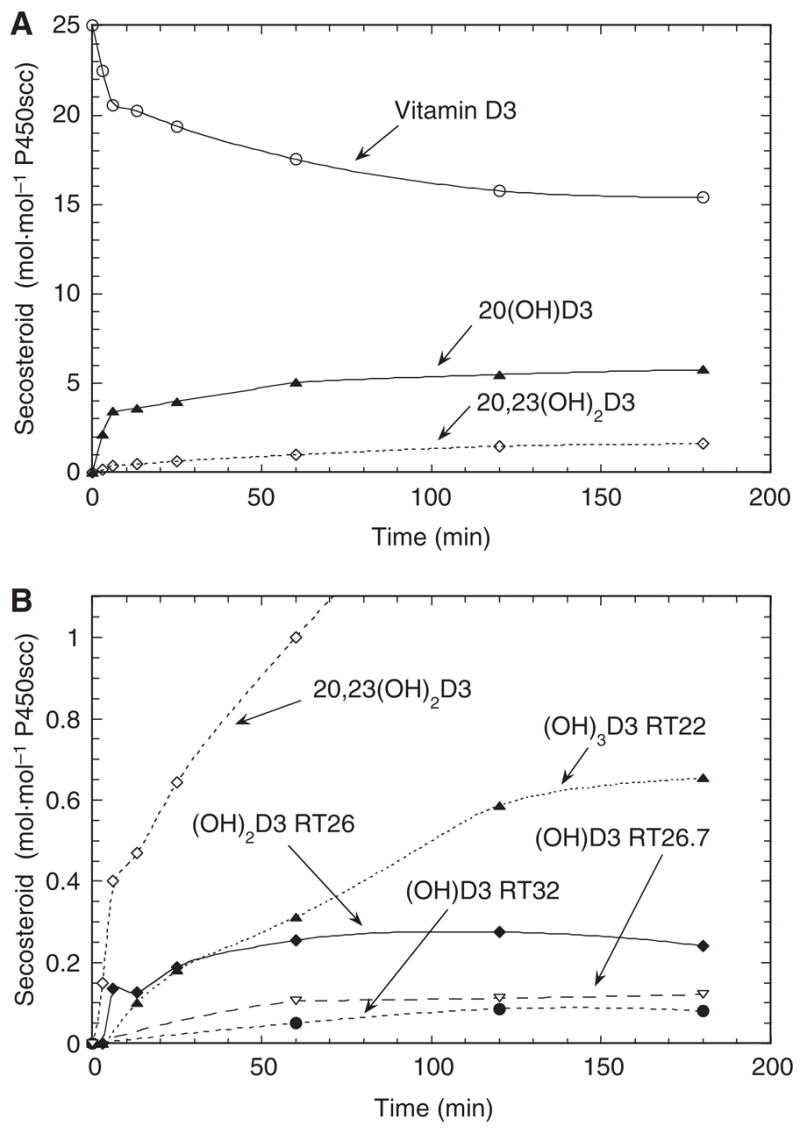
Time course for the metabolism of vitamin D3 in phospholipid vesicles. Vesicles containing 0.2 mol vitamin D3·mol−1 phospholipid were incubated with 2.0 μM P450scc for the indicated times. (A) Consumption of vitamin D3 and major metabolites produced; (B) minor metabolites of vitamin D3.
Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by P450scc
Incubation of 20-hydroxyvitamin D3 in cyclodextrin with P450scc resulted in the formation of 20,23-dihydroxyvitamin D3 (RT = 30 min) and trihydroxyvitamin D3 (RT = 22 min) (Fig. 4A). A small lag (0–3 min) was seen in the time course for formation of trihydroxyvitamin D3, consistent with accumulation of 20,23-dihydroxyvitamin D3 being required before the trihydroxyvitamin D3 can be produced. A product with RT = 26 min was also observed, as seen for the metabolism of vitamin D3, and identified as a dihydroxy derivative (Fig. 1). There was no lag in its time course, consistent with it being formed by a single hydroxylation of 20-hydroxyvitamin D3. The products with retention times of 26.7 min and 32 min as shown in Fig. 1, identified as monohydroxyvitamin D3 derivatives by MS (as described above), were not seen as products from 20-hydroxyvitamin D3, as expected.
Fig. 4.
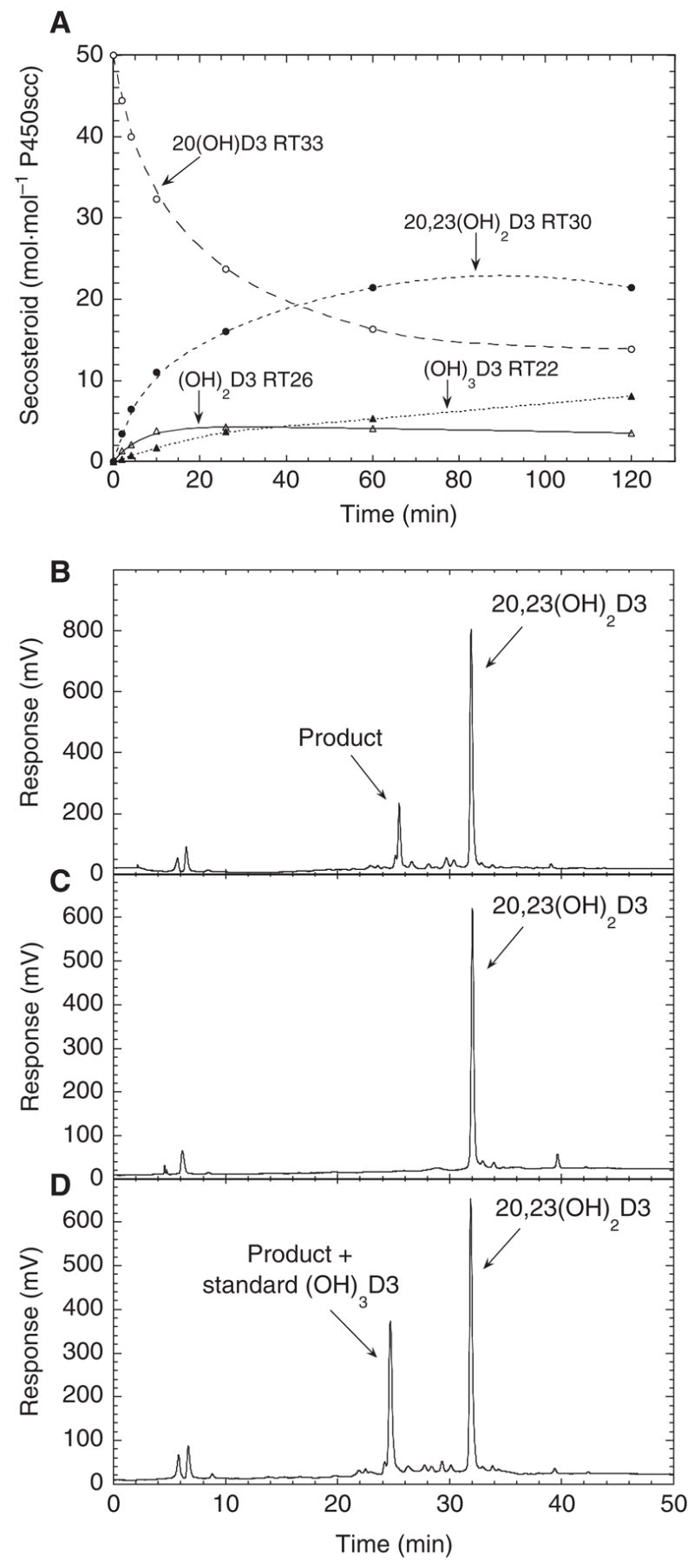
Hydroxylation of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by P450scc. (A) Time-course for the metabolism of 20-hydroxyvitamin D3 (50 μM) dissolved in 0.45% cyclodextrin and incubated with 1.0 μM P450scc. (B–D) HPLC chromatograms showing metabolism of 20,23-dihydroxyvitamin D3 by P450scc from a 1 h incubation in 0.45% cyclodextrin. (B) Test reaction; (C) zero-time control where the reaction mixture was extracted at the end of the pre-incubation; (D) test reaction spiked with 1 nmol standard trihydroxyvitamin D3 purified as for the NMR experiments.
Incubation of 20,23-dihydroxyvitamin D3 with cytochrome P450scc in cyclodextrin resulted in one major product with RT = 25.5 min, identical to that for the trihydroxyvitamin D3 standard added to the test reaction following sample extraction (Fig. 4B,D). This demonstrates that the trihydroxyvitamin D3 can be made from 20,23-dihydroxyvitamin D3 and thus provides the sites of two of the three hydroxyl groups added to vitamin D3 by P450scc.
Identification of dihydroxyvitamin D3 as 20,23- dihydroxyvitamin D3 by NMR
NMR was performed on two preparations of the major dihydroxyvitamin D3 metabolite: one synthesized directly from vitamin D3 and the other from the purified intermediate, 20α-hydroxyvitamin D3. Both gave essentially identical NMR spectra. We started the identification of the hydroxylation sites in both dihydroxyvitamin D3 and trihydroxyvitamin D3 by comparing their 1D proton NMR to that of the parent vitamin D3 (Fig. 5A–C). For both dihydroxyvitamin D3 and trihydroxyvitamin D3, the chemical shifts for 6-CH, 7-CH, 3-CH(OH), 19-CH2 and 9-CH2 were the same as those in vitamin D3. 21-Me shifted downfield from 0.95 p.p.m. (proton, doublet)/19.54 p.p.m. (carbon) in vitamin D3 to 1.36 p.p.m. (singlet)/26.3 p.p.m. in the dihydroxy metabolite and 1.39 p.p.m. (singlet)/23.2 p.p.m. in the trihydroxy metabolite. This is a classical indication of 20-hydroxylation. Furthermore, compared with vitamin D3, besides 3-CH at 3.76 p.p.m./70.7 p.p.m., another hydroxylated CH group at 4.06 p.p.m./67.9 p.p.m. appears in both metabolites (expansions of HSQC spectra are shown in Fig. 5D,E). This clearly indicates that the second hydroxylation occurs on a methylene group, either in the C-ring, D-ring or the side chain.
Fig. 5.
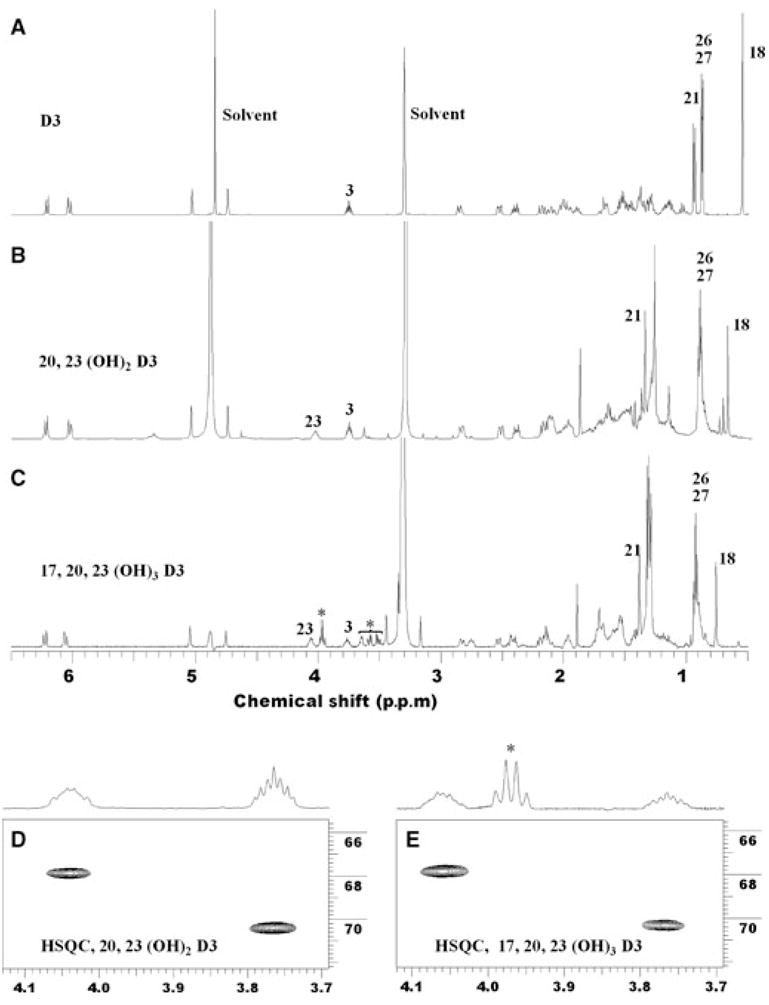
Proton NMR spectra of vitamin D3, dihydroxyvitamin D3 and trihydroxyvitamin D3. (A) Vitamin D3; (B) dihydroxy metabolite identified as 20,23-dihydroxyvitamin D3; (C) trihydroxy metabolite identified as 17α,20,23-trihydroxyvitamin D3. The peaks marked with an asterisk are from unidentified impurities. (D,E) Expansion of proton–carbon HSQC of the two metabolites for 3-CH and 23-CH, respectively.
To identify the exact position for the second hydroxylation, we analysed 2D COSY, TOCSY and HSQC spectra. Figure 6 summarizes our analysis. In the COSY spectrum (Fig. 6A), the correlation between 3-CH and 4-CH2 (2.53 p.p.m. and 2.19 p.p.m.), and the correlation between 3-CH and 2-CH2 (1.97 p.p.m. and 1.52 p.p.m.), are clearly intact. In the TOCSY spectrum (Fig. 6B), all the expected correlations from 3-CH in the A-ring are the same as in the parent vitamin D3. This further confirms that no hydroxylation occurs in the A-ring. The new hydroxylated CH shows correlations in the COSY spectrum to four protons, and analysis of HSQC indicates that these protons belong to two methylene groups (Fig. 6C). The complete spin system revealed by the TOCSY spectrum unambiguously indicates that these two methylene groups are at positions 22 and 24, as two methyl groups (C26 and C27) and a methine group (C25) are in this spin network. Therefore, this hydroxylation must be at C23. The TOCSY spectrum for the dihydroxy metabolite also confirms the first hydroxylation is at position 20 because there is no additional correlation assignable to 20-CH (1.36 p.p.m. for proton in parent vitamin D3). Hydroxylation at position 20 transforms it to a tertiary alcohol that does not participate in the spin system of the side chain. Full 500-MHz proton NMR, COSY, TOCSY, NOESY, HSQC and heteronuclear multiple bond correlation (HMBC) spectra of 20,23-dihydroxyvitamin D3 are presented in the supplementary Fig. S1. The structure of 20,23-dihydroxyvitamin D3 is presented in the Discussion.
Fig. 6.
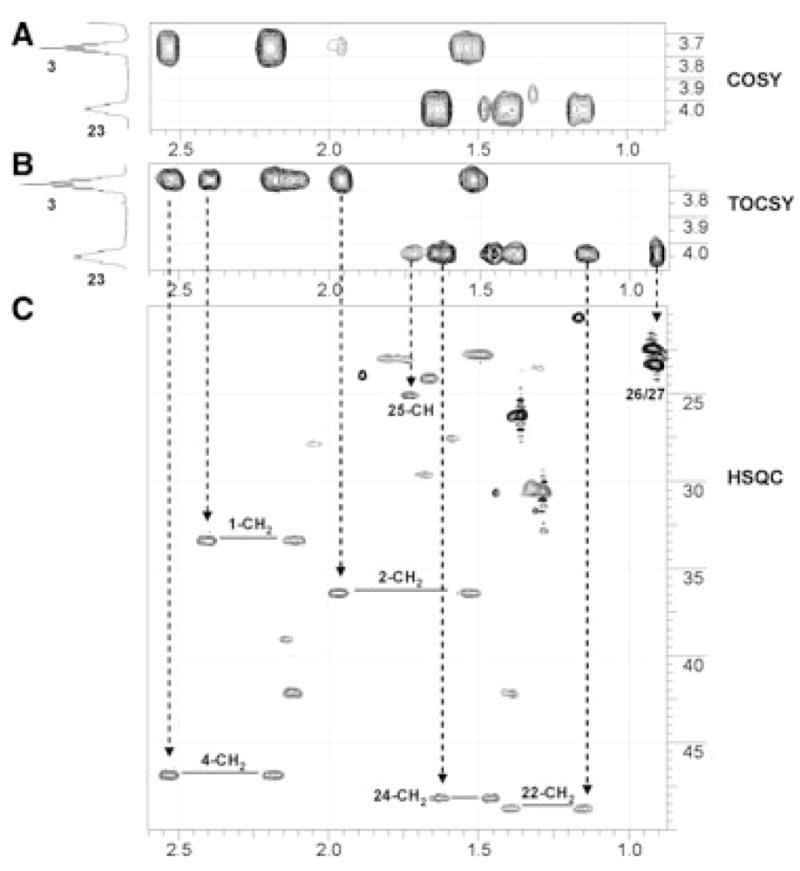
Identification of 20,23-dihydroxyvitamin D3. (A) Expansion of proton–proton COSY correlations for 3-CH and 23-CH; (B) expansion of proton–proton TOCSY correlations for 3-CH and 23-CH; (C) expansion of proton–carbon HSQC showing groups having correlation to 3-CH and 23-CH (for details, see text).
Identification of trihydroxyvitamin D3 as 17α,20,23-trihydroxyvitamin D3 by NMR
The NMR analysis is shown in Fig. 7. A critical difference in the proton 1D NMR is the appearance of a triplet peak at 2.75 p.p.m. (Fig. 7B). HSQC indicates that this is from is a methine group. Besides 3-CH and 23-CH, no additional CH bearing a hydroxyl group is present, ruling out possible hydroxylation at methylene groups. Because all four methyl groups are accounted for, the third hydroxyl group must exist as a tertiary alcohol from hydroxylation of a methine group. The candidates are 14-CH (2.0 p.p.m./57.7 p.p.m.), 17-CH (1.64 p.p.m./62.0 p.p.m.) and 25-CH (1.74 p.p.m./25.2 p.p.m.), with their proton/carbon chemical shifts for the dihydroxy precursor indicated in parenthesis. Analysis of the 2D HSQC NMR clearly indicates that 25-CH is intact. As additional evidence, there are virtually no changes in chemical shifts for 24-CH2 (Fig. 7C) and 26/27-CH3 (Fig. 5). Furthermore, hydroxylation at 25-CH is unlikely to cause 14 or 17 downshift to 2.75 p.p.m. due to its remoteness. The third hydroxyl group is therefore in positions 14 or 17.
Fig. 7.
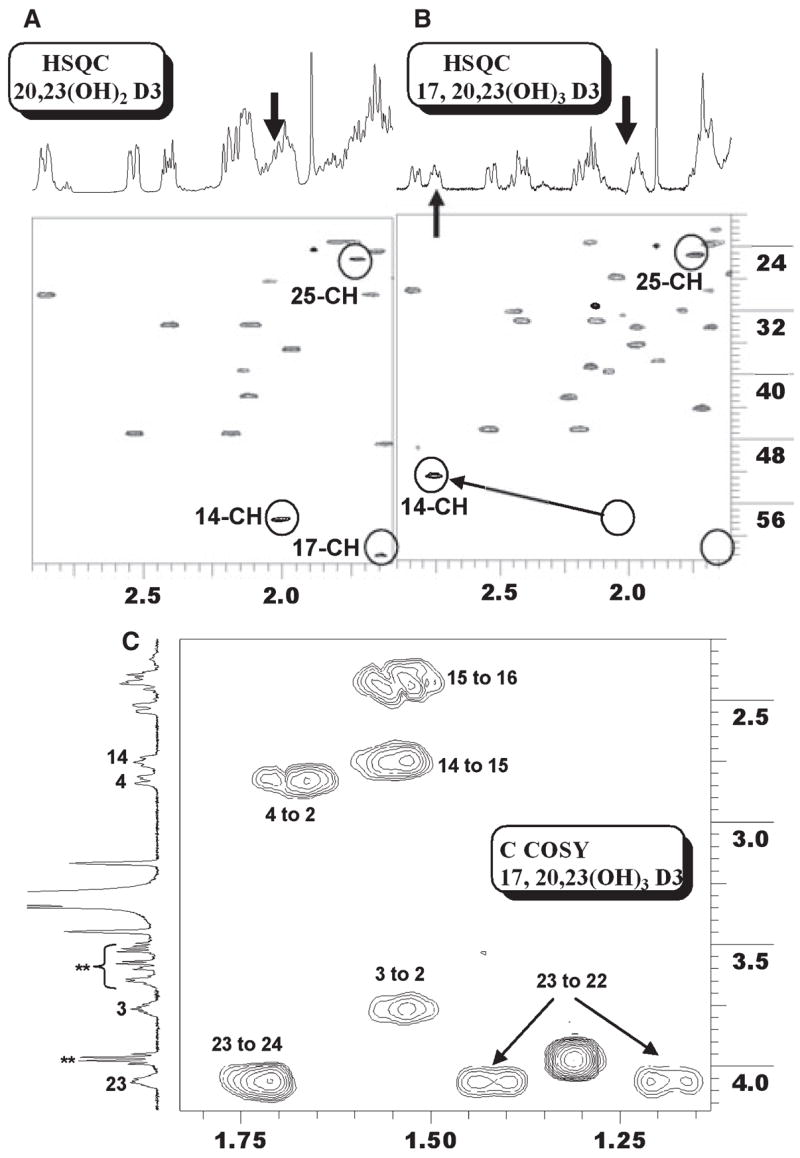
Identification of 17α,20,23-trihydroxyvitamin D3. (A) Expansion of proton–carbon HSQC of 20,23-dihydroxyvitamin D3 showing the three methine groups. (B) Expansion of the same region of 17α,20, 23-trihydroxyvitamin D3. 25-CH is intact, 17-CH is missing and 14-CH is shifted. (C) Expansion of proton–proton COSY showing the correlation from 14-CH to 15-CH2, and from 15-CH2 to 16-CH2.
The only COSY correlation detected from 2.75 p.p.m. is to a 15-CH2 group at 1.53/21.9 p.p.m. Further analysis indicates that the 16-CH2 signals have shifted to 1.80 and 2.44 p.p.m. (protons) and 32.0 p.p.m. (carbon), as indicated by the COSY correlation between 1.53 p.p.m. (15-CH2) and 2.44 p.p.m. (one proton on 16-CH2). This strongly suggests that a third hydroxylation occurs at position 17. Consistent with this assignment, the proton chemical shifts for both 18-Me (0.69 p.p.m. to 0.75 p.p.m.) and 21-Me (1.36 p.p.m. to 1.39 p.p.m.) have shifted downfield slightly. Similar results for 17-hydroxylation were previously shown on identifying 17α,20-dihydroxyvitamin D2 as a product of vitamin D2 metabolism by P450scc [6]. Finally, we were unable to collect a workable HMBC spectrum, which theoretically should have unambiguously indicated the third hydroxylation position, due to the limited amount of trihydroxyvitamin D3 available. Despite this, analysis of all the spectra collectively indicates that this trihydroxy metabolite is 17α,20,23-trihydroxyvitamin D3. Full 500-MHz proton, COSY, TOCSY, HSQC and HMBC NMR spectra of 17α,20,23-trihydroxyvitamin D3 are presented in the supplementary Fig. S2.
Identification of minor products of vitamin D3 metabolism by P450scc
To further identify the minor products of vitamin D3 metabolism by P450scc, each was tested as a substrate and the products were identified by comparison of their HPLC retention times with purified standards. When the monohydroxyvitamin D3 product with RT = 32 min in Fig. 1 was tested as a substrate for P450scc, it was converted to a product with the same retention time as 20,23-dihydroxyvitamin D3 (Fig. 8A–C); thus, we identified this product as 23-hydroxyvitamin D3. A small amount of a product with RT = 24.8 min corresponding to 17α,20,23-trihydroxyvitamin D3 was also seen. The metabolite observed with RT = 28.2 min could be 17α,23-dihydroxyvitamin D3, but insufficient product was generated to investigate this further.
Fig. 8.
Identification of minor metabolites of vitamin D3. Products of P450scc action on vitamin D3 with retention times of 32, 26 and 26.7 min in Fig. 1 were purified, incorporated into phospholipid vesicles at a molar ratio to phospholipid of 0.025, incubated with P450scc (1 μM) for 1 h and the products were analyzed by HPLC with gradient elution. (A) Test reaction of the RT = 32 min monohydroxyvitamin D3 product shown in Fig. 1, identified in this experiment as 23-hydroxyvitamin D3; (B) zero-time control for this substrate where the reaction mixture was extracted at the end of the pre-incubation; (C) 20,23-dihydroxyvitamin D3 standard. (D) Test reaction of the RT = 26 min dihydroxyvitamin D3 product shown in Fig. 1, identified in this experiment as 17α,20-dihydroxyvitamin D3; (E) zero-time control for this substrate; (F) 17α,20,23-trihydroxyvitamin D3 standard. (G) Test reaction of the RT = 26.7 min monohydroxyvitamin D3 product shown in Fig. 1, identified in this experiment as 17α-hydroxyvitamin D3; (H) zero-time control for this substrate; (I) zero-time control spiked with 17α, 20-dihydroxyvitamin D3 standard. Note that the retention times for reactants and products here are different from those described in Fig. 1 due to the use of a new HPLC column.
The dihydroxyvitamin D3 product with RT = 26 min in Fig. 1 is produced from both vitamin D3 and 20-hydroxyvitamin D3 (Figs 1–4); thus, one of the hydroxyl groups must be in position 20. When this product was incubated with P450scc, it was converted to one major product with a retention time the same as that for 17α,20,23-trihydroxyvitamin D3 (Fig. 8D–F). Spiking of the reaction mixture with purified 17α,20,23-trihydroxyvitamin D3 confirmed that the peaks were coincident (not shown). Because this dihydroxyvitamin D3 derivative is clearly different from 20,23-dihydroxyvitamin D3, the above data enabled us to identify it as 17α,20-dihydroxyvitamin D3.
The monohydroxyvitamin D3 product with RT = 26.7 min in Fig. 1 was almost completely converted to a product with an identical retention time to 17α,20-dihydroxyvitamin D3 by P450scc (Fig. 8G–I). A small amount of product with a retention time the same as purified 17α,20,23-trihydroxyvitamin D3, run under identical conditions, was also seen (20.4 min; Fig. 8G), consistent with the major product being 17α,20-dihydroxyvitamin D3. We have therefore identified this monohydroxyvitamin D3 as 17α-hydroxyvitamin D3. This product is only seen for the metabolism of D3, and not for the metabolism of 20-hydroxyvitamin D3 or 23-hydroxyvitamin D3, consistent with it being 17α-hydroxyvitamin D3. Reaction chemes with structures for the formation of the various products observed in Figs 1–4 and 8 are presented in the Discussion.
Discussion
The present study reveals that, in addition to C20, vitamin D3 can be hydroxylated by P450scc in positions 23 and 17α. P450scc can hydroxylate in these three positions regardless of whether vitamin D3 is dissolved in cyclodextrin or the bilayer of phospholipid vesicles. In agreement with previous studies [2,4], 20-hydroxyvitamin D3 was the major product of vitamin D3 metabolism by P450scc. However, in contrast to these studies, we have identified the second major product as 20,23-dihydroxyvitamin D3, and not 20,22-dihydroxyvitamin D3. NMR on two independent samples (one prepared directly from vitamin D3 and the other prepared from purified 20-hydroxyvitamin D3) were in agreement on this new designation. Re-interpretation of our original NMR spectra [4] indicates that our original interpretation was incorrect. At that time, we did not have access to the 3 mm probe and used a high-resolution magic angle spinning probe. The spinning sidebands from high speed sample spinning generated numerous artefacts in the COSY spectra. The use of a 3 mm Shigemi tube in the present study dramatically improved spectral quality without too much compromise in sensitivity, especially for COSY and TOCSY spectra. In addition, unlike the present study where we used methanol-D4 as solvent, our previous study used CDCl3. We have since found that the hydroxylated derivatives of vitamin D3 are unstable in CDCl3 if tiny amounts of acid residue are present, which we observed even in high purity preparations of this solvent.
It is unlikely that the major dihydroxy product observed by Guryev et al. [2], reported as 20,22-dihydroxyvitamin D3, is different to the one we identified as 20,23-dihydroxyvitamin D3 in the present study because they were both produced in cyclodextrin under almost identical conditions. The only difference, other than minor differences in the concentrations of enzymes and substrate used, is that Guryev et al. [2] used cytochrome b5 in their incubations to improve the yield of products. Because the same dihydroxyvitamin D3 product was observed in their study in the absence of cytochrome b5, this does not provide an explanation for hydroxylation at C22 rather than C23. More likely, the difference in reported structures is associated with the collection and interpretation of the NMR data. Their spectra were recorded in CDCl3 and COSY spectra lacked fine detail. HSQC and expanded COSY were not provided by Guryev et al. [2] and, without these, it is difficult to distinguish between hydroxylation at C22 and C23.
The six products seen for the metabolism of vitamin D3 by P450scc in the present study can be explained by the various possible combinations of the three hydroxylations that we identified (Fig. 9). All products and/or intermediates can ultimately be converted to 17α,20,23-trihydroxyvitamin D3. Thus, minor products could be identified with NMR structures of only 20-hydroxyvitamin D3, 20,23-dihydroxyvitamin D3 and 17α,20,23-trihydroxyvitamin D3. The major pathway of vitamin D3 metabolism by cytochrome P450scc involves initial hydroxylation at C20 followed by hydroxylations at C23 and C17, respectively (Fig. 9, pathway indicated in bold). There are additional minor pathways where the relative order of the three hydroxylations differ. All products shown in Fig. 9 were detected in both cyclodextrin and phospholipid vesicles, demonstrating that all pathways occur with both methods of vitamin D3 solubilization. The only combination of hydroxylations not identified is for the potential product, 17α,23-dihydroxyvitamin D3. This is likely due to hydroxylation at position 20 being favoured as the first site of hydroxylation, or the second if initial hydroxylation is at C17 or C23. An unidentified small peak (RT = 28.2 min) in the HPLC chromatogram for the reaction of 23-hydroxyvitamin D3 with P450scc may, however, correspond to this product (Fig. 8A).
Fig. 9.
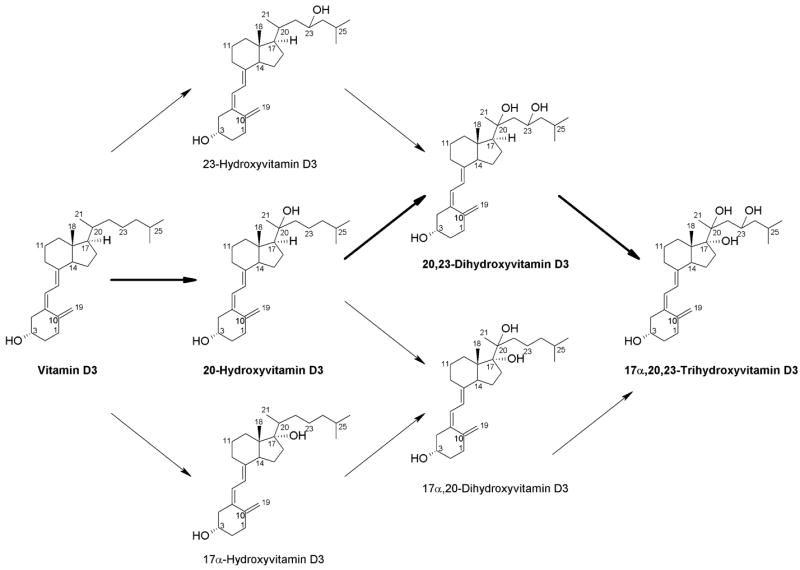
Pathways for the metabolism of vitamin D3 by P450scc. The major pathway is indicated in bold.
Comparing the major pathways of vitamin D3 and cholesterol metabolism by P450scc, the initial hydroxylation of vitamin D3 at C20 is clearly different to that for cholesterol, where initial hydroxylation occurs at C22 [1,14]. The lack of hydroxylation of vitamin D3 at position 22 now provides a clear explanation for the inability of P450scc to cleave the side chain of vitamin D3. Hydroxylation of the side chain of cholesterol at the adjacent carbons C22 and C20, to produce 20α,22R-dihydroxycholesterol as an intermediate, is a prerequisite for cleavage of the bond between C20 and C22 [1,14]. Another difference between the metabolism of vitamin D3 and cholesterol by P450scc is that the dissociation of intermediates occurs for the metabolism of vitamin D3, but not for the metabolism of cholesterol [15,16]. 20-Hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 clearly dissociate from the active site of P450scc because they accumulate at a concentration well above that of P450scc. Their subsequent metabolism is therefore in competition with vitamin D3. 17α,20-Dihydroxyvitamin D3 and 17α,20,23-trihydroxyvitamin D3 also dissociate from the P450scc in cyclodextrin, reaching a concentration in excess of the P450scc concentration, but this is not observed in phospholipid vesicles where less of these metabolites are produced. Dissociation of intermediates from P450scc is not confined to vitamin D3 metabolism because intermediates have also been observed to accumulate in the metabolism of cholestanone (cholest-4-en-3-one) by P450scc [17].
The present study on vitamin D3, when considered together with our previous study involving vitamin D2 [6], indicates that P450scc is able to hydroxylate the D-ring of both secosteroids at C17. The configuration of the newly introduced hydroxyl group was assigned as 17α based on the preferential hydroxylation by P450scc from the less hindered ‘bottom’ (α) side of the steroid molecule. Such stereochemistry of the reaction is in accordance with an accepted mechanism of oxidation by cytochrome P450 [18], where the relatively large molecule has to approach the steroidal substrate for both abstraction of a hydrogen atom and insertion of the hydroxyl group. Thus, C17 must be able to come close to the heme iron, presumably due to the side chain occupying a slightly different position in the active site compared to that of cholesterol. Hydroxylation at C17 is not dependent on prior hydroxylation of the side chain because 17α-hydroxyvitamin D3 is seen as a minor product. Ergosterol, the vitamin D2 precursor with an intact B-ring, is also hydroxylated by P450scc at C17 [5]. In comparison to vitamin D3, neither vitamin D2, nor ergosterol are hydroxylated at C23, most likely due to the double bond between C22 and C23 of these molecules.
The biological significance of P450scc-catalysed metabolism of vitamin D and its precursors cannot be underestimated because P450scc-generated hydroxy derivatives of ergosterol and vitamin D2 have biological activity in skin cells [5,6]. Of note, we have recently documented that 20-hydroxyvitamin D3, a direct precursor of 20,23-dihydroxyvitamin D3, can regulate proliferation and differentiation of human epidermal keratinocytes cultured in vitro [10]. Thus, the vitamin D3 derivatives produced by the action of P450scc are good candidates for use in the therapy of hyperproliferative disorders. Furthermore, this pathway may have wider physiological implications because skin, the site of vitamin D3 production [8], expresses functional P450scc [3], and steroidogenic tissues such as the adrenal gland may receive vitamin D from the bloodstream for further metabolism [4].
In conclusion, the identification of new metabolites of vitamin D3 made by the action of P450scc will enable us to proceed with further biological testing of these compounds and the investigation of whether this pathway operates in the human body in vivo.
Experimental procedures
Preparation of enzymes
Cytochrome P450scc and adrenodoxin reductase were purified from bovine adrenal mitochondria [19,20]. Adrenodoxin was expressed in Escherichia coli and purified as described previously [21].
Measurement of vitamin D3 metabolism in phospholipid vesicles
Vesicles were prepared from dioleoyl phosphatidylcholine and bovine heart cardiolipin (Sigma, Castle Hill, NSW, Australia) in the ratio 85 : 15 (mol·mol−1). Buffer comprising 20 mm Hepes (pH 7.4), 100 mM NaCl, 0.1 mM dithiothreitol and 0.1 mM EDTA was added to 1.25 μmol of phospholipid and vesicles prepared by sonication for 10 min in a bath-type sonicator [22]. Vitamin D3, cholesterol and hydroxyvitamin D3 derivatives were included in the mixture for sonication as required. Purified P450scc was incorporated into the vesicle membrane by incubation with the vesicles for 20 min at room temperature [22]. The incubation mixture comprised 510 μM phospholipid vesicles, cytochrome P450scc (1–2 μM), 15 μM adrenodoxin, 0.2 μM adrenodoxin reductase, 2 mM glucose 6-phosphate, 2 U·mL−1 glucose 6-phosphate dehydrogenase and 50 μM NADPH, in the buffer used for sonication. Samples were pre-incubated for 8 min, reactions started by the addition of NADPH and incubations carried out at 37 °C with shaking. Typical incubation volumes were 0.25–1.0 mL. Reactions were stopped by the addition of 2 mL of ice-cold dichloromethane. After shaking and centrifugation, the lower phase was retained and the upper aqueous phase was extracted twice more with 2 mL aliquots of dichloromethane. The dichloromethane was removed under nitrogen and samples dissolved in 64% methanol in water for HPLC analysis.
Measurement of vitamin D3 metabolism by substrates dissolved in cyclodextrin
Incubations were carried out as described for phospholipid vesicles except that the vesicles were replaced by 2-hydroxypropyl-β-cyclodextrin (Sigma) at a final concentration of 0.45%. Substrates were initially dissolved in 45% cyclodextrin (typically 5 mM) [23].
HPLC analysis of vitamin D3 metabolites
HPLC was carried out using a Perkin-Elmer HPLC (Perkin Elmer Life Sciences Inc., Walthan, MA, USA) equipped with a C18 column (Brownlee Aquapore, 22 cm × 4.6 mm, particle size 7 μm). Samples were applied in 64% methanol and eluted with a 64–100% methanol gradient in water, at a flow rate of 0.5 mL·min−1. Products were detected with a UV monitor at 265 nm.
Large-scale preparation of vitamin D3 metabolites for NMR
20-Hydroxyvitamin D3 was prepared enzymatically from 50 mL incubations of 2 μM P450scc with 100 μM vitamin D3 in 0.9% cyclodextrin in a scaled-up version of the method described above, and purified by preparative TLC as described previously [4]. 20,23-Dihydroxyvitamin D3 (90 μ) and 17α,20,23-trihydroxyvitamin D3 (60 μg) were similarly prepared from 50 mL incubations of 50 μM TLC-purified 20-hydroxyvitamin D3 with 1 μM P450scc in 0.45% cyclodextrin. The two products were purified by HPLC, as described above, approximately 10–20 μg at a time. UV spectra of products were recorded to check that they had the same typical vitamin D3 spectrum as the substrate and were quantitated using an extinction coefficient of 18 000 M−1·cm−1 at 263 nm [24]. Initial NMR of the trihydroxyvitamin D3 indicated the presence of some impurities; thus, the sample was further purified by reverse-phase HPLC on an Atlantis C18 column (Waters Associates, Milford, MA, USA) running an isocratic mobile phase of 62.5% methanol in water at 1.5 mL·min−1. This step removed three minor contaminants. A separate enzymatic synthesis of 20,23-dihydroxyvitamin D3 (80 μg) for structure determination by NMR was performed using a 50 mL incubation of 50 μM vitamin D3 with 2 μM P450scc in 0.45% cyclodextrin, with the product being purified by TLC [4], and then by gradient HPLC as above.
Preparation of minor products to test as substrates for P450scc
Minor metabolites of vitamin D3 were purified from 50 mL incubations of 100 μM vitamin D3 with 2 μM P450, as described above for the preparation of 20-hydroxyvitamin D3. The TLC plate was divided into sections and products eluted from the silica gel for each section with CHCl3:CH3OH (1 : 1). Products were analysed and purified by reverse-phase HPLC with gradient elution as above.
NMR
All NMR measurements were performed on a Varian Unity Inova-500 MHz spectrometer equipped with a 3 mm inverse probe (Varian NMR, Inc, Palo Alto, CA, USA). We used deuterated methanol as solvent and susceptibility matched 3 mm Shigemi NMR tubes were used for maximum sensitivity (Shigemi, Inc., Allison Park, PA, USA). Temperature was regulated at 20 °C and was controlled with a general accuracy of ± 0.1 °C. Chemical shifts were referenced to 3.31 p.p.m. for proton and 49.15 p.p.m. for carbon from solvent peaks. Initial NMR measurements on the trihydroxy metabolite revealed that impurities were still present. This metabolite was further purified by HPLC as outlined above and all the NMR measurements repeated under similar conditions. Peaks that either grossly changed their chemical shifts or substantially changed their calibrated intensities before and after repurification were assigned as impurities.
Other procedures
The concentration of cytochrome P450scc was determined from the CO-reduced minus reduced difference spectrum using an extinction coefficient of 91 000M−1·cm−1 for the absorbance difference in the range 450–490 nm [25]. Electron impact ionization mass spectra were recorded with a Micromass VG Autospec Mass Spectrometer (Micromass Inc., Beverly, MA, USA) as described previously [6]. Mass spectra were recorded by positive electrospray ionization using a Micromass VG Autospec Mass Spectrometer at 8 kV. The sample was run in 100% methanol at a sample flow rate of 30 μL·min−1. Nitrogen was used as both the bath (250 L·h−1) and nebulizing gas (12 L·h−1). The scan range for the mass spectrum was m/z 100–1000.
Supplementary Material
The 500-MHz NMR spectra of 20,23-dihydroxyvitamin D3.
The 500-MHz NMR spectra of 17α,20,23-trihydroxyvitamin D3.
This material is available as part of the online article from http://www.blackwell-synergy.com
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Acknowledgments
We thank Dr Tony Reeder for recording mass spectra. This work was supported by NIH grant R01AR052190 to A. S. and by the University of Western Australia.
Abbreviations
- cyclodextrin
2-hydroxypropyl-β-cyclodextrin
- HMBC
heteronuclear multiple bond correlation
- HSQC
heteronuclear single quantum correlation
- RT
retention time
References
- 1.Tuckey RC. Progesterone synthesis by the human placenta. Placenta. 2005;26:273–281. doi: 10.1016/j.placenta.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Guryev O, Carvalho RA, Usanov S, Gilep A, Esta-brook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci USA. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slominski A, Zjawiony J, Wortsman J, Semak I, Stew-art J, Pisarchik A, Sweatman T, Marcos J, Dunbar C, Tuckey RC. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271:4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slominski A, Semak I, Zjawiony J, Wortsman J, Gandy MN, Li J, Zbytek B, Li W, Tuckey RC. Enzymatic metabolism of ergosterol by cytochrome P450scc to biologically active 17α,24-dihydroxyergosterol. Chem Biol. 2005;12:931–939. doi: 10.1016/j.chembiol.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. An alternative pathway of vitamin D2 metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–2901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res. 2003;164:3–28. doi: 10.1007/978-3-642-55580-0_1. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D: a millenium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 9.Bikle DD. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 10.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman T, Jones E, Nguyen MN, Slominski AT. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450sc, stimulates keratinocytes differentiation. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merz K, Sternberg B. Incorporation of vitamin D3-derivatives into liposomes of different lipid types. J Drug Target. 1994;2:411–417. doi: 10.3109/10611869408996817. [DOI] [PubMed] [Google Scholar]
- 12.Kazanci N, Toyran N, Haris PI, Svercan F. Vitamin D2 at high and low concentrations exert opposing effects on molecular order and dynamics of dipalmitoyl phosphatidylcholine membranes. Spectroscopy. 2001;15:47–55. [Google Scholar]
- 13.Headlam MJ, Wilce MCJ, Tuckey RC. The F-G loop region of cytochrome P450scc (CYP11A1) interacts with the phospholipid membrane. BBA Biomembr. 2003;1617:97–108. doi: 10.1016/j.bbamem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Hume R, Kelly RW, Taylor PL, Boyd GS. The catalytic cycle of cytochrome P-450scc and intermediates in the conversion of cholesterol to pregnenolone. Eur J Biochem. 1984;140:583–591. doi: 10.1111/j.1432-1033.1984.tb08142.x. [DOI] [PubMed] [Google Scholar]
- 15.Lambeth JD, Kitchen SE, Farooqui AA, Tuckey R, Kamin H. Cytochrome P-450scc-substrate interactions. Studies of binding and catalytic activity using hydroxycholesterols. J Biol Chem. 1982;257:1876–1884. [PubMed] [Google Scholar]
- 16.Tuckey RC. Side chain cleavage of cholesterol sulfate by ovarian mitochondria. J Steroid Biochem Mol Biol. 1990;37:121–127. doi: 10.1016/0960-0760(90)90380-4. [DOI] [PubMed] [Google Scholar]
- 17.Sugano S, Morishima N, Sone Y, Horie S. Cytochrome P-450scc-catalyzed production of progesterone from cholestenone. Biochem Mol Biol Int. 1995;35:31–36. [PubMed] [Google Scholar]
- 18.Meunier B, de Visser SP, Shaik S. Mechanism of oxidation reactions catalyzed by cytochrome P450 enzymes. Chem Rev. 2004;104:3947–3980. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]
- 19.Tuckey RC, Stevenson PM. Properties of ferredoxin reductase and ferredoxin from the bovine corpus luteum. Int J Biochem. 1984;16:489–495. doi: 10.1016/0020-711x(84)90165-4. [DOI] [PubMed] [Google Scholar]
- 20.Tuckey RC, Stevenson PM. Properties of bovine luteal cytochrome P-450scc incorporated into artificial phospholipid vesicles. Int J Biochem. 1984;16:497–503. doi: 10.1016/0020-711x(84)90166-6. [DOI] [PubMed] [Google Scholar]
- 21.Woods ST, Sadleir J, Downs T, Triantopoulos T, Head-lam MJ, Tuckey RC. Expression of catalytically active human cytochrome P450scc in Escherichia coli and mutagenesis of isoleucine-462. Arch Biochem Bio-phys. 1998;353:109–115. doi: 10.1006/abbi.1998.0621. [DOI] [PubMed] [Google Scholar]
- 22.Tuckey RC, Kamin H. Kinetics of the incorporation of adrenal cytochrome P-450scc into phosphatidylcholine vesicles. J Biol Chem. 1982;257:2887–2893. [PubMed] [Google Scholar]
- 23.De Caprio J, Yun J, Javitt NB. Bile acid and sterol solubilization in 2-hydroxypropyl-β-cyclodextrin. J Lipid Res. 1992;33:441–443. [PubMed] [Google Scholar]
- 24.Hiwatashi A, Nishii Y, Ichikawa Y. Purification of cytochrome P-450D1α (25-hydroxyvitamin D3-1α-hydroxylase) of bovine kidney mitochondria. Biochem Biophys Res Commun. 1982;105:320–327. doi: 10.1016/s0006-291x(82)80047-8. [DOI] [PubMed] [Google Scholar]
- 25.Omura T, Sato R. The carbon monoxide binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 500-MHz NMR spectra of 20,23-dihydroxyvitamin D3.
The 500-MHz NMR spectra of 17α,20,23-trihydroxyvitamin D3.
This material is available as part of the online article from http://www.blackwell-synergy.com
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.