Abstract
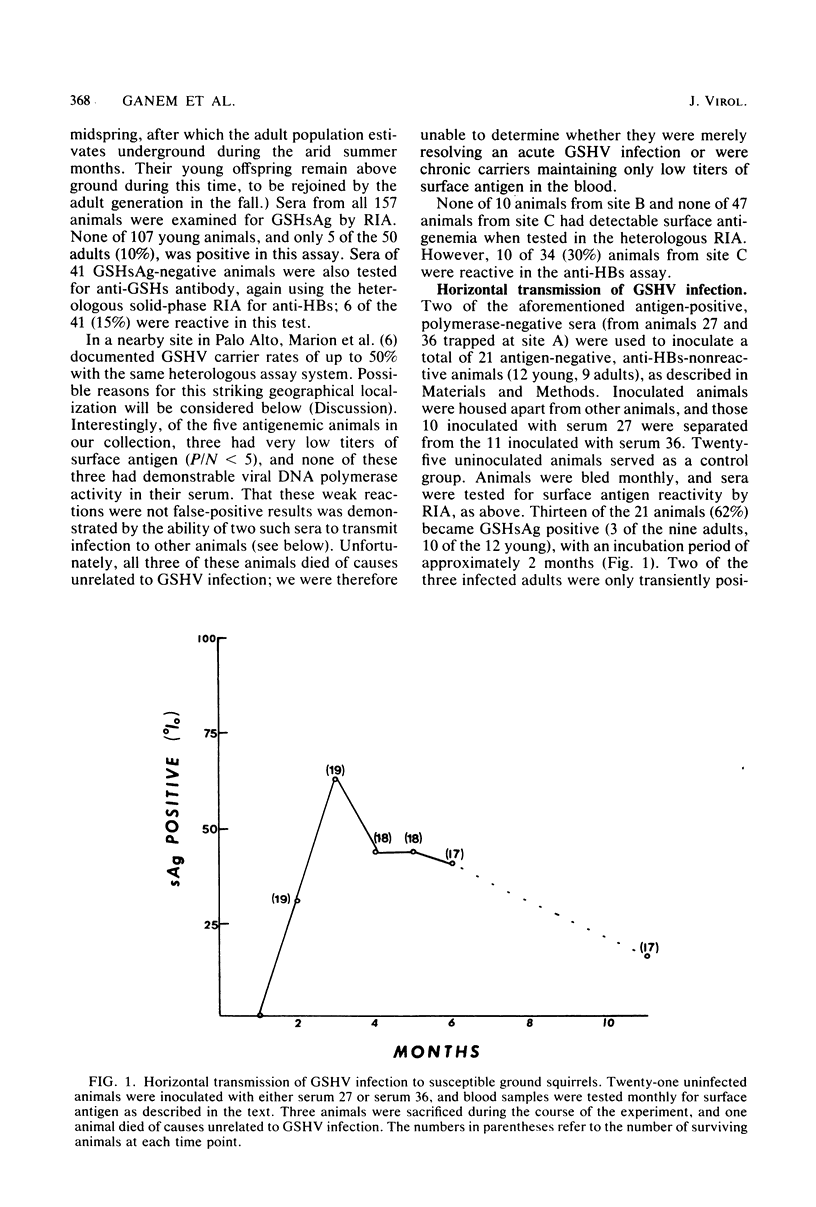
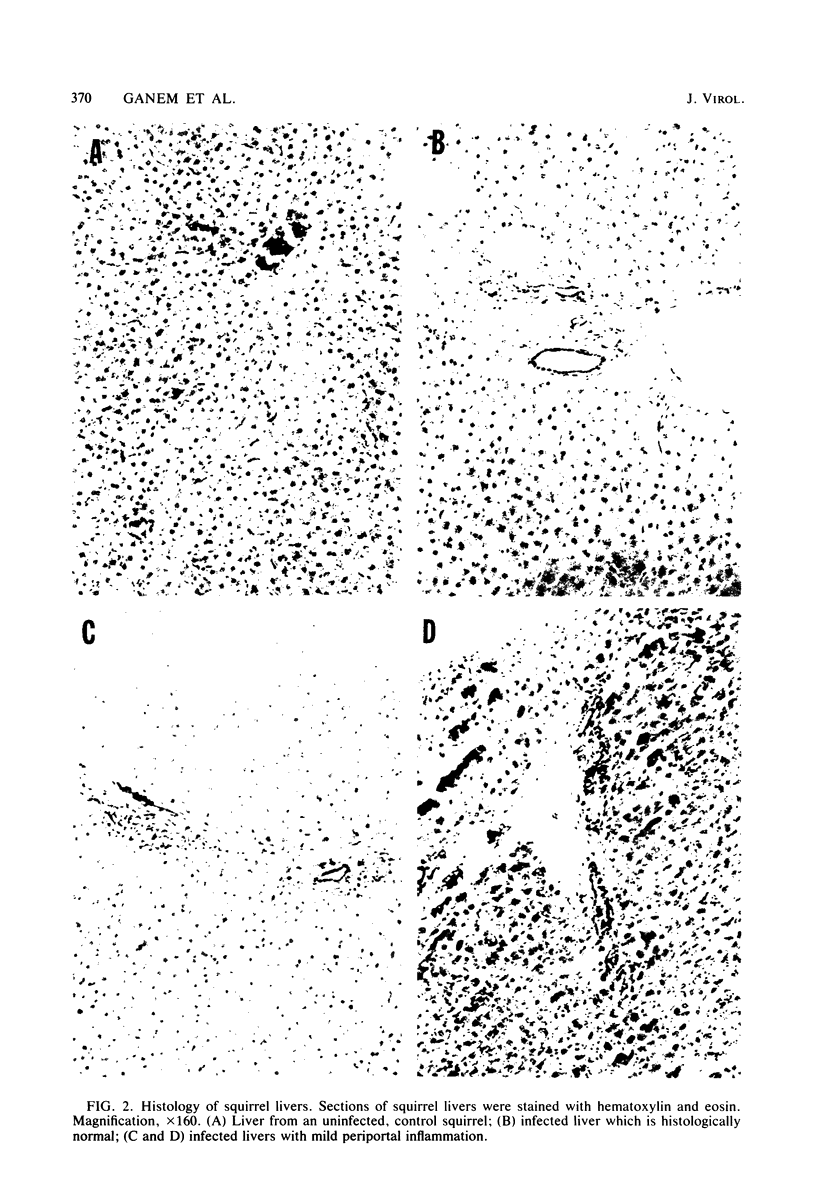
Ground squirrel hepatitis virus (GSHV) is a small DNA virus, structurally and antigenically related to the human hepatitis B virus, which occurs naturally among certain wild populations of ground squirrels (P. L. Marion et al., Proc. Natl. Acad. Sci. U.S.A. 77:2941-2945, 1980). Serum from naturally infected animals was used to transmit GSHV in the laboratory by parenteral inoculation of susceptible squirrels. Sixty percent of recipient animals developed viral surface antigenemia after a latent period of 2 to 3 months; three of these animals have remained viremic for over 9 months. Like hepatitis B virus, GSHV demonstrates marked hepatotropism, with viral DNA detected in significant quantities only in the liver, where an average of 6 X 10(2) to 6 X 10(3) viral DNA molecules per cell were found by molecular hybridization. However, histological signs of liver injury after acute infection are minimal. In contrast to infection of its natural host, parenteral administration of GSHV to rats, mice, guinea pigs, and hamsters did not result in demonstrable antigenemia, suggesting that the host range of GSHV, like that of hepatitis B virus, is narrow.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ganem D., Greenbaum L., Varmus H. E. Virion DNA of ground squirrel hepatitis virus: structural analysis and molecular cloning. J Virol. 1982 Oct;44(1):374–383. doi: 10.1128/jvi.44.1.374-383.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlich W. H., Feitelson M. A., Marion P. L., Robinson W. S. Structural relationships between the surface antigens of ground squirrel hepatitis virus and human hepatitis B virus. J Virol. 1980 Dec;36(3):787–795. doi: 10.1128/jvi.36.3.787-795.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers T. A., Greenberg H. B., Robinson W. S. Structure of hepatitis B Dane particle DNA and nature of the endogenous DNA polymerase reaction. J Virol. 1977 Aug;23(2):368–376. doi: 10.1128/jvi.23.2.368-376.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Wu J. R., Bonner J. Analysis of rat repetitive DNA sequences. Biochemistry. 1978 Jan 10;17(1):51–59. doi: 10.1021/bi00594a008. [DOI] [PubMed] [Google Scholar]
- Peterson D. L., Roberts I. M., Vyas G. N. Partial amino acid sequence of two major component polypeptides of hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1530–1534. doi: 10.1073/pnas.74.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker A. G. Viral hepatitis: clinical aspects. Am J Med Sci. 1975 Jul-Aug;270(1):9–16. doi: 10.1097/00000441-197507000-00003. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Greenman R. L. DNA polymerase in the core of the human hepatitis B virus candidate. J Virol. 1974 Jun;13(6):1231–1236. doi: 10.1128/jvi.13.6.1231-1236.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W. S., Lutwick L. I. The virus of hepatitis, type B (first of two parts). N Engl J Med. 1976 Nov 18;295(21):1168–1175. doi: 10.1056/NEJM197611182952105. [DOI] [PubMed] [Google Scholar]
- Sattler F., Robinson W. S. Hepatitis B viral DNA molecules have cohesive ends. J Virol. 1979 Oct;32(1):226–233. doi: 10.1128/jvi.32.1.226-233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Siddiqui A., Marion P. L., Robinson W. S. Ground squirrel hepatitis virus DNA: molecular cloning and comparison with hepatitis B virus DNA. J Virol. 1981 Apr;38(1):393–397. doi: 10.1128/jvi.38.1.393-397.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui A., Sattler F., Robinson W. S. Restriction endonuclease cleavage map and location of unique features of the DNA of hepatitis B virus, subtype adw2. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4664–4668. doi: 10.1073/pnas.76.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]