Abstract
Positron emission tomography (PET) was used to investigate differences in neural plasticity associated with learning a unique motor task in patients with schizophrenia and healthy volunteers. Working with a robotic manipulandum, subjects learned reaching movements in a force field. Visual cues were provided to guide the reaching movements. PET rCBF measures were acquired while participants learned the motor skill over successive runs. The groups did not differ in behavioral performance but did differ in their rCBF activity patterns. Healthy volunteers displayed blood flow increases in primary motor cortex and supplementary motor area with motor learning. The patients with schizophrenia displayed an increase in the primary visual cortex with motor learning. Changes in these regions were positively correlated with changes in each group’s motor accuracy, respectively. This is the first study to employ a unique arm-reaching motor learning test to assess neural plasticity during multiple phases of motor learning in patients with schizophrenia. The patients may have an inability to rapidly tune motor cortical neural populations to a preferred direction. The visual system, however, appears to be highly compensated in schizophrenia and the inability to rapidly modulate the motor cortex may be substantially corrected by the schizophrenic group’s visuomotor adaptations.
Keywords: PET, Visuomotor, Arm reaching, Neural plasticity, rCBF, Motor learning
1. Introduction
Practiced reaching movements, performed in a novel force field will adapt to the physical constraints that characterize the field. The direction, force magnitude, and acceleration applied by the field will dictate the accommodations required of a person’s nervous system and musculature (Shadmehr et al., 1993; Shadmehr and Wise, 2005). By integrating information obtained from peripheral joint and muscle sensors, a person’s spinal cord and brain generate activity patterns in conjunction with performance feedback (Shadmehr and Mussa-Ivaldi, 1994; Shadmehr and Holcomb, 1997). Motor learning is time- and experience-dependent, and requires a minimum amount of practice time and protection from alternative, interfering motor experiences (Karni and Sagi, 1993; Brashers-Krug et al., 1996; Shadmehr and Brashers-Krug, 1997; Smith et al., 2006). Consequently, both the acquisition of an adaptive movement or motor skill and its consolidation require blocks of time and protection from interfering alternative programs. The psychophysics of motor learning has been extensively characterized in humans (Shadmehr and Mussa-Ivaldi, 1994) and non-human primates (Gandolfo et al., 2000). But it is largely unknown to what extent specific brain regions are dedicated to motor program acquisition at specific stages of performance (Karni et al., 1995, 1998; Floyer-Lea and Matthews, 2004, 2005). The studies cited concern motor movement sequences and visual-motor tracking. Motor adaptation to a force field has not been extensively investigated in neuroimaging research (Shadmehr and Holcomb, 1997; Doyon et al., 2003). As a result, it is not known how one’s brain changes as an internal model of movement in a force field is created.
Schizophrenic persons are often unable to construct accurate representations of their environments (Frith et al., 2000b; Danckert et al., 2004; Shergill et al., 2005). Their inability to generate stable, representative models may arise from a failure to dedicate, or use, appropriate brain regions that provide salient information (Wolpert et al., 1995). This could contribute to delusions of control (Frith et al., 2000a), misperceptions, and hallucinations. At the very least it is likely to promote imprecise, error-prone responses to rapidly changing environmental demands. It is important to ascertain the adaptive capacity of schizophrenic persons. Similarly it is important to determine to what extent practice at a difficult task can help a schizophrenic person develop skilled responses to unpredictable demands. Reaching movements in a novel force field may provide a model for studies of this problem.
One prior report showed behavioral and biological “normalization” in persons with schizophrenia who practiced a motor (Kodama et al., 2001) task. Improved performance was associated with a relatively “normalized” neural activity pattern. Those subjects practiced for an extended time period that lasted at least 1 week. It is unclear whether neural activity in persons with schizophrenia will change from an abnormal pattern to a normal pattern in a short time period (min), when practice at a task results in a normal performance or accuracy. This study was undertaken to demonstrate whether (and how) volunteers with schizophrenia use appropriate brain regions to acquire the skills needed to make effective movements in a force field.
Reaching, grasping, and pointing constitute motor behaviors that are over learned and highly adaptive with respect to different physical and social circumstances (Shadmehr and Wise, 2005). Schizophrenic subjects may be unable to adapt their reaching movements to novel force fields as quickly or precisely as healthy volunteers (Malenka et al., 1986; Sullivan et al., 1994; Schröder et al., 1999; Kumari et al., 2002; Exner et al., 2006). Their ability to use feedback regarding joint angles, limb movement direction and velocity/acceleration information may be compromised. An extensive literature documenting their failure to monitor various types of error and error likelihood correctly (Carter et al., 2001; Alain et al., 2002; Holcomb, 2004) is consistent with reports of diminished motor skills. It is not known to what extent they rely on primary and secondary motor cortex when adapting to novel forces. It is also unclear to what extent schizophrenic subjects develop neural representations of limb dynamics or object dynamics (Cothros et al., 2006). Perceptual studies using functional neuroimaging methods have shown that schizophrenic subjects may be able to make difficult sensory judgments using “alternative” neural systems (Hong et al., 2005; Gur et al., 2007). The predisposition to rely on one system over another may help research scientists and clinicians better appreciate the adaptive limits and possibilities characteristic of schizophrenia.
The task used in this study permitted us to study the neurobiology of reaching movements in a force field during skill acquisition. We predicted that (1) healthy volunteers would exhibit a shift in rCBF activity from prefrontal cortex to motor regions with motor learning; and (2) volunteers with schizophrenia would exhibit increased rCBF activity in motor cortical regions with motor learning but initial frontal rCBF activity was expected to be diminished or absent.
2. Methods
2.1. Participants
Eight patients with schizophrenia (females; mean age 35.7 (S.D.=9.3) and eight healthy volunteers (two females, mean age 22.3 (S.D.=2.7)) participated in this study. All subjects were right-handed. Patients with a DSM-IIIR diagnosis of schizophrenia were recruited from the Maryland Psychiatric Research Center outpatient clinics. Patients were clinically stable (BPRS total mean=28.9 S.D.=5.7) and were treated with clozapine (n=4) or olanzapine (n=4). Concomitant medications included lorazepam (n=1), klonazepam (n=1), risperidone (n=1), and sertraline (n=1). Patients were excluded if they had a diagnosis of a neurological disorder, mental retardation, history of severe head trauma, or substance use disorder not fully remitted. Volunteers with schizophrenia were evaluated for their ability to provide informed consent before signing consent documents. Healthy volunteers had no past or present psychiatric or neurological disorder, no substance use disorder, and no first-degree relatives with a diagnosis of a psychotic disorder.
All volunteers gave written informed consent. The University of Maryland Internal Review Board and the Johns Hopkins University Joint Committee on Clinical Investigation provided oversight and clinical approval. All subjects were paid for their participation.
2.2. PET image acquisition
PET scans were obtained by using the General Electric 4096+ system, which produces 15 brain image slices at an intrinsic resolution of 6.1 mm in each dimension. The bolus [15O] H2O method (Raichle et al., 1983; Herscovitch et al., 1983) was used without arterial blood sampling; radiolabeled water was administered through a catheter inserted into the left antecubital vein. Approximately 62 mCi of [15O] H2O were administered 20 s before each scan. Accumulated radioactivity in the 90 s after initiation of the scan was used as an index of rCBF. Scans were acquired at 10-min intervals. The motor task was initiated 90 s before administration of the bolus and continued until completion of the scan.
2.3. Motor task
The methods used in this study have been previously published (Shadmehr and Mussa-Ivaldi, 1994; Shadmehr and Holcomb, 1997, 1999; Nezafat et al., 2001). The following description reflects those earlier publications.
One to five days before the experiment, participants were trained on the task. Participants moved the handle of a robotic arm using their dominant hand (all subjects were right-handed) while lying in the scanner. A monitor displayed a cursor indicating the handle’s position. The robotic arm was attached to a motor that delivered a novel dynamic force against the participant’s movement (Shadmehr and Mussa-Ivaldi, 1994). Subjects gripped the handle of the robot with their right hand and viewed a monitor that displayed a cursor corresponding to the handle’s position. The task was to take the handle to a series of targets. Subjects were told to reach for the target. In order to be successful the subject was required to reach the target within 500±50 ms. Targets were placed 10 cm from the starting position. A target appeared at one of eight directions. The target turned blue if the subject reached it too late, red if he/she reached it too soon, and “exploded” with a distinctive sound if the target was reached within the allotted time. One second after a target was reached, the next target appeared. During a pre-training session, the robot motor was turned off while subjects practiced 400 targets. On the day of the PET study, subjects practiced the task again with the robot motors turned off. After that practice we acquired rCBF measures on two repetitions of five successive conditions. This report concerns three of those conditions (indicated with an asterisk), Random (R), Early Learning (A1) and Late Learning (A2). The order of conditions was the following:
1. During a null field condition in which the robot’s motors were off.
2*. During a random field condition in which the robot produced a random, non-stationary velocity-dependent force field representing an unlearnable mechanical system. This condition was designated “R.”
3*. During an early learning condition in which the robot produced a stationary force field (A). This field was a linear function of the hand velocity vector and produced a force that was at all times perpendicular to the actual direction of hand motion. Early learning in field A was designated “A1.”
4*. During a late learning condition in which participants performed the task with skill after additional practice in field A. Late learning in field A was designated “A2.”
5. During a second, learnable field (B). This field was mathematically anticorrelated with field A. The forces were rotated 180°. Subjects learned field B 10 min after completion of field A practice. This condition is not discussed here.
Manipulandum joint angles and joint velocities were sampled at a rate of 100 Hz. Hand positions and velocities were computed. The performance measure was the similarity between the hand trajectory in the force field and a baseline trajectory in the null field measured for each subject. A detailed description of this procedure was presented by Shadmehr and Mussa-Ivaldi (1994).
2.4. Statistical analysis
Movement distance was analyzed with a 2(group)×2 (learning phase) mixed factorial ANOVA with repeated measures for learning. Alpha (P) was set at 0.05.
PET: All scans were realigned and spatially normalized into the stereotaxic space of Talairach and Tournoux (1988). Images were smoothed (Poline et al., 1995, 1997) with a full width at half maximum (FWHM) of 10×10×10 mm in the x, y, and z planes. Before anatomical normalization voxels were 2×2×4.25 (millimeters) and after normalization were 2×2×2 millimeters. Pixel rCBF values were scaled using the ratio adjustment method. The image data were analyzed using Statistical Parametric Mapping (SPM99; Welcome Department of Cognitive Neurology, London, England), where voxel by voxel comparisons determined significant changes in rCBF (P≤0.005, uncorrected). Activity change clusters of spatially contiguous voxels (20 voxels in a cluster above a statistical threshold of T=2.35) were assessed on the basis of activation magnitude and spatial extent (P≤0.05) (Worsley et al., 1995; Poline et al., 1997).
3. Results
3.1. Changes in motor behavior accuracy with practice
Motor learning was exhibited by both groups, as indicated by significant main effect of learning phase (F(1,12)=9.7, P<0.01). Both groups decreased in movement length from early [SZ:116 mm (S.D.=20); NV:119 mm (S.D.=11)] to late [SZ:109 mm (S.D.=15); NV:114 mm (S.D.=10)] learning phases. Subjects with schizophrenia and normal volunteers did not significantly differ in motor learning performance (F (1,12)=0.3, P>0.05). The group by learning phase interaction was not significant (F(1,12)=0.21, P>0.05).
Table 1 contains SPM rCBF differences described by region, Talairach coordinate location, voxel extent (Ke) and statistic (Z).
Table 1.
Motor task contrasts, regional cerebral blood flow changes
| Group | Region | Location | Voxel extent | Statistic |
|---|---|---|---|---|
| Early learning minus random (A1-R) | ||||
| NV | Left anterior cingulate | x=-4, y=30, z=-2 | Ke=388 | Z=3.11, P<0.001 |
| Left visual cortex | x=-26, y=-74, z=12 | Ke=109 | Z=3.25, P<0.001 | |
| Left ventrolateral thalamus | x=-20, y=-16, z=4 | Ke=100 | Z=3.29, P<0.001 | |
| SZ | Right para/hippocampus | x=42, y=-30, z=-12 | Ke=185 | Z=4.75, P<0.001 |
| Random minus early learning (R-A1) | ||||
| NV | Right motor cortex | x=68, y=-34, z=40 | Ke=289 | Z=3.77, P<0.001 |
| Left motor cortex | x=-48, y=-32, z=30 | Ke=587 | Z=3.42, P<0.001 | |
| Right middle frontal cortex | x=44, y=26, z=42 | Ke=198 | Z=3.48, P<0.001 | |
| SZ | Left inferior occipital gyrus | x=-40, y=-92, z=-8 | Ke=98 | Z=3.72, P<0.001 |
| Left inferior parietal lobe | x=-68, y=-40, z=22 | Ke=174 | Z=3.65, P<0.001 | |
| Late minus early learning (A2-A1) | ||||
| NV | Supplementary motor area | x=6, y=-2, z=46 | Ke=205 | Z=3.42, P<0.001 |
| Right motor cortex | x=52, y=-22, z=46 | Ke=110 | Z=3.27, P<0.001 | |
| Left motor cortex | x=-54, y=-6, z=42 | Ke=200 | Z=2.83, P<0.002 | |
| SZ | Bilateral occipital cortex | x=6, y=-90, z=14 | Ke=497 | Z=3.35, P<0.001 |
| Early minus late learning (A1-A2) | ||||
| NV | Right ventral mid-frontal | x=42, y=54, z=-8 | Ke=662 | Z=3.86, P<0.0001 |
| Left middle temporal | x=-62, y=-26, z=-14 | Ke=354 | Z=3.85, P<0.0001 | |
| Right middle temporal | x=68, y=-24, z=-14 | Ke=212 | Z=3.58, P<0.0001 | |
| Left superior temporal | x=-42, y=-30, z=6 | Ke=121 | Z=3.70, P<0.0001 | |
| SZ | Right para/hippocampal | x=40, y=-30, z=-14 | Ke=177 | Z=4.19, P<0.0001 |
| Double subtraction with A2-A1 | ||||
| NV-SZ | Right motor cortex | x=46, y=-10, z=48 | Ke=121 | Z=2.99, P<0.005 |
| SZ-NV | Occipital cortex | x=6, y=-88, z=14 | Ke=14 | Z=3.19, P<0.001 |
| SZ-NV | Occipital cortex | x=24, y=-72, z=10 | Ke=62 | Z=2.89, P<0.002 |
3.2. rCBF activity changes with early learning versus control (A1 - R) and (R - A1)
Normal volunteers exhibited greater activity during early learning (A1) than the control condition (R) in the left anterior cingulate, left visual cortex, and left ventrolateral thalamus. Schizophrenics exhibited greater activity during early learning (A1) than the control condition (R) in the right para/hippocampal region. See Fig. 1 for illustration of increased activity with early learning.
Fig. 1.
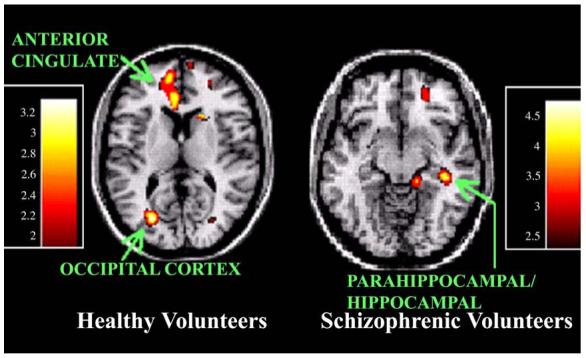
Increased rCBF activity with early motor learning versus control (A1 - R). Healthy volunteers exhibit greater ventral medial frontal activity and occipital activity during early learning than during the random field condition. Schizophrenic subjects show greater activity in right hippocampal and parahippocampal areas during early learning than during the random field condition.
Normal volunteers exhibited diminished activity with early learning (A1) compared to the control condition (R) in the right motor cortex, left motor cortex, and right middle frontal cortex. Schizophrenic volunteers exhibited diminished activity with early learning (A1) compared to the control condition (R) in the left inferior occipital gyrus, and left inferior parietal lobule. See Fig. 2 for illustration of diminished activity with early learning.
Fig. 2.
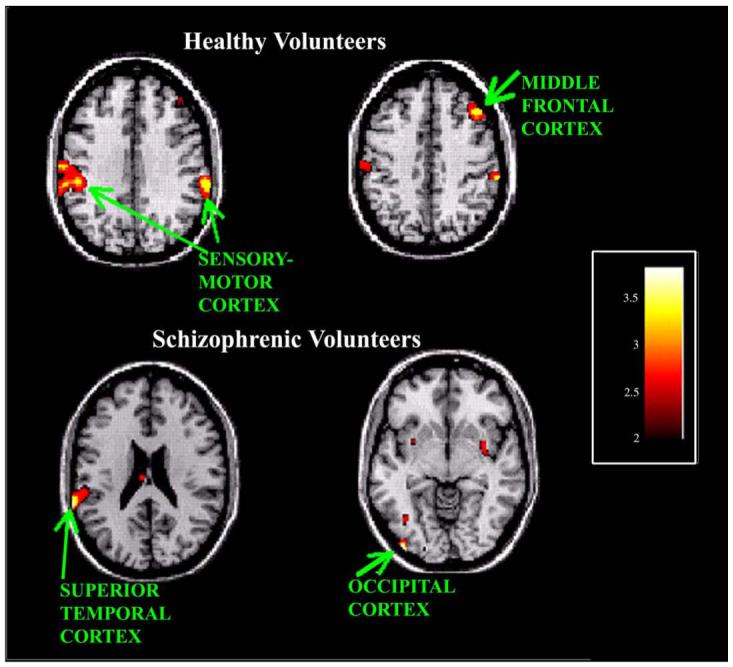
Decreased rCBF activity with early motor learning versus control (R - A1). Healthy volunteers exhibit greater rCBF in sensory-motor regions, bilaterally, during the random field condition. Activity in the right dorsolateral prefrontal region is also greater in healthy volunteers during the random field condition. Schizophrenic subjects show greater activity in the left inferior parietal lobule and the left occipital region during the random field condition than the early learning condition.
3.3. rCBF changes associated with late motor learning (A2 - A1) and (A1 - A2)
Normal volunteers exhibited greater activity during A2 than A1 in the supplementary motor area, right motor cortex, and left motor cortex. Schizophrenic volunteers exhibited greater activity during A2 than A1 only in bilateral occipital cortex. See Fig. 3 for illustration of increased activity with late learning.
Fig. 3.
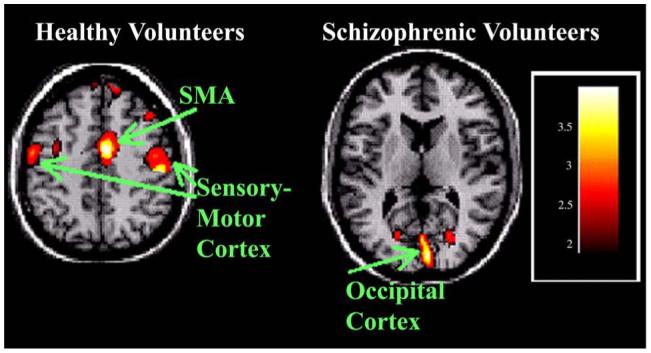
Increased rCBF activity with late motor learning (A2 - A1). Healthy volunteers exhibit greater rCBF in primary motor regions, bilaterally, during the final, late learning phase, than during the early learning phase. This is accompanied by a marked increase in the supplementary motor area (SMA) as well. Schizophrenic subjects show greater activity in the occipital region only during late learning.
Normal volunteers sustained greater activity in the early learning phase (A1) than the late phase (A2) in the right ventral mid-frontal, left middle temporal, right middle temporal, and left superior temporal. Schizophrenic volunteers exhibited greater activity during early learning (A1) than late learning (A2) in the right hippocampal/parahippocampal. See Fig. 4 for illustration of diminished activity with late learning.
Fig. 4.
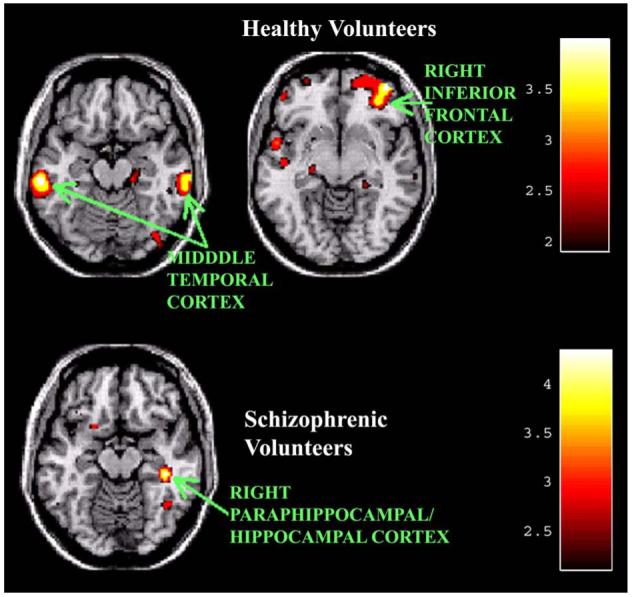
Decreased rCBF activity with late motor learning (A1 - A2). Healthy volunteers exhibit greater rCBF in middle temporal cortical regions bilaterally, right ventral frontal, and left superior temporal cortex during early learning than late learning. Schizophrenic subjects show greater activity in the right hippocampal/parahippocampal region during early learning than late learning.
3.4. Normal versus schizophrenia volunteers rCBF activity differences with motor learning
A double subtraction (NV minus SZ, A2 minus A1) with masking of decreased activity in the schizophrenia group was obtained. This contrast showed that normal volunteers have greater right motor cortex activity than schizophrenic participants, with motor learning.
A double subtraction (SZ minus NV, A2 minus A1) with masking of decreased activity in the normal group was also generated. It showed that schizophrenic volunteers have greater occipital activity than healthy controls with motor learning. The brain regions that revealed group differences are in comparable locations to observations of rCBF increases with learning in normal and schizophrenia volunteers, separately (see Fig. 3).
4. Discussion
The purpose of this study was to investigate neural changes associated with learning a unique motor task that required subjects to make visually guided reaching movements in a force field. The serial blood flow studies showed us how the activity in multiple brain regions responded to different dynamic environments across time. The robotic manipulandum gave us a means to assess reaching movements involving multiple joints, multiple muscle groups, and multiple stages of neural adaptation. The robotic manipulandum was particularly well suited to the PET environment. Its motorized, electromechanical interface makes it unsuitable in the magnetic environment required for functional magnetic resonance (fMRI) research. Though recent technical advances have made it possible to perform some reaching studies with fMRI (Diedrichsen et al., 2005) only healthy control subjects have participated in this new technology thus far.
Healthy control and schizophrenic volunteers showed similar learning patterns when the force field dynamics were fixed. In spite of these learning similarities, schizophrenic volunteers showed markedly different patterns of neural plasticity across the six scans considered here.
When subjects switched from a Random force field to a predictable force field (A1), healthy volunteers exhibited a significant rCBF decline in primary motor cortex, sensory-motor cortex, and right frontal. In contrast, volunteers with schizophrenia declined in the lateral occipital (visual cortex) and parietal regions. The switch from Random forces to predictable forces (A1) was accompanied by increased activity in ventrolateral thalamus, rostral/anterior cingulate, and medial primary visual cortex in the healthy volunteers. Schizophrenia volunteers responded to the switch from Random to A1 with increased activity in the right parahippocampal region.
The switch from early learning (A1) to late learning (A2) was associated with significantly greater blood flow in primary motor cortex and the supplementary motor area of healthy volunteers. In volunteers with schizophrenia this switch was associated with a marked increase in midline primary visual cortex blood flow. The increased blood flow associated with late learning (A2 - A1) in the healthy volunteers’ primary motor cortex, and the schizophrenia volunteers’ primary visual cortex were positively correlated with their respective accuracy changes from early to late learning trials.
The physiological changes associated with these switches reflect the influences of practice, time, and predictability. By practicing movements in a learnable force field subjects diminish the need for some neural systems and increase the need for others. This dynamic activity pattern has been described with learning (Ungerleider et al., 2002) and appears to be skill and time dependent (Karni et al., 1998; Shadmehr et al., 1998; Gandolfo et al., 2000; Nezafat et al., 2001; Korman et al., 2003). These changes presumably reflect processes associated with long-term potentiation and long-term depression, the principal physiological mechanisms responsible for plasticity and adaptation (Rioult-Pedotti et al., 1998; Monfils and Teskey, 2004). The data presented here are compatible with rapid switches in regional activity patterns. We believe these changes are required for motor performance learning and reflect the extent to which different neural assemblies accommodate the unique dynamics specific to this task (Chen and Wise, 1996; Laubach et al., 2000).
The pattern changes in these two groups are discussed in parts. First, we consider the switch from the random dynamic field trials to the early learning (A1) trials. Second, we consider the switch from the early learning (A1) trials to the late learning (A2) trials, their activity changes and the correlations they exhibited in conjunction with practice.
4.1. Random to A1, early learning pattern changes
Healthy volunteers exhibited marked neural activity reductions in the contralateral sensory-motor cortex and the right middle frontal cortex when they switched from random to early learning (A1) trials. In contrast, schizophrenia volunteers exhibited only occipital and parietal reductions but no frontal cortex changes. When initially confronted with the random force field, healthy volunteers may have relied on connections between the frontal and sensory-motor cortex to adapt to these unpredictable forces. Frontal activity likely reflects rapid judgments and kinetic predictions in a novel environment. This is in agreement with studies that reported marked blood flow declines in frontal activity during a shift from an unpredictable to a predictable motor task (Deiber et al., 1997; Grafton et al., 1998; Mushiake et al., 2006). It is also in agreement with this group’s prior study (Shadmehr and Holcomb, 1997).
The sensory-motor cortex decline in the healthy volunteers may represent a diminished demand for motor output when subjects shift from a random (R) to a learnable force field (A1). Schizophrenic volunteers showed reduced activity in the lateral occipital region when switching from random to early learning (A1). Given this group’s subsequent recruitment of greater visual resources during late learning (A2), the initial drop associated with the R to A1 shift may prefigure their marked reliance on visual cues. This is consistent with the idea that motor trajectory prediction, which uses substantial visual cues, precedes motor trajectory control (Flanagan et al., 2003). It is, however, also consistent with their generating a different kind of internal model, one that is primarily associated with the object being moved and not their own limbs, which are doing the moving (Cothros et al., 2006). This study cannot answer that question. But we can anticipate studies with schizophrenic subjects that will explicitly monitor brain activity changes associated with two different types of error, execution errors or target-based errors (Diedrichsen et al., 2005).
The A1 condition also reveals a significant shift to rostral anterior cingulate and ventrolateral thalamic activity in healthy volunteers (higher in A1 than R). The substantial inter-regional connections between thalamus and cingulate may support initial learning (Alexander et al., 1986; Hoover and Strick, 1993; Middleton and Strick, 2002). In the schizophrenia group, reliance on parahippocampal regions may reflect this group’s tendency to compensate through visually guided navigational strategies (Wiener et al., 1989; Dragoi and Buzsaki, 2006) rather than kinesthetic planning and adaptation. This is compatible with the group’s reliance on a target-based internal model.
Healthy subjects learn this task by emphasizing the relationship between motor errors and the proprioceptive state of their arm (Hwang and Shadmehr, 2005). As a consequence, they generalize their adaptation from one configuration of the arm to another in coordinates of the joints and muscles, and not the coordinate system of the visual feedback (Shadmehr and Moussavi, 2000). The results in the schizophrenic patients suggest that the learning was an association between motor errors and the visual state of the cursor. This suggests that generalization in this group would be fundamentally different than in the healthy population. This prediction remains to be tested.
4.2. A1 to A2, late learning pattern changes
Healthy volunteers exhibited large neural activity reductions associated with learning. Ventrolateral frontal cortex, middle temporal cortex, and superior frontal cortex were significantly lower during A2 than A1. Previous reports from our research group emphasized an important role for the ventral frontal cortex during shifts from initial learning to late (5.5 h later) motor task performance (Shadmehr and Holcomb, 1997). The ventral frontal cortex may be particularly important during early learning. Its activity seems to be essential during the initial acquisition of a task characterized by a high error rate. Lesions of this region show that non-human primates are greatly diminished in their ability to learn a new visuomotor association within a session, but not across multiple sessions (Bussey et al., 2001). GABAergic antagonist infusion to this region is particularly potent in reducing a primate’s new strategies needed to sustain a task with novel-pattern response associations (Wang et al., 2000). These studies are consistent with the marked decline in ventral frontal activity observed in healthy volunteers during late learning when errors have substantially diminished (i.e. shift A1 to A2).
The schizophrenic group showed a marked decline in parahippocampal activity with the shift from A1 to A2. This occurred in conjunction with an activity rise in primary visual cortex. This combination suggests that visual neurons are being effectively tuned to assist with hand-trajectory planning. Parahippocampal assemblies may become less important for reaching as the visual system becomes better “trained.”
Middle temporal regions have been studied extensively with respect to motion perception and eye movements (Ungerleider and Desimone, 1986; Komatsu and Wurtz, 1988). Those studies confirm a robust role for this region when subjects follow objects in motion and make predictions of their trajectories. Furthermore, investigations have confirmed a prominent role for superior temporal cortex in motion perception and motor planning (Komatsu and Wurtz, 1988; Geesaman et al., 1997; Nelissen et al., 2006). The marked reductions in middle and superior temporal cortical blood flow associated with the shift from A1 to A2 may reflect the healthy volunteers’ ability to ultimately represent the internal model (Wolpert et al., 1995) of this task in primary motor cortex and the supplementary motor area (Gandolfo et al., 2000; Li et al., 2001; Muellbacher et al., 2002; Padoa-Schioppa et al., 2004).
The schizophrenic group’s failure to engage middle temporal regions is surprising. Parahippocampal neurons may provide some of the directional information that healthy volunteers obtain from the middle temporal system. Future studies should provide a clearer picture of how this diagnostic group relies on alternative systems for perceptual and motor skill acquisition (Mather and Putchat, 1984; Schröder et al., 1995; Schwartz et al., 1996; Keil et al., 1998; Schröder et al., 1999; Weickert et al., 2002; Exner et al., 2006). To what extent does activity in a “secondary” system result in diminished skill acquisition? Can prolonged practice induce a switch from secondary to primary regions? Do those schizophrenic subjects who use “primary” systems benefit by acquiring greater skill? These questions go to the heart of the cognitive deficit associated with this syndrome. As new therapeutic interventions become available it will be important to ascertain how perceptual and motor skills benefit and how those skills are supported by primary and secondary systems.
Optimal motor performance occurred during A2 for both groups. But the shift from early learning to late learning (from A1 to A2) was associated with a marked increase in motor system pathways for healthy volunteers and a marked increase in visual system pathways for schizophrenic subjects. These groups may be adapting to this visuomotor task with motor (healthy volunteers) and visual cortex (schizophrenic subjects) in order to improve motor precision. Each group is tuning neural assemblies to code direction, orientation, and trajectory (Chen and Wise, 1996; Laubach et al., 2000; Paz et al., 2004; Poggio and Bizzi, 2004). By increasing the relevant information contained in neural activity, it is likely that interactions between spinal cord and cortex are optimized, but it is also likely that greater information improves the fidelity of an efference copy (corollary discharge) of the movement. The corollary discharge, in turn, could help provide better predictions of motor action consequences (Sommer and Wurtz, 2002; Flanagan et al., 2003). Visual and motor neural tuning could improve the subject’s ability to adapt to the force field and anticipate the consequences of his own actions.
The finding that the schizophrenic group relies more on the adaptive properties of the visual cortex, and the healthy volunteers rely more on the properties of motor cortex, is revealing. Several studies have shown sensory-motor abnormalities during finger movement tasks in schizophrenic subjects (Schröder et al., 1995; Mattay et al., 1997; Müller et al., 2002; Rogowska et al., 2004) but none has provided insight into neural plasticity over time. Our results emphasize the capacity of two different brain regions to support learning by tuning neural assemblies in an adaptive, dynamic manner. It also emphasizes the capacity of those neural systems to respond in a manner that generalizes from trial to trial, across a range of directions. Neural theoreticians suggest that perceptual and motor skills may need similar properties of flexibility and generalizability from visual and motor cortical regions (Paz et al., 2003; Shadmehr, 2004; Fahle, 2005).
4.3. Limitations and future directions
There are several limitations of this study. First, the sample size is small. A larger group of patients may reveal greater heterogeneity in activity patterns. It would be extremely useful to know if some subgroups of subjects with schizophrenia exhibit normal adaptive responses to motor training in a force field. Second, the presence of antipsychotic medication is always problematic in physiological studies of schizophrenic subjects. Because of their dopamine receptor antagonist properties antipsychotics are likely to perturb and bias motor learning physiology. But clozapine and olanzapine (the antipsychotics used by this group of schizophrenic subjects) appear to be less disruptive to motor physiology than typical, first-generation antipsychotics. Rogawska has summarized numerous motor activation studies in the schizophrenic population (Rogowska et al., 2004). Several general patterns emerge from publications of the last ten years. First, unmedicated, first-episode schizophrenics exhibit normal motor system integrity (Braus et al., 2000). Second, first-generation antipsychotics diminish activity in the primary motor cortex (Rogowska et al., 2004) and the supplementary motor cortex (SMA) (Braus et al., 1999), but second-generation antipsychotics are less likely to suppress the primary sensory-motor cortex (Braus et al., 1999; Müller et al., 2002) during a self-paced finger sequence task. Third, when engaged in a pronation/supination task schizophrenics taking clozapine exhibit diminished activity in the sensory-motor cortex. Fourth, following a week of finger sequence training schizophrenic subjects enhance neural activity in the premotor cortex. Healthy controls, in contrast, reduce activity in premotor cortex following a week of training (Kodama et al., 2001).
These motor studies with schizophrenic subjects used fMRI, finger sequencing or hand pronation, and restricted their studies to single occasions (excepting Kodama et al., 2001). It is difficult to extrapolate between studies but it is likely that antipsychotic medications, whether typical or atypical, reduce sensory-motor and SMA responsivity. It is not known to what extent these medications interfere with motor system activity during a prolonged series of learning exercises. The impact of chronically administered antipsychotic medications on LTP and LTD remains to be elucidated in human subjects (Gemperle and Olpe, 2004).
4.4. General summary
This is the first study to employ a unique arm-reaching test to assess neural plasticity during multiple stages of motor learning in subjects with schizophrenia. Schizophrenics may have an inability to rapidly tune motor cortical neural populations. The visual system appears to be highly compensated in schizophrenia, but the inability to rapidly modulate the motor cortex may diminish the schizophrenic subject’s capacity to rapidly acquire complex motor skills. This group’s reliance on the visual system may reflect a tendency to build internal models of target characteristics instead of limb dynamics when adapting to a unique motor problem.
Acknowledgements
The authors acknowledge the excellent assistance provided by Nancy Kakoyannis of the University of Maryland Department of Psychiatry. The Johns Hopkins University Cyclotron Facility staff was essential and helpful at all times, especially Robert F. Dannals, Robert Smoot, and David Clough. Kurt A. Thoroughman, Ning Ma, Hongye Wang, Raimi Quition, and Kristin Frey provided expert data analyses. The NARSAD Foundation provided funding for this study.
References
- Alain C, McNeely HE, He Y, Christensen BK, West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cerebral Cortex. 2002;12:840–846. doi: 10.1093/cercor/12.8.840. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Braus DF, Ende G, Weber-Fahr W, Sartorius A, Krier A, Hubrich-Ungureanu P, Ruf M, Stuck S, Henn FA. Antipsychotic drug effects on motor activation measured by functional magnetic resonance imaging in schizophrenic patients. Schizophrenia Research. 1999;39:19–29. doi: 10.1016/s0920-9964(99)00032-8. [DOI] [PubMed] [Google Scholar]
- Braus DF, Ende G, Hubrich-Ungureanu P, Henn FA. Cortical response to motor stimulation in neuroleptic-naive first episode schizophrenics. Psychiatry Research. 2000;98:145–154. doi: 10.1016/s0925-4927(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, Murray EA. The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115:971–982. doi: 10.1037//0735-7044.115.5.971. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. American Journal of Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Chen LL, Wise SP. Evolution of directional preferences in the supplementary eye field during acquisition of conditional oculomotor associations. Journal of Neuroscience. 1996;16:3067–3081. doi: 10.1523/JNEUROSCI.16-09-03067.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cothros N, Wong JD, Gribble PL. Are there distinct neural representations of object and limb dynamics? Experimental Brain Research. 2006;173:689–697. doi: 10.1007/s00221-006-0411-0. [DOI] [PubMed] [Google Scholar]
- Danckert J, Saoud M, Maruff P. Attention, motor control and motor imagery in schizophrenia: implications for the role of the parietal cortex. Schizophrenia Research. 2004;70:241–261. doi: 10.1016/j.schres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M. Frontal and parietal networks for conditional motor learning: a positron emission tomography study. Journal of Neurophysiology. 1997;78:977–991. doi: 10.1152/jn.1997.78.2.977. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. Journal of Neuroscience. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50:145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Exner C, Weniger G, Schmidt-Samoa C, Irle E. Reduced size of the pre-supplementary motor cortex and impaired motor sequence learning in first-episode schizophrenia. Schizophrenia Research. 2006;84:386–396. doi: 10.1016/j.schres.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: specificity versus generalization. Current Opinions in Neurobiology. 2005;15:154–160. doi: 10.1016/j.conb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Flanagan JR, Vetter P, Johansson RS, Wolpert DM. Prediction precedes control in motor learning. Current Biology. 2003;13:146–150. doi: 10.1016/s0960-9822(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Changing brain networks for visuomotor control with increased movement automaticity. Journal of Neurophysiology. 2004;92:2405–2412. doi: 10.1152/jn.01092.2003. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Distinguishable brain activation networks for short- and long-term motor skill learning. Journal of Neurophysiology. 2005;94:512–518. doi: 10.1152/jn.00717.2004. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Research: Brain Research Reviews. 2000a;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philosophical Transactions of the Royal Society of London: B Biological Sciences. 2000b;355:1771–1788. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo F, Li C, Benda BJ, Schioppa CP, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2259–2263. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geesaman BJ, Born RT, Andersen RA, Tootell RB. Maps of complex motion selectivity in the superior temporal cortex of the alert macaque monkey: a double-label 2-deoxyglucose study. Cerebral Cortex. 1997;7:749–757. doi: 10.1093/cercor/7.8.749. [DOI] [PubMed] [Google Scholar]
- Gemperle A, Olpe HR. Effects of subchronic clozapine treatment on long-term potentiation in rat prefrontal cortex. European Neuropsychopharmacology. 2004;14:340–346. doi: 10.1016/j.euroneuro.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA. Dorsal premotor cortex and conditional movement selection: A PET functional mapping study. Journal of Neurophysiology. 1998;79:1092–1097. doi: 10.1152/jn.1998.79.2.1092. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M, Pratiwadi R, Ragland JD, Bilker WB, Siegel SJ, Kanes SJ, Arnold SE, Gur RC. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. American Journal of Psychiatry. 2007;164:442–449. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous H215O. I. Theory and error analysis. Journal of Nuclear Medicine. 1983;24:782–789. [PubMed] [Google Scholar]
- Holcomb HH. Practice, learning, and the likelihood of making an error: how task experience shapes physiological response in patients with schizophrenia. Psychopharmacology. 2004;174:136–142. doi: 10.1007/s00213-004-1834-6. [DOI] [PubMed] [Google Scholar]
- Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hwang EJ, Shadmehr R. Internal models of limb dynamics and the encoding of limb state. Journal of Neural Engineering. 2005;2:S266–S278. doi: 10.1088/1741-2560/2/3/S09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Elbert T, Rockstroh B, Ray WJ. Dynamical aspects of motor and perceptual processes in schizophrenic patients and healthy controls. Schizophrenia Research. 1998;33:169–178. doi: 10.1016/s0920-9964(98)00069-3. [DOI] [PubMed] [Google Scholar]
- Kodama S, Fukuzako H, Fukuzako T, Kiura T, Nozoe S, Hashiguchi T, Yamada K, Takenouchi K, Takigawa M, Nakabeppu Y, Nakajo M. Aberrant brain activation following motor skill learning in schizophrenic patients as shown by functional magnetic resonance imaging. Psychological Medicine. 2001;31:1079–1088. doi: 10.1017/s0033291701004196. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. Journal of Neurophysiology. 1988;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12492–12497. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SC, Ng VW, Vythelingum GN, Simmons A, Suckling J, Corr PJ, Sharma T. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophrenia Research. 2002;57:97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- Laubach M, Wessberg J, Nicolelis MA. Cortical ensemble activity increasingly predicts behaviour outcomes during learning of a motor task. Nature. 2000;405:567–571. doi: 10.1038/35014604. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Angel RW, Thiemann S, Weitz CJ, Berger PA. Central error-correcting behavior in schizophrenia and depression. Biological Psychiatry. 1986;21:263–273. doi: 10.1016/0006-3223(86)90047-8. [DOI] [PubMed] [Google Scholar]
- Mather JA, Putchat C. Motor control of schizophrenics-II. Manual control and tracking: sensory and motor deficits. Journal of Psychiatric Research. 1984;18:287–298. doi: 10.1016/0022-3956(84)90019-0. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Callicott JH, Bertolino A, Santha AK, Tallent KA, Goldberg TE, Frank JA, Weinberger DR. Abnormal functional lateralization of the sensorimotor cortex in patients with schizophrenia. Neuroreport. 1997;8:2977–2984. doi: 10.1097/00001756-199709080-00034. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia ‘projections’ to the prefrontal cortex of the primate. Cerebral Cortex. 2002;12:926–935. doi: 10.1093/cercor/12.9.926. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Teskey GC. Skilled-learning-induced potentiation in rat sensorimotor cortex: a transient form of behavioural long-term potentiation. Neuroscience. 2004;125:329–336. doi: 10.1016/j.neuroscience.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Müller JL, Roder CH, Schuierer G, Klein H. Motor-induced brain activation in cortical, subcortical and cerebellar regions in schizophrenic inpatients. A whole brain fMRI finger-tapping study. Progress in Neuropsychopharmacology and Biological Psychiatry. 2002;26:421–426. doi: 10.1016/s0278-5846(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Saito N, Sakamoto K, Itoyama Y, Tanji J. Activity in the lateral prefrontal cortex reflects multiple steps of future events in action plans. Neuron. 2006;50:631–641. doi: 10.1016/j.neuron.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W, Orban GA. Charting the lower superior temporal region, a new motion-sensitive region in monkey superior temporal sulcus. Journal of Neuroscience. 2006;26:5929–5947. doi: 10.1523/JNEUROSCI.0824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Experimental Brain Research. 2001;140:66–76. doi: 10.1007/s002210100787. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Li CS, Bizzi E. Neuronal activity in the supplementary motor area of monkeys adapting to a new dynamic environment. Journal of Neurophysiology. 2004;91:449–473. doi: 10.1152/jn.00876.2002. [DOI] [PubMed] [Google Scholar]
- Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nature Neuroscience. 2003;6:882–890. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- Paz R, Wise SP, Vaadia E. Viewing and doing: similar cortical mechanisms for perceptual and motor learning. Trends in Neuroscience. 2004;27:496–503. doi: 10.1016/j.tins.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Poggio T, Bizzi E. Generalization in vision and motor control. Nature. 2004;431:768–774. doi: 10.1038/nature03014. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Holmes AP, Frackowiak RS, Friston KJ. Estimating smoothness in statistical parametric maps: variability of P values. Journal of Computer Assisted Tomography. 1995;19:788–796. doi: 10.1097/00004728-199509000-00017. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ. Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage. 1997;5:83–96. doi: 10.1006/nimg.1996.0248. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. Journal of Nuclear Medicine. 1983;24:790–798. [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nature Neuroscience. 1998;1:230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rogowska J, Gruber SA, Yurgelun-Todd DA. Functional magnetic resonance imaging in schizophrenia: cortical response to motor stimulation. Psychiatry Research: Neuroimaging. 2004;130:227–243. doi: 10.1016/j.pscychresns.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Schröder J, Wenz F, Schad LR, Baudendistel K, Knopp MV. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. British Journal of Psychiatry. 1995;167:197–201. doi: 10.1192/bjp.167.2.197. [DOI] [PubMed] [Google Scholar]
- Schröder J, Essig M, Baudendistel K, Jahn T, Gerdsen I, Stockert A, Schad LR, Knopp MV. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: a study with functional magnetic resonance imaging. Neuroimage. 1999;9:81–87. doi: 10.1006/nimg.1998.0387. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Rosse RB, Veazey C, Deutsch SI. Impaired motor skill learning in schizophrenia: implications for corticostriatal dysfunction. Biological Psychiatry. 1996;39:241–248. doi: 10.1016/0006-3223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Human Movement Science. 2004;23:543–568. doi: 10.1016/j.humov.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. Journal of Neuroscience. 1994;14:3208–3224. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. Journal of Neuroscience. 1997;17:409–419. doi: 10.1523/JNEUROSCI.17-01-00409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Inhibitory control of competing motor memories. Experimental Brain Research. 1999;126:235–251. doi: 10.1007/s002210050733. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Moussavi ZM. Spatial generalization from learning dynamics of reaching movements. Journal of Neuroscience. 2000;20:7807–7815. doi: 10.1523/JNEUROSCI.20-20-07807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Wise SP. The Computational Neurobiology of Reaching and Pointing. Cambridge, Massachusetts: 2005. [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA, Bizzi E. Postural force fields of the human arm and their role in generating multijoint movements. Journal of Neuroscience. 1993;13:45–62. doi: 10.1523/JNEUROSCI.13-01-00045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Brandt J, Corkin S. Time-dependent motor memory processes in amnesic subjects. Journal of Neurophysiology. 1998;80:1590–1597. doi: 10.1152/jn.1998.80.3.1590. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. American Journal of Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biology. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A. A deficit profile of executive, memory, and motor functions in schizophrenia. Biological Psychiatry. 1994;36:641–653. doi: 10.1016/0006-3223(94)91173-8. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers, Inc.; New York: 1988. [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. Journal of Comparative Neurology. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiology of Learning and Memory. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhang H, Li BM. Deficit in conditional visuomotor learning by local infusion of bicuculline into the ventral prefrontal cortex in monkeys. European Journal of Neuroscience. 2000;12:3787–3796. doi: 10.1046/j.1460-9568.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Malley JD, Hyde T, Egan MF, Weinberger DR, Goldberg TE. Habit and skill learning in schizophrenia: evidence of normal striatal processing with abnormal cortical input. Learning and Memory. 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SI, Paul CA, Eichenbaum H. Spatial and behavioral correlates of hippocampal neuronal activity. Journal of Neuroscience. 1989;9:2737–2763. doi: 10.1523/JNEUROSCI.09-08-02737.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Poline JB, Vandal AC, Friston KJ. Tests for distributed, nonfocal brain activations. Neuroimage. 1995;2:183–194. doi: 10.1006/nimg.1995.1024. [DOI] [PubMed] [Google Scholar]


