Abstract
OBJECTIVE
Little is known about temporal patterns of diet change within interventions, nor about predictors of early and sustained successful change. Social cognitive theory asserts that early successes in achieving behavior change increase self-efficacy, leading to longer-term success.
DESIGN
We conducted exploratory cluster analyses using dietary data from the first month of the telephone counseling intervention of the Women’s Healthy Eating and Living (WHEL) Study.
MAIN OUTCOME MEASURE
Change in dietary pattern at three early intervention timepoints.
RESULTS
Three clusters were identified: Cluster 1 (25%) was close to meeting study goals at baseline, but still made major changes; Cluster 2 (49%) and Cluster 3 (26%) were not achieving study goals at baseline, but Cluster 2 made substantial immediate changes while Cluster 3 changed their diet more gradually. Baseline demographic and behavioral variables were associated with cluster membership; however, the strongest predictors of cluster were self-efficacy, motivation, and approaches to study goals. Cluster membership predicted dietary pattern at 12 months.
CONCLUSION
These data suggest that a one-on-one telephone counseling intervention that is intensive in the early weeks may maximize the level of change achieved in a study.
Keywords: behavior change, diet intervention, social cognitive theory, cluster analysis, tailoring
INTRODUCTION
A number of chronic diseases have been associated with dietary patterns that involve an under-consumption of vegetables, fruits, and whole grains and an over-consumption of saturated fat (Kris-Etherton et al., 2002; Lichtenstein et al., 2006; West, 2000). However, in the United States, a minority of the population consume the recommended dietary pattern (USDHHS, 2000). Numerous behavior change studies have aimed at improving dietary patterns; however, most have achieved only small amounts of change (Ammerman, Lindquist, Lohr, & Hersey, 2002). Successful studies have not published detailed patterns of change that might inform the design of more effective interventions. Little is known about the trajectory of dietary change occuring within an intervention context, particularly in the earliest stages of a diet program.
Studies with marked change in dietary pattern include the DASH feeding study, which achieved a dietary pattern high in vegetables, fruit and fiber, and low in energy from fat. The intervention was limited to 8 weeks; however, this was sufficient to show an impact of the dietary pattern in reducing blood pressure levels (Conlin et al., 2000). The follow-up PREMIER study achieved and maintained a similar dietary pattern in a free-living population for 6 months, replicating the reduced hypertension effect (Appel et al., 2003). The Women’s Healthy Eating and Living (WHEL) Study has also reported achieving major changes in participants’ dietary pattern that were maintained for at least 12 months (Pierce et al., 2004). This paper uses the large WHEL sample to explore how participants changed their dietary pattern immediately at the start of the intervention, in order to make inferences for improving the tailoring of future interventions (Campbell et al., 1994).
The WHEL Study was designed to test the effect of dietary pattern on the probability of additional breast cancer events among women previously diagnosed with early stage breast cancer (Pierce et al., 2002). There is considerable evidence from pre-clinical studies indicating that plant foods contain anti-carcinogens (Steinmetz & Potter, 1991), and a comprehensive literature review concludes that a diet high in vegetables and fruit probably decreases breast cancer risk and a diet high in total fat possibly increases risk (WCRF, 1997).
The WHEL Study intervention arm achieved major baseline-12-month changes in dietary intake, including an 82% increase in vegetables, 18% increase in fruit, 37% increase in fiber, and 17% decrease in energy from fat (Pierce et al., 2004). These changes were accompanied by a 51% increase in total plasma carotenoids, a biomarker of vegetable and fruit intake, a change that was achieved by increasing both solid food and juice intake with no significant contribution from dietary supplements (Pierce et al., 2006).
Telephone counseling was the main component of the study intervention. Compared to clinic interventions, this approach significantly reduces participant burden and allows the timing of assistance to accommodate the participant’s needs (Zhu et al., 1996). The intervention was based on social cognitive theory (Bandura, 1977, 1986) and focused on helping participants implement optimal self-regulatory skills (proximal goal setting, self-monitoring and performance judgments that maintained perseverance with the change attempt). The protocol emphasized participant decision-making and the change agents (counselors) used motivational interviewing techniques (Miller & Rollnick, 1991, 2002).
The intervention was divided into three phases. (Newman et al., 2005). Phase 1 focused on rapidly developing self-efficacy to achieve the dietary targets as quickly as possible. After randomization, study counselors contacted participants for an introductory call, during which the counselor reviewed the counseling protocol and described the behavioral targets (daily servings of 5 vegetables, 3 fruits, 16 oz of vegetable juice, 30 g of fiber and 20% energy from fat), as well as the suggested telephone call protocol. The introductory call also included a series of questions designed to help the counselor identify the participants’ self-efficacy for dietary change, preferred approach to meeting the study targets, and beliefs about diet change and breast cancer recurrence risk. Each Phase 1 counseling call included a 24-hour dietary assessment to help calibrate self-monitoring skills, a review of the participants’ performance in meeting dietary targets, and identification of potential areas of difficulty. In addition, short-term dietary goals were set for the following call. Both the number of calls and their frequency were tailored to the self-efficacy needs of the participant. Assistance was offered as frequently as on a daily basis. The protocol suggested between 3 and 8 calls during Phase 1, allowing additional calls if the participant did not feel ready to advance to Phase 2. During Phase 2, counselors encouraged participants to modify their environment to help maintain the dietary changes achieved in Phase 1. Phase 3 was designed to minimize relapse, through monthly calls followed by quarterly calls throughout the remainder of the study. Cooking classes were offered during the first year of the study and monthly newsletters were sent to all participants.
METHODS
Study population
The WHEL Study randomized 3088 women aged 18 to 70 years who were early breast cancer survivors from seven clinical sites (University of California at San Diego and Davis, Stanford University, Kaiser Permanente at Oakland and Portland, University of Arizona, and MD Anderson Cancer Center) between 1995 and 2000. Details of the study protocol have been presented elsewhere (Pierce et al., 2002), and the study has Human Subjects approval by each of the local Institutional Review Boards, as well as oversight by a Data and Safety Monitoring Committee. The mean age of participants at baseline was 52 years, mean body mass index (BMI) was 27.3 kg/m2, and over 50% had attended college. Approximately 40% of participants were diagnosed with a stage I (>1 cm) breast cancer, 55% with Stage II, and 5% with Stage IIIA.
In this paper, we focus on the 1267 intervention participants (82% of study arm) who had dietary data at both baseline and 12 months and who also had three or more dietary adherence scores in Phase 1. Of those excluded, 100 did not have adherence scores on at least three Phase 1 calls, and 170 did not have 12-month dietary data (74 of these experienced a study endpoint and 96 did not complete their 12-month 24-hour recall within the study protocol). We also provide data for the 1380 comparison group participants who provided dietary data at baseline, 6 months (a random half sample), and 12 months.
Formal dietary assessment
The study used two types of dietary assessments: formal assessments conducted by trained diet assessors, and less formal assessments conducted as part of the intervention by the study counselors. Formal assessments included a set of four 24-hour dietary recalls conducted by blinded telephone assessors on a random set of days (stratified for weekend and weekdays) within a three-week period. At the initial interview before enrollment, participants were trained to estimate serving sizes with food models to optimize their ability to accurately describe food intake. The formal study assessments used a standardized computer-driven multi-pass protocol; dietary intake data was collected by telephone prior to randomization and at 12 months. A 50% random sample was assessed also at 6 months. The Minnesota Nutritional Data System software was used for data collection and for estimating dietary and nutrient intakes (NDS-R version 4.01, 2001, University of Minnesota, Minneapolis MN).
Self-reported dietary intake was validated by plasma carotenoid levels from blood samples drawn at baseline and 12 months (Natarajan et al., 2006; Pierce et al., 2006). Plasma carotenoids were separated and quantified using a high performance liquid chromatography (HPLC) method that has been previously described (Pierce et al., 2006; Rock et al., 1997).
A single index of dietary pattern
For a number of analytic purposes, it is advantageous to have a single measure of dietary adherence. The WHEL Study protocol paper for (Pierce et al., 2002), outlined a WHEL Adherence Score (WAS) that was weighted to reflect the study’s emphasis on total vegetable intake (servings + juice). Using dietary assessment data, participants were awarded 30 points per serving of vegetables or fruit, and 10 points per ounce of vegetable juice. The score for % energy from fat was prorated from 0 to 100, so that intakes of 40+% energy from fat scored zero points and intakes of 20% or less scored 100 points (this fat measure was capped, since lower fat levels may influence carotenoid uptake). A pro-rated score was also used for fiber intake with an intake of 5g/1000 kcals scoring 0 points and each additional g/1000 kcals scoring 7.7 points. At baseline, the mean WAS (± standard errors) for the WHEL intervention and comparison groups were 283 (± 3) and 280 (± 3) respectively (Pierce et al., 2002). An individual achieving all of the dietary targets would score 600 points.
Baseline demographic and health behavior variables
At the baseline clinic visit, demographic data (including age, education, and race/ethnicity) were collected by interview. Participants also completed several questionnaires, including measures of (1) current smoking status and a brief smoking history, and (2) usual physical activity from the Women’s Health Initiative 9-item questionnaire (WHI Study Group, 1998), previously demonstrated to be both reliable and valid with this population (Johnson-Kozlow, Rock, Gilpin, Hollenbach, & Pierce, 2007; Johnson-Kozlow, Sallis, Gilpin, Rock, & Pierce, 2006). Height and weight were measured at clinic visits using a standard clinical research center protocol, and BMI was calculated (weight[kg]/height[m2]).
Intervention assessments
Potential predictors of performance
During the Introductory telephone call, in addition to answering participant queries, the counselor elicited responses to a series of single-question measures hypothesized to be related to participant performance on the study. These included:
After having some time to think about the study and your role as a participant, how are you feeling about it? (response options: Excited, somewhat excited, good, not sure/other).
What is your primary motivation for participating in this dietary study? (response options: To prevent recurrence, eat healthier, help others, improve health/lose weight/other).
How strongly do you believe that your dietary intake could play an important role in reducing your chances of recurrence of breast cancer? (response options: very strongly, strongly, somewhat/none/don’t know).
Since you were diagnosed with breast cancer, have you attempted to modify your diet? (response options: yes, no)
Which of the study dietary components, if any, do you expect to be the most difficult to achieve? (response options: vegetable juice or vegetables, all others)
What approach do you plan on taking towards the study goals? (response options: All targets at once, intermediate goals for each target, one target at a time, other)
How confident are you that you can reach the study goals? (response options: very confident, confident, somewhat confident, not confident). Due to a technical problem, data were not available for <10% of participants; these were coded as “not assessed”.
Dietary assessments during Phase 1
Less formal dietary assessments were undertaken by study counselors. During all Phase 1 calls, counselors conducted a single-pass 24-hour recall using dietary analysis software (FoodProcessor 7.4, Salem, OR). These assessments were less burdensome than the formal study assessments, with an emphasis on helping participants monitor their performance.
Metrics of intervention implementation during Phase 1
Both the timing and the number of Phase 1 calls were tailored to participant needs and the counselor and participant mutually determined the call schedule. To explore the relationship between the dose of Phase 1 intervention and dietary change, we report on the following five metrics: (1) time (days) between randomization and the introductory call, (2) time (days) between introductory call and first Phase 1 call, (3) number of Phase 1 calls, (4) number of days in Phase 1, and (5) mean interval between calls (days).
Statistical methods
Determination and validation of clusters
A cluster analysis was undertaken to identify early patterns of behavior change in the intervention group using the WAS obtained from the following dietary assessments: (1) the formal dietary assessment at baseline; (2) the dietary assessment from the first counseling call (i.e., the call following the introductory call); and (3) an average of the two highest (peak) scores from the remaining dietary assessments in Phase 1. These three scores were converted to standardized z-scores, and outliers (z-scores > +/- 4) were winsorized to a z-score of +/-4.
Using these three variables, the CLUSTER procedure in SAS (SAS v 9.1 Cary, NC) suggested an ideal number of clusters according to the Pseudo-F statistic, cubic-clustering criterion (CCC) and pseudo-t2 statistic (Milligan & Cooper, 1985). Two of the three statistics (CCC and pseudo-t2) suggested that three clusters provided the best solution, and the pseudo-F statistic suggested that either two or three clusters would fit the data. The k-means cluster procedure in SPSS (SPSS for Windows, v11.0.1. 2001. Chicago: SPSS Inc.) was used, forcing a 3-cluster solution. To validate the 3-cluster solution, the k-means cluster procedure was again run in a random half sample of the intervention group (test sample). Clusters were then formed using the remaining half of the subjects (validation sample), first without forced cluster centroids, and then using the cluster centroids determined by the test sample. We compared the two separate solutions in the validation sample and calculated the kappa statistic for agreement.
Comparison of clusters
We compared the clusters on diet pattern and the metrics of intervention delivery using ANOVA, with Tukey’s HSD test for post-hoc comparisons. We used categories for demographic and health behavior variables as well as the variables measured during the introductory call. On these variables we used χ-square tests to assess differences between clusters. Effect sizes are reported for comparisons of categorical variables (Cramer’s V) and continuous variables (eta2).
RESULTS
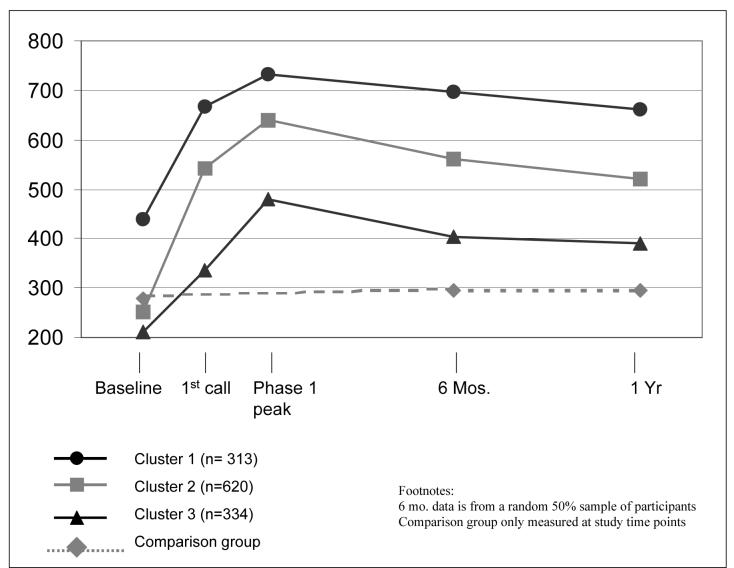
WHEL adherence scores over time for the clusters are presented in Figure 1. Cluster assignments were very robust; the kappa statistic for agreement between the validation sample cluster solutions was 0.991. Cluster assignment was highly predictive of the 6-and 12-month adherence score (ANOVA for mean WAS by cluster membership at 6 months, F(2, 595)=150.3, p<0.001, eta2=.336; at 12 months, F(2, 1264)=238.8, p<0.001, eta2=.274). Cluster was also predictive of 12 month intake for each component of the target WHEL diet pattern (Table 1).
Figure 1.
Patterns of dietary change by cluster as measured by the WHEL Study Adherence Score (WAS)
Table 1.
Change in WHEL dietary components and plasma carotenoids from baseline to one year by cluster
| Cluster | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | F-statistic | p-value | post-hoc differences | ||
| Vegetables (serv/day) | Baseline | 4.5 | (2.0) | 2.6 | (1.2) | 2.2 | (1.1) | |||
| One year | 6.1 | (2.2) | 4.6 | (1.8) | 3.5 | (1.7) | 156.2 | <0.001 | 1>2>3 | |
| Change | 35% | 79% | 61% | |||||||
| Fruit (serv/day) | Baseline | 3.7 | (1.8) | 2.1 | (1.3) | 1.7 | (1.3) | |||
| One year | 4.3 | (1.8) | 3.1 | (1.5) | 2.4 | (1.5) | 126.2 | <0.001 | 1>2>3 | |
| Change | 17% | 50% | 43% | |||||||
| Vegetable juice (serv/day) | Baseline | 0.2 | (0.5) | 0.1 | (0.3) | 0.1 | (0.3) | |||
| One year | 2.3 | (1.3) | 1.9 | (1.3) | 1.2 | (1.2) | 57.4 | <0.001 | 1>2>3 | |
| Change | 1328% | 2152% | 1906% | |||||||
| Fiber (g/day) | Baseline | 29.0 | (9.8) | 19.6 | (5.9) | 17.3 | (5.7) | |||
| One year | 36.3 | (9.6) | 29.0 | (9.1) | 23.0 | (8.3) | 175.8 | <0.001 | 1>2>3 | |
| Change | 25% | 48% | 33% | |||||||
| Percent calories from fat | Baseline | 24.3 | (6.2) | 29.3 | (6.7) | 30.5 | (7.2) | |||
| One year | 19.8 | (6.3) | 22.7 | (6.8) | 26.5 | (7.9) | 75.1 | <0.001 | 3>2>1 | |
| Change | -19% | -22% | -13% | |||||||
| Plasma carotenoids (μmol/L) | Baseline | 2.8 | (1.5) | 2.2 | (1.2) | 1.9 | (1.1) | |||
| One year | 4.7 | (2.7) | 3.7 | (2.3) | 2.9 | (1.9) | 48.1 | <0.001 | 1>2>3 | |
| Change | 68% | 67% | 46% | |||||||
Cluster 1 contained 25% of the study population (n=313) and had a baseline mean (SD) adherence score of 439(124); this was almost 75% greater than Cluster 2 and over twice the score of Cluster 3. At the first Phase 1 call, the score increased to 668 (117) and additional counseling increased the score by a further 10% to a Phase 1 peak of 733 (99). The mean score declined to 662 (156) at 12 months, 51% higher than the baseline score and comparable to the first Phase 1 call score.
Cluster 2 comprised 49% of the study sample (n=620), and had a baseline score of 251 (82) that more than doubled to 542 (98) following the introductory call. At the peak of Phase 1 counseling, they achieved a mean score of 640(67), an increase of 18% over the level achieved by the first Phase 1 call. At 12 months, cluster 2 participants had a score of 520 (158), slightly below that reported at the first Phase 1 call. This score was over double that of baseline, although a decline of 19% from the peak Phase 1 score.
Cluster 3 contained 26% of the sample (n=334). This cluster had consistently lower scores than the other two clusters. The baseline score [mean(SD) = 210(87)] indicated that the WAS needed to increase threefold to meet the behavioral targets. This cluster increased their score by 50% by the first Phase 1 counseling call [mean(SD) = 337(108)]. The peak score achieved in Phase 1 counseling was 480 (90), a further increase of 50% of the level achieved by the first call. By 12 months, the mean score had decreased 19% to 389 (161), just less than double the baseline score.
The adherence scores for the comparison group remained relatively unchanged from baseline over the first year (Figure 1). At baseline, the comparison group had a mean dietary score of 284 (161) that was comparable with the combined intervention sample used in this study [285 (159)]. At 6 months, this score was 302 (156) and at 12 months was 293 (158). Plasma carotenoids in the comparison group were 2.33(1.47) μmol/L at baseline and 2.28(1.37) at 12 months. Thus, there was no significant change in dietary pattern in the comparison group by either self-report or biomarker measure.
Cluster differentiation on intervention metrics
The counseling protocol specified that the introductory call be conducted within 2 weeks of randomization. The mean time between randomization and introductory call was significantly different across clusters (see Table 2): Introductory calls were completed within 2 weeks in 77% of Cluster 1, 61% of Cluster 2, and 61% of Cluster 3 (Table 2). While Cluster 3 also had a longer time between the introductory call and the first counseling call, the difference was not as marked as the delay in receiving the introductory call.
Table 2.
Intensity and frequency of the first phase of the WHEL tailored intervention
| Cluster Number | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||||
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | F | P | Eta2 | Post-Hoc | |
| Number of Phase 1 calls | 4.4 (1.5) | 312 | 4.7 (1.6) | 617 | 5.3 (2.3) | 334 | 19.2 | <0.001 | 0.03 | 1<2<3 |
| Days in Phase 1 | 22.2 (47.8) | 312 | 32.1 (104.9) | 617 | 49.6 (127.7) | 334 | 6.2 | 0.002 | 0.01 | 1,2 <3 |
| Call interval (days) | 4.6 (8.2) | 312 | 5.9 (13.7) | 617 | 8.6 (20.4) | 334 | 6.6 | 0.001 | 0.01 | 1,2 <3 |
| Time from randomization to introductory call (days) | 11.3 (8.9) | 313 | 12.5 (11.0) | 619 | 16.3 (20.2) | 333 | 12.4 | <0.001 | 0.02 | 1,2 <3 |
| Time from introductory call to first Phase 1 call (days) | 7.5 (10.3) | 313 | 7.0 (8.5) | 617 | 9.2 (14.3) | 331 | 4.5 | 0.011 | 0.01 | 1,2 <3 |
The protocol suggested 3-8 calls during Phase 1, but allowed for individual variation which was evident as this variable was significantly different across clusters. Cluster 1 had a mean of 4.4 calls, with only 10% receiving 7 or more calls. Cluster 2 had a mean of 5.7 calls, with 14% receiving 7 or more calls; and Cluster 3 had a mean of 5.3 calls, with 22% receiving 7 or more calls. The interval between Phase 1 calls also differed significantly between groups, with the mean number of interval days and proportion taking 10+ days distributed as follows: Cluster 1=4.6 days between calls and 5% taking 10+ days; Cluster 2=5.9 days between calls and 8% 10+ days; and Cluster 3=8.6 days between calls, and 14% taking 10+ days. As a result, time from randomization to completion of Phase 1 also differed by cluster, with Phase 1 for Clusters 1, 2, and 3, lasting 40 days, 49 days and 65 days respectively.
Cluster differentiation by demographic and baseline lifestyle variables
Age was significantly related to cluster, with those younger than 50 years being less likely to be in Cluster 1 and more likely to be in Cluster 3 (Table 3). The reverse pattern was seen for those older than50 years. Hispanics and African Americans were also less likely to be in Cluster 1 and more likely to be in Cluster 3. Education level was also associated with cluster, with college graduates being more likely to be in Cluster 1 and less likely to be in Cluster 3, with those who did not graduate from college having the reverse pattern.
Table 3.
Differences in cluster membership by baseline characteristics and health behaviors
| Total N (%) | Cluster 1 % | Cluster 2 % | Cluster 3 % | χ2 | P | Cramer’s V | ||
|---|---|---|---|---|---|---|---|---|
| Age | ≤45 | 237 (18.7) | 14.4 | 18.7 | 22.8 | 16.9 | 0.031 | 0.08 |
| 46-50 | 274 (21.6) | 17.3 | 22.6 | 24.0 | ||||
| 51-55 | 281 (22.2) | 25.6 | 21.6 | 20.1 | ||||
| 56-60 | 195 (15.4) | 16.9 | 15.0 | 14.7 | ||||
| ≥61 | 280 (22.1) | 25.9 | 22.1 | 18.6 | ||||
| Ethnicity | White | 1080 (85.2) | 88.5 | 86.5 | 79.9 | 19.4 | 0.013 | 0.09 |
| Hispanic | 71 (5.6) | 3.8 | 5.6 | 7.2 | ||||
| Black | 47 (3.7) | 1.6 | 3.2 | 6.6 | ||||
| Asian/Pac. Islander | 49 (3.9) | 4.8 | 3.4 | 3.9 | ||||
| Other | 20 (1.6) | 1.3 | 1.3 | 2.4 | ||||
| Education | Less than college | 167 (13.2) | 7.7 | 13.5 | 17.7 | 30.3 | <0.001 | 0.11 |
| Some college | 372 (29.4) | 23.0 | 30.8 | 32.6 | ||||
| College graduate | 384 (30.3) | 37.7 | 28.9 | 26.0 | ||||
| Post graduate | 344 (27.2) | 31.6 | 26.8 | 23.7 | ||||
| BMI | Healthy (<25) | 541 (42.9) | 50.3 | 42.9 | 35.9 | 16.2 | 0.003 | |
| Overweight (25-29.9) | 396 (31.4) | 30.1 | 30.0 | 35.0 | ||||
| Obese (30+) | 325 (25.8) | 19.6 | 27.1 | 29.0 | ||||
| Smoking status | Current smoker | 37 (3.0) | 2.3 | 3.2 | 3.5 | 1.6 | 0.815 | |
| Former smoker | 520 (42.7) | 44.0 | 41.3 | 43.8 | ||||
| Never smoked | 662 (54.3) | 53.6 | 55.5 | 52.7 | ||||
| Physical activity (weekly METs) | Least activity | 248 (21.1) | 8.2 | 22.0 | 31.5 | 89.9 | <0.001 | 0.20 |
| 2ndquintile | 223 (19.0) | 14.1 | 19.9 | 21.8 | ||||
| 3rd quintile | 249 (21.2) | 22.0 | 21.7 | 19.5 | ||||
| 4th quintile | 237 (20.2) | 23.4 | 20.5 | 16.6 | ||||
| Most activity | 219 (18.6) | 32.3 | 15.9 | 10.7 |
BMI was also strongly related to cluster. Those who were overweight and obese were overrepresented in Cluster 3, although 35% of the members of this cluster were also of healthy weight. Few smokers participated in this study, and smoking status was not associated with cluster membership. We observed a strong trend for the more physically active to be represented in Cluster 1, although 11% of the most active people were in Cluster 3.
Cluster differentiation by potential predictors from introductory call
Given that baseline adherence scores were much higher in Cluster 1 participants than in Clusters 2 and 3, we were particularly interested in differences between the latter two clusters. Compared to participants in Cluster 2, those in Cluster 3 were more likely to report that the vegetable target (including vegetable juice) would be the most difficult; they were much less likely to report that they could achieve all of the targets at once and they had lower self-efficacy levels. They were also less likely to have reported having changed their diet since diagnosis.
DISCUSSION
We have identified three distinct groups of breast cancer survivors based on their early patterns of dietary change in a dietary intervention trial.. One group already consumed a healthy diet at baseline, a second group substantially changed their diet with an early, intensive intervention, and a third group made substantial changes in dietary pattern, but at a slower rate and to a lesser extent than the other groups. Perhaps the most important finding is that even the least motivated women in the intervention group (Cluster 3) substantially changed their diet pattern. This is encouraging because it suggests that the theory-based telephone intervention is structured enough to achieve consistent changes in all dietary targets, but flexible enough for individual tailoring. This behavior change paradigm may be a promising approach for other populations, including those who are less ready to change than breast cancer survivors.
The women in Cluster 1 had likely improved their diets before enrolling in the WHEL Study, as evidenced by their higher baseline adherence score and previous self-reports of post-diagnosis dietary change (Thomson et al, 2002). This previous success may have led to their greater self-efficacy and a more aggressive approach to meeting the study’s dietary targets. Also, these women reported stronger beliefs that diet plays a role in breast cancer recurrence. Overall, women in Cluster 1 had the lowest dose of the counseling intervention and proceeded to Phase 2 more rapidly. This is not unexpected, since the intervention is tailored to the level of success of the participant in meeting the WHEL targets. The women in Cluster 2 also improved their dietary pattern considerably, evidenced by both their self-reported dietary intake patterns and increased plasma carotenoid concentrations. Indeed, the percentage increase in plasma carotenoids from baseline to one year (67%) is the same as that observed in Cluster 1 (68%).
Interestingly, many breast cancer survivors do not believe that diet played a causative role in their breast cancer, but report that following a healthy diet will reduce the chance that their breast cancer will recur (Lavery & Clarke, 1996; Stewart et al., 2001). Many breast cancer survivors improve their diet following diagnosis, according to previous studies (Maunsell, Drolet, Brisson, Robert, & Deschenes, 2002; Salminen, Lagstrom, Heikkila, & Salminen, 2000), including the WHEL Study (Thomson et al., 2002). However, a recent study found that while breast cancer survivors reported qualitatively changing dietary intakes following their diagnosis, the absolute amount of change was quite small (Wayne et al., 2004). Major changes in dietary pattern are more likely to occur through intervention than through self-initiated means.
Numerous behavioral interventions, including dietary interventions have used social cognitive theory (SCT) as a conceptual framework(Sahay, Ashbury, Roberts, & Rootman, 2006). The theory postulates that one of the key concepts in achieving behavior change is self-efficacy (Bandura, 1977, 1986). Believing one’s self to be capable of a behavior is a consistent predictor of undertaking the behavior, not only in studies of dietary change but of other health behaviors as well. Outcome expectancies are also important in SCT; if one perceives a future benefit to undertaking a behavior, they will be more likely to perform the behavior. The SCT also suggests that proximal goals are easier to achieve than distal goals and that positive reviews of performance will in turn increase the motivation to continue. Our results reflect the importance of these SCT constructs in a dietary change context, as our items that assessed motivation and self-efficacy were strongly associated with cluster membership.
There are limitations regarding the use of cluster analysis as our method of identifying groups. Cluster analysis assigns membership such that the within-group variance is minimized and the between-group variance is maximized; thus, there is a reasonable degree of homogeneity within each cluster. However, there are individuals whose dietary patterns do not actually fit the profile of any of these three clusters; indeed, there are conceivably as many clusters as there are individuals. While cluster analysis is a useful tool for identifying homogeneous subsets of individuals with similar characteristics or patterns, it should be recognized that not all individuals will precisely fit an identified pattern.
We conducted these analyses on 1267 (82%) of the 1537 women in the WHEL intervention arm. The 18% that were excluded comprised 100 women without adequate adherence score data, 96 women without 12-month adherence score data, and 74 women who had reached a study endpoint. A disproportionate number of women with incomplete 12-month data were in the intervention arm compared to the control arm of the study, suggesting that some women found the intervention to be too difficult. This subset likely represents a “fourth cluster” with unique characteristics.
The 24-hour recalls used to determine the adherence score were measured by diet assessors at scheduled study assessments (baseline, 6 months, and 1 year) and by trained lay counselors during the Phase 1 counseling calls. It is unlikely that this difference would change our results substantially, since both groups are trained extensively in diet recall methodology, and the same formula is used to calculate the adherence score. While using a single score as a measure of diet pattern may have limitations in other contexts, the WAS is a specific measure of the WHEL Study targets and is a useful measure for behavioral studies looking at overall adherence to specific goals. We have previously shown that self-reports of fruit and vegetable intake in the WHEL study were quite accurate, per our validation using carotenoid levels (Natarajan et al 2004, 2006 and Pierce et al, 2006), so it is unlikely that our results reflect differential self-reporting as opposed to a real dietary change achieved by participants.
There is little in the literature to compare with the present study. Urban and colleagues (1992) used a recursive model to show that experiences during the Women’s Health Trial (WHT) predicted maintenance of a low-fat diet following the completion of the trial (Urban, White, Anderson, Curry, & Kristal, 1992). Importantly, that study did find that baseline diet predicted adherence during the WHT and adherence during the WHT predicted long-term maintenance of the low-fat diet. Steptoe (2004) showed that in a dietary intervention study, early changes (at 8 weeks) in behavioral constructs (such as self-efficacy, perceived benefits, and encouragement from others) were associated with dietary change in the longer-term (12 months) (Steptoe, Perkins-Porras, Rink, Hilton, & Cappuccio, 2004). These studies support our finding that early intervention experiences predict diet pattern during the maintenance phase of interventions. Using a stage-of-change model, Kristal (2000) discussed the finding of greater dietary change (within intervention studies) amongst individuals in the “action” or “maintenance” stage at baseline (Kristal, Glanz, Tilley, & Li, 2000). Those findings are consistent with those in the present study; it is likely that those individuals who had already improved their dietary intake in the past (thus increasing self-efficacy) were highly motivated to continue their trajectory.
Identifying a few key variables that can predict the likely trajectory of an individual is appealing, since these early patterns were strong predictors of longer-term maintenance of behavioral change. This is a potential benefit for the design of future behavioral interventions in that a more tailored approach can be implemented; resources can be better allocated by reducing the amount of intervention provided to those who need it least while enriching the intervention for those who have a greater amount of change to achieve.
As expected, the intervention employed by the WHEL Study led to a major change in dietary pattern in the intervention group as a whole (Pierce et al., 2004). However, even among those who had to make the most change and who had the lowest level of self-efficacy and motivation, a major change was achieved.
Table 4.
Differences in cluster membership by introductory call variables
| Total N (%) | Cluster 1 % | Cluster 2 % | Cluster 3 % | χ2 | P | Cramer’s V | ||
|---|---|---|---|---|---|---|---|---|
| Feelings about participation | Excited | 619 (49.9) | 57.3 | 49.8 | 43.2 | 18.5 | 0.005 | 0.09 |
| Somewhat excited | 225 (18.1) | 16.3 | 17.4 | 21.3 | ||||
| Good | 265 (21.4) | 20.2 | 21.8 | 21.6 | ||||
| Not sure/other | 132 (10.6) | 6.2 | 11.1 | 14.0 | ||||
| Why participate? | Prevent recurrence | 444 (36.2) | 40.4 | 36.7 | 31.5 | 10.3 | 0.113 | |
| Eat healthier | 297 (24.2) | 23.5 | 23.4 | 26.5 | ||||
| Help others | 230 (18.8) | 18.5 | 19.9 | 16.8 | ||||
| Health/weight/other | 254 (20.7) | 17.5 | 19.9 | 25.2 | ||||
| Strength of belief that diet plays a role in recurrence | Very strongly | 444 (36.6) | 45.2 | 35.2 | 31.0 | 14.7 | 0.005 | 0.08 |
| Strongly | 347 (28.6) | 25.1 | 28.7 | 32.0 | ||||
| Somewhat/none/dk | 421 (34.7) | 29.7 | 36.1 | 37.0 | ||||
| Diet change since diagnosis | Yes | 918 (73.7) | 82.2 | 74.2 | 64.7 | 25.2 | <0.001 | 0.14 |
| No | 328 (26.3) | 17.8 | 25.8 | 35.3 | ||||
| Hardest anticipated study goal | Juice or vegetable | 495 (40.3) | 36.2 | 37.5 | 49.7 | 15.8 | <0.001 | 0.11 |
| All others | 732(59.7) | 63.8 | 62.5 | 50.3 | ||||
| Approach to goals | All at once | 586 (48.8) | 71.7 | 48.7 | 27.7 | 123.1 | <0.001 | 0.23 |
| Intermediate goals | 349 (29.1) | 13.5 | 29.7 | 42.5 | ||||
| One goal at a time | 219 (18.3) | 11.4 | 18.5 | 24.2 | ||||
| Other | 46 (3.8) | 3.4 | 3.1 | 5.7 | ||||
| Confidence in achieving goals | Very confident | 535 (42.3) | 53.7 | 43.1 | 30.0 | 75.8 | <0.001 | 0.17 |
| Confident | 418 (33.0) | 30.4 | 35.5 | 30.9 | ||||
| Somewhat confident | 167 (13.2) | 8.0 | 11.3 | 21.6 | ||||
| Not confident | 37 (2.9) | 0.0 | 2.6 | 6.3 | ||||
| Not assessed | 108 (8.5) | 8.0 | 7.4 | 11.1 |
Acknowledgements
WHEL Study Coordinating Center: University of California, San Diego, Cancer Prevention and Control Program, Moores UCSD Cancer Center, San Diego, CA (John P. Pierce, PhD; Susan Faerber, BA; Barbara A. Parker, MD; Loki Natarajan, PhD, Cheryl L. Rock, PhD, RD; Vicky A. Newman, MS, RD; Shirley W. Flatt, MS; Sheila Kealey, MPH; Linda Wasserman, MD, PhD; Wayne A. Bardwell, PhD; Lisa Madlensky, PhD.)
Dietary Counselors: Sheila Fisher, Joyce Bertaux, Leslie Barbier, Sharon Bonner, Prudy Galagan, Carrie Gonzales, Kaylene Grove, Pam Herskovitz, Susie Newmiller, Lita Simmons, Susan Wancewicz
Dietary Assessors: Andrea Jackson, Lita Simmons, Denice Murillo, Sophie Levy, Nichole Brumley
Laboratory Analysis: Dennis Heath, MS, Mila Pruitt
Funding support: This study was initiated with the support of the Walton Family Foundation and continued with funding from NCI grants CA 69375 and CA 72092. Some of the data were collected from General Clinical Research Centers, NIH grants M01-RR00070 and M01-RR00827.
Clinical Sites
Center for Health Research-Portland, Portland, OR (Njeri Karanja, PhD, Mark U. Rarick, MD, Lucy Fulton, DTR, RD); Kaiser Permanente Northern California, Oakland, CA (Bette J. Caan, DrPH, Lou Fehrenbacher, MD, Sarah Josef, RD); Stanford Prevention Research Center, Stanford University, CA (Marcia L. Stefanick, PhD, Robert Carlson, MD, Charlene Kranz, RD, Gwen D’Antoni, RD, Natalie Ledesma, MS, RD, Monique Schloetter, MS, RD,); University of Arizona, Tucson & Phoenix, AZ (Cynthia Thomson, PhD, RD, James Warneke, MD, Cheryl Ritenbaugh, PhD, MPH, Tina Green, MS, RD, Emily Nardi, MPH, RD); University of California, Davis, Davis, CA (Ellen B. Gold, PhD, Sidney Scudder, MD, Stephanie Burns, Linda Bresnick); University of California, San Diego, Moores UCSD Cancer Center, San Diego, CA (Kathryn A. Hollenbach, PhD, Vicky Jones, M.D., Michelle McKinney, Diana Wiggins, RD); University of Texas M.D. Anderson Cancer Center, Houston, TX (Lovell A. Jones, PhD, Richard Hajek, PhD, Richard Theriault, DO, Taylor Tran, RD, LD)
The authors would like to thank all WHEL participants for their continued dedication to the study, and Christine Hayes and Sheila Kealey for their assistance with manuscript preparation.
REFERENCES
- Ammerman AS, Lindquist CH, Lohr KN, Hersey J. The efficacy of behavioral interventions to modify dietary fat and fruit and vegetable intake: a review of the evidence. Prev Med. 2002;35(1):25–41. doi: 10.1006/pmed.2002.1028. [DOI] [PubMed] [Google Scholar]
- Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: a social cognitive theory. Prentice Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Campbell MK, DeVellis BM, Strecher VJ, Ammerman AS, DeVellis RF, Sandler RS. Improving dietary behavior: the effectiveness of tailored messages in primary care settings. Am J Public Health. 1994;84(5):783–787. doi: 10.2105/ajph.84.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlin PR, Chow D, Miller ER, Svetkey LP, Lin PH, Harsha DW, et al. The effect of dietary patterns on blood pressure control in hypertensive patients: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Am J Hypertens. 2000;13(9):949–55. doi: 10.1016/s0895-7061(99)00284-8. [DOI] [PubMed] [Google Scholar]
- Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31(2):193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7. doi: 10.1186/1479-5868-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- Kristal AR, Glanz K, Tilley BC, Li S. Mediating factors in dietary change: understanding the impact of a worksite nutrition intervention. Health Educ Behav. 2000;27(1):112–125. doi: 10.1177/109019810002700110. [DOI] [PubMed] [Google Scholar]
- Lavery JF, Clarke VA. Causal attributions, coping strategies, and adjustment to breast cancer. Cancer Nurs. 1996;19(1):20–28. doi: 10.1097/00002820-199602000-00003. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- Maunsell E, Drolet M, Brisson J, Robert J, Deschenes L. Dietary change after breast cancer: extent, predictors, and relation with psychological distress. J Clin Oncol. 2002;20(4):1017–1025. doi: 10.1200/JCO.2002.20.4.1017. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. Guilford Press; New York: 1991. [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. The Guilford Press; New York, NY: 2002. [Google Scholar]
- Milligan GW, Cooper MC. An Examination of Procedures for Determining the Number of Clusters in a Data Set. Psychometrika. 1985;50:159–179. [Google Scholar]
- Natarajan L, Flatt SW, Sun X, Gamst AC, Major JM, Rock CL, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol. 2006;163(8):770–778. doi: 10.1093/aje/kwj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan L, Rock CL, Major JM, Thomson CA, Caan BJ, Flatt SW, Chilton JA, Hollenbach KA, Newman VA, Faerber S, Ritenbaugh CK, Gold E, Stefanick ML, Jones LA, Marshall JR, Pierce JP. On the importance of using multiple methods of dietary assessment. Epidemiology. 2004;15(6):738–45. doi: 10.1097/01.ede.0000135178.36362.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman VA, Thomson CA, Rock CL, Flatt SW, Kealey S, Bardwell WA, et al. Achieving substantial changes in eating behavior among women previously treated for breast cancer--an overview of the intervention. J Am Diet Assoc. 2005;105(3):382–391. doi: 10.1016/j.jada.2004.12.008. quiz 488. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23(6):728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Natarajan L, Sun S, Al-Delaimy W, Flatt SW, Kealey S, et al. Increases in plasma carotenoid concentrations in response to a major dietary change in the women’s healthy eating and living study. Cancer Epi Biomarkers Prev. 2006;15(10):1886–92. doi: 10.1158/1055-9965.EPI-05-0928. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Newman VA, Flatt SW, Faerber S, Rock CL, Natarajan L, et al. Telephone counseling intervention increases intakes of micronutrient-and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. J Nutr. 2004;134(2):452–8. doi: 10.1093/jn/134.2.452. [DOI] [PubMed] [Google Scholar]
- Rock CL, Flatt SW, Wright FA, Faerber S, Newman V, Kealey S, et al. Responsiveness of carotenoids to a high vegetable diet intervention designed to prevent breast cancer recurrence. Cancer Epidemiol Biomarkers Prev. 1997;6(8):617–623. [PubMed] [Google Scholar]
- Sahay TB, Ashbury FD, Roberts M, Rootman I. Effective components for nutrition interventions: a review and application of the literature. Health Promot Pract. 2006;7(4):418–427. doi: 10.1177/1524839905278626. [DOI] [PubMed] [Google Scholar]
- Salminen EK, Lagstrom HK, Heikkila S, Salminen S. Does breast cancer change patients’ dietary habits? Eur J Clin Nutr. 2000;54(11):844–848. doi: 10.1038/sj.ejcn.1601103. [DOI] [PubMed] [Google Scholar]
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control. 1991;2(6):427–442. doi: 10.1007/BF00054304. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Perkins-Porras L, Rink E, Hilton S, Cappuccio FP. Psychological and social predictors of changes in fruit and vegetable consumption over 12 months following behavioral and nutrition education counseling. Health Psychol. 2004;23(6):574–81. doi: 10.1037/0278-6133.23.6.574. [DOI] [PubMed] [Google Scholar]
- Stewart D, Cheung A, Duff S, Wong F, McQuestion M, Cheng T, et al. Attributions of cause and recurrence in long-term breast cancer survivors. Psychooncology. 2001;10:179–183. doi: 10.1002/pon.497. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Flatt SW, Rock CL, Ritenbaugh C, Newman V, Pierce JP. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J Am Diet Assoc. 2002;102(6):801–808. doi: 10.1016/s0002-8223(02)90180-x. [DOI] [PubMed] [Google Scholar]
- Urban N, White E, Anderson GL, Curry S, Kristal AR. Correlates of maintenance of a low-fat diet among women in the Women’s Health Trial. Prev Med. 1992;21(3):279–91. doi: 10.1016/0091-7435(92)90027-f. [DOI] [PubMed] [Google Scholar]
- USDHHS Healthy People 2010 (Conference Edition, in Two Volumes) 2000 Retrieved May 17, 2006, from http://www.healthypeople.gov/Publications/
- Wayne SJ, Lopez ST, Butler LM, Baumgartner KB, Baumgartner RN, Ballard-Barbash R. Changes in dietary intake after diagnosis of breast cancer. J Am Diet Assoc. 2004;104(10):1561–1568. doi: 10.1016/j.jada.2004.07.028. [DOI] [PubMed] [Google Scholar]
- WCRF . Food, nutrition and the prevention of cancer: A global perspective. World Cancer Research Fund, American Institute for Cancer Research; Washington DC: 1997. [DOI] [PubMed] [Google Scholar]
- West IC. Radicals and oxidative stress in diabetes. Diabet Med. 2000;17(3):171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- WHI. The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996;64(1):202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]