To the Editor:
Hereditary and acquired forms of hypophosphatemia result in metabolic bone disease with significant degrees of disability in children, adolescents and adults. Linear sebaceous nevus syndrome (LSNS) is a rare sporadic congenital phakomatosis of unknown etiology with a variable phenotype that includes hypophosphatemia [Carey et al., 1986].
We have previously reported on a patient with LSNS and hypophosphatemia where the plasma phosphatonin, fibroblast growth factor-23 (FGF-23), was inversely related to the serum phosphorous prior to and after treatment with octreotide and excision surgery [Hoffman et al., 2005]. We now extend that observation by reporting on the positive correlation between serum phosphorus and matrix extracellular phosphoglycoprotein (MEPE), a downstream member of phosphate homeostasis and mineralization.
Briefly, the patient, a 7-year-old boy of Korean ancestry with LNSS (which followed Blaschko’s lines and involved the left side of his face, trunk, upper and lower extremities and epidermal nevi of the limbus) began treatment for hypophosphatemic rickets with phosphate and calcitriol for vitamin D-resistant rickets. Octreotide (Sandostatin LAR) was added to the phosphate and calcitriol therapy when he was 16 years of age, due to increasing musculoskeletal symptoms and frequent stress fractures and persistent hypophosphatemia [Hoffman et al., 2005]. Further details of the treatment protocol are given in the legend for Figure 1. The competitive enzyme-linked immunosorbent (ELISA) assays for MEPE were performed as previously described [Jain et al., 2004]. The antibody employed in the competitive ELISA (LF-155, a kind gift of Dr. L.W. Fisher, N.I.D.C.R., N.I.H.) was raised against exon 4, the last coding exon of human MEPE, and was affinity-purified using recombinantly expressed protein. The assay entails an anion exchange column chromatography step to clean up the sample and detects intact, full length MEPE. The bioactive acidic serine-aspartate-rich MEPE (ASARM) peptide was assayed by a competitive ELISA specific for ASARM-peptide epitopes [Bresler et al., 2004] in the laboratory of Dr. P Rowe. Associations between MEPE and phosphorus were analyzed by Pearson regression analysis.
Figure 1.
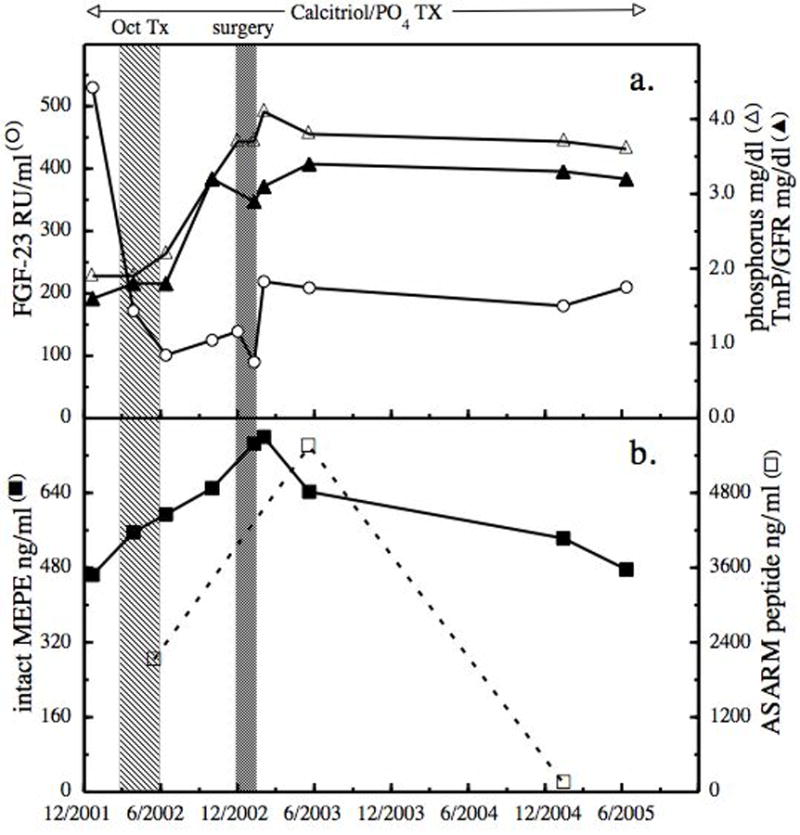
Treatment effects on MEPE, FGF-23 and phosphorus. At 16 years of age, with a history of hypophosphatemia, musculoskeletal symptoms, recurrent insufficiency fractures and an elevated plasma level of FGF-23, octreotide therapy (Oct TX) was begun along with ongoing phosphate and calcitriol therapy. Initially octreotide acetate 50 mcg was administered subcutaneously daily for 3 days, followed by Sandostatin LAR depot 10 mg intramuscularly every 2 weeks for 4 months. Six months after completing the Sandostatin LAR, excision surgery was performed for cosmetic reasons. The levels of FGF-23 (open circle), serum phosphorus (open triangle), calculated TmP/GFR (solid triangle) (panel a) and total plasma MEPE (solid square) and ASARM peptide (open square) (panel b) were followed for up to 2.5 years after surgery.
FGF-23 decreased within 1 month following the first injection of depot octreotide, was suppressed during the 4 months of injections, and remained in the normal range over the following 6 months. After a further decrease following surgery, FGF-23 increased 2 months post surgery and remained stable for the next 2.5 years during phosphate and calcitriol therapy and in the absence of octreotide (Fig 1). This somewhat elevated level of FGF-23 was most likely due to incomplete excision of the tumor [Zimering et al., 2005]. It is of interest that tubular reabsorption of phosphate became normal 4 months after octreotide; serum phosphorus did not increase during octreotide, but began increasing immediately after octreotide, remained slightly below normal until after surgery, and remained normal throughout the remainder of follow up [Hoffman et al., 2005]. Thus phosphaturia continued after FGF-23 had returned to normal. The initial level of MEPE prior to octreotide was 464 ng/ml when serum phosphorus was 1.9 mg/dl. This MEPE value was significantly below aged-matched normal subjects whose mean values were 880 ± 160 ng/ml [Jain et al., 2004]. MEPE increased throughout the depot octreotide therapy, continued to increase and reached a maximal value of 760 ng/ml 2 months after incomplete excisional surgery for cosmetic reasons. MEPE was decreased 6 months after surgery and continued to gradually decline, reaching a pre octreotide level 2.5 years after surgery (Fig 1). MEPE and phosphorus had a significant positive correlation prior to and throughout treatment (Fig 2). During octreotide treatment intact MEPE was increasing, indicating that unlike FGF-23, MEPE was not suppressed by octreotide, suggesting a differential effect of octreotide on the phosphatonins and that MEPE had a phosphaturic effect. The latter seems unlikely since the MEPE values did not exceed the reported normal range [Jain et al., 2004].
Figure 2.
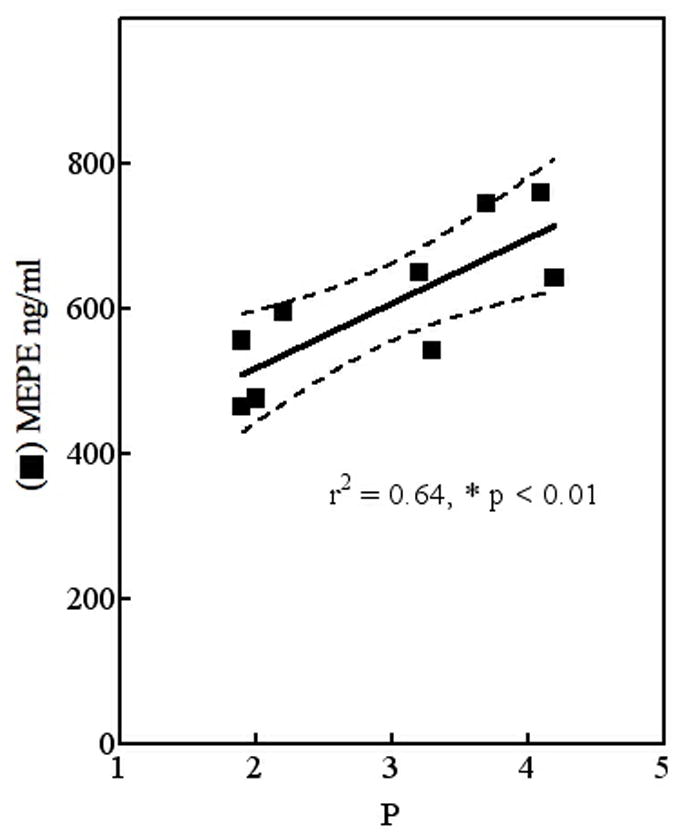
MEPE, but not FGF-23, correlates with serum phosphorus. Determined values for serum phosphorus (P) and total MEPE across the 3 year follow-up were analyzed by regression analysis.
However, it is of interest that serum ASARM, the biological active fragment of MEPE, exceeded the normal range of 3,250 ng/ml and paralleled the increase of MEPE, which could explain the phosphaturia [Bresler et al., 2004]. An alternative possibility is the presence of an additional phosphaturic peptide [White et al., 2006]. This possibility has recently been suggested by Elston et al., [2007] to explain persistent hypophosphatemia in a patient with oncogenic osteomalacia after a five- day course of octreotide had suppressed FGF-23. It is important to note that in the report of Elston et al., although the intact FGF-23 was suppressed, the C-fragment of FGF-23 increased for 16 days following surgery and then decreased over the following 5 months. Following octreotide treatment, our patient had significant improvement in the musculoskeletal symptoms, and no further stress fractures occurred. By the age of 20, the patient’s T-Score of bone density had improved by −1.2 in the lumbar spine (from −3.2 to −2.1); by −1.0 in the femoral neck (from −4.4 to −3.4); and by −0.8 in the total hip (from −3.8 to −3.0). However, the values remained in the osteopenic (−1.0 to −2.5) and osteoportic (below −2.5) ranges.
The term “phosphatonin” was introduced in 1994 for the protein which has since come to be known as FGF-23 [Econs and Drezner, 1991]. MEPE, also known as osteoclast/osteocyte factor 45, is a glycoprotein which has more recently been identified as a putative phosphatonin [Rowe et al., 2000] based on its ability to inhibit phosphate-uptake and mineralization in vivo and in vitro [Rowe et al., 2004]. These phosphate-regulating hormones have rapidly gained recognition for their importance in influencing phosphate homeostasis and mineralization through a bone-kidney axis. MEPE and FGF-23 mediate the molecular mechanisms of the bone-kidney axis of phosphate metabolism by controlling the genes encoded for sodium phosphate cotransporters such as Npt2a [Rowe et al., 2004; Marsell et al., 2008], and by regulating vitamin D-1alpha-hydroxylase activity [Sommer et al., 2007; Barthel et al., 2007].
As with many peptide-hormones the biological functions of MEPE are linked to specific peptide fragments that differentially influence processing and handling of phosphate by the kidney [Martin et al., 2007]. Cleavage of MEPE releases acidic serine-aspartate-rich motif (ASARM peptide), the carboxy-terminal peptide that is responsible for MEPE activity in vivo [Rowe et al., 2005]. The positive correlation that we observed between MEPE and serum phosphorus prior to and following octreotide therapy is consistent with a cross-sectional study of normal subjects [Jain et al., 2004]. Also the hyperphosphatemia in a MEPE-TRG model correlates with up-regulation of Npt2a [Rowe et al., 2004] and contrasts with infusions of recombinant intact MEPE which result in increased fractional excretion of phosphate and hypophosphatemia in mice and rats [Dobbie et al., 2007].
We have no direct data to support that the positive correlation between MEPE and serum phosphorus is due to octreotide influencing the processing of MEPE. However MEPE, ASARM and serum phosphorus all increased with the initiation of octreotide treatment and decreased following excision of the LSN tumor. We posit the following sequence occurred in this case: first, octreotide resulted in a significant reduction in FGF-23 and a gradual increase in serum phosphorus; second, the dramatic decrease of FGF-23 resulted in an overcompensation of MEPE expression due to chronic and excessive suppression by FGF-23; (An additional explanation for the increase in MEPE could be that octreotide influences the processing of MEPE and possibly increases its expression.); and third, partial excision of the nevus resulted in a decrease and stabilization of MEPE, and after a brief decrease, FGF-23 stabilized followed by gradual stabilization of serum phosphorus, and urine phosphate excretion. Whether this stabilization is a long-term result of octreotide treatment [Eriksson and Oberg, 1999; Garcia de la Torre et al., 2002; Latuada et al., 2002] and/or the result of the natural course of the disease is uncertain. While the number of points studied for ASARM is limited, the correlation between MEPE and ASARM prior to and following surgery supports that octreotide directly or indirectly influenced the processing of MEPE. The two values at six months (12 months after octreotide therapy) and 24 months post partial excisional surgery suggest that MEPE and ASARM had different processing after an increased period off octreotide therapy.
It has been posited that phakomatoses are caused by dysregulation of cellular paracrine growth factors and the extracellular matrix [Kousseff, 1992]. The phosphatonins are an example of such growth factors. FGF-23, and to a somewhat lesser extent, MEPE, have been extensively studied in conditions of abnormal phosphate metabolism including the phakomatoses McCune-Albright syndrome [Yamamoto et al., 2005] and phakomatosis pigmentokeratotica [Saraswat et al., 2003; Bouthors et al., 2006]. The latter condition, along with LSNS [Hoffman et al., 2005; Heike et al., 2005], belongs to the group of epidermal nevus syndromes (ENS) which have a high prevalence of hypophosphatemic rickets [Vidaurri-de la Cruz et al., 2004]; and therefore, the phosphatonins likely have increased relevance in the abnormal phosphate metabolism. Whether the phosphatonin phenotypes are the same in the various forms of ENS remains to be determined.
This follow-up report is the first longitudinal measurement of both MEPE and FGF-23 and their response to medical and surgical treatment in tumor-induced hypophosphatemia, and illustrates the advances in the understanding of phosphate metabolism beyond that of parathyroid hormone and 1,25-dihydroxyvitamin D [Rowe, 2004]. The follow-up also indicates the need for continued close monitoring of urine phosphate excretion and serum phosphorus in patients who have FGF-23 (phosphatonin) tumor-induced hypophosphatemia, such as LSNS, to determine the short-and long-term benefit of octreotide when complete excision is not possible. Additional considerations are including FGF-23 and MEPE as part of the initial evaluation of hypophosphatemia and/or rare forms of chondrodysplasias [Zeger et al., 2007], as well as to direct and monitor treatment plans [Garabedian, 2007].
Acknowledgments
The authors are grateful to Dr. Peter Rowe for his critical review of the manuscript and performing ASARM assay. This research was supported in part by Department of Defense grants DAMD 17-02-0684 (N.S.F.) and NIH grant CA113865 (N.S.F.).
Footnotes
Conflicts of interest: None
References
- Barthel TK, Mathern DR, Whitfield GK, Haussler CA, Hopper HA, 4th, Hsieh JC, Slater SA, Hsieh G, Kaczmarska M, Jurutka PW, Kolek OI, Ghishan FK, Haussler MR. 1-25-dihydroxyvitamin D3/VDR-mediated induction of FGF23 as well as transcriptional control of bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol. 2007;103:381–388. doi: 10.1016/j.jsbmb.2006.12.054. [DOI] [PubMed] [Google Scholar]
- Bouthors J, Vantyghem MC, Manouvrier-Hanu S, Soudan B, Proust E, Happle R, Piette F. Phacomatosis pigmentokeratotica associated with hypophosphataemic rickets, pheochromocytoma and multiple basal cell carcinomas. Br J Dermatology. 2006;155:225–226. doi: 10.1111/j.1365-2133.2006.07313.x. [DOI] [PubMed] [Google Scholar]
- Bresler D, Bruder J, Mohnike K, Fraser WD, Rowe PS. Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): Implications for phosphaturia and rickets. J Endocrinol. 2004;183:R1–9. doi: 10.1677/joe.1.05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey DE, Drezner MK, Hamdan JA, Mange M, Ahmad MS, Mubarak S, Nyhan WL. Hypophosphatemic rickets/osteomalacia in linear sebaceous nevus syndrome: a variant of tumor-induced osteomalacia. J Pediatr. 1986;109:994–1000. doi: 10.1016/s0022-3476(86)80283-9. [DOI] [PubMed] [Google Scholar]
- Dobbie H, Unwin RJ, Faria NJ, Shirley DG. Matrix extracellular phosphoglycoprotein causes phosphaturia in rats by inhibiting tubular phosphate reabsorption. Nephrol Dial Transplant. 2007 Nov 23; doi: 10.1093/ndt/gfm535. Epub. [DOI] [PubMed] [Google Scholar]
- Econs MJ, Drezner MK. Tumor-induced osteomalacia--unveiling a new hormone. N Engl J Med. 1994;330:1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- Elston MS, Stewart IJ, Clifton-Bligh R, Conaglen JV. A case of oncogenic osteomalacia with preoperative secondary hyperparathyroidism: description of the biochemical response of FGF23 to octreotide therapy and surgery. Bone. 2007;40:263–241. doi: 10.1016/j.bone.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Eriksson B, Oberg K. Summing up 15 years of somatostatin analog therapy in neuroendocrine tumors: Future outlook. Ann Oncol. 1999;10(Suppl 2):S31–S38. doi: 10.1093/annonc/10.suppl_2.s31. [DOI] [PubMed] [Google Scholar]
- Garabedian M. Regulation of phosphate homeostasis in infants, children, and adolescents, and the role of phosphatonins in this process. Current Opinion in Pediatrics. 2007;19:488–491. doi: 10.1097/MOP.0b013e328270b902. [DOI] [PubMed] [Google Scholar]
- Garcia de la Torre N, Waas JAH, Turner HE. Antiangiogenic effects of somatostatin analogues. Clin Endocrinol. 2002;57:425–441. doi: 10.1046/j.1365-2265.2002.01619.x. [DOI] [PubMed] [Google Scholar]
- Heike CL, Cunningham ML, Steiner RD, Wenkert D, Hornung RL, Gruss JS, Gannon FH, McAlister WH, Mumm S, Whyte MP. Skeletal changes in epidermal nevus syndrome: does focal bone disease harbor clues concerning pathogenesis. Am J Med Genet Part A. 2005;139A:67–77. doi: 10.1002/ajmg.a.30915. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Jueppner HW, DeYoung BR, O’Dorisio MS, Given KS. Elevated fibroblast growth factor-23 in hypophosphatemic linear nevus sebaceous syndrome. Am J Med Genet Part A. 2005;134A:233–236. doi: 10.1002/ajmg.a.30599. [DOI] [PubMed] [Google Scholar]
- Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemia rickets. J Bone Miner Res. 2007;22:520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- Jain A, Fedarko NS, Collins MT, Gelman R, Ankrom MA, Tayback M, Fisher LW. Serum levels of matrix extracellular phosphoglycoprotein (MEPE) in normal humans correlate with serum phosphorus, parathyroid hormone and bone mineral density. J Clin Endocrinol Metab. 2004;89:4158–4161. doi: 10.1210/jc.2003-032031. [DOI] [PubMed] [Google Scholar]
- Kousseff BG. Hypothesis: Jadassohn nevus phakomatosis: a paracrinopathy with variable phenotype. Am J Med Genet. 1992;43:651–661. doi: 10.1002/ajmg.1320430402. [DOI] [PubMed] [Google Scholar]
- Lattuada D, Casnici C, Venuto A, Marelli O. The apoptotic effect of somatostatin analogue SMS 201–992 on human lymphocytes. J Neuroimmunol. 2002;133:211–216. doi: 10.1016/s0165-5728(02)00364-8. [DOI] [PubMed] [Google Scholar]
- Marsell R, Krajisnik T, Goransson H, Ohlsson C, Ljunggren O, Larsson TE, Jonsson KB. Gene expression analysis of kidneys from transgenic mice expressin fibroblast growth factor-23. Nephrol Dial Transplant. 2008;23:827–833. doi: 10.1093/ndt/gfm672. [DOI] [PubMed] [Google Scholar]
- Martin A, David V, Laurence JS, Schwarz PM, Lafer EM, Hedge AM, Rowe PS. Degradation of MEPE, DMP1 and release of SIBLING ASARM-peptides (minhibins). ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2007 Dec 27; doi: 10.1210/en.2007-1205. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PSN, de Zoysa PA, Dong R, Wang HR, White KE, Econs MJ, Oudet CL. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- Rowe PSN. The wrickkened pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med. 2004;15:264–281. doi: 10.1177/154411130401500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PSN, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, Cundy J, Navvab S, Chen D, Drezner MK, Quarles LD, Mundy GR. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PSN, Garrett IR, Schwarz PM, Carnes DL, Lafer EM, Mundy GR, Gutierrez GE. Surface plasmon resonance (SPR) confirms MEPE binds to PHEX via the MEPE-ASARM-motif: A model for impaired mineralization in X-linked rickets (HYP) Bone. 2005;36:33–46. doi: 10.1016/j.bone.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswat A, Dogra S, Bansali A, Kumar B. Phakomatosis pigmentokeratotica associated with hypophosphatemic vitamin D-resistant rickets: improvement in phophate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074–1076. doi: 10.1046/j.1365-2133.2003.05273.x. [DOI] [PubMed] [Google Scholar]
- Sommer S, Berndt T, Craig T, Kumar R. The phosphatonins and the regulation of phosphate transport and vitamin D metabolism. J Steroid Biochem Mol Biol. 2007;103:497–503. doi: 10.1016/j.jsbmb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Vidaurri-de la Cruz H, Tamayo-Sanchez L, Duran-McKinster C, de la Luz Orozco-Covarrubias M, Ruiz-Maldonado R. Epidermal nevus syndromes: clinical findings in 35 patients. Pediatr Dermatol. 2004;21:432–439. doi: 10.1111/j.0736-8046.2004.21402.x. [DOI] [PubMed] [Google Scholar]
- White KE, Larsson TE, Econs MJ. The roles of specific genes implicated as circulating factors involved in normal and disordered phosphate homeostasis: frizzled related protein-4, matrix extracellular phosphoglycoprotein, and fibroblast growth factor 23. Endocr Rev. 2006;27:221–241. doi: 10.1210/er.2005-0019. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Imanishi Y, Kinoshita E, Nakagomi Y, Shimizu N, Miyauchi A, Satomura K, Koshiyama H, Inaba M, Nishizawa Y, Juppner H, Ozono K. The role of fibroblast growth factor 23 for hypophosphatemia and abnormal regulation of vitamin D metabolism in patients with McCune-Albright syndrome. Bone and Mineral Metabolism. 2005;23:231–237. doi: 10.1007/s00774-004-0589-9. [DOI] [PubMed] [Google Scholar]
- Zimering MB, Caldarella FA, White KE, Econs MJ. Persistent tumor-induced osteomalacia confirmed by elevated postoperative levels of serum fibroblast growth factor-23 and 5-year follow-up of bone density changes. Endocr Pract. 2005;11:108–114. doi: 10.4158/EP.11.2.108. [DOI] [PubMed] [Google Scholar]


