Abstract
The diploid hybrid species Helianthus deserticola inhabits the desert floor, an extreme environment relative to its parental species Helianthus annuus and Helianthus petiolaris. Adaptation to the desert floor may have occurred via selection acting on transgressive, or extreme, traits in early hybrids between the parental species. We explored this possibility through a field experiment in the hybrid species’ native habitat using H. deserticola, H. annuus, H. petiolaris, and two populations of early-generation (BC2) hybrids between the parental species, which served as proxies for the ancestral genotype of the ancient hybrid species. Character expression was evaluated for each genotypic class. Helianthus deserticola was negatively transgressive for stem diameter, leaf area, and flowering date, and the latter two traits are likely to be advantageous in a desert environment. The BC2 hybrids contained a range of variation that overlapped these transgressive trait means, and an analysis of phenotypic selection revealed that some of the selective pressures on leaf size and flowering date, but not stem diameter, would move the BC2 population toward the H. deserticola phenotype. Thus, H. deserticola may have originated from habitat-mediated directional selection acting on hybrids between H. annuus and H. petiolaris in a desert environment.
Keywords: natural selection, adaptation, Helianthus, hybridization, speciation, transgressive segregation
The processes of speciation and its counterpart, hybridization, are of central importance to our understanding of evolution. Hybridization and hybrid zones have long been studied because of their potential to inform us about species boundaries and permanence (e.g., Anderson 1948b; Arnold 1997; Barton 2001). Hybrid zones can also provide unique insight into the speciation process through the production of hybrid species. Many species that result from hybridization events are polyploid, but some are diploid hybrid species with the same number of chromosomes as the parental species (reviewed in Rieseberg 1997). These diploid, or homoploid, hybrid species are of interest not only because of their novel origins, but also because they are highly amenable to experimental studies of the speciation process. For most other taxa, an understanding of speciation can be gained only through studies of modern-day species and their close relatives. Homoploid hybrid species offer a marked advantage in that it is possible to approximate the ancestral genotype and phenotype simply by hybridizing the parental species (Rieseberg et al. 1996, 2003; Lexer et al. 2003a, 2003b).
In general, speciation might result from genetic drift, natural selection, polyploidy, hybridization, or a combination of any of these factors. The relative importance of each element in speciation events is frequently argued, but natural selection resulting from divergent ecological pressures is acknowledged as an important force in plant speciation (Hodges and Arnold 1994; Fulton and Hodges 1999; Schemske and Bradshaw 1999; Levin 2000; Bradshaw and Schemske 2003). Diploid hybrid speciation events are no exception; although the chromosomal differences between early hybrids and their parental species may provide partial reproductive isolating barriers, ecological divergence appears to be essential for the establishment a new lineage (Buerkle et al. 2000). Habitat differentiation is especially important in light of the initially precarious position of hybrid neospecies. Hybrids are necessarily in close physical proximity to at least one parental species, so their early evolution is essentially a form of parapatric or sympatric speciation. Strong ecological selection can alleviate direct competition between hybrids and the parental species while simultaneously allowing for genetic divergence in the face of gene flow with the progenitors. Studying the role of selection in hybrid speciation may be especially important because the process of differentiation is thought to be quite rapid. Estimates based on the genomic architecture of an ancient hybrid species suggest that the speciation process may occur in as little as 60 generations (Ungerer et al. 1998), and simulations predict a similarly brief interval of around 50 generations (McCarthy et al. 1995; Ungerer et al. 1998; Buerkle et al. 2000). Thus, the ecological divergence of a hybrid species must occur quickly if it is to play a role in the speciation process, and ecological selection must be strong. The selective divergence of hybrid neospecies can be studied with relative ease by examining patterns of phenotypic selection in populations of contemporary synthetic hybrids between parental species.
Some cases of ecological divergence between hybrids and their progenitors appear to result from a recombination of parental traits that allow hybrids to colonize intermediate habitats (Anderson 1948a). Other hybrid species, however, are found in environments that are unique compared to the environments of the parental species (Rieseberg et al. 2003). Such habitats likely require the acquisition of novel traits relative to progenitors, and the origin of these traits is of interest in the context of adaptive evolution and speciation. It has been proposed that these traits are the product of directional selection acting on extreme phenotypes present in early-generation hybrids (Rieseberg et al. 1999; Schwarzbach et al. 2001; Lexer et al. 2003a, 2003b). Trait values found in segregating hybrids often exceed the phenotypes of both parents in either a positive or negative direction. These extreme or transgressive trait values are common in both natural hybrid populations and controlled crosses and are thought to result from the complementary action of alleles from two divergent parents, that is, the “stacking” of alleles with the same directional effect on a given trait (deVincente and Tanksley 1993). Note that these new phenotypes are heritable (unlike those that result from heterosis), and selection on transgressive individuals during a speciation episode could easily generate a hybrid species with a new, extreme phenotype for a given trait.
Several lines of evidence would support the supposition that transgressive segregation was important in the formation of a hybrid species. First, the hybrid species in question should possess traits that are transgressive relative to parental species. Second, contemporary hybrids between parental species must contain a range of variation that encompasses the mean phenotype of the existing hybrid species. Third, habitat-mediated selection on hybrids between parental species must act in the direction of the hybrid species.
Helianthus deserticola, a confirmed diploid hybrid species, has several novel traits that may be the product of directional selection in an extreme environment. Evidence from isozymes, chloroplast DNA, and nuclear ribosomal DNA indicate that the parental species are Helianthus annuus and Helianthus petiolaris (Rieseberg 1991). The three species share an annual outcrossing life history, and all have a haploid chromosome number of 17, but they are divergent in their habitat preferences. Helianthus annuus is distributed throughout the central and western United States and typically inhabits heavy, clay-based soils. Helianthus petiolaris, the smaller of the two parental species, is distributed mainly through the central United States and inhabits sandier soils than H. annuus. Helianthus deserticola is a xerophytic species found in sandy soils of the desert floor and is restricted to small populations located in western Nevada, west central Utah, and along the border of Utah and Arizona (Heiser 1947; Heiser et al. 1969; Rogers et al. 1982).
A recent greenhouse study revealed that H. deserticola is transgressive for several traits that are predicted to confer a fitness advantage in a desert environment (Rosenthal et al. 2002). The hybrid species flowers earlier than either parental species, has smaller leaves, and also takes up less boron than either parent. Although no fitness comparisons could be made in the greenhouse, all of these traits are associated with desert-adapted plants, suggesting that they may have been selected for as early hybrids colonized the desert floor. In this study, we focus on the following questions in accordance with the requirements proposed above: What traits are transgressive for H. deserticola in the field? Does the variation present in hybrids between H. annuus and H. petiolaris encompass the current H. deserticola phenotype? How is selection acting on traits in the desert environment, especially those that are currently transgressive in H. deserticola?
Material and Methods
Field Site
The experiment was conducted in the Little Sahara Recreation Area, Utah, administered by the Utah State Office of the Bureau of Land Management. Helianthus deserticola occurs naturally in the Little Sahara, and the experimental plot at 281.3°N, 112.3°W was located several meters from wild individuals. Annual rainfall at the park averages 298 mm, although annual rainfall during the year of the experiment (2002) was much lower, at only 120 mm (Western Regional Climate Center 2003).
Populations
Five genotypic classes were employed in the experiment: Helianthus annuus, Helianthus petiolaris, their hybrid derivative H. deserticola, and experimental second-generation backcross (BC2) populations crossed toward H. annuus (BC2Ann) and H. petiolaris (BC2Pet), respectively. Achenes of H. deserticola and the two parental species were collected from wild populations: ANN1308 (51.5 km east of Kanab, Kane County, Utah), PET1324 (42 km southeast of Page, Coconino County, Ariz.), and DES1321 (Little Sahara Recreation Area, 6.8 km east of visitor center, Juab County, Utah). The H. deserticola population was selected because of its geographic proximity to the field site, whereas the pure parental populations were in close proximity to the parental populations employed in experimental crosses ANN1295 and PET1277 (locality data in Lexer et al. 2003a). The BC2 populations were initiated by backcrossing a single F1 plant to a single individual each from the PET1277 and ANN1295 populations. Thirty-eight BC1Pet progeny were generated and crossed to a third individual from PET1277 to generate the BC2Pet population. Likewise, 54 BC1Ann progeny were generated and crossed to a third individual from ANN1295 to generate the BC2Ann population. This crossing design was chosen to facilitate quantitative trait locus (QTL) analysis but has the possible drawback of confounding inter- and intraspecific variation; that is, some of the phenotypic variation observed in the BC2 populations is specific to the individuals in the original cross rather than to interspecific differences. Although outbreeding depression is a concern for some interspecific crosses, there was no evidence of hybrid inviability in greenhouse-grown plants from the same cross (Rieseberg et al. 2003; L. H. Rieseberg, unpublished data). That is, the greenhouse BC2’s did not differ significantly from parental species for all fitness-related traits measured, including plant height, shoot biomass, flower biomass, and relative growth rate. Possibly, outbreeding depression was reduced because of purging prior to the BC2 generation (Rieseberg et al. 1996). Regardless of the explanation, phenotypic character expression in this cross seems unlikely to be strongly influenced by this phenomenon.
Plant Propagation
Plants were propagated in the Indiana University greenhouse to ensure maximum sample size for DNA extraction and future QTL analysis of the BC2 populations. Although planting seeds directly into the field would have provided important information regarding early selection pressures, individuals subject to such selection may have failed to germinate or produce true leaves and would therefore have been underrepresented during future genetic work.
Helianthus annuus and BC2Ann individuals have a rapid germination and growth rate as compared with H. deserticola, H. petiolaris, and BC2Pet individuals. While these germination and growth rates may be advantageous for the different species in natural settings, they result in large size differences if the germination protocol is initiated simultaneously in a laboratory setting. Thus, germination was staggered according to growth rate to insure that all individuals were of a similar size at the time of planting. Germination of H. petiolaris and H. deserticola was initiated on April 2, germination of BC2Pet was initiated on April 4, germination of BC2Ann was initiated on April 8, and germination of H. annuus was initiated on April 9.
Achenes were soaked for 10 min in a 2% bleach, 1% Triton-X solution for sterilization and then rinsed with distilled water. After removing the blunt end of each achene, seeds were placed on moist filter paper inside a petri dish and left in darkness overnight. The fruit wall was removed the following day, and seeds were transferred to clean, moist filter paper. Seeds were transferred to clean, moist filter paper daily and kept in darkness until the hypocotyls began to elongate. At this point, the seedlings were moved into natural light and were planted when the cotyledons began to green. The filter paper was moistened with ddH2O for the H. annuus, H. petiolaris, BC2Ann, and BC2Pet populations. Helianthus deserticola is exceptionally difficult to germinate (Heiser et al. 1969), so a 200-ppm solution of gibberellic acid was used in place of the pure water to promote germination. All populations were planted in a 50:50 sand/soil mixture. Eighty-eight 6 × 6 × 10-cm Jiffypots containing a single plant per pot were placed in plastic bins lined with 7 cm of Strong-Lite vermiculite.
Transplantation
On May 8, all plants were placed in a truck for transport to the Little Sahara Recreation Area. Plants arrived on May 10 and were placed outside and watered as necessary to prevent wilting. On May 13, the seedlings were transplanted into the H. deserticola desert floor habitat. The experimental plot was divided into 10 blocks, each of which contained one row of 23 plants and one row of 24 plants for a total of 55 plants per block and an overall total of 550 plants in the garden. Each block contained 24 BC2Ann, 23 BC2Pet, four H. deserticola, two H. annuus, and two H. petiolaris arranged in a regular pattern. In sum, 240 BC2Ann, 230 BC2Pet, 40 H. deserticola, 20 H. annuus, and 20 H. petiolaris seedlings were planted on May 13. Blocks were spaced 1 m apart, and plants within the blocks were spaced at a distance of 0.4 m. Individual seedlings were chosen haphazardly from each genotypic class and planted in assigned positions within blocks. The garden was fenced using both barbed wire and plastic fencing to deter cattle and vehicles. Plants were watered immediately after transplantation and were watered daily until May 20. Dead plants were replaced on May 14 and 15, and we assumed that death was a result of transplantation shock. No further replacements were made, and selection started on May 16.
Measurements
Leaf measurements were made on the most recent fully expanded leaf between June 18 and 19. Branching and absence of apical dominance in wild Helianthus make it impossible to collect leaves from a standard position after the first weeks of development. Collecting the most recent fully expanded leaf from each plant is therefore the best alternative. Note that this method was employed in a previous greenhouse-based QTL study (Rosenthal et al. 2002; Rieseberg et al. 2003), which successfully identified up to five significant QTLs for individual leaf traits. This result implies that heritable variation in leaf traits is not fully masked by this collection strategy. Also, because growth and flowering are indeterminate in Helianthus, collecting leaves before or after the date of first flowering yields comparable information. Leaves were collected from each plant before sunrise to insure the leaf was maximally hydrated, and they were measured for wet weight and area. Leaves were then dried at 60°C for a minimum of 24 h before dry weight was assessed. Leaf succulence was calculated as [(fresh wt. − dry wt.)/fresh wt.] × 100 to provide an estimate of the percentage of water in the leaf tissue.
Stem diameter was measured on July 9. Plants were checked weekly for the date of floral initiation. Flowering heads were collected as ligules senesced and achenes became mottled, indicating maturity. Mortality was surveyed on a weekly basis, and aboveground biomass was collected after senescence. Surviving plants were harvested on September 4 at the termination of the experiment. All flowering heads and harvested plants were dried for a minimum of 24 h at 60°C before they were weighed for a measure of aboveground biomass. Total number of flowering heads was calculated based on heads collected before harvest and heads present on the plants at harvest. Because heads were collected after achenes were fully mature and the heads had senesced, it seems unlikely that collection throughout the season would affect overall head production. Even if it did, this is unlikely to adversely influence data analysis because all plants were treated identically (i.e., all plants were subject to continuous head removal).
Elemental concentration (P and B) was assessed for leaves from the BC2Pet population after measurements were completed. Elemental concentration was determined by means of inductively coupled argon plasma spectrometry (Midwest Laboratories, Omaha, Nebr.). Due to the high cost of analysis, elemental concentration for the BC2Ann population was not determined.
Data Analysis
Basic statistics such as trait means and variances were calculated using SPSS (SPSS 2002). The H. deserticola population trait means were compared with those of the parental species, and H. deserticola means that exceeded both parental means (in either a positive or negative direction) were considered for further analysis. Traits were transgressive in the hybrid species if they differed significantly from the closer parental mean based on the nonparametric Mann-Whitney U-test.
Bivariate correlations between pairs of traits were calculated in SPSS (SPSS 2002) using Pearson’s correlation coefficient in order to evaluate the degree of phenotypic integration among traits. Also, a principal component analysis (PCA) was performed on the trait correlation matrix using the PCA module of ADE-4 (Thioulouse et al. 1997). The first two principal components were graphed on a correlation circle using the Scatter module of ADE-4.
Directional selection was measured in the BC2 populations according to the methods of Lande and Arnold (1983). All phenotypic traits were standardized to a mean of 0 (SD = 1). No other transformations were applied because data did not violate the distributional assumptions of multiple regressions. Absolute fitness measures, in the form of flowering head number, were converted to relative fitness measures (fitness was assigned to each plant based on head number, regardless of mortality date). While seed number may provide a more accurate measure of fitness than head number, the labor involved in counting seeds is prohibitive, and the two measures have been found to be highly and tightly correlated (F. Ludwig, unpublished data). The BC2Ann and BC2Pet crosses were analyzed separately due to large differences in mean phenotypes between the populations. Standardized directional selection differentials were estimated as the covariance between relative fitness and the standardized trait of interest. Significance levels were assigned by nonparametric Spearman rank correlations of relative fitness and the standardized trait of interest. Standardized directional selection gradients were estimated using a multiple regression analysis with relative fitness as the dependent variable and standardized traits as covariates (“Regression” module, “Linear” option in SPSS). The gradients were estimated as the partial regression coefficients from these regressions, and significance levels were assigned using parametric t-tests.
Results
The parental species, Helianthus annuus and Helianthus petiolaris, showed comparable survival in the desert environment. In both cases, 15 of 20 individuals (75%) survived to the end of the experiment on September 4. In contrast, only 21 of the 40 Helianthus deserticola individuals (48%) survived until September 4. Survival of the BC2 populations were similar to those in parental populations; 75% of the BC2Ann individuals and 85% of the BC2Pet individuals survived to until September 4 (fig. 1). The average numbers of heads produced by H. annuus, H. petiolaris, and H. deserticola individuals were 8.3, 17.2, and 9.7, respectively. The average numbers of heads produced by BC2Ann and BC2Pet individuals were 7.6 and 35.2, respectively.
Figure 1.
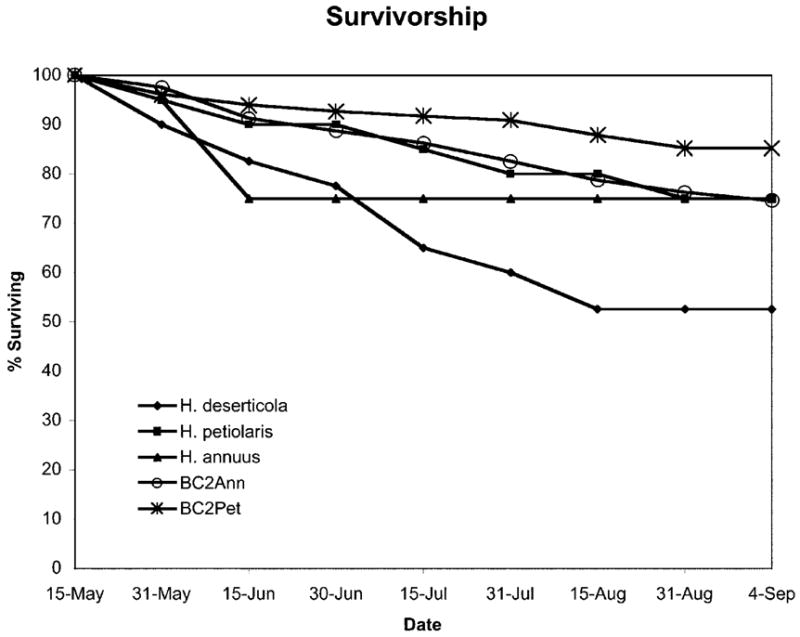
Survival of Helianthus deserticola, Helianthus petiolaris, Helianthus annuus, BC2Ann, and BC2Pet populations over the course of the experiment (May 15–September 4). Survival is shown as percentage individuals living at dates indicated by tick marks.
Helianthus deserticola displayed several transgressive traits when compared with the parental species, based on the nonparametric Mann-Whitney U-test (table 1). Note that classifying a trait as transgressive defines only the relationship between trait means in a progenitor species and its hybrid derivative and does not indicate how a trait evolved (i.e., a transgressive trait may evolve through transgressive segregation or through the accumulation of adaptive mutations). In the field, H. deserticola was negatively transgressive for leaf area, stem diameter, and flowering date, while succulence was intermediate. These patterns of trait expression corresponded to a previous greenhouse study of character expression where leaf area and flowering date were also negatively transgressive in H. deserticola compared with parental species (Rosenthal et al. 2002). The intermediate trait, leaf succulence, was not transgressive in the greenhouse study. Stem diameter was negatively transgressive in this study but not in the greenhouse study. The phenotypic range of the BC2 populations overlapped the mean phenotype of H. deserticola for all traits (table 1; fig. 3).
Table 1.
Trait means and flowering head number for Helianthus annuus, Helianthus deserticola, Helianthus petiolaris, BC2Ann, and BC2Pet populations in the field
| Trait | H. annuus (n = 15) | H. deserticola (n = 26) | H. petiolaris (n = 15) | BC2Ann (n = 201) | BC2Pet (n = 193) | Fielda | Greenhouseb |
|---|---|---|---|---|---|---|---|
| Succulence (no units) | 79.9 (.6) | 81.7 (.4) | 82.8 (.7) | 79.8 (.2) | 82.8 (.2) | Intermediate | Ann/Pet-like |
| 73.6–82.6 | 78.1–86.2 | 77.0–86.2 | 73.8–84.7 | 77.1–87.6 | |||
| Leaf area (cm2) | 13.02 (1.3) | 4.41 (.50)* | 8.31 (1.0) | 12.73 (.50) | 7.92 (.21) | Negatively transgressive | Negatively transgressive |
| 7.11–23.39 | 1.21–11.48 | 2.78–17.8 | 2.11–42.48 | 1.94–15.00 | |||
| Stem diameter (cm) | 5.95 (.28) | 3.41 (.17)* | 5.08 (.40) | 4.94 (.10) | 5.78 (.13) | Negatively transgressive | Pet-like |
| 4.28–7.89 | 1.93–5.19 | 1.71–8.41 | 1.65–10.92 | 1.23–12.08 | |||
| Flowering date (d) | 48.9 (2.1) | 30.9 (2.0)* | 47.5 (3.0) | 49.5 (.7) | 34.3 (.93) | Negatively transgressive | Negatively transgressive |
| 39–74 | 12–51 | 19–65 | 22–99 | 8–92 | |||
| Phosphorus (%) | .111 (.006) | .153 (.012) | .129 (.007) | … | .121 (.004) | Pet-like | Pet-like |
| .07–.15 | .06–.26 | .09–.19 | .03–.36 | ||||
| Boron (ppm) | 169.6 (13.8) | 172.2 (11.3) | 155.0 (7.3) | … | 98.4 (3.6) | Ann-like | Negatively transgressive |
| 96–248 | 91–327 | 116–220 | 15–375 | ||||
| Head number | 8.3 (1.8) | 9.7 (1.5) | 17.2 (3.4) | 7.6 (.7) | 35.2 (1.9) | … | … |
| 0–29 | 0–33 | 0–52 | 0–86 | 0–159 |
Note: The trait values in bold are transgressive in H. deserticola compared with parental species. Transgression was tested only if the trait mean for H. deserticola exceeded both parental means and H. deserticola was then compared to the closest parental species. SEs are in parentheses, followed by the range of each phenotypic trait.
This column shows the direction of transgression.
This column gives the direction of statistically significant transgression in a previous greenhouse study; traits that are Ann/Pet-like or Pet-like were not transgressive (Rosenthal et al. 2002).
P ≤ .001 based on nonparametric Mann-Whitney U-tests comparing the mean of H. deserticola to that of closer parent.
Figure 3.
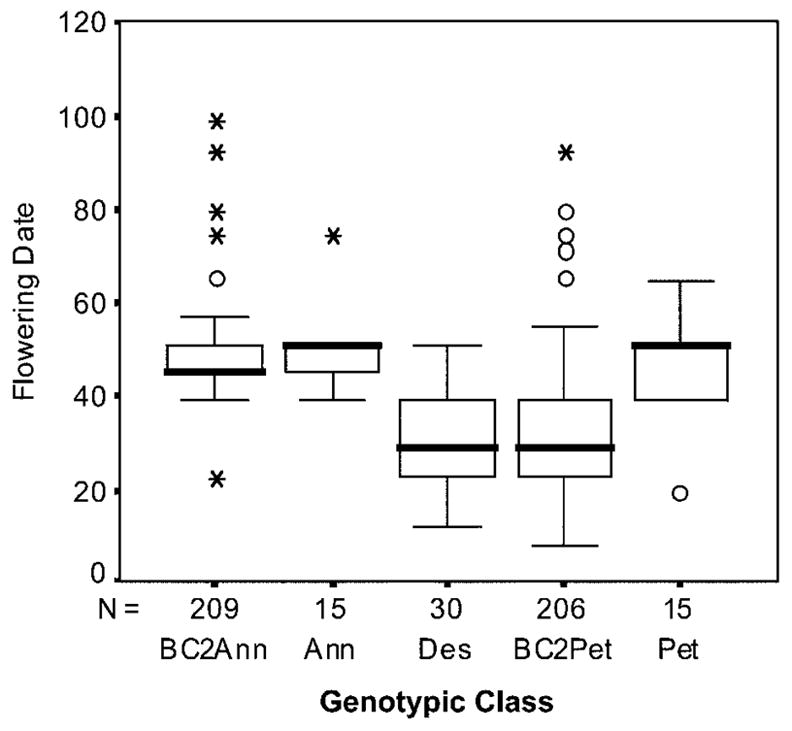
Boxplot showing the distribution of flowering date for the five populations included in the experimental garden (Helianthus deserticola, Helianthus annuus, Helianthus petiolaris, the BC2Ann population, and the BC2Pet population). Mean is shown with a heavy bar, the box represents the interquartile range, and bars represent the largest observation within 1.5 interquartile ranges from the top or bottom of the box. Circles represent outliers and asterisks represent extremes.
Several traits included in the analysis were significantly correlated; the highest correlation was between stem diameter and leaf area in the BC2Ann population (0.762; table 2). All significant correlations were in the same direction in both populations. Circle graphs representing the PCA analyses reveal that traits are more highly correlated in the BC2Pet population than in the BC2Ann population (fig. 2). Interestingly, flowering date and boron concentration appear to be highly correlated according to the PCA despite the fact that the numerical correlation is only 0.052 (not statistically significant). However, the arrow representing flowering date is very short, indicating that the trait is not well represented by the two principal components shown in this graphic. Stem diameter and leaf area are highly correlated in both populations.
Table 2.
Bivariate correlations between phenotypic traits in the two hybrid populations
| Succulence | Leaf area | Stem diameter | Flowering date | Phosphorous | Boron | |
|---|---|---|---|---|---|---|
| Succulence | 1 | .426*** | .564*** | −.349*** | … | … |
| Leaf area | .433*** | 1 | .762*** | −.452*** | … | … |
| Stem diameter | .631*** | .583*** | 1 | −.476*** | … | … |
| Flowering date | .064 | −.024 | −.400*** | 1 | … | … |
| Phosphorus | −.007 | −.180** | −.112 | .066 | 1 | … |
| Boron | −.179** | −.189** | −.199** | .052 | .585*** | 1 |
Note: The BC2Ann population is shown above the diagonal, and the BC2Pet is shown below the diagonal.
P ≤.05.
P ≤.01.
P ≤.001.
Figure 2.
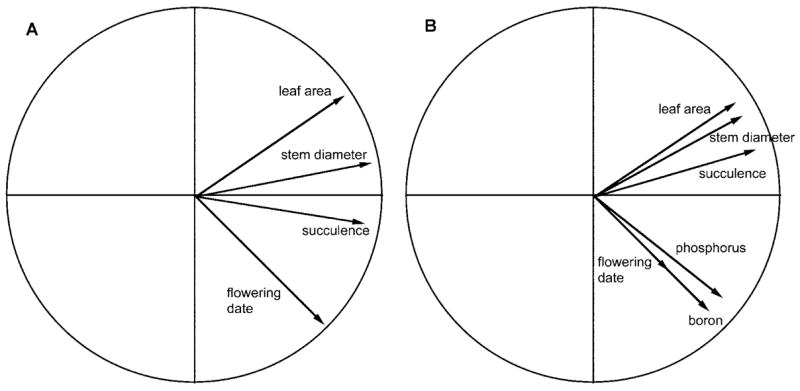
Correlations between phenotypic traits in the BC2 populations. A, BC2Ann. B, BC2Pet. The correlation circle was obtained by a principal component analysis of the trait correlation matrix. The X- and Y-axes correspond to the first two principal components. Traits are depicted as arrows, with the angle between arrows expressing the strength of the correlation (small angle = strong correlation) and the length of each arrow symbolizing the degree to which a particular trait is represented by the first two principal components.
Measures of covariance between standardized traits and relative fitness yielded statistically significant selection differentials for all traits measured in both crosses, with the exception of leaf phosphorus concentration (table 3). Differentials were positive for leaf area, leaf succulence, and stem diameter in both populations. Differentials for flowering date and leaf concentrations of phosphorus and boron were negative.
Table 3.
Standardized selection differentials and gradients for the BC2Ann and BC2Pet populations
| Differential | Gradient (SE) | |
|---|---|---|
| BC2Ann: | ||
| Succulence | .474*** | .0566 (.096)+ |
| Leaf area | .454*** | −.316 (.123)** |
| Stem diameter | .801*** | 1.083 (.135)*** |
| Flowering date | −.161*** | .229 (.091)** |
| BC2Pet: | ||
| Succulence | .259*** | −.209 (.048)*** |
| Leaf area | .303*** | −.0981 (.043)* |
| Stem diameter | .621*** | .877 (.060)*** |
| Flowering date | −.229*** | .122 (.042)** |
| Phosphorus | −.150 | .0995 (.042)* |
| Boron | −.125** | −.0759 (.42)a |
P ≥.10.
P ≤.05.
P ≤.01.
P ≤.001.
P = .07.
Selection gradients were derived from the partial regression coefficients of the linear regression of fitness against standardized traits (table 3). Selection gradients for leaf area, stem diameter, and flowering date were statistically significant in both crosses; leaf succulence was also significant in the BC2Pet population. In the BC2Pet cross, the selection gradient for leaf phosphorus concentration was significant, and the gradient for leaf boron concentration was marginally significant (P = .07). Two of the traits that exhibited significant selection differentials did not have statistically significant selection gradients (succulence in the BC2Ann population and boron concentration in the BC2Pet population). Conversely, the gradient for phosphorus concentration was statistically significant, while the differential was not. These changes suggest that significance levels of selection differentials were influenced by selection on correlated characters.
Selection gradients generally had the same sign in the two populations; the gradient for leaf area was negative while the gradients for stem diameter and flowering date were positive in both populations. However, the selection gradient for leaf succulence was positive in the BC2Ann population and negative in the BC2Pet population. Such differences are expected if the phenotypic optimum for succulence level is intermediate relative to the mean phenotypes of the two populations. Alternatively, this difference may not be relevant, given the fact that the selection gradient is nonsignificant and also quite small in the BC2Ann population. Sign changes among selection differentials and gradients within a single trait were also observed; signs for leaf succulence, leaf area, flowering date, and phosphorus all changed signs in at least one of the populations. This change in sign can be attributed to the effects of correlated characters. The magnitude of selection differentials and gradients varied between the populations, as is expected for populations with different fitness means and variance.
Discussion
We proposed that three criteria must be fulfilled in order to argue convincingly that Helianthus deserticola is the product of directional selection on transgressive hybrids. The requirements are that traits in the hybrid species must be transgressive relative to parental species, hybridization between the parental species must produce some individuals with similarly extreme trait values, and selection pressures on such a hybrid population must be in the direction of the hybrid species. In this study, we found that H. deserticola was negatively transgressive for leaf area, flowering date, and stem diameter in the field. The trait variation present in the BC2 populations did overlap the transgressive traits that characterize the hybrid species phenotype. Finally, the negative selection gradient for leaf area and the negative selection differential for flowering date were in accordance with the modern trait values of H. deserticola although the remaining measures of selection were not. The relevance of our study to the origins of this diploid hybrid species is discussed below.
Fitness of Pure Species
A large percentage (75%) of both Helianthus annuus and Helianthus petiolaris individuals survived until the termination of the experiment in September, suggesting that survival on the desert floor is not restricted to the hybrid species, at least within the temporal and spatial scale of this experiment. Indeed, a smaller percentage (48%) of H. deserticola individuals than for either parental species survived until the termination of the experiment (fig. 1). Taken at face value, this result implies that the hybrid species is not well adapted to the desert environment. Helianthus deserticola seedlings failed to thrive in the greenhouse, however, which resulted in the transplantation of weak, etiolated plants into the desert garden. High mortality may have been the result of this poor start rather than an accurate reflection of the effects of habit-specific selection. Furthermore, H. deserticola likely has a more rapid life cycle than its parental species (a phenomenon that is common in desert annuals), or the hybrid species may hold an advantage in terms of germination success and early survival (not documented in this study). In either case, survival to the termination of the experiment would not be an informative fitness proxy and was not used in the selection analysis. Indeed, H. deserticola individuals produced a greater number of heads, on average, than did H. annuus individuals (despite their shorter lives), suggesting that the hybrid species would have greater fitness in the desert habitat.
Transgression in the Hybrid Species
Several traits in H. deserticola were transgressive relative to the parental species in the field. Leaf area, flowering date, and stem diameter were negatively transgressive, while leaf succulence, phosphorus, and boron content were intermediate (table 1). Small leaves and early flowering are both characteristic of desert annuals and are thought to be of adaptive significance. In general, small leaves can reduce water loss and prevent heat stress while early flowering enables seed production before the onset of strong summer drought (Givnish 1979; Fox 1989; Aronson et al. 1992, 1993; Smith et al. 1997; Gibson 1998). Both of these traits were negatively transgressive in a previous greenhouse study, implying strong genetic control (Rosenthal et al. 2002).
The exceedingly slender stems of H. deserticola in the field differed from an earlier greenhouse study where the stem thickness resembled H. petiolaris. While it is possible that expression of this trait may have been influenced by germination procedures for H. deserticola (see above), plants in the field also have slender stems and a small stature (B. Gross, personal observation), so this result cannot be considered aberrant. Helianthus deserticola did not differ for leaf boron concentration compared to parental species even though it was negatively transgressive for this trait in the greenhouse (Rosenthal et al. 2002). Differences between trait expression in the greenhouse and the field are not surprising given that the diploid hybrid species Helianthus paradoxus also showed striking differences in trait expression between the two environments (Lexer et al. 2003b). Such findings accentuate the importance of G × E interactions and of studying trait expression under natural conditions. Overall, the transgressive aspects of the H. deserticola phenotype indicate that its origin required the acquisition of novel traits, not just the combination of parental features.
Trait Variation in Artificial Hybrids
The range of variation in both the BC2Pet and BC2Ann populations overlapped the phenotype of the hybrid species for every trait in this study, indicating that it would be possible to derive the H. deserticola phenotype from a backcross hybrid population. Both of the BC2 populations contained individuals that overlapped the mean H. deserticola phenotype for leaf succulence, leaf area, flowering date, and stem diameter (table 1). However, only the BC2Pet population contained individuals with flowering dates as early as the bulk of the H. deserticola individuals (fig. 3). The phosphorus and boron content of the BC2Pet population also overlapped the mean levels of mineral content in H. deserticola.
Based on the differences in flowering date between the two crosses, the BC2 toward H. petiolaris appears to be the best approximation of the modern H. deserticola phenotype. Indeed, several of the BC2Pet individuals had both small leaves and an early flowering date; the average flowering date of BC2Pet individuals with leaves as small as those in the hybrid species is 32.4 days, a figure very close to the mean flowering date for H. deserticola of 30.9 days. This level of phenotypic integration for extreme traits exceeds those found in a previous selection experiment (Lexer et al. 2003b), although recombination among genotypes in a hybrid population would no doubt be necessary to produce a phenotype that resembled H. deserticola across all traits. Combined with the extensive overlap between the BC2Pet cross and H. deserticola, it seems that generating the phenotype of the hybrid species would not only be possible but also rather simple.
As noted in “Material and Methods,” the crossing design used to generate these BC2 populations was limited to a small number of founding individuals in order to facilitate future QTL mapping, and thus some of the patterns we observe in this study may be specific to the genotypes employed in the original crosses. While this situation is regrettable, it has the effect of making the results from the experiment quite conservative; that is, it seems unlikely that the individuals chosen to generate the crosses would be the only ones to harbor the genetic variation necessary to produce transgressive phenotypes overlapping those of the hybrid species. The requirement that the range of variation the BC2 hybrids overlap the mean phenotype of the hybrid species is also rather conservative in that transgressive segregation in the hybrids might be responsible for only a portion of the ultimate phenotype, with gradual adaptive mutations taking the hybrid species the rest of the distance. However, this requirement is appropriate if speciation and adaptation is thought to be rapid, as is the case for diploid hybrid species (McCarthy et al. 1995; Ungerer et al. 1998).
Alternatively, it is possible that phenotypic expression in the BC2 hybrid populations overlapped the H. deserticola phenotype only because it was influenced by environmental variation or outbreeding depression. It is impossible to rule out the effects of environmental variation in field experiments, but previous results from greenhouse experiments have also shown that trait variation of BC2 hybrids overlaps the H. deserticola phenotype for leaf size, flowering date, and stem diameter (Rieseberg et al. 2003). Likewise, the effects of outbreeding depression are always a concern when dealing with hybrid populations. However, both of the BC2 populations produced either a similar or greater number of seed heads per plant as with parental species (large differences in sample size preclude statistical analysis), and no evidence of reduced hybrid viability was observed in a greenhouse BC2 population (Rieseberg et al. 2003). Both results suggest that outbreeding depression is not adversely affecting plant fitness in these populations. Further, preharvest mortality of the BC2 populations did not exceed mortality of the parental species (fig. 1). Thus, it seems unlikely that trait expression in the BC2 populations was strongly influenced by outbreeding depression.
Note that despite the morphological similarities between the BC2Pet population and the hybrid species, there is no doubt that H. deserticola is a “good species.” Helianthus deserticola, H. petiolaris, and H. annuus are all reproductively isolated from each other due to chromosomal rearrangements that result in low pollen fertility in inter-specific hybrids (Rieseberg 2000). However, crosses between geographically disparate populations of H. deserticola show almost no fertility barriers (Gross et al. 2003). Also, it is noteworthy that extensive fieldwork by the authors has failed to detect natural hybrids between H. deserticola and either of its parental species.
Selection
Selection on transgressive traits was significant and strong, although not always in the expected direction (table 3). Gradients for leaf size and succulence were negative, with the exception of the nonsignificant coefficient for succulence in the BC2Ann population. Selection for small leaves is in accordance with common desert phenotypes, and this selective pressure would move the hybrids toward the H. deserticola phenotype. Selection against succulence is logical, given that the trait is unlikely to be important in the rapid life cycle typical of desert annuals such as H. deserticola (Givnish 1979; Gibson 1998). The mean succulence of the H. deserticola population is lower than that for the BC2Pet population (table 1), so selection to lower succulence would move the population in the direction of the hybrid species. Given a propensity for rapid cycling, one would expect a negative gradient for flowering date. When the effects of stem diameter, leaf size, and leaf succulence are accounted for in the selection gradient, however, selection appears to favor later flowering. The positive sign of this coefficient likely reflects the fact that it is not advantageous to flower early in an unqualified sense but only for plants that also grow quickly or allocate strongly to reproduction.
Of all the traits measured in this study, the occurrence of slender stems in H. deserticola is the most puzzling, given the strong selection that was detected for large stems in the BC2 hybrids. The selection gradient for stem diameter was positive and large in both populations, suggesting strong selection for large stems that would move the hybrid populations away from the H. deserticola phenotype. Clearly, this aspect of the H. deserticola phenotype cannot be explained based on our measures of selective pressures. It is possible that stem size in the hybrid species is constrained either developmentally or genetically or that germination procedures resulted in smaller than optimal stems (although plants in nearby natural populations also had slender stems). It is also noteworthy that the pure populations H. deserticola and H. petiolaris have smaller stems than H. annuus, but both of the former species produced a greater number of heads per plant than did H. annuus (table 1). It therefore appears that relatively larger stems are advantageous in hybrids (perhaps for increased nutrient transport, etc.) but that massive stems are not required for high fitness in the desert environment.
Selection for increased phosphorus concentration in the BC2Pet population is in the direction of H. deserticola and may be a consequence of selective pressures that promote uptake of necessary mineral elements. Selection for reduced leaf boron concentration is in the direction predicted from ecological considerations; the element can be toxic in high concentrations and is a serious problem in arid environments (Gupta et al. 1995; Jefferies et al. 1999, 2000). However, the mean boron concentration for H. deserticola in the desert garden is actually higher than in the BC2Pet population, so the negative selection observed for this trait would not generate the H. deserticola phenotype.
It is also interesting to consider the selection differentials, a measure of selection that does not correct for the effects of correlated traits (table 3). The differential for leaf succulence was positive, while the differential for flowering date was negative; both are in the direction that would be predicted for survival in a desert environment. In contrast, the differential for leaf area was positive despite the fact that large leaves would likely be detrimental in an arid habitat. Selection differentials for stem diameter and boron concentration were positive and negative, respectively, and in the same direction as the gradients.
A prominent pattern in this study was sign changes between the selection gradients and differentials, something that occurs frequently in selection analyses (Lande and Arnold 1983; Kingsolver et al. 2001). This situation means that the ultimate outcome of selection depends on the genetic architecture underlying trait variation. In the absence of pleiotropy, selection can act on each trait independently, and only the selection gradient need be taken into account when considering long-term selection response. Although it may be appropriate to ignore genetic correlations in populations close to linkage equilibrium and with minimal pleiotropy, early-generation hybrids exhibit extreme linkage disequilibrium, necessitating careful consideration of correlated characters. Thus, hybrid speciation likely represents a situation in which selection differentials provide the most accurate measure for predicting the ultimate outcome of natural selection.
The potential importance of linkage disequilibrium and pleiotropy are underscored by current genetic maps of the hybrid species, which confirm that the genome of H. deserticola is a combination of large chromosomal segments from the two parents (Rieseberg et al. 2003). This structure renders each chromosomal segment into a single pleiotropic unit and reduces the potential for natural selection to act on characters independently. Strong selection on any trait controlled by loci within a particular chromosomal block might easily drag linked alleles to fixation in the neospecies. Helianthus deserticola’s unique genetic composition requires that both measures of selection be given equal consideration in reference to the origin of the species.
Implications
This study was designed to increase our understanding of the role of ecological selection in the early stages of homoploid hybrid speciation. The diploid hybrid species in this article, H. deserticola, is of particular interest because it was previously shown to be transgressive for several traits that are characteristic of desert-adapted plants. By transplanting pure parental species, the ancient hybrid species H. deserticola and synthetic BC2 hybrids into the native habitat of H. deserticola, we are able to make inferences regarding the critical traits and the role of habitat-mediated selection in this speciation event. Three traits were found to be negatively transgressive for H. deserticola in the field; leaf area, flowering date, and stem diameter. The BC2 populations contained a range of variation that overlapped the mean H. deserticola phenotype for all three of these traits, and the BC2Pet population most closely matched the H. deserticola phenotype. Although selection gradients and differentials sometimes differed in sign, at least some measures of selection favored BC2 hybrids resembling H. deserticola for leaf area and flowering date. Our findings indicate that several of the extreme aspects of the modern H. deserticola phenotype could have originated via ecological selection acting on hybrids in the desert floor habitat.
While a focus on ecological factors makes sense given the habitat differentiation among the five hybrid and parental Helianthus species, past work has shown that fertility selection alone generates a genomic composition that is quite similar to that of Helianthus anomalus, one of the three hybrid species (Rieseberg et al. 1996). If fertility selection were the only important force determining genomic content, we might not expect the three hybrid species to be so distinct, differing in morphology, habitat preference, and karyotype (Chandler et al. 1986). Presumably, genomic composition is also shaped by strong habitat-mediated selection, which contributes to modern species differences. Indeed, Rieseberg et al. (2003) demonstrate that certain aspects of the genomic composition of H. anomalus may be accounted for by phenotypic rather than fertility selection. Moreover, genome content in H. deserticola can be predicted by the QTL analyses of synthetic hybrids (Rieseberg et al. 2003), implying an important role for phenotypic selection in this species as well. Thus, this study details one of two strong selective episodes known to shape H. deserticola genome content and evolution.
Perhaps the most interesting result of this study is the finding that the H. deserticola phenotype could be so easily recreated from a BC2Pet population, a result that accords well with an earlier greenhouse-based study (Rieseberg et al. 2003). In a previous article, we documented patterns of cpDNA and microsatellite variation in H. deserticola and parental species that were consistent with multiple origins of the hybrid species (Gross et al. 2003). The current experiment supports the possibility of multiple origins because the phenotypic variation required to produce H. deserticola is likely to be present in nearly any hybrid zone where backcrosses toward H. petiolaris are present. The parental populations used in this study were from the southwestern U.S.A., near or within the geographic range of H. deserticola. While this is thought to be the native range of both parental species, both parents are now found throughout a large part of the U.S.A. (Heiser et al. 1969). Hybrids between parental individuals from more mesic areas might not show the same degree of transgressive segregation toward a desert-adapted phenotype documented for this cross. Future studies will assess the impact of intraspecific variation in the parental species on conclusions about the role of transgressive segregation in ecological divergence and the repeatability of hybrid speciation.
Acknowledgments
The authors would like to thank J. L. Durphy, J. Johnston, J. Lance, C. Morjan, B. Ridenhour, and M. L. Woods for their help with data collection and manuscript preparation. This work was supported by National Science Foundation (NSF) Integrative Graduate Education and Research Traineeship and Predoctoral Fellowships to B.L.G. and N.C.K., NSF grant IBN-0131078 to L.A.D., and National Institutes of Health grant ROI-G59065 to L.H.R.
Literature Cited
- Anderson E. Hybridization of the habitat. Evolution. 1948a;2:1–9. [Google Scholar]
- Anderson E. Introgressive hybridization. Wiley; New York: 1948b. [Google Scholar]
- Arnold ML. Oxford series in ecology and evolution. Oxford University Press; New York: 1997. Natural hybridization and evolution. [Google Scholar]
- Aronson J, Kigel J, Shmida A, Klein J. Adaptive phenology of desert and Mediterranean populations of annual plants grown with and without water stress. Oecologia (Berlin) 1992;89:17–26. doi: 10.1007/BF00319010. [DOI] [PubMed] [Google Scholar]
- Aronson J, Kigel J, Shmida A. Reproductive allocation strategies in desert and Mediterranean populations of annual plants grown with and without water stress. Oecologia (Berlin) 1993;93:336–342. doi: 10.1007/BF00317875. [DOI] [PubMed] [Google Scholar]
- Barton NH. The role of hybridization in evolution. Molecular Ecology. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw HD, Jr, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Buerkle CA, Morris RJ, Asmussen MA, Rieseberg LH. The likelihood of homoploid hybrid speciation. Heredity. 2000;84:441–451. doi: 10.1046/j.1365-2540.2000.00680.x. [DOI] [PubMed] [Google Scholar]
- Chandler J, Jan C, Beard B. Chromosomal differentiation among the annual Helianthus species. Systematic Botany. 1986;11:353–371. [Google Scholar]
- deVincente M, Tanksley S. QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics. 1993;134:585–596. doi: 10.1093/genetics/134.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GA. Consequences of flowering-time variation in a desert annual: adaptation and history. Ecology. 1989;70:1294–1306. [Google Scholar]
- Fulton M, Hodges SA. Floral isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the Royal Society of London B. 1999;266:2247–2252. [Google Scholar]
- Gibson AC. Photosynthetic organs of desert plants. BioScience. 1998;48:911–920. [Google Scholar]
- Givnish T. On the adaptive significance of leaf form. In: Solbrig OT, Jain S, Johnson GB, Raven PH, editors. Topics in plant population biology. Columbia University Press; New York: 1979. pp. 373–407 . [Google Scholar]
- Gross BL, Schwarzbach AE, Rieseberg LH. Origin(s) of the diploid hybrid species Helianthus deserticola (Asteraceae) American Journal of Botany. 2003;90:1708–1719. doi: 10.3732/ajb.90.12.1708. [DOI] [PubMed] [Google Scholar]
- Gupta U, Jame Y, Campbell C, Leyshon A, Nicholaichuk W. Boron toxicity and deficiency: a review. Canadian Journal of Soil Science. 1995;65:381–409. [Google Scholar]
- Heiser C. Hybridization between the sunflower species Helianthus annuus and H. petiolaris. Evolution. 1947;1:249–262. [Google Scholar]
- Heiser C, Smith D, Clevenger S, Martin W. The North American sunflowers (Helianthus) Memoirs of the Torrey Botanical Club. 1969;22:1–218. [Google Scholar]
- Hodges SA, Arnold ML. Floral and ecological isolation between Aquilegia formosa and Aquilegia pubescens. Proceedings of the National Academy of Sciences of the USA. 1994;91:2493–2496. doi: 10.1073/pnas.91.7.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies S, Barr A, Karakousis A, Kretschmer J, Manning S, Chalmers K, Nelson J, et al. Mapping of chromosome regions conferring boron toxicity tolerance in barley (Hordeum vulgare L.) Theoretical and Applied Genetics. 1999;98:1293–1303. [Google Scholar]
- Jefferies S, Pallotta M, Paull J, Karakousis A, Kretschmer J, Manning S, Islam A, et al. Mapping and validation of chromosome regions conferring boron toxicity tolerance in wheat (Triticum aestivum) Theoretical and Applied Genetics. 2000;101:767–777. [Google Scholar]
- Kingsolver J, Hoekstra H, Hoekstra J, Berrigan D, Vignieri S, Hill C, Hoang A, et al. The strength of phenotypic selection in natural populations. American Naturalist. 2001;157:245–261. doi: 10.1086/319193. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Levin DA. Oxford series in ecology and evolution. Oxford University Press; New York: 2000. The origin, expansion, and demise of plant species. [Google Scholar]
- Lexer C, Welch ME, Durphy JL, Rieseberg LH. Natural selection for salt tolerance quantitative trait loci (QTLs) in wild sunflower hybrids: implications for the origin of Helianthus paradoxus, a diploid hybrid species. Molecular Ecology. 2003a;12:1225–1235. doi: 10.1046/j.1365-294x.2003.01803.x. [DOI] [PubMed] [Google Scholar]
- Lexer C, Welch ME, Raymond O, Rieseberg LH. The origin of ecological divergence in Helianthus paradoxus (Asteraceae): selection on transgressive characters in a novel hybrid habitat. Evolution. 2003b;57:1989–2000. doi: 10.1111/j.0014-3820.2003.tb00379.x. [DOI] [PubMed] [Google Scholar]
- McCarthy EM, Asmussen MA, Anderson WW. A theoretical assessment of recombinational speciation. Heredity. 1995;74:502–509. [Google Scholar]
- Rieseberg LH. Homoploid reticulate evolution in Helianthus (Asteraceae): evidence from ribosomal genes. American Journal of Botany. 1991;78:1218–1237. [Google Scholar]
- Rieseberg LH. Hybrid origins of plant species. Annual Review of Ecology and Systematics. 1997;28:359–389. [Google Scholar]
- Rieseberg LH. Crossing relationships among ancient and experimental sunflower hybrid lineages. Evolution. 2000;54:859–865. doi: 10.1111/j.0014-3820.2000.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Sinervo B, Linder CR, Ungerer MC, Arias DM. Role of gene interaction in hybrid speciation: evidence from ancient and experimental hybrids. Science. 1996;272:741–745. doi: 10.1126/science.272.5262.741. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Archer MA, Wayne RK. Transgressive segregation, adaptation and speciation. Heredity. 1999;83:363–372. doi: 10.1038/sj.hdy.6886170. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, Durphy JL, et al. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 2003;301:1211–1216. doi: 10.1126/science.1086949. [DOI] [PubMed] [Google Scholar]
- Rogers C, Thompson T, Seiler GJ. Sunflower species of the United States. National Sunflower Association; Bismarck, N.D: 1982. [Google Scholar]
- Rosenthal DM, Schwarzbach AE, Donovan LA, Raymond O, Rieseberg LH. Phenotypic differentiation between three ancient hybrid taxa and their parental species. International Journal of Plant Sciences. 2002;163:387–398. [Google Scholar]
- Schemske DW, Bradshaw HD., Jr Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus) Proceedings of the National Academy of Sciences of the USA. 1999;96:11910–11915. doi: 10.1073/pnas.96.21.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbach AE, Donavan LA, Rieseberg LH. Transgressive character expression in a hybrid sunflower species. American Journal of Botany. 2001;88:270–277. [PubMed] [Google Scholar]
- Smith SD, Monson RK, Anderson JE. Physiological ecology of North American desert plants. Springer; New York: 1997. Desert annuals. [Google Scholar]
- SPSS. SPSS for Mac., Version 11.0. SPSS; Chicago: 2002. [Google Scholar]
- Thioulouse J, Chessel D, Doledec S, Olivier JM. ADE-4: a multivariate analysis and graphical display software. Statistics and Computing. 1997;7:75–83. [Google Scholar]
- Ungerer MC, Baird SJE, Pan J, Rieseberg LH. Rapid hybrid speciation in wild sunflowers. Proceedings of the National Academy of Sciences of the USA. 1998;95:11757–11762. doi: 10.1073/pnas.95.20.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western Regional Climate Center. Period of record general climate summary: precipitation, August 6, 2003. 2003 http://www.wrcc.dri.edu/cgi-bin/cliMAIN.pl?utlitt.


