Summary
To contribute to the understanding of ecological differentiation in speciation, we compared salinity responses of the halophytic diploid hybrid species Helianthus paradoxus and its glycophytic progenitors Helianthus annuus and Helianthus petiolaris.
Plants of three populations of each species were subjected to a control (nonsaline) and three salinity treatments, including one simulating the ion composition in the habitat of H. paradoxus.
Relative to the control, saline treatments led to a 17% biomass increase in H. paradoxus while its progenitors suffered 19–33% productivity reductions and only in H. paradoxus, leaf contents of potassium, calcium, and magnesium were strongly reduced. Under all treatments, H. paradoxus allocated more resources to roots, was more succulent, and had higher leaf contents of sodium (> 200 mmol l−1 tissue water) and sulfur than its progenitor species.
These results suggest that salt tolerance and thus speciation of H. paradoxus is related to sodium replacing potassium, calcium and magnesium as vacuolar osmotica. The evolutionary and genetic mechanisms likely to be involved are discussed.
Keywords: hybrid speciation, ion compartmentation, salt tolerance, sodium, sulfur, Helianthus (sunflower)
Introduction
Apart from geographic or spatial isolation, ecological divergence has long been suspected to play an important role in plant speciation (Stebbins, 1950; Grant, 1981) and new experimental studies implicate ecological differences as drivers of speciation (reviewed by Coyne & Orr, 2004; Levin, 2004; Gross & Rieseberg, 2005). In homoploid hybrid speciation in particular, ecological divergence is believed to be instrumental to the speciation process: only by acquiring new ecological attributes can incipient hybrid species colonize novel habitats and escape interbreeding and competition with parental genotypes (Rieseberg & Carney, 1998; Buerkle et al., 2000; Rieseberg et al., 2003). Genetic methods to study genes involved in ecological adaptation have recently become available. To render such studies meaningful it is vital to first investigate ecological responses on a whole-plant basis. This study contributes such a comparative assessment of the response to saline conditions in the halophytic sunflower hybrid species Helianthus paradoxus and its glycophytic progenitor species Helianthus annuus and Helianthus petiolaris.
Molecular evidence indicates that the highly salt-tolerant H. paradoxus has arisen rapidly through natural hybridization of a moderately salt-tolerant species, H. annuus, and a salt-sensitive species, H. petiolaris, without a change in ploidy (Rieseberg, 1991; Welch & Rieseberg, 2002b). These data are also consistent with a single origin of H. paradoxus rather than multiple hybridization events throughout its range (Welch & Rieseberg, 2002b). Helianthus paradoxus occurs exclusively in salt seeps and has a limited range in the south-western USA whereas both parental species have broad ranges in North America and are found in comparatively mesic habitats (Rogers et al., 1982). Helianthus annuus and H. petiolaris cannot survive in the habitat of the hybrid species (Lexer et al., 2003b) and both parental species are competitively inferior to the hybrid species under saline conditions (Bush & Van Auken, 2004). Investigating the responses to salinity in H. paradoxus and its progenitors is therefore an important step toward understanding the origin of the ecological differences that allowed the hybrid species to become reproductively isolated from its progenitors.
Responses to salinity in H. paradoxus and its progenitors have previously been compared by Welch & Rieseberg (2002a) and by Lexer et al. (2003b). The two studies highlight different aspects of the salinity response. Welch & Rieseberg (2002a) conducted a growth chamber experiment with the H. paradoxus and its parental species, and suggested that the ability to grow in the presence of high leaf sodium contents is crucial for the salt tolerance of the hybrid species. Lexer et al. (2003b) transplanted the three species and a back-cross generation between the parental species into the saline habitat of H. paradoxus and concluded that sodium exclusion and high calcium uptake might be important for salt tolerance in Helianthus (Lexer et al., 2003b). The divergent conclusions of the two experiments are likely related to differences in conditions and procedures, but, because each experiment used different populations, the discrepancies could also result from differences among populations within species. In cultivars of H. annuus, a wide range of responses to salinity has been reported (Ashraf & Tufail, 1995) implying that wild Helianthus populations could also differ in salt tolerance. Differences in the response to salinity between populations of the parental species would suggest that only certain preadapted populations could have given rise to H. paradoxus. In both previous experiments the parental species exhibited severe growth reductions and high mortality (Welch & Rieseberg, 2002a; Lexer et al., 2003b) demonstrating that they are ecologically excluded from the habitat of H. paradoxus. However, such severe conditions are not ideal for investigating the reaction to saline conditions in the glycophytic parental species. When experimental conditions jeopardize survival, phenomena occurring near plant death are difficult to distinguish from genuine responses to salinity (Munns, 2002).
Salinity can adversely affect plant growth by reducing water availability and causing ion toxicity, as well as by inducing nutrient deficiencies, particularly of potassium and calcium (Greenway & Munns, 1980; Niu et al., 1995; Maathuis & Amtmann, 1999; Cramer, 2002; Munns, 2002). Moderate potassium supplements can alleviate the effects of salinity in various crops, such as spinach and lettuce (Kaya et al., 2002). On a functional level, salinity-induced potassium deficiency has been related to the uptake of sodium through a high-affinity potassium uptake system in roots (Yeo, 1998). In halophytes, however, a sharp decrease in potassium levels can occur without signs of potassium deficiency as sodium is thought to replace potassium as a vacuolar osmoticum in these plants (Munns et al., 1983). In many cultivated plants, including wheat, bean and tomato calcium is also used to mitigate the effects of salt stress, but such calcium–sodium interactions are not observed universally (Cramer, 2002). High sodium concentrations reduce calcium activity both in the external medium and at membranes, and can disturb calcium functions in turgor maintenance, cell elongation, membrane stabilization, and signaling (Bressan et al., 1998; Liu & Zhu, 1998; Cramer, 2002). Moreover, nitrogen and phosphate supplementation improve salt tolerance in some species and various interactions between multiple nutrients and salinity have been reported (Niu et al., 1995; Cramer, 2002).
Salt tolerance in plants is often related to the storage of excess ions, namely sodium, in the vacuole rather than in the cytoplasm (ion compartmentation; Niu et al., 1995). As a result, halophytes can support much higher vacuolar (and thus total) leaf sodium loads than glycophytes (Greenway & Munns, 1980). Plant cytoplasm generally has low sodium–potassium ratios and sodium can disturb the function of potassium-activated enzymes as well as protein synthesis (Wyn Jones & Gorham, 2002; Tester & Davenport, 2003). In addition to cellular processes, transport mechanisms on a larger scale, such as sodium extrusion by roots, sodium retention at xylem loading, and sodium recirculation via the phloem can contribute to salt tolerance (Munns, 2002; Tester & Davenport, 2003). Furthermore, an important role for calcium-dependent signaling pathways has recently been suggested (Liu & Zhu, 1998; Tester & Davenport, 2003).
In the present study, we examine the salinity response of the hybrid species H. paradoxus and its progenitors H. annuus and H. petiolaris using three populations per species. In many saline habitats, ions other than sodium and chloride (namely calcium, magnesium and sulfate) contribute to salinity or interact with the salinity response (Cramer, 2002; Tanji, 2002; Bush & Van Auken, 2004). For this reason, we simulated the ion composition of the soil solution of the H. paradoxus habitat (Lexer et al., 2003b; Bush & Van Auken, 2004) rather than using a sodium chloride solution to induce salinity responses. To further emulate field-like conditions, we exposed plants to salinity at germination and slowly increased salt concentrations. In salt marshes, decreasing precipitation and rising temperatures lead to an increase in salt concentrations in the soil solution, particularly of sodium, over the season (Tanji, 2002). In order to avoid mortality and near-fatal stress in the parental species, we used moderate final salinities, comparable to field conditions during the establishment phase of H. paradoxus. Specifically, our study addresses the following questions: (1) How do H. paradoxus, H. annuus and H. petiolaris respond to saline conditions, in terms of growth and leaf ion contents? (2) Do saline conditions cause potassium deficiency in these taxa? (3) Do the high calcium concentrations observed in the H. paradoxus habitat improve salt tolerance? (4) Does the response to salinity differ among populations of each species? (5) What are the implications of these salinity responses for the evolution of salt tolerance in H. paradoxus?
Materials and Methods
Plant material, growth conditions, and experimental design
Seeds from three populations of each of the study species, H. annuus L., H. paradoxus Heiser and H. petiolaris Nutt., were used in the experiment (Table 1). We selected populations from locations that were well distributed across the ranges of each species. The experiment was conducted within the natural growing season in the glasshouse at Indiana University, Bloomington, IN, USA, using ambient light and climate. Seeds were germinated on 14 June, 2004 in the four treatment solutions with 0.5 or 25 mmol l−1 NaCl (see later) and were otherwise treated according to the protocol of Schwarzbach et al. (2001) that involves scarification and hull removal. On June 18, 2004 germinants of each population were planted into 10 × 10 × 10 cm pots filled with a 1 : 2 mixture (by volume) of compost soil and sand. Thirty-four plants that died within the first week after planting were replaced by extra or new germinants. Each pot received treatment solution (see later) every third day until 10 d after planting and on alternate days thereafter. In order to replace the larger part of the substrate solution, we applied approx. 75 ml of the treatment solution to each pot, resulting in considerable flow-through. Between treatments, distilled water was given approximately to saturation to avoid increased salt concentrations caused by evaporation from the substrate.
Table 1.
Populations of three annual sunflower species, the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris that were used in salt tolerance experiments; all are from within the USA
| Name | Reference | Nearest location | County | State | |
|---|---|---|---|---|---|
| H. annuus | ANN1 | EJB 2003 | Hermosa | Custer | South Dakota |
| ANN2 | PI 413024 | Limon | Lincoln | Colorado | |
| ANN3 | Ames 14400 | Tucson | Pima | Arizona | |
| H. paradoxus | PAR1 | LHR 1300 | Grants | Cibola | New Mexico |
| PAR2 | LHR 1302 | Santa Rosa | Guadeloupe | New Mexico | |
| PAR3 | LHR 1303 | Bitter Lake | Caves | New Mexico | |
| H. petiolaris | PET1 | PI 586912 | Terry | Prairie | Montana |
| PET2 | PI 586920 | Carr | Weld | Colorado | |
| PET3 | PI 435809 | Channing | Hartley | Texas |
Populations ANN2, ANN3, and PET1–3 were obtained from the National Germplasm Resources Laboratory (Beltsville, MD, USA); ANN1 was collected by Eric J. Baack (Indiana University), and PAR1–3 are collections of Loren H. Rieseberg.
Plants were arranged in split-plot design with 17 randomized complete blocks (whole plots) of four treatments (see later). Plants of the same treatment were grouped in rows (subplots) with each row containing a random array of one plant of each of the nine populations with a total of 612 plants. The experiment was set up along a single, south-facing glasshouse bench.
Treatment solutions
To develop treatment solutions, we compared soil analyses from the habitats of the three species. Soil samples were collected in November 2002 in the Southern Great Plains region within seven populations of H. annuus (Colorado, Kansas and Texas, USA) and six populations of H. petiolaris (Colorado, Nebraska and Texas, USA) with a soil auger (25 cm). Three samples from each population were pooled and analysed for total ionic strength (electrical conductivity, EC) using a 1 : 1 water–soil extract and for exchangeable cations and sulfur using ammonium acetate extraction followed by inductively coupled argon plasma (ICAP) detection (Midwest Laboratories, Omaha, NE, USA). Similar results for a H. paradoxus habitat (Bitter Lake, NM, USA) from March and May 2002 were already available (Lexer et al., 2003b; C. Lexer, unpublished). Soil extracts from the H. paradoxus habitat had a higher EC, higher contributions of sodium, calcium, and magnesium to the cation exchange capacity (CEC) and higher sulfur contents than soil extracts from H. annuus or H. petiolaris habitats (Table 2). Furthermore, EC and the contribution of sodium to CEC strongly increased over the season in the saline habitat (Table 2), as is characteristic of many saline soils (Tanji, 2002). Note that for the May measurements of the H. paradoxus habitat, CEC is unreasonably high, possibly owing to the extraction procedure and ion percentages must therefore be interpreted with caution (K. Tanji, pers. comm.). Similar results were found in analysis of spring water from a H. paradoxus habitat (Bush & Van Auken, 2004).
Table 2.
Electrical conductivity (EC), cation exchange capacity (CEC) with contributions of four cations, and sulfur concentrations for habitats of Helianthus annuus, Helianthus paradoxus and Helianthus petiolaris
| H. annuus (n = 7) | H. paradoxus, March (n = 4) | H. paradoxus, May (n = 20) | H. petiolaris (n = 6) | |
|---|---|---|---|---|
| EC (dS m−1) | 0.2 (0.1–0.4) | 4.6 (2.7–5.8) | 22.9 (17.0–34.0) | 0.1 (0.1–0.2) |
| CEC (mEq (100 g)−1) | 14.0 (8.7–21.4) | 42.4 (32.4–55.7) | 91.5 (74.9–99.9) | 7.5 (4.6–8.8) |
| % Na | 2.4 (0.5–11.7) | 22.6 (11.2–31.4) | 46.6 (40.8–58.0) | 0.6 (0.5–0.9) |
| % K | 6.0 (2.4–9.4) | 1.2 (0.7–1.6) | 0.5 (0.4–0.8) | 4.7 (2.2–9.7) |
| % Ca | 69.4 (47.9–89.1) | 67.6 (6.5–82.3) | 44.0 (31.4–51.1) | 79.6 (69.2–90.3) |
| % Mg | 22.1 (7.8–38.0) | 8.6 (5.8–10.6) | 8.9 (6.9–12.1) | 15.1 (6.1–21.2) |
| Sulfur (p.p.m.) | 28.7 (10.0–96.0) | 95.5 (94.0–97.0) | na | 10.5 (10.0–13.0) |
Values are means and range in parentheses; n, total number of samples analysed; na, not available.
We developed treatment solutions (Table 3) to simulate nonsaline conditions (nonsaline treatment) and field-like conditions of the salt-tolerant hybrid species H. paradoxus, high in sodium, calcium, magnesium, and sulfate (field-like treatment). To investigate whether potassium deficiency contributes to salt stress we used a field-like solution with added potassium (K addition treatment). To test whether the high calcium concentrations in the H. paradoxus habitat improve salt tolerance, we used a field-like solution with reduced calcium and magnesium concentrations (Ca & Mg reduction treatment). Both calcium and magnesium concentrations were reduced in this treatment because maintaining the high magnesium concentrations of the field-like treatment in a low-calcium treatment would lead to potentially harmful magnesium–calcium ratios (Madhok & Walker, 1969). Because ion balance had to be maintained when preparing treatment solutions from salts, the K addition and the Ca & Mg reduction treatment solutions differed slightly from the field-like solutions also in Cl− and concentrations SO4 (Table 3). All solutions contained the following micronutrients (μmol l−1): CuSO4, 0.5; FeSO4, 40; H3BO4, 25; H2MoO4, 0.5; MnSO4, 2; ZnSO4, 2.
Table 3.
Composition of final treatment solutions with the estimated electrical conductivity (EC, Tanji, 2002) used in an experiment on the salt tolerance mechanism of the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris
| Composition (mEq l−1) | Nonsaline | Field-like | K addition | Ca & Mg reduction |
|---|---|---|---|---|
| Na+ | 0.5 | 90 | 90 | 90 |
| K+ | 1 | 1 | 4 | 1 |
| Ca2+ | 3.5 | 16 | 16 | 3.5 |
| Mg2+ | 1 | 11 | 11 | 1 |
| NH4+ | 0.5 | 0.5 | 0.5 | 0.5 |
| Cl− | 2.5 | 97 | 100 | 78.5 |
| SO42− | 1 | 16 | 16 | 12 |
| HCO3− | 0.5 | 3 | 3 | 3 |
| NO3− | 2 | 2 | 2 | 2 |
| PO43− | 0.5 | 0.5 | 0.5 | 0.5 |
| EC (dS m−1) | 0.65 | 11.85 | 12.15 | 9.60 |
Concentrations of sodium chloride were increased from 28 mmol l−1−40, 70 and 90 mmol−1 beginning 14, 19 and 25 d after planting, respectively. Until NaCl concentration were raised to 70 mmol l−1, calcium and magnesium concentrations in the field-like and K addition treatments were kept at 11 mmol l−1 and 6 mmol l−1 to avoid large differences in osmotic potential in comparison to the field-like treatment. After reaching the final concentrations the experiment was continued for two more weeks.
Measurements
Plant heights were measured 13, 21, and 27 d after planting and at harvest, 40–42 d after planting. Upon harvest, the six oldest turgid leaves were sampled. Their fresh mass was determined and the combined area of these six leaves was measured using a Li-Cor 1300 area meter (Li-Cor Biosciences, Lincoln, NE, USA). Shoots were cut off at the root–shoot transition zone and roots were thoroughly cleaned. Six-leaf samples, shoots with all remaining leaves, and roots were oven-dried in paper bags at 75°C for 3 d and their dry mass was determined. Ten entire blocks were chosen from the 17, and their six-leaf samples from all population-by-treatment combinations were analysed for sodium, potassium, calcium, magnesium and sulfur contents, determined by ICAP detection (Midwest laboratories).
Losses caused by mortality were minimal in this experiment: 13 out of 612 plants died after the first week (six of H. annuus and seven of H. petiolaris); all but one H. annuus were from salt treatments. Six of these plants died within the first 3 wk of the experiment, before final salt concentration had been reached. In addition, two plants of H. paradoxus were excluded from the dataset because they appeared to be infected with a virus.
Data analysis
Four growth traits were calculated from the measurements: height growth rate, total plant biomass, root mass fraction and leaf succulence. Height growth rates (hGR) were calculated over the last 2 wk of the experiment (when the final salt concentrations were applied), as (height at harvest − height day 27)/number of days. Total plant biomass was obtained by adding dry masses of the six-leaf sample, remaining shoot and root. Because the harvest took place over 3 d and 34 plants had been replaced within the first week, biomass values were linearly adjusted to 45 d of growth using (biomass/days of growth) ×45. We determined the root mass fraction as root mass/total biomass. Leaf succulence was expressed as the amount of tissue water per leaf area in the six-leaf sample as (fresh mass − dry mass) leaf−1 area (compare Reimann & Breckle, 1995).
Ion contents expressed as mmol g−1 DW were used for further analysis, because this allows comparison with most other studies. We also calculated sodium, potassium and sulfur concentrations in tissue water (mmol l−1) using the water content of the six-leaf sample. To investigate whether sodium or potassium was preferentially transported to the leaves we determined potassium–sodium selectivity as sK,Na = (potassium/sodium leaf concentrations)/(potassium/sodium concentrations in the treatment solution) (compare Pitman, 1976; Wilson et al., 2002).
Plant growth traits and ion contents were analysed with hierarchical linear models and a two-level error structure according to the split-plot design of the study (Cochran & Cox, 1957; Venables & Ripley, 2002). Using the entire dataset, the effects of treatments, species, species-by–treatment interaction, and population nested in species on growth traits and leaf ion contents were analysed. The treatment effect was tested over the mean square of the treatment-by-block interaction (errorwhole plot), the species effect over the population mean square and all other effects over the subplot error mean square (errorsubplot, Venables & Ripley, 2002). The block effect, and all other effects were treated as fixed (Newman et al., 1997). The distribution of the residuals of these models was tested for normality with the Wilk–Shapiro test (Zar, 1999). Root mass fraction, and contents of sodium, magnesium and sulfur were log-transformed before analysis to yield residuals that conformed to the normal distribution. Means and 95% confidence intervals of these log-transformed data were back-transformed to the original scale (corresponding to medians on the original scale). Missing values were predicted according to Cochran and Cox (1957) and degrees of freedom reflect the actual number of data points (Cochran & Cox, 1957). Because treatment-by-species interaction effects were significant in most cases, separate models for each species were calculated in a second step. Where the population-by-treatment interaction was significant, models for individual populations were calculated in a third step. Following these analyses, multiple comparisons of treatment means on the level of species and population, where needed, were performed using multiple t-tests with P-value adjustment to an overall α = 0.05 by the Benjamini and Hochberg procedure (Verhoeven et al., 2005).
To investigate relationships between leaf contents of the three main ions – potassium, sodium and calcium – and between contents of these ions and total biomass, we calculated Pearson product-moment correlations. The false discovery rate for significance tests of correlation coefficients was controlled at α = 0.05 following the Benjamini and Hochberg procedure (Verhoeven et al., 2005). All analyses were performed in the program R 2.10 (R Development Core team, 2005).
Results
Response to saline conditions
All four growth traits – height growth rate, biomass production, root mass fraction, and leaf succulence – were significantly impacted both by experimental salt treatments and by constitutive differences between species (Table 4a). The interaction effect of species and treatment was significant in all cases, indicating that the species differed in their reaction to salt treatments. Significant treatment effects remained when each species was analysed separately (Table 5a).
Table 4.
Anova results for the growth traits height growth rate (hGR, cm d−1), total biomass (g), root mass fraction (g g−1) and leaf succulence (g H2O cm−2) (a) and for leaf ion contents (mmol g−1 DW) (b) in split-plot experiments on the salt tolerance of the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris
| Source of variation | |||||||
|---|---|---|---|---|---|---|---|
| Treatment | Errorwhole plot | Species | Population (sp.) | Treatment ×sp. | Errorsubplot | ||
| (a) Growth traits | |||||||
| df | 3 | 45–48a | 2 | 6 | 6 | ||
| hGR | SS | 2317.9 | 996.2 | 1520.9 | 345.3 | 891.2 | 7255.8 |
| F | 34.903*** | 13.214** | 3.951*** | 10.195*** | df = 475 | ||
| Biomass | SS | 5.096 | 7.029 | 48.292 | 3.996 | 12.685 | 79.329 |
| F | 11.301*** | 36.255*** | 4.4330*** | 14.072*** | df = 508 | ||
| Root mass fraction | SS | 3.735 | 3.735 | 33.893 | 18.120 | 6.772 | 31.478 |
| F | 16.036*** | 5.611 ns | 50.848*** | 19.003*** | df = 498 | ||
| Succulence | SS | 641.6 | 476.4 | 56521.0 | 1126.0 | 201.00 | 5828.0 |
| F | 21.518*** | 150.589*** | 17.072*** | 3.048** | df = 497 | ||
| (b) Leaf ion contents | |||||||
| df | 3 | 27 | 2 | 6 | 6 | ||
| Sodium | SS | 279.78 | 26.65 | 680.86 | 14.93 | 30.04 | 149.91 |
| F | 94.481*** | 136.720*** | 5.079*** | 10.221*** | df = 295 | ||
| Potassium | SS | 15.757 | 5.915 | 162.890 | 17.678 | 77.680 | 57.667 |
| F | 23.974*** | 27.645*** | 15.634*** | 68.700*** | df = 297 | ||
| Calcium | SS | 9.214 | 7.413 | 8.458 | 7.756 | 11.233 | 34.414 |
| F | 11.186*** | 3.270 ns | 11.494*** | 16.647*** | df = 296 | ||
| Magnesium | SS | 0.953 | 1.532 | 11.689 | 2.293 | 2.010 | 8.694 |
| F | 5.600** | 15.296** | 13.449*** | 11.790*** | df = 297 | ||
| Sulfur | SS | 3.531 | 1.084 | 302.151 | 6.265 | 5.481 | 14.44 |
| F | 29.328*** | 144.700*** | 22.122*** | 19.353*** | df = 295 | ||
Parentheses denote nested effects, asterisks stand for an interaction effect. The species effect was tested over the population mean square; all other effects were tested over the residual error. Effect significance levels:
P < 0.001;
P < 0.01;
P < 0.05; ns, not significant. SS, sum of squares. Error df are noted below the error SS for each anova.
Whole-plot error df were 45 for hGR and 48 for all other growth traits.
Table 5.
Anova results for the growth traits height growth rate (hGR), biomass, root mass fraction and leaf succulence (a) and for leaf ion contents (b) in a salt tolerance experiment (split-plot design) with the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris
| Treatment | Errorwhole plot | Population | Population × treatment | Errorsubplot | ||
|---|---|---|---|---|---|---|
| (a) Growth traits | ||||||
| df | 3 | 45–48a | 2 | 6 | 113–127 | |
| hGR | ||||||
| H. annuus | SS | 691.72 | 957.86 | 68.29 | 106.80 | 1511.82 |
| F | 10.832*** | 2.710 ns | 1.413 ns | df = 122 | ||
| H. paradoxus | SS | 164.21 | 535.73 | 56.09 | 46.68 | 1683.05 |
| F | 4.598** | 1.999 ns | 0.555 ns | df = 126 | ||
| H. petiolaris | SS | 2353.17 | 850.03 | 220.97 | 148.45 | 1962.92 |
| F | 41.525*** | 6.754** | 1.513 ns | df = 113 | ||
| Biomass | ||||||
| H. annuus | SS | 5.602 | 9.280 | 0.134 | 1.718 | 9.280 |
| F | 9.658*** | 0.359 ns | 1.536 ns | df = 120 | ||
| H. paradoxus | SS | 3.039 | 4.916 | 3.002 | 2.426 | 15.297 |
| F | 9.891*** | 12.561*** | 3.383** | df = 127 | ||
| H. petiolaris | SS | 9.140 | 4.584 | 0.860 | 0.713 | 15.999 |
| F | 31.901*** | 3.441* | 0.951 ns | df = 114 | ||
| Root mass fraction | ||||||
| H. annuus | SS | 3.356 | 2.689 | 11.062 | 0.605 | 8.387 |
| F | 19.967*** | 84.416*** | 1.538 ns | df = 118 | ||
| H. paradoxus | SS | 2.372 | 1.776 | 5.171 | 0.661 | 9.252 |
| F | 21.366*** | 35.769*** | 1.523 ns | df = 126 | ||
| H. petiolaris | SS | 4.778 | 3.939 | 1.887 | 0.313 | 5.728 |
| F | 19.410*** | 21.083*** | 1.165 ns | df = 108 | ||
| Succulence | ||||||
| H. annuus | SS | 116.07 | 329.88 | 371.44 | 44.37 | 987.99 |
| F | 5.630** | 24.061*** | 0.958 ns | df = 118 | ||
| H. paradoxus | SS | 611.42 | 836.31 | 724.61 | 42.29 | 2401.65 |
| F | 11.697*** | 19.310*** | 0.376 ns | df = 121 | ||
| H. petiolaris | SS | 115.16 | 280.10 | 30.41 | 45.92 | 280.101 |
| F | 6.578*** | 2.023 ns | 1.018 ns | df = 112 | ||
| (b) Leaf ion contents | ||||||
| df | 3 | 27 | 2 | 6 | 66–71 | |
| Sodium | ||||||
| H. annuus | SS | 33.955 | 24.457 | 14.008 | 10.958 | 48.559 |
| F | 12.495*** | 10.385*** | 2.708* | df = 67 | ||
| H. paradoxus | SS | 159.875 | 3.763 | 0.305 | 0.631 | 6.980 |
| F | 382.35*** | 1.57 ns | 1.08 ns | df = 71 | ||
| H. petiolaris | SS | 115.991 | 27.491 | 0.617 | 5.489 | 38.952 |
| F | 37.973*** | 0.570 ns | 1.691 ns | df = 67 | ||
| Potassium | ||||||
| H. annuus | SS | 3.058 | 3.032 | 3.470 | 0.065 | 6.585 |
| F | 9.078*** | 18.971*** | 0.119 ns | df = 69 | ||
| H. paradoxus | SS | 89.322 | 6.039 | 13.741 | 2.671 | 18.218 |
| F | 133.120*** | 27.154*** | 1.760 ns | df = 71 | ||
| H. petiolaris | SS | 1.057 | 8.584 | 0.466 | 0.783 | 14.261 |
| F | 1.108 ns | 1.177 ns | 0.629 ns | df = 67 | ||
| Calcium | ||||||
| H. annuus | SS | 0.6805 | 7.7781 | 1.0349 | 0.7848 | 10.7930 |
| F | 0.787 ns | 3.452* | 0.873 ns | df = 69 | ||
| H. paradoxus | SS | 16.148 | 2.671 | 0.473 | 0.564 | 6.839 |
| F | 54.412*** | 2.490 ns | 0.989 ns | df = 71 | ||
| H. petiolaris | SS | 3.6187 | 3.1477 | 6.2480 | 0.7536 | 6.2152 |
| F | 10.347*** | 36.190*** | 1.455 ns | df = 67 | ||
| Magnesium | ||||||
| H. annuus | SS | 0.567 | 1.324 | 1.356 | 0.242 | 2.435 |
| F | 3.857* | 20.105*** | 1.194 ns | df = 69 | ||
| H. paradoxus | SS | 2.028 | 0.571 | 0.820 | 0.166 | 1.758 |
| F | 31.965*** | 16.789*** | 1.131 ns | df = 70 | ||
| H. petiolaris | SS | 0.369 | 1.441 | 0.113 | 0.114 | 1.621 |
| F | 2.302 ns | 2.518 ns | 0.845 ns | df = 68 | ||
| Sulfur | ||||||
| H. annuus | SS | 2.121 | 0.637 | 4.567 | 0.464 | 2.811 |
| F | 29.950*** | 58.357*** | 1.981 ns | df = 69 | ||
| H. paradoxus | SS | 0.5274 | 0.6775 | 1.5487 | 0.7033 | 2.4711 |
| F | 7.007** | 22.263*** | 3.416** | df = 70 | ||
| H. petiolaris | SS | 6.364 | 1.054 | 0.160 | 0.505 | 5.113 |
| F | 54.364*** | 1.123 ns | 1.185 ns | df = 66 | ||
Significance is indicated by asterisks:
P < 0.001;
P < 0.01;
P < 0.05; ns, P > 0.05.
Whole-plot error df were 45 for hGR and 48 for all other growth traits.
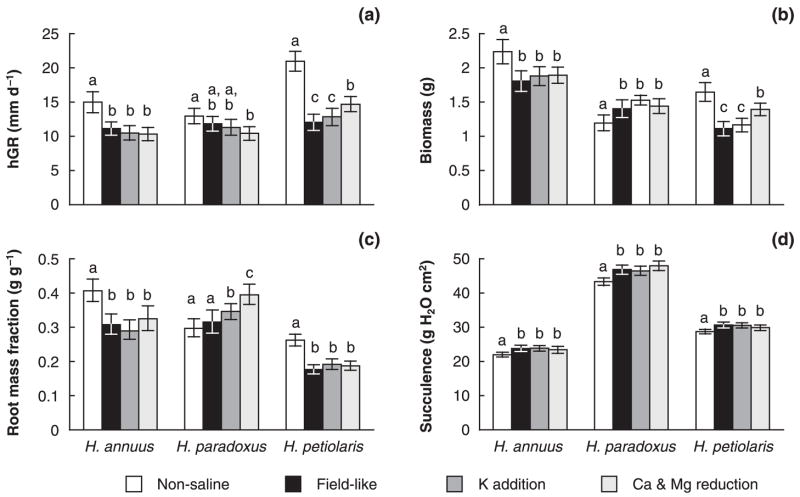
The response to salt treatment in terms of height growth rate (hGR) and biomass production differed sharply between the three species (Fig. 1a,b, Table 4). Height growth rates over the last 2 wk of the experiment, when final salt concentrations had been reached, were largest in H. petiolaris and smaller in H. paradoxus and H. annuus. In the parental species, a considerable reduction in hGR occurred under the field-like salt treatment compared with nonsaline conditions; this decrease was more severe in H. petiolaris (42%) compared with H. annuus (26%). In H. paradoxus, by contrast, hGR did not change significantly in response to conditions resembling its habitat, leading to similar growth rates for all three species under these conditions. Comparable results were obtained when hGR was calculated over different periods, including the 4-wk period from 1 wk after transplantation to the end of the experiment (data not shown). Similar to results for hGR, total biomass production decreased in both parental species in response to saline conditions (Fig. 1b). This decrease was smaller in H. annuus (19%) compared with H. petiolaris (33%). The hybrid species, by contrast, produced approx. 17% more biomass after treatment with saline treatment than under nonsaline conditions.
Fig. 1.
(a) Height growth rate (hGR, (b) biomass, (c) root mass fraction and (d) leaf succulence in the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris as affected by four treatments in a salt tolerance experiment. Means (a, b, d) or medians (c) are given with 95% confidence intervals. Within species, different letters denote significant differences between means or means of log-transformed data (= medians of original data).
The growth reduction in H. annuus and H. petiolaris affected roots more than shoots, as indicated by a decrease of root mass fraction (Fig. 1c). Helianthus petiolaris generally allocated fewer resources to roots than the other two species under nonsaline conditions, and further reduced biomass allocation to roots under saline conditions. In H. paradoxus, root biomass allocation did not change in response to the field-like treatment (Table 5a). Leaf succulence was much greater in H. paradoxus than in either parental species regardless of the treatment (Fig. 1d). In all three species, the succulence ratio increased significantly after saline treatment.
Leaf contents of sodium, potassium, magnesium and sulfur were significantly affected by treatment and species identity, and by the interaction effect between species and treatment (Table 4b). In a model for leaf calcium content, treatment and the treatment-by-species interaction effect were significant, but not the overall species effect. In individual models for each species, the salt treatments significantly impacted leaf contents of all ions except for potassium and magnesium in H. petiolaris and calcium in H. annuus (Table 5b).
Under nonsaline conditions, average leaf sodium contents (mmol g−1 DW) in H. paradoxus were more than nine times higher than in H. annuus and nearly six times higher than in H. petiolaris (Fig. 2a). Results were similar when using sodium concentrations in tissue water (mmol l−1; compare Appendix, Table A1). In response to the field-like treatment, a surge in sodium content was observed in all three species; sodium content (mmol g−1 DW) increased approximately fourfold in H. annuus, more than 11-fold in H. petiolaris, and 14-fold in H. paradoxus. Average potassium contents under nonsaline conditions were higher in H. petiolaris and H. paradoxus than in H. annuus (Fig. 2b). In reaction to salt treatment H. annuus exhibited moderately elevated potassium contents (by 12%) and H. petiolaris did not show a significant change in potassium content with salt treatment. In H. paradoxus, by contrast, we observed a significant reduction in potassium content (by nearly 60%). Similar results were obtained using potassium concentrations in tissue water (compare Appendix, Table A1). Potassium–sodium selectivity was highest in H. annuus and lowest in H. paradoxus (Table 6). In all three species potassium–sodium selectivity increased in response to saline conditions but this increase was larger in the parental species. Leaf calcium contents under nonsaline condition were c. 30% higher in the hybrid species compared with the parental species (Fig. 2c). Under salt treatment, however, leaf calcium contents in the hybrid species dropped significantly to concentrations similar to the parental species. Helianthus petiolaris also had slightly, but significantly reduced calcium levels under salt treatment while H. annuus exhibited no significant change in calcium levels. Magnesium levels under nonsaline conditions were higher in H. petiolaris than in H. annuus and exceeded levels of both parental species in H. paradoxus (Fig. 2d). In both parental species, the field-like treatment led to an increase in magnesium contents. In H. paradoxus, by contrast, magnesium levels decreased significantly in response to salt treatment. Sulfur levels were generally elevated by a factor of four to five in H. paradoxus, compared with the parental species (Fig. 2e). While sulfur levels in the parental species decreased in response to salt treatment, we observed significantly increased sulfur levels in the hybrid species. Results were similar when sulfur concentrations in tissue water were considered (compare Appendix, Table A1).
Fig. 2.
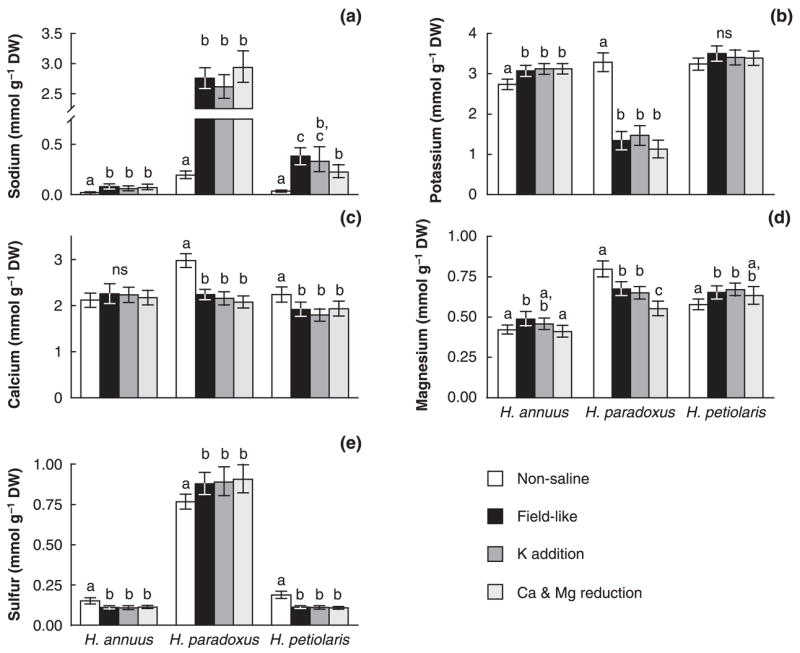
(a) Leaf contents of sodium, (b) potassium, (c) calcium, (d) magnesium and (e) sulfur in the hybrid species Helianthus paradoxus and its progenitors H. annuus and H. petiolaris as affected by four treatments in a salt tolerance experiment. Means (b,c) or medians (a,d,e) are given with 95% confidence intervals. Within species, different letters denote significant differences between means or means of log-transformed data (= medians of original data).
Table 6.
Potassium-sodium selectivities (sK,Na) calculated from ion concentrations in tissue water in a salt tolerance experiment with the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris
| H. annuus | H. paradoxus | H. petiolaris | |
|---|---|---|---|
| Nonsaline | 262 (197–348) | 34 (27–41) | 196 (141–272) |
| Field-like | 3409 (2295–5065) | 39 (31–49) | 836 (627–1116) |
| K addition | 1287 (850– 1948) | 11 (9–14) | 238 (157–360) |
| Ca & Mg reduction | 3971 (2708–5823) | 30 (23–40) | 1354 (986–1859) |
Medians with 95% confidence intervals are given.
K addition and Ca & Mg reduction treatments
The K addition treatment had little effect on growth traits and leaf ion composition in comparison to the field-like treatment (Figs 1 and 2). The Ca & Mg reduction treatment generally produced results similar to the field-like treatment in H. annuus (Figs 1 and 2). In H. paradoxus, hGR and biomass remained unchanged with Ca & Mg reduction, but root biomass allocation was significantly increased and leaf magnesium content was significantly reduced (Figs 1c and 2d). Unlike H. annuus and H. paradoxus, H. petiolaris exhibited a significantly elevated hGR, produced significantly more biomass and had significantly reduced leaf sodium content under the Ca & Mg reduction treatment compared with the field-like treatment (Figs 1a,b and 2a).
Population differentiation
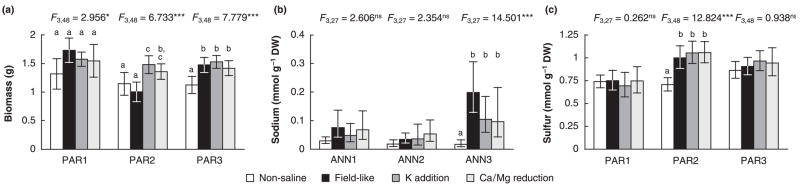
The effect of population identity varied between species and traits. Helianthus annuus populations were not differentiated in terms of hGR or biomass, while H. paradoxus exhibited a significant population effect for biomass but not for hGR, and H. petiolaris for biomass and hGR (Table 5a). For H. paradoxus, we also detected a significant population-by-treatment interaction for biomass (Table 5a). The overall H. paradoxus pattern of increased biomass production in reaction to all three salt treatments (Fig. 1b) was present only in populations PAR3, whereas in population PAR2, biomass was lower in both the nonsaline treatment and field-like treatment compared with the two other saline treatments and population PAR1 exhibited no significant differences in biomass caused by saline treatments (Fig. 3a). In all three species, root mass fraction exhibited strong population differentiation. Population differentiation in leaf succulence was also strong in H. annuus and H. paradoxus.
Fig. 3.
(a) Total plant biomass and leaf contents of (b) sodium and (c) and sulfur for different populations of Helianthus annuus or H. paradoxus in a salt tolerance experiment. For these parameter-species combinations significant population-by-treatment interaction effects were detected (compare Table 5). Means (a) or medians (b,c) are given with 95% confidence intervals. Within populations, different letters denote significant differences between means or means of log-transformed data (= medians of original data). Population-level anova results for treatment effects are given above each group of columns. Significant effects levels: ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, P > 0.05.
Contents of all five ions measured differed significantly between populations of H. annuus (Table 5b), especially sodium content. In the field-like treatment sodium contents were five times higher in population ANN3 than in population ANN1 (Fig. 3b). Moreover, populations of H. annuus differed in their reaction to treatments in terms of sodium content, indicated by the significant population-by-treatment interaction (Table 5b, Fig. 3b).
Populations of H. paradoxus showed significant differences in potassium, magnesium and sulfur contents, whereas H. petioaris populations differed significantly only in calcium content. In H. paradoxus a significant population-by-treatment interaction for sulfur content was detected (Table 5b). Salt treatment did not have a significant effect on sulfur content in two H. paradoxus populations (PAR1 and PAR3), whereas sulfur content increased with salt treatment the third population, PAR2 (Fig. 3d).
Correlations among leaf ion contents and between ion contents and biomass production
Under nonsaline conditions, we detected no significant correlations among ion contents in any species after correction for false discovery rate (Table 7a). Saline conditions produced negative associations of potassium content with either calcium content (H. annuus) or sodium content (H. paradoxus and H. petiolaris). In H. annuus, the negative correlation of potassium and calcium content occurred only after treatment with a field-like solution, but not in the other two saline treatments. Helianthus paradoxus exhibited the sodium–potassium correlation in all three salt treatments, while this correlation was detected in H. petiolaris only in the field-like and the Ca & Mg reduction treatment but not in the K addition treatment.
Table 7.
Product-moment correlations among leaf contents of sodium, potassium and calcium (a) and between these leaf ion contents and plant biomass (b) in a salt tolerance experiment with the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris
| H. annuus
|
H. paradoxus
|
H. petiolaris
|
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| (a) Leaf ion contents (mmol g−1 DW) | ||||||
| Nonsaline | ||||||
| Na–K | 0.2580 | 0.1850 | 0.0259 | 0.8925 | −0.2965 | 0.1116 |
| Na–Ca | −0.0050 | 0.9797 | −0.0971 | 0.6096 | 0.1297 | 0.4946 |
| K–Ca | −0.1922 | 0.3273 | −0.4215 | 0.0204 | −0.1088 | 0.5669 |
| Field-like | ||||||
| Na–K | −0.2795 | 0.1347 | −0.5865 | 0.0007 | −0.6658 | < 0.001 |
| Na–Ca | 0.1398 | 0.4611 | −0.0904 | 0.6346 | −0.3687 | 0.0535 |
| K–Ca | −0.7759 | < 0.001 | 0.1088 | 0.5673 | 0.0685 | 0.7291 |
| K addition | ||||||
| Na–K | −0.5244 | 0.0042 | −0.6498 | 0.0002 | −0.2780 | 0.1690 |
| Na–Ca | −0.3060 | 0.1132 | −0.2549 | 0.1905 | −0.3970 | 0.0446 |
| K–Ca | −0.3060 | 0.1132 | 0.1273 | 0.5186 | −0.2279 | 0.2628 |
| Ca & Mg reduction | ||||||
| Na–K | −0.2369 | 0.2160 | −0.6817 | < 0.001 | −0.4913 | 0.0058 |
| Na–Ca | 0.0573 | 0.7678 | 0.0068 | 0.9719 | −0.3431 | 0.0634 |
| K–Ca | −0.4243 | 0.0218 | 0.0046 | 0.9811 | 0.0356 | 0.8518 |
| (b) Leaf ion contents and total plant biomass | ||||||
| Nonsaline | ||||||
| Na | 0.2728 | 0.1601 | −0.0309 | 0.8712 | 0.0928 | 0.6257 |
| K | −0.1339 | 0.4970 | 0.1582 | 0.4039 | −0.2598 | 0.1656 |
| Ca | −0.2313 | 0.2364 | −0.2472 | 0.1878 | −0.2758 | 0.1402 |
| Field-like | ||||||
| Na | −0.0727 | 0.7026 | −0.4761 | 0.0078 | −0.6035 | < 0.001 |
| K | −0.4304 | 0.0176 | 0.6653 | < 0.001 | 0.3911 | 0.0396 |
| Ca | 0.3999 | 0.0286 | 0.5778 | < 0.001 | 0.4996 | 0.0068 |
| K addition | ||||||
| Na | 0.0622 | 0.7533 | −0.4540 | 0.0152 | −0.3102 | 0.1230 |
| K | −0.2094 | 0.2850 | 0.3922 | 0.0390 | −0.1705 | 0.4049 |
| Ca | 0.6022 | < 0.001 | 0.2174 | 0.0999 | 0.5162 | 0.0069 |
| Ca & Mg reduction | ||||||
| Na | −0.2536 | 0.1843 | −0.4096 | 0.0273 | 0.2518 | 0.1794 |
| K | 0.1893 | 0.3254 | 0.2007 | 0.3966 | −0.0521 | 0.7845 |
| Ca | −0.1038 | 0.5922 | 0.4987 | 0.0059 | −0.1359 | 0.4740 |
The number of replicates for nonsaline, field-like, K addition and Ca & Mg reduction treatments, respectively, was 28, 30, 28 and 29 for H. annuus, 30, 30, 28 and 28 for H. paradoxus and 30, 28, 26 and 30 for H. petiolaris. Correlation coefficients (r) and P-values are given. Coefficients that were significant after correction for false discovery rate at α < 0.05 are indicated in bold type.
Under nonsaline conditions, total biomass was not significantly correlated with any of the ion contents (Table 7b). In plants grown under saline conditions, however, we a detected significant association between ion content and biomass that differed between species and treatments (Table 7b). Under the field-like treatment, calcium was positively associated with biomass in all three species, whereas potassium content was positively correlated with biomass in H. paradoxus and H. petiolaris but negatively correlated with biomass in H. annuus. Sodium content was negatively correlated with biomass under all three salt treatments in H. paradoxus and in the field-like treatment in H. petiolaris. In H. annuus, sodium content and biomass were not significantly associated.
Discussion
Helianthus paradoxus performs best in saline conditions
The hybrid species H. paradoxus produced, on average, 17% more biomass under moderately saline conditions compared with nonsaline conditions, but its parental species, H. annuus and H. petiolaris, suffered 19% and 33% reductions in productivity (Fig. 1a). These results show that optimal conditions for H. paradoxus, as for many other halophytes, include some degree of soil salinity (Greenway & Munns, 1980; Flowers et al., 1986) and corroborate results of Welch & Rieseberg (2002a) and of Bush & Van Auken (2004). Moreover, the hybrid species was distinguished from its progenitors by a higher root biomass allocation and higher leaf succulence indicating that anatomical differences might allow H. paradoxus, like many other halophytes, to increase water use efficiency under saline conditions and to support large sodium loads (compare Flowers et al., 1986; Rosenthal et al., 2002; Welch & Rieseberg, 2002a).
Biomass produced in H. annuus and H. paradoxus is roughly comparable across the studies of Welch & Rieseberg (2002a), that of Bush & Van Auken (2004), and our experiment but the productivity of H. petiolaris in the present study is three- to four-fold higher under nonsaline conditions and more than 20-fold higher under saline conditions. We used top watering and a sandy substrate, while the other two experiments used conditions more similar to the wet clay soils of H. paradoxus habitat (i.e. subirrigation) (Welch & Rieseberg, 2002a) or clayey soil from a H. paradoxus site (Bush & Van Auken, 2004). Thus, our results do not necessarily reflect the growth potential in the H. paradoxus habitat, particularly for H. petiolaris, but conditions are ideal to compare responses to salinity among the three species. Under such moderate stress physiological integrity is preserved and near-death phenomena, such as a sudden massive increase in leaf sodium content, can be avoided (Greenway & Munns, 1980).
Ion contents suggest that sodium replaces potassium, calcium and magnesium as vacuolar osmotica in H. paradoxus but not in H. annuus and H. petiolaris
The hybrid species H. paradoxus appeared to achieve greater salt tolerance than its parental species by increasing leaf contents of sodium and sulfur and decreasing leaf contents of potassium, calcium and magnesium under saline conditions as opposed to nonsaline conditions (Fig. 2). These results are similar to findings in other halophytes and suggest that sodium replaces potassium, and possibly calcium and magnesium as vacuolar osmotica (Greenway & Munns, 1980; Munns et al., 1983; Reimann & Breckle, 1995; Köhl, 1997; Wyn Jones & Gorham, 2002). This interpretation is corroborated by two other results: a negative correlation of sodium and potassium under saline conditions only (H. paradoxus and H. petiolaris, Table 7a) and high sodium concentrations in tissue water in H. paradoxus (> 200 mmol l−1, compare Appendix, Table A1). Sodium concentrations above 100 mmol l−1 are thought to inhibit protein synthesis in most plants when present in the cytoplasm (Flowers et al., 1986; Munns, 2002).
Sulfur contents are high only in the hybrid species
In addition to high sodium concentrations, the H. paradoxus habitat and the treatment solutions used in this study (Tables 2 and 3) were also high in sulfate, which can itself be toxic (Harborne, 1975; Tanji, 2002; Rajakaruna et al., 2003). In H. paradoxus, sulfur levels were generally elevated four to five times above the levels of the parental species (Fig. 2e), suggesting that sulfate uptake also plays a role in the salinity response. Sulfate detoxification through the production of sulfated flavonoids that are stored in the vacuole has been suggested for plants from sulfate-rich saline habitats (Harborne, 1975; Rajakaruna et al., 2003), but further studies are needed to investigate whether this is the case in H. paradoxus but not its parental species.
Potassium deficiency or high calcium concentrations do not play a role in the salinity responses of H. paradoxus and its progenitor species
A major aim of this study was to elucidate mechanisms of salt tolerance by amending field-like treatment solutions with added potassium or reduced calcium and magnesium. Potassium deficiency did not appear to contribute to growth reduction in the parental species under the saline conditions in this experiment as it does in other salt-sensitive species (Maathuis & Amtmann, 1999; Kaya et al., 2002) and neither species exhibited an increase in potassium content in the potassium addition treatment compared with the field-like treatment (Fig. 2b). However, all three species increased their selective uptake of potassium under saline conditions (sNa,K, Table 6). Similar results were reported for sunflower cultivars (Ashraf & Tufail, 1995) but not for wheat and quinoa (Wilson et al., 2002).
Calcium contents in H. paradoxus and H. petiolaris but not in H. annuus were reduced under saline compared with nonsaline conditions (Fig. 2c), indicating that in the first two species saline conditions interfered with calcium nutrition, possibly by reducing calcium activity or by impacting calcium uptake (Cramer, 2002). Positive associations of calcium contents and biomass under saline conditions but not under nonsaline conditions (Table 7b) further suggest that calcium might be involved in the response to salinity, as has been suggested by Lexer et al. (2003b). Nevertheless, a calcium concentration of 1.75 mmol l−1 (= 3.5 meq l−1, Table 3) in the Ca & Mg reduction treatment was sufficient for calcium nutrition under saline conditions in all three species as no biomass or hGR reductions were observed under this treatment compared with the field-like treatment. In most other species tested at similar salinities, the calcium concentration needed for maximal growth in moderately saline conditions was 4.5 mmol l−1 or more (Cramer, 2002). In our experiment, any adverse effects of low calcium concentration might have been balanced by growth increases caused by the lower total salinity in the Ca & Mg reduction treatment and this effect could have been responsible for the growth increase in the Ca & Mg reduction treatment observed in H. petiolaris. Experiments with a large range of both total salinities and sodium–calcium ratios are needed to further resolve the role of calcium for salt tolerance in the three species.
Patterns of population differentiation are consistent with specific parental populations giving rise to H. paradoxus and with further evolution of H. paradoxus after speciation
Population differentiation was most pronounced in H. annuus and H. paradoxus, whereas H. petiolaris populations were more uniform (Table 5, Fig. 3). Of the nine traits measured in this study, seven differed significantly between populations in H. annuus and six differed significantly between populations of H. paradoxus, but only four exhibited significant differences between populations of H. petiolaris. However, salt tolerance did not appear to differ between populations of H. annuus as hGR and biomass did not exhibit population differences or population-by-treatment interactions. Similar results of population differences in leaf ion contents without differences in salt tolerance have been reported for Armeria maritima (Köhl, 1997) and for sunflower cultivars (Ashraf & Tufail, 1995). Differences between populations in the three species may be the result of local adaptation to edaphic and climatic conditions or caused by selectively neutral population divergence. However, we cannot exclude maternal effects, because we have not controlled the maternal environment for the seeds used. Similarly, differences in the maternal environments could also have influenced our results on the species level. Only experiments with seeds produced in multiple environments could reveal whether maternal effects are important for the salt stress response in Helianthus species.
Population differentiation in H. annuus and, to a lesser extent, in H. petiolaris, leaves open the possibility that only certain – and presumably rare – combinations of parental populations could have given rise to H. paradoxus by hybridization. Indeed, more salt-tolerant populations of the parental species than those investigated here may remain to be discovered, as broad population and habitat surveys are not available for either species. The involvement of preadapted populations of the parental species in the hybrid origin of H. paradoxus is consistent with molecular data indicating a single origin of H. paradoxus in contrast to apparent multiple origins of the two other sunflower hybrid species, H. anomalus and H. deserticola (Schwarzbach & Rieseberg, 2002; Welch & Rieseberg, 2002b; Gross et al., 2003). Moreover, morphological and genetic analyses indicate that the multitrait H. paradoxus phenotype is only rarely recovered in artificial crossing experiments between the parental species (Rieseberg et al., 2003; Rosenthal et al., 2005). This is due both to problematic genetic correlations and to the fact that certain extreme phenotypes in H. paradoxus occur only rarely (or not at all) in synthetic hybrids. Conversely, population differentiation in H. paradoxus is suggestive of further adaptive or neutral evolution of H. paradoxus after hybrid speciation was completed. This constitutes an alternative explanation for the lower congruence in the genomic composition of artificial hybrids and H. paradoxus and the difficulty of recreating the H. paradoxus phenotype.
Helianthus paradoxus differs from its progenitor species both in constitutive trait expression and in reaction norms
For many traits likely to be relevant for salt tolerance, such as leaf succulence, leaf sodium content (Greenway & Munns, 1980; Flowers et al., 1986), and leaf sulfur content, H. paradoxus trait values were not similar to or intermediate between the parental species (Figs 1 and 2). Rather, the mean values of these traits in H. paradoxus exceeded levels of trait expression in both parental species regardless of the treatment (transgressive traits; Rosenthal et al., 2002; Lexer et al., 2003b). Such transgressive trait expression is thought to be the result of the segregation of genes of opposing effects (de Vincente & Tanksley, 1993), a prediction recently confirmed in Helianthus (Rieseberg et al., 2003). Potassium, calcium and magnesium contents have also previously been described as transgressive traits in H. paradoxus (Rosenthal et al., 2002; Lexer, 2003b). In this study, calcium and magnesium contents were positively transgressive under nonsaline conditions only and potassium content was negatively transgressive under saline conditions indicating that for these traits the reaction norm (rather than constitutive trait expression) differentiates H. paradoxus from its progenitor species. Such differences in reaction norms point to the physiological mechanisms involved in the salt stress response of H. paradoxus, as discussed above, and might allow this species to outcompete its progenitors in saline habitats (Bush & Van Auken, 2004). Constitutive responses that likely promote salt tolerance of H. paradoxus, such as high sodium uptake, might involve a physiological cost under nonsaline conditions and thus contribute to the lower competitive ability of this species under nonsaline conditions (Bush & Van Auken, 2004). These results underline the importance of using multiple and realistic conditions when making comparisons of species from divergent habitats.
Implications for the molecular basis of salt tolerance in H. paradoxus
The salinity response in H. paradoxus parallels findings in other halophytes despite its evolution via hybridization of two nonhalophytic species. Thus, it may be possible to make inferences about the likely molecular basis of salt tolerance in H. paradoxus based on studies of salt tolerance in other plants. In particular, we expect increased activity of tonoplast ion carriers leading to sodium compartmentation in H. paradoxus compared with the parental species. Such ion carriers might influence contents of other positively charged ions directly or indirectly through altered osmotic gradients (Yeo, 1998). This notion is supported by correlations between ion contents in this study (Table 7a) and in a study by Lexer et al. (2003b), as well as by the detection of quantitative trait loci (QTL) with pleiotropic effects on contents of several ions in a backcross between H. annuus and H. petiolaris (Lexer et al., 2003a; Rieseberg et al., 2003). The system of a halophytic hybrid species with salt-sensitive progenitors provides a promising avenue of research to determine which genetic changes are required to produce salt tolerance and thus speciation of H. paradoxus. Candidate genes for salt marsh adaptation in H. paradoxus, including a calcium-dependent protein kinase and a transcriptional regulator, have recently been proposed (Lexer et al., 2004), and gene expression studies in the three species are currently underway.
Acknowledgments
We thank E.J. Baack for sunflower seeds and J. L. Strasburg, Y. Sapir, K. D. Whithney, and D. J. van der Nat for comments on the manuscript. This research was supported by grant 1777/1-1 of the Deutsche Forschungsgemeinschaft (DFG) to SK, Erwin-Schrödinger grant J 2148 of the Austrian Science Foundation (FWF) to C.L. and grants R01 G059065 of NIH and DEB 0314654 of NSF to L.H.R.
Appendix
Table A1.
Concentrations in tissue water of sodium, potassium, and sulfur in a salt tolerance experiment with the hybrid species Helianthus paradoxus and its progenitors Helianthus annuus and Helianthus petiolaris
| H. annuus | H. paradoxus | H. petiolaris | |
|---|---|---|---|
| Sodium (mmol l−1) | |||
| Nonsaline | 2.608 (1.816–3.745) | 16.28 (13.66–19.40) | 4.298 (3.107–5.944) |
| Field-like | 11.818 (8.239–16.842) | 242.1 (216.2–271.1) | 52.435 (41.312–66.552) |
| K addition | 8.059 (5.380–12.073) | 252.0 (219.6–289.4) | 45.908 (30.570–68.492) |
| Ca & Mg reduction | 9.675 (6.966–13.437) | 268.6 (238.7–302.3) | 31.197 (23.164–42.015) |
| Potassium (mmol l−1) | |||
| Nonsaline | 369.5 (323.8–415.3) | 286.6 (251.3–321.8) | 430.7 (394.4–467.0) |
| Field-like | 492.1 (420.3–563.9) | 121.3 (96.4–146.2) | 503.7 (452.6–554.8) |
| K addition | 493.9 (432.9–555.0) | 145.4 (116.0–174.7) | 497.2 (455.0–539.4) |
| Ca & Mg reduction | 470.3 (402.3–538.4) | 106.3 (84.9–127.8) | 481.5 (444.0–520.0) |
| Sulfur (mmol l−1) | |||
| Nonsaline | 18.95 (15.27–23.50) | 64.68 (57.40–72.90) | 24.54 (21.77–27.66) |
| Field-like | 16.33 (13.37–19.94) | 77.09 (67.00–88.70) | 15.97 (14.29–17.86) |
| K addition | 16.50 (13.41–20.30) | 85.66 (72.55–101.15) | 16.54 (15.64–18.69) |
| Ca & Mg reduction | 15.57 (12.88–18.82) | 82.78 (72.45–94.58) | 15.16 (13.55–16.95) |
Means (potassium) or medians (sodium and sulfur) are given with 95% confidence intervals.
References
- Ashraf M, Tufail M. Variation in salinity tolerance in sunflower (Helianthus annuus L.) Journal of Agronomy and Crop Science. 1995;174:351–362. [Google Scholar]
- Bressan RA, Hasegawa PM, Pardo JM. Plants use calcium to resolve salt stress. Trends in Plant Science. 1998;3:411–412. [Google Scholar]
- Buerkle CA, Morris RJ, Asmussen MA, Rieseberg LH. The likelihood of homoploid hybrid speciation. Heredity. 2000;84:441–451. doi: 10.1046/j.1365-2540.2000.00680.x. [DOI] [PubMed] [Google Scholar]
- Bush JK, Van Auken OW. Relative competitive ability of Helianthus paradoxus and its progenitors, H. annuus and H. petiolaris (Asteraceae), in varying soil salinities. International Journal of Plant Sciences. 2004;165:303–310. [Google Scholar]
- Cochran WG, Cox GM. Experimental designs. New York, NY, USA: John Wiley & Sons, Inc; 1957. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA, USA: Sinauer; 2004. [Google Scholar]
- Cramer GR. Sodium–calcium interactions under salinity stress. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht, the Netherlands: Kluwer; 2002. pp. 205–227. [Google Scholar]
- Flowers TJ, Hajibagheri MA, Clipson NJW. Halophytes. Quarterly Review of Biology. 1986;61:313–336. [Google Scholar]
- Grant V. Plant speciation. New York, NY, USA: Columbia University Press; 1981. [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance in non-halophytes. Annual Review of Plant Physiology. 1980;31:149–190. [Google Scholar]
- Gross BL, Rieseberg LH. The ecological genetics of homoploid hybrid speciation. Journal of Heredity. 2005;96:241–252. doi: 10.1093/jhered/esi026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross BL, Schwarzbach AE, Rieseberg LH. Origin(s) of the diploid hybrid species Helianthus deserticola (Asteraceae) American Journal of Botany. 2003;90:1708–1719. doi: 10.3732/ajb.90.12.1708. [DOI] [PubMed] [Google Scholar]
- Harborne JB. Flavonoid sulphates: a new class of sulphur compounds in higher plants. Phytochemistry. 1975;14:1147–1155. [Google Scholar]
- Kaya C, Higgs D, Sakar E. Response of two leafy vegetables grown at high salinity to supplementary potassium and phosphorus during different growth stages. Journal of Plant Nutrition. 2002;25:2663–2676. [Google Scholar]
- Köhl KI. The effect of NaCl on growth, dry matter allocation and ion uptake in salt marsh and inland populations of Armeria maritima. New Phytologist. 1997;135:213–225. [Google Scholar]
- Levin DA. Ecological speciation: crossing the divide. Systematic Botany. 2004;29:807–816. [Google Scholar]
- Lexer C, Welch ME, Durphy JL, Rieseberg LH. Natural selection for salt tolerance quantitative trait loci (QTLs) in wild sunflower hybrids: Implications for the origin of Helianthus paradoxus, a diploid hybrid species. Molecular Ecology. 2003a;12:1225–1235. doi: 10.1046/j.1365-294x.2003.01803.x. [DOI] [PubMed] [Google Scholar]
- Lexer C, Welch ME, Raymond O, Rieseberg LH. The origin of ecological divergence in Helianthus paradoxus (Asteraceae): Selection on transgressive characters in a novel hybrid habitat. Evolution. 2003b;57:1989–2000. doi: 10.1111/j.0014-3820.2003.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Lexer C, Lai Z, Rieseberg LH. Candidate gene polymorphisms associated with salt tolerance in wild sunflower hybrids: implications for the origin of Helianthus paradoxus, a diploid hybrid species. New Phytologist. 2004;161:225–233. doi: 10.1046/j.1469-8137.2003.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- Maathuis FJ, Amtmann A. K+ nutrition and Na+ toxicity: the basis of cellular K+/Na+ ratios. Annals of Botany. 1999;84:123–133. [Google Scholar]
- Madhok OP, Walker RB. Magnesium nutrition in two species of sunflower. Journal of Ecology. 1969;44:1016–1022. doi: 10.1104/pp.44.7.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant, Cell & Environment. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Munns R, Greenway H, Kirst GO. Halotolerant eukaryotes. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Encyclopedia of plant physiology. Berlin, Germany: Springer; 1983. pp. 59–135. [Google Scholar]
- Newman JA, Bergelson J, Grafen A. Blocking factors and hypothesis tests in ecology: Is your statistics text wrong? Ecology. 1997;78:1312–1320. [Google Scholar]
- Niu X, Bressan RA, Hasegawa PM, Pardo JM. Ion homeostasis in NaCl stress environments. Plant Physiology. 1995;109:735–742. doi: 10.1104/pp.109.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman MG. Ion uptake by plant roots. In: Lüttge U, Pitman MG, editors. Encyclopedia of plant physiology. Transport in higher plants. Berlin, Germany: Springer; 1976. pp. 95–128. [Google Scholar]
- R Development Core Team. Royal: a language and environment for statistical computing. Vienna, Austria: Royal Foundation for Statistical Computing; 2005. http://www.Royal-project.org. [Google Scholar]
- Rajakaruna N, Siddiqi MY, Whitton J, Bohm BA, Glass ADM. Differential responses to Na+/K+ and Ca2+/Mg2+ in two edaphic races of the Lasthenia californica (Asteraceae) complex: a case for parallel evolution of physiological traits. New Phytologist. 2003;157:93–103. doi: 10.1046/j.1469-8137.2003.00648.x. [DOI] [PubMed] [Google Scholar]
- Reimann C, Breckle SW. Salt tolerance and ion relations of Salsola kali L. – differences between ssp. tragus (L.) Nyman and ssp. ruthenica (Iljin) Soo. New Phytologist. 1995;130:37–45. [Google Scholar]
- Rieseberg LH. Homoploid reticulate evolution in Helianthus (Asteraceae) – evidence from ribosomal genes. American Journal of Botany. 1991;78:1218–1237. [Google Scholar]
- Rieseberg LH, Carney SE. Plant hybridization. New Phytologist. 1998;140:599–624. doi: 10.1046/j.1469-8137.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Raymond O, Rosenthal DM, Lai Z, Livingstone K, Nakazato T, Durphy JL, Schwarzbach AE, Donovan LA, Lexer C. Major ecological transitions in wild sunflowers facilitated by hybridization. Science. 2003;301:1211–1216. doi: 10.1126/science.1086949. [DOI] [PubMed] [Google Scholar]
- Rogers CE, Thompson TE, Seiler GE. Sunflower species of the United States. Fargo, ND, USA: The National Sunflower Association; 1982. [Google Scholar]
- Rosenthal DM, Schwarzbach AM, Donovan LA, Raymond O, Rieseberg LH. Phenotypic differentiation between three ancient and hybrid taxa and their parental species. International Journal of Plant Sciences. 2002;163:387–398. [Google Scholar]
- Rosenthal DM, Rieseberg LH, Donovan LA. Recreating ancient hybrid species’ complex mulitrait phenotypes from early generation synthetic hybrids: three examples using wild sunflowers. American Naturalist. 2005;166:26–41. doi: 10.1086/430527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbach AE, Rieseberg LH. Likely multiple origins of a diploid hybrid sunflower species. Molecular Ecology. 2002;11:1703–1715. doi: 10.1046/j.1365-294x.2002.01557.x. [DOI] [PubMed] [Google Scholar]
- Schwarzbach AE, Donovan LA, Rieseberg LH. Transgressive character expression in a hybrid sunflower species. American Journal of Botany. 2001;88:270–277. [PubMed] [Google Scholar]
- Stebbins GL. Variation and evolution in plants. New York, NY, USA: Columbia University Press; 1950. [Google Scholar]
- Tanji KK. Salinity in the soil environment. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht, the Netherlands: Kluwer; 2002. pp. 21–52. [Google Scholar]
- Tester M, Davenport R. Na+ tolerance and Na+ transport in higher plants. Annals of Botany. 2003;91:503–527. doi: 10.1093/aob/mcg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S-Plus. New York, NY, USA: Springer; 2002. [Google Scholar]
- Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–647. [Google Scholar]
- de Vincente MC, Tanksley SD. QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics. 1993;134:585. doi: 10.1093/genetics/134.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch ME, Rieseberg LH. Habitat divergence between a homoploid hybrid sunflower species, Helianthus paradoxus (Asteraceae), and its progenitors. American Journal of Botany. 2002a;89:472–478. doi: 10.3732/ajb.89.3.472. [DOI] [PubMed] [Google Scholar]
- Welch ME, Rieseberg LH. Patterns of genetic variation suggest a single, ancient origin for the diploid hybrid species Helianthus paradoxus. Evolution. 2002b;56:2126–2137. doi: 10.1111/j.0014-3820.2002.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Wilson C, Read JJ, Abo-Kassem E. Effect of mixed-salt salinity on growth and ion relations of a quinoa and a wheat variety. Journal of Plant Nutrition. 2002;25:2689–2704. [Google Scholar]
- Wyn Jones G, Gorham J. Intra- and inter-cellular compartmentation of ions. In: Läuchli A, Lüttge U, editors. Salinity: environment–plants–molecules. Dordrecht, the Netherlands: Kluwer; 2002. pp. 159–180. [Google Scholar]
- Yeo A. Molecular biology of salt tolerance in the context of whole-plant physiology. Journal of Experimental Botany. 1998;49:915–929. [Google Scholar]
- Zar JH. Biostatistical analysis. Upper Saddle River, NJ, USA: Prentice Hall; 1999. [Google Scholar]