Abstract
Epithelial organs including the lung are known to possess regenerative abilities through activation of endogenous stem cell populations but the molecular pathways regulating stem cell expansion and regeneration are not well understood. Here we show that Gata6 regulates the temporal appearance and number of bronchioalveolar stem cells (BASCs) in the lung leading to the precocious appearance of BASCs and concurrent loss in epithelial differentiation in Gata6 null lung epithelium. This expansion of BASCs is the result of a dramatic increase in canonical Wnt signaling in lung epithelium upon loss of Gata6. Expression of the non-canonical Wnt receptor Fzd2 is down-regulated in Gata6 mutants and increased Fzd2 or decreased (β-catenin expression rescues, in part, the lung epithelial defects in Gata6 mutants. During lung epithelial regeneration, we show that canonical Wnt signaling is activated in the niche containing BASCs and forced activation of Wnt signaling leads to a dramatic increase in BASC numbers. Moreover, Gata6 is required for proper lung epithelial regeneration and postnatal loss of Gata6 leads to increased BASC expansion and decreased differentiation. Together, these data demonstrate that Gata6 regulated Wnt signaling controls the balance between stem/progenitor expansion and epithelial differentiation required for both lung development and regeneration.
INTRODUCTION
Organ regeneration requires the proper balance between differentiation and self-renewal of tissue specific stem/progenitor cells. The transcriptional and signaling pathways required to direct this balance are largely unknown but it is thought that many of the pathways involved in regulating embryonic development are recapitulated in stem/progenitor expansion and tissue regeneration. Characterization of these pathways and methods to modulate them are of great interest as they may reveal important new therapeutic targets for tissue replacement and regeneration.
The lung is a complex organ consisting of multiple distinct epithelial and mesodermal cell lineages patterned in a proximal-distal manner including specialized cell types that produce surfactant proteins and lipids as well as form the thin gas exchange interface required for postnatal respiration (reviewed in 1,2). Developmental defects in this patterning lead to defective differentiation and postnatal respiratory distress, a hallmark of congenital lung disease. A small core of transcription factors, including Gata6, Nkx2.1, and Foxa1/2, are known to be important for lung airway epithelial differentiation and development3–5. Altered expression or activity of any of these factors leads to severe defects in airway epithelial differentiation and development. However, it is unclear whether any of these factors play critical roles in regulating the balance between differentiation and stem/progenitor cell development and regeneration in the lung. Moreover, the molecular pathways acting down-stream of these factors are largely unknown.
In adult mammals, lung epithelium undergoes slow homeostatic turn-over resulting in the replacement of much of the epithelium after approximately four months in rats 6. In addition, lung epithelium possesses a significant ability to regenerate after injury. Both homeostatic turn-over and regeneration after injury are thought to involve endogenous lung stem/progenitor cells. The stem/progenitor cell niches within the lung are poorly characterized but recent studies have identified several niches located in distinct positions along the proximal-distal axis of the airways and each of these niches contains a specific stem/progenitor cell capable of regenerating the epithelial cell types in that region 7,8. Bronchioalveolar stem cells (BASCs) represent one of these regional stem/progenitor cell populations and are located in the bronchioalveolar duct junction (B ADJ) at the terminal ends of distal bronchiols, a region containing a classic stem cell niche where lung epithelial cell types change significantly 7,9. BASCs are thought to regenerate both bronchiolar and alveolar epithelium during homeostatic turn-over and in response to injury 9. The transcriptional and signaling pathways required for the differentiation and expansion in BASCs are largely unknown. Given the proposed requirement for BASCs and other stem/progenitor cells in normal homeostatic turn-over in the lung as well as regeneration and repair after injury, characterization of the regulatory mechanisms controlling the expansion and differentiation these cells could have a profound impact on our understanding and treatment of lung disease in humans.
In this study we have examined the role of the zinc finger transcription factor Gata6 in regulating the balance between lung epithelial differentiation and stem/progenitor cell expansion and regeneration. Specific ablation of Gata6 in lung epithelium leads to a dramatic loss in airway epithelial differentiation and neonatal death. Surprisingly, this is associated with the ectopic and premature appearance of BASCs, which are not normally present prior to birth. Gata6 acts through direct regulation of Fzd2, which inhibits the canonical Wnt pathway in lung epithelial cells and loss of Gata6 leads to activation of Wnt/β-catenin signaling in the lung. Postnatal deletion of Gata6 results in compromised airway regeneration associated with defective BASC expansion and differentiation, which also occurs upon postnatal activation of canonical Wnt signaling. These studies define a novel GATA6-Wnt mediated pathway that controls the balance between progenitor/stem cell expansion and epithelial differentiation and regeneration in the lung. Furthermore, these studies reveal important cross-talk between canonical and non-canonical Wnt pathways in epithelial differentiation and stem/progenitor cell development in vivo.
RESULTS
Loss of Gata6 in lung epithelium leads to loss of epithelial differentiation and precocious appearance of BASCs
Gata6 is expressed robustly in both the developing distal airway epithelium and in the more proximal bronchiolar epithelium (Supplemental Fig. 1 and 10). To examine the role of Gata6 in lung epithelial development, we crossed mice with a conditional null allele of Gata6 (Gata6Δ/Δ) to the SP-C/rtta:teto-cre bigenic system (hereafter referred to as SP-C/cre) to generate Gata6Δ/Δ:SP-C/cre mutants which lack Gata6 in airway epithelium n. Of note, the SP-C/rtta system has been shown to inducibly express transgenes in both distal airway epithelium as well as in bronchiolar epithelium 12–14. Treatment of pregnant dams from E0.5 to birth with doxycycline (dox) resulted in lethality in all Gata6Δ/Δ:SP-C/cre mutants within minutes after birth due to respiratory failure. Examination of Gata6Δ/Δ:SP-C/cre mutants earlier in gestation showed that loss of Gata6 in lung epithelium leads to increased airway dilation, which can be observed as early as E12.5 (Fig. 1A–F). However, lung size and lobation was normal in Gata6Δ/Δ:SP-C/cre mutants (Fig. 1A–F and data not shown). Normal lobe patterning suggests Gata6 is not required for some of the earliest stages of lung branching.
Figure 1. Loss of Gata6 leads to lung epithelial differentiation defects.
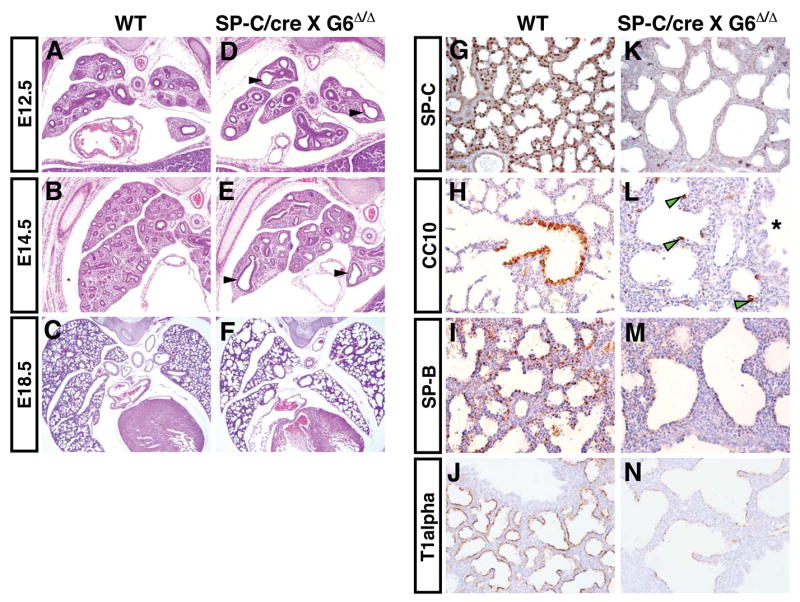
Wild-type (WT) and Gata6 Δ/Δ :SP-C/cre mutants (SP-C/cre X G6 Δ/Δ) were examined by H+E staining (A–F) or by immunohistochemical staining for lung epithelial marker proteins.(G–N). Gata6 Δ/Δ :SP-C/cre mutants displayed dilated airways beginning as early as E12.5 (D and E, arrowheads). Gata6 Δ/Δ :SP-C/cre mutants showed decreased or absent expression of SP-C (G and K), CC10 (H and L), SP-B (I and M), and T lalpha (J and N). However, ectopic expression of the Clara cell marker protein CC10 was observed in distal regions of the airways of Gata6 Δ/Δ :SP-C/cre mutants (L, arrowheads). Asterisk indicates a large bronchiolar airway, which has reduced levels of CC10 expression in the mutants.
To determine the extent of epithelial differentiation in Gata6Δ/Δ:SP-C/cre mutants, immunohistochemistry was performed using antibodies recognizing markers of distinct epithelial cell lineages within the lung airways including surfactant protein C (SP-C) a marker of alveolar epithelial type 2 cells (AEC-2), Clara cell 10 kD protein (CC10) a marker of non-ciliated Clara cells of the bronchiolar airways, surfactant protein B (SP-B) which is expressed by both AEC-2 and Clara cells, and Tlalpha, a marker of type 1 alveolar epithelial cells (AEC-1). While wild-type animals at E18.5 expressed typical spatial patterns and levels of all of these proteins, Gata6Δ/Δ:SP-C/cre mutants showed little or no expression of these proteins (Fig. 1G–N). Interestingly, sporadic expression of CC10 was observed in cells within the distal airways of Gata6Δ/Δ:SP-C/cre mutants, a region of the lung that normally does not contain Clara cells (Fig. 1L, arrowheads). These data demonstrate that loss of Gata6 expression leads to defective lung epithelial differentiation and ectopic expression of CC10 in distal airway epithelium.
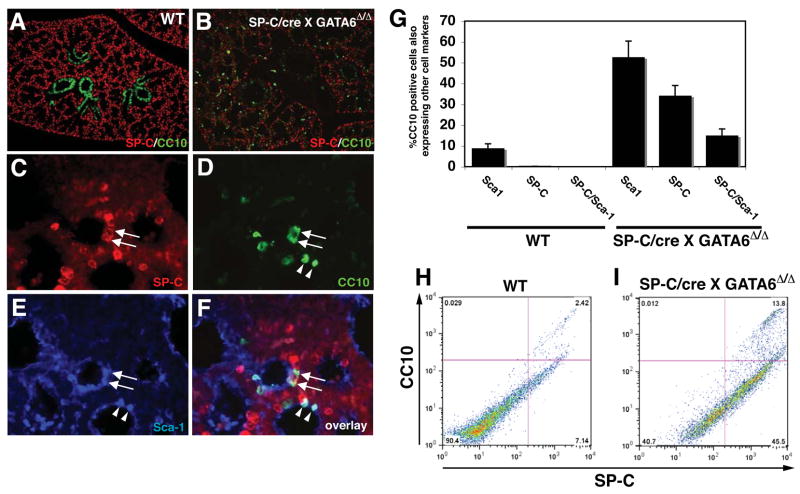
The appearance of CC10 expression in distal airway epithelium suggested that either proximal-distal airway patterning was disrupted or that there was an increase in specific cells types expressing CC10 in the distal airway epithelium. Loss of CC10 expression in the bronchiolar epithelium argues against a simple loss of proximal-distal patterning. CC10+ Clara cells are thought to harbor several stem/progenitor cell populations in the lung airway including BASCs, which express both SP-C and CC10 proteins, a trait not shared by other epithelial cell types within the lung 9. To assess whether the cells ectopically expressing CC10 in Gata6Δ/Δ:SP-C/cre mutants also expressed SP-C, double immunofluorescent staining was performed at E18.5. A large percentage of cells expressing CC10 within the distal airways also expressed SP-C, indicating that these represented BASCs (Fig. 2A–D). Moreover, a high percentage of the SP-C/CC10 double positive BASCs in Gata6Δ/Δ:SP-C/cre mutants also expressed Sca1, an additional marker of BASCs (Fig. 2E and F and 9). There appeared to be at least two populations of Sca1 expressing cells: CC10+/Sca1+ and SP-C+/CC10+/Sca1+. Whereas approximately 8–10% of CC10+ cells expressed Sca1 in wild-type embryos at E18.5, Gata6Δ/Δ: SP-C/cre mutants exhibited a five-fold increase in CC10+/Sca1+ cells (Fig. 2D–G). Importantly, at this stage of development, we were unable to identify significant numbers of SP-C+/CC10+ BASCs in wild-type littermates by immunostaining (Fig. 2G). However, using FACS analysis for SP-C and CC10 expression on permeablized lung epithelial cells, we did observe a small percentage of SP-C+/CC10+ BASCs in wild-type animals at E18.5 which was dramatically increased in Gata6Δ/Δ:SP-C/cre mutants (Fig. 2H and I). These data demonstrate that a lung epithelial specific loss of Gata6 leads to the premature appearance and increased numbers of BASCs.
Figure 2. Loss of Gata6 in lung epithelium results in the precocious appearance of BASCs.
Expression of SP-C and CC10 was analyzed at E18.5 in wild-type (A) and Gata6 Δ/Δ :SP-C/cre mutants (B–F) using double and triple immunofluorescent staining. Gata6 Δ/Δ :SP-C/cre mutants contained a large number of SP-C/CC10 double positive BASCs in airways (C and D, arrows and G) while wild-type lungs did not (A and G). Many of these double positive cells also expressed Sca1 (E and F, arrows). In addition to the SP-C/CCl0/Scal triple positive population, a CCl0/Scal population was also up-regulated in Gata6 Δ/Δ :SP-C/cre mutants (D–F, arrowheads, and G). FACS analysis was performed using SP-C and CC10 antibodies on both wild-type (H) and Gata6 Δ/Δ :SP-C/cre mutant (I) permeabilized lung epithelial cells at E18.5 and showed a significant increase in BASC numbers in the mutant lungs (2.4% versus 13.8%).
Gata6 regulates bronchiolar epithelial proliferation
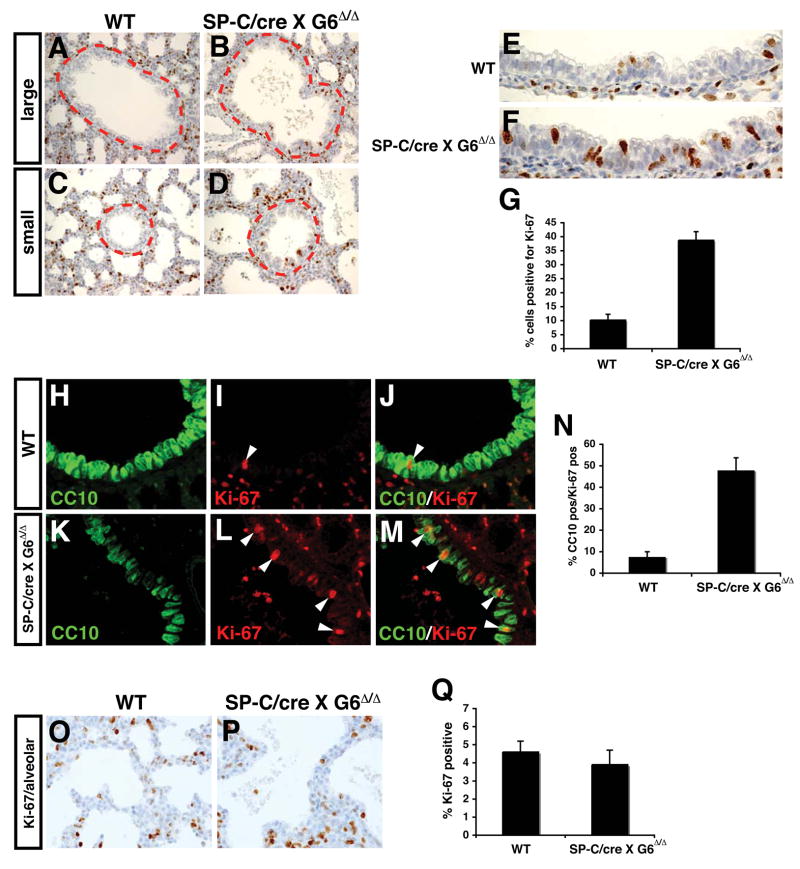
To further elucidate the cellular defects arising from loss of Gata6 expression in lung epithelium, cell proliferation was assessed. In wild-type lungs, cell proliferation is normally very low in bronchiolar epithelium (Fig. 3A and C and 6). By contrast, cell proliferation was significantly increased in bronchiolar epithelium of Gata6Δ/Δ:SP-C/cre mutants (Fig. 3A–G). Moreover, the bronchiolar epithelium had a more disorganized appearance with cells growing on top of each other (Fig. 3E and F). This phenotype is likely the result of increased proliferation of Clara cells as shown by double immunostaining for Ki-67 and CC10, which was expressed at very low levels in the bronchiolar epithelium of mutants (Fig. 3H–N). Remarkably, cell proliferation was relatively unaltered in the distal airways of Gata6Δ/Δ:SP-C/cre mutants (Fig. 3O–Q). Coupled with the loss in epithelial differentiation and increased numbers of BASCs, these data are consistent with a model where Gata6 regulates differentiation and proliferation of the bronchiolar epithelial niche, expanding the BASC population.
Figure 3. Increased proliferation in bronchiolar epithelium of Gata6 Δ/Δ :SP-C/cre mutants.
Increased staining for Ki-67 was observed in Gata6 Δ/Δ :SP-C/cre mutants versus wild-type lungs in bronchiolar epithelium of both medium and small caliber airways (A–D, inside dotted line). This corresponded to a more disorganized appearance in this epithelium with multiple layers of cells growing on top of each other (E and F). Quantitation revealed an approximate four-fold increase in proliferation in bronchiolar epithelium (G). Double immunofluorescent microscopy showed that most of the increased cell proliferation was observed in cells weakly positive for CC10 in Gata6 Δ/Δ :SP-C/cre mutants (H–M, arrowheads, and N; green-CC10, red-Ki-67). Of note, CC10 expression as detected by immunofluorescent microscopy in these studies was very low and the data represent a longer exposure than used in wild-type samples. No increase in distal airway epithelial proliferation was observed in Gata6 Δ/Δ :SP-C/cre mutants as detected by Ki-67 immunostaining (O–Q).
Fzd2 is a target of Gata6 and antagonizes Wnt/β-catenin signaling in lung epithelium
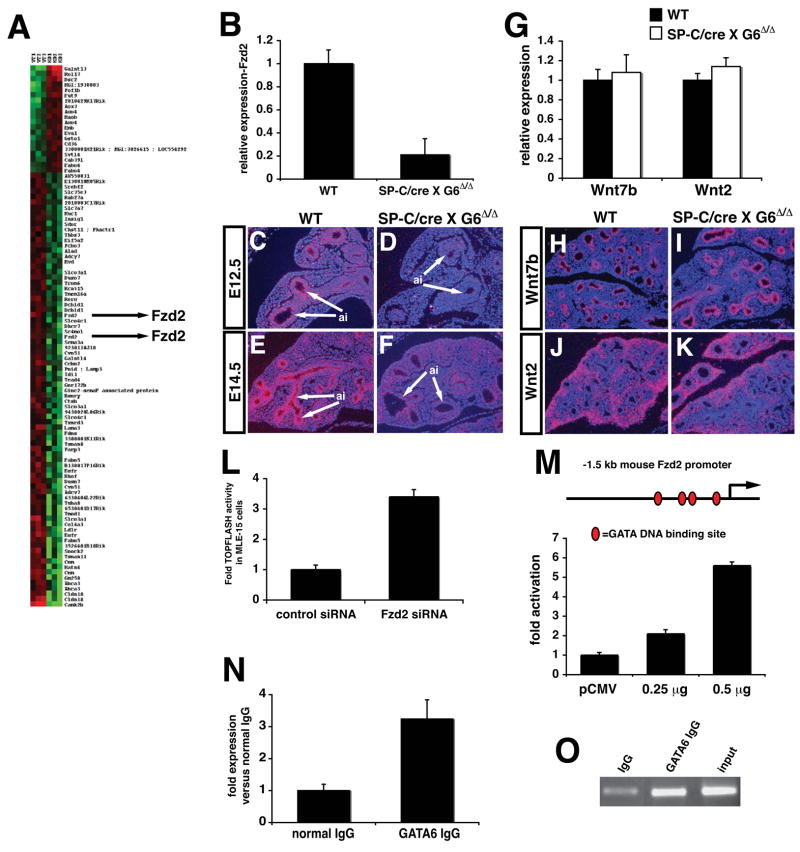
The precocious appearance of BASCs and increased proliferation in CC10+ cells suggested that Gata6 regulates a molecular pathway(s) required for stem/progenitor cell development in the lung. Microarray analysis was performed to identify Gata6 dependent pathways in lung epithelium (Fig. 4A). One of the genes identified from these analyses was Fzd2, a Wnt receptor previously reported to activate the non-canonical Wnt pathway 15,16. Fzd2 expression was specifically down-regulated in airway epithelium of Gata6Δ/Δ:SP-C/cre mutants as determind by RT-PCR and in situ hybridization analyses (Fig. 4B–F). In contrast, expression of Wnt7b and Wnt2, two Wnt ligands expressed in the lung, was not affected (Fig. 4G–K). Although in vitro studies have indicated that non-canonical Wnt signaling antagonizes the (β-catenin dependent canonical Wnt pathway, less is known about what function this plays in vivo 16–18. To assess whether loss of Fzd2 led to altered canonical Wnt signaling in lung epithelia, Fzd2 expression was inhibited in the lung epithelial cell line MLE-15, which expresses Fzd2, using siRNA mediated knock-down. Fzd2 expression is reduced by approximately 75% as compared to control siRNA transfected cells based on quantitative PCR (data not shown). Surprisingly, co-transfection of the TOPFLASH Wnt reporter revealed that inhibition of Fzd2 expression led to a significant increase in TOPFLASH activity (Fig. 4L). To determine whether Gata6 could directly activate Fzd2 transcription, a luciferase reporter construct containing the −1.5 kb proximal mouse Fzd2 promoter, which contains four Gata DNA binding sites, was used in trans-activation assays along with a Gata6 expression vector. Moreover, chromatin immunoprecipitation (ChIP) assays shows a direct association of Gata6 with the mouse Fzd2 promoter in the lung (Fig. 4N and O). Data from these assays show that Gata6 activates the mouse Fzd2 promoter in a dose-dependent manner, providing further evidence that Fzd2 is a direct target of Gata6 (Fig. 4M).
Figure 4. Fzd2 is a target of Gata6 in lung epithelium and negatively regulates canonical Wnt signaling.
Microarray studies were performed to compare gene expression profiles in wild-type versus Gata6 Δ/Δ :SP-C/cre mutants. A heat map representing a portion of the genes up or down regulated in Gata6 Δ/Δ :SP-C/cre mutants, highlighting Fzd2, is shown in (A). Q-PCR shows that Fzd2 expression is decreased approximately five-fold in Gata6 Δ/Δ :SP-C/cre mutants (B). In situ hybridization shows that Fzd2 expression is lost in airway epithelium but is still observed in mesenchyme of the lung in Gata6 Δ/Δ :SP-C/cre mutants (C–F, arrows). Q-PCR shows that Wnt7b and Wnt2 expression remains unchanged in Gata6 Δ/Δ :SP-C/cre mutants (G) while in situ hybridization shows that spatial expression of these Wnt ligands also remains unchanged (H–K). Knock-down of Fzd2 expression by siRNA in MLE-15 cells results in increased TOPFLASH activity (L). The proximal −1.5 kb Fzd2 promoter is trans-activated by Gata6 in a dose-dependent manner in NIH-3T3 cells (M). ChIP assays show that Gata6 is associated with the mouse Fzd2 promoter using Q-PCR (N) and as shown by agarose gel electrophoresis (O). ai-airways.
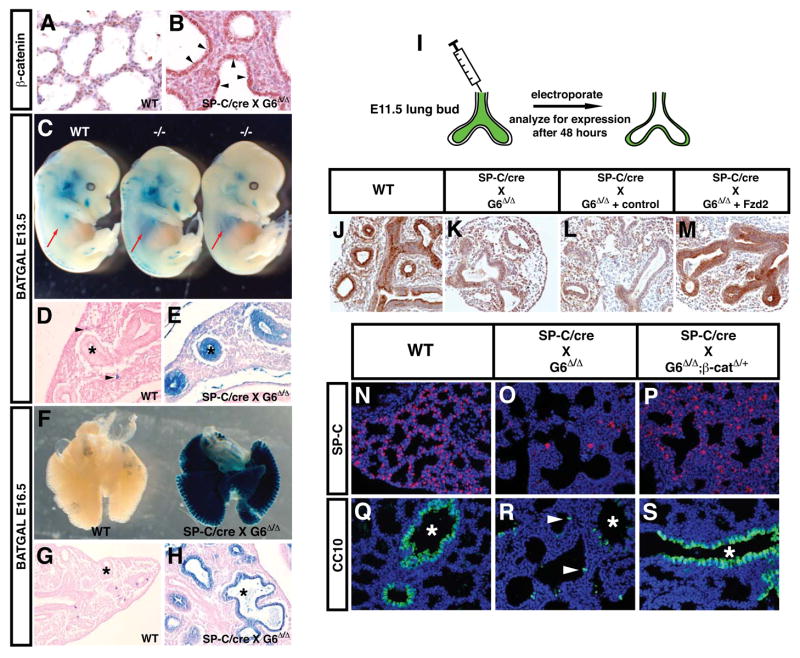
Based on these data, we predicted that Gata6Δ/Δ:SP-C/cre mutants would exhibit increased canonical Wnt signaling in airway epithelia in vivo. Given that canonical Wnt signaling has been demonstrated to promote expansion of stem/progenitor cells in other tissues 19–22, increased activity in this pathway could help explain the increased proliferation of CC10+ cells and expansion of BASCs that we observed. Therefore, we determined the levels and activity of canonical Wnt signaling in Gata6Δ/Δ:SP-C/cre mutants. Increased activated (β-catenin protein expression was readily observed in Gata6Δ/Δ:SP-C/cre mutants at E18.5 (Fig. 5A and B). Next, the transgenic Wnt/(β-catenin reporter line BAT-GAL was crossed into the Gata6Δ/Δ:SP-C/cre mutants and embryos were stained for lacZ expression at E13.5 and E16.5. We have previously reported that lacZ expression from this Wnt reporter declines rapidly in airway epithelium after E11.5 12. Remarkably, a dramatic increase in lacZ expression was observed in the airway epithelium of Gata6Δ/Δ:SP-C/cre mutants indicating that Wnt/(β-catenin signaling was up-regulated in airway epithelium upon loss of Gata6 expression (Fig. 5C–H). In wild-type mice, lacZ expression is observed in scattered lung mesenchymal cells at E13.5 (Fig. 5D, arrowheads) and in a few epithelial cells at E16.5 (Fig. 5G) as previously reported 12. This dramatic increase in canonical Wnt signaling is also the likely cause of the increased proliferation observed in Clara cells of Gata6Δ/Δ:SP-C/cre mutants (Fig. 3). Previously described targets of canonical Wnt signaling such as FGFR2, N-myc, and BMP4 are also up-regulated in Gata6Δ/Δ:SP-C/cre mutants (Supplemental Figure 2). However, intestinal genes previously shown to be up-regulated upon forced activation of (β-catenin signaling in the lung were relatively unaltered suggesting distinct differences between this model of hyper-activated canonical Wnt signaling and the up-regulation of canonical Wnt signaling we observe in the Gata6Δ/Δ:SP-C/cre mutants (Supplemental Figure 2). This could be in part due to the lack of endogenous Lef 1 expression in airway epithelium. Together, these data demonstrate that canonical Wnt signaling is dramatically up-regulated by loss of Gata6 and Fzd2, which may lead to the imbalance in BASC proliferation and differentiation.
Figure 5. Increased canonical Wnt signaling in lung epithelium upon loss of Gata6 expression and rescue of these defects by re-expression of Fzd2 or decreased β-catenin expression.
Activated (β-catenin protein expression is increased in Gata6 Δ/Δ :SP-C/cre mutant airway epithelium (A and B, arrows). The BAT-GAL lacZ Wnt reporter mice show increased canonical Wnt activity in the lungs of Gata6 Δ/Δ :SP–C/cre mutants at both E13.5 (C-E) and E16.5 (F–H). This activity is dramatic and can be observed through the chest wall of the mutants at E13.5 (C, arrows). This increase in Wnt signaling is confined to airway epithelium (E and H, asterisks). Residual lacZ expression is observed in wild-type littermates in scattered mesenchymal or epithelial cells (D and G, arrowheads). The airway lumen of E11.5 lung buds from both wild-type and Gata6 Δ/Δ :SP-C/cre mutants was injected with either control plasmid or an expression plasmid encoding mouse Fzd2 and then electroporated as described in Materials and Methods (I). SP-C expression was used as a indication of rescue of epithelial defects in Gata6 Δ/Δ :SP-C/cre mutants. As expected, SP-C expression was observed in the airway epithelium of the wild-type explants (J). Gata6 Δ/Δ:SP-C/cre mutants lacked SP-C expression (K). Electroporation of the control vector did not result in increased SP-C expression (L) whereas the Fzd2 expression plasmid resulted in re-expression of SP-C (M). Gata6 Δ/Δ :SP-C/cre mutants were crossed into the β-catenirflox/+ background and expression of SP-C and CC10 was analyzed in wild-type (N and Q), Gata6 Δ/Δ :SP-C/cre mutants (O and R), and Gata6 Δ/Δ:β-catenirflox/+:SP-C/cre mutants (P and S). As expected, Gata6 Δ/Δ:SP-C/cre mutants have significantly decreased SP-C and CC10 expression with ectopic CC10 expression in distal airways (O and R, arrowheads). However, loss of one allele of β-catenin leads to a marked increase in SP-C and CC10 expression in Gata6 Δ/Δ:SP-C/cre mutants and a decrease in ectopic CC10 expression (P and S). Asterisks-airway epithelium.
Re-expression of Fzd2 and decreased β-catenin expression rescues epithelial defects caused by loss of Gata6
To determine whether loss of Fzd2 was responsible for the lung epithelial differentiation defects observed in Gata6Δ/Δ:SP-C/cre mutants, we tested whether re-expression of Fzd2 could rescue the block in epithelial differentiation as noted by loss of SP-C expression in Gata6Δ/Δ:SP-C/cre mutants (Fig. 5I–M). As expected, electroporation of a control plasmid into lung explants did not rescue the loss of SP-C expression observed in Gata6Δ/Δ:SP-C/cre mutants (Fig. 5L). By contrast, re-expression of Fzd2 in Gata6Δ/Δ:SP-C/cre mutant lungs by electroporation of a Fzd2 expression plasmid led to a significant increase in SP-C expression (Fig. 5M). Expression of FGFR2, N-myc, and BMP4 were also up-regulated upon loss of Gata6 expression and this increase is reversed upon rescue with Fzd2 re-expression in lung explants (Supplemental Figure 3).
Since increase in Wnt/(β-catenin signaling in Gata6Δ/Δ:SP-C/cre mutants could contribute to the increase in BASC expansion and epithelial differentiation defects, we generated Gata6Δ/Δ:SP-C/cre:β-catenin+/Δ mutants to reduce (β-catenin expression and signaling by 50% in a Gata6 null background. Remarkably, loss of one copy of (β-catenin led to increased SP-C expression, re-establishment of proper CC10 expression in bronchiolar epithelium, and reduced numbers of SP-C/CC10 double positive BASCs as compared to Gata6Δ/Δ:SP-C/cre mutants (Fig. 5N–S and data not shown). Although this level of rescue was significant, these animals still succumbed to respiratory failure at birth indicating that rescue was not complete (data not shown). Together, these data indicate that Gata6 regulates Fzd2, which antagonizes Wnt/(β-catenin signaling in lung epithelium to control the balance between BASC expansion and epithelial differentiation.
Canonical Wnt/β-catenin signaling is activated during lung epithelial regeneration and regulates BASC expansion
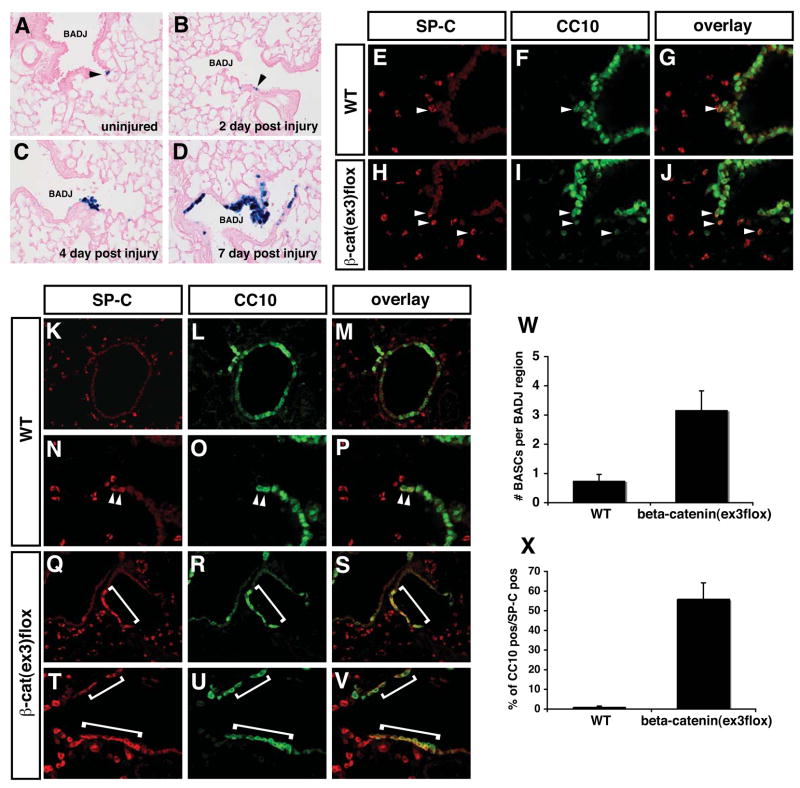
The above data suggests that canonical Wnt/(β-catenin signaling promotes expansion of BASCs. However, it is unknown whether Wnt/(β-catenin signaling is activated during lung epithelial regeneration in the adult or whether forced activation of this pathway leads to BASC expansion. To determine whether canonical Wnt/(β-catenin signaling is activated upon lung epithelial regeneration, we performed naphthalene injury on BAT-GAL Wnt/(β-catenin adult reporter mice and examined the activity of Wnt signaling using lacZ histochemical staining 23. Bronchiolar Clara cells, except for BASCs, express cytochrome P4502F2, making them susceptible to naphthalene toxicity 24. Depletion of Clara cells with naphthalene induces expansion and differentiation of BASCs as they regenerate the bronchiolar epithelium and this injury is normally resolved after 10–14 days 7,9. Thus, naphthalene injury is a good model in which to study injury, repair, and regeneration of this region of the lung. In uninjured animals and two days post injury, we observed small clusters of one to three lacZ positive cells within the BADJ region (Fig. 6A and B). By four days post-injury, we see a significant increase in the number of cells staining for lacZ expression and by seven days post-injury, we observed a dramatic increase in lacZ staining in the BADJ niche where BASCs are located (Fig. 6C and D). Thus, Wnt/(β-catenin activity is induced in the BADJ niche where BASCs are located during the airway epithelial regeneration process.
Figure 6. Canonical Wnt signaling is activated upon lung regeneration and forced activation of Wnt/β-catenin signaling leads to expansion of BASCs.
BAT-GAL mice were subjected to naphthalene based lung airway injury to induce airway regeneration. In uninjured mice, only rare lacZ positive cells were observed in the BADJ region of the airways (A, arrowhead). After naphthalene injury and during the epithelial regeneration process, a dramatic increase in lacZ staining indicating increased canonical Wnt signaling is observed in cells within the BADJ niche were BASCs reside (B–D). SP-C/CC10 co-staining shows that BASC numbers are increased in the BADJ region of β-cateninΔex3:CC10/cre mice (E–J, arrows and W). Seven days after naphthalene induced lung injury and regeneration, a significant increase in BASC number is observed in β-cateninΔex3:CC10/cre mice (K–V, compare arrowheads to brackets). Quantitation reveals that approximately 50% of the cells regenerating in the bronchiolar airways are SP-C/CC10 double positive BASCs (X).
To determine whether increased activity of Wnt/(β-catenin signaling would lead to increased numbers of BASCs, we crossed a CC10/cre mouse line, which targets cre expression specifically to Clara cells, to the β-cateninΔex3 allele which produces a constitutively activated form of (β-catenin that activates canonical Wnt signaling to high levels 25. Cre expression from this mouse line is initiated at approximately E18.5 and spares alveolar epithelium thus allowing us to target a more specific pool of cells containing BASCs and circumventing perinatal lethality due to severe respiratory failure observed in Gata6Δ/Δ:SP-C/cre mutants 26. β-cateninΔex3:CC10/cre adult mice are viable and express CC10 normally (data not shown). However, β-cateninΔex3:CC10/cre mice have increased numbers of BASCs located in the BADJ region (Fig. 6E–J). Using naphthalene injury to induce epithelial regeneration, we compared wild-type and (β-cateninΔex3:CC10/cre mice to observe differences in the number of BASCs during airway regeneration. Remarkably, in β-cateninΔex3:CC10/cre mice, a majority of cells found in the regenerating airways after injury were SP-C/CC10 double positive BASCs (Fig. 6K–X). This was supported by a dramatic increase in double positive BASCs using FACS analysis (Supplemenmtal Figure 4). These data show that Wnt signaling is activated in the niche containing BASCs and that forced activation of Wnt/(β-catenin signaling results in a dramatic increase in BASCs.
Gata6 is essential for lung epithelial regeneration by regulating BASC expansion and differentiation
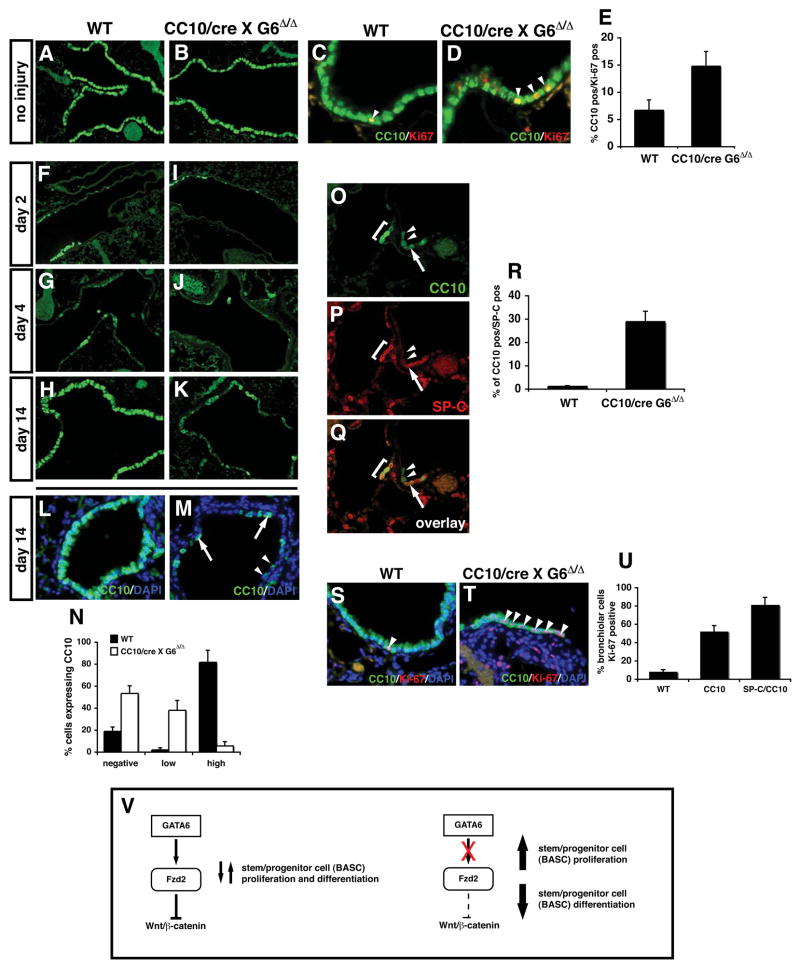
Based on the data showing increased Wnt signaling after loss of Gata6 in lung epithelium and that activated Wnt signaling leads to increased BASC numbers, we predicted that postnatal loss of Gata6 would lead to an increase in BASC number and decreased airway regeneration. Since the Gata6Δ/Δ:SP-C/cre mutants are lethal, we crossed the Gata6Δ/Δ mice to the CC10/cre mice to delete Gata6 in Clara cells. Gata6 Δ/Δ:CC10/cre mutants had similar viability as wild-type littermates (data not shown). However, Gata6 Δ/Δ:CC10/cre mutant mice exhibited increased proliferation in CC10 positive cells similar to that observed in Gata6Δ/Δ:SP-C/cre mutants at E18.5 (Fig. 7A–E).
Figure 7. Gata6 regulates BASC expansion and differentiation in airway epithelial regeneration.
Both wild-type and adult Gata6 Δ/Δ :CC10/cre mutants show normal expression of CC10 (A and B). However, Gata6 Δ/Δ :CC10/cre mutants showed increased proliferation (C–E). Napthalene injury of Gata6 Δ/Δ :CC10/cre mutants resulted in decreased Clara cell regeneration by 10 days after injury (F–K). CC10 positive cells that did regenerate were divided into two categories: low expressing cells and high expressing cells (L–N). Few if any CC10 high expressing cells regenerated in Gata6 Δ/Δ:CC10/cre mutants (N). This loss in Clara cell regeneration was accompanied by an increase in BASCs (O–R) and increased proliferation in the CC10 expressing cells that did regenerate (S–U). Gata6 regulates Fzd2 expression which in turn antagonizes canonical Wnt signaling in lung epithelia and allows proper epithelial differentiation and development of BASCs which is required for lung regeneration (V).
To determine whether BASC differentiation and airway regeneration was affected in Gata6Δ/Δ:CC10/cre mutants, naphthalene induced acute lung injury studies were performed. The initial response in both wild-type and Gata6Δ/Δ:CC10/cre mutants was similar at two and four days after naphthalene treatment with both control and mutants demonstrating severe loss of CC10 expressing cells (Fig. 7F–J). However, by 14 days post injury, the Gata6Δ/Δ:CC10/cre mutants exhibited a significant impairment in Clara cell regeneration in the bronchiolar airways (Fig. 7H and K). The CC10 positive cells within the bronchi of Gata6Δ/Δ:CC10/cre mutants were also qualitatively different: instead of uniform CC10 expression as observed in wild-type mice, CC10 expression in the mutants fell into two categories: high level expression as observed in wild-type lungs or low level CC10 expression (Fig. 7L and M). More than 50% of bronchiolar epithelial cells were negative for CC10 expression and less than 10% expressed high levels of CC10 in Gata6Δ/Δ:CC10/cre mutants (Fig. 7N). The different levels of CC10 expression observed in these mice may reflect abnormal development of Clara cells repopulating the airways due to the disruption in BASC expansion and differentiation. The majority of the remaining non-CC10 positive cells in the bronchiolar airways expressed (β-tubulin IV indicating that they were ciliated epithelium (data not shown). These data indicated a severe inhibition of Clara cell regeneration and a block in epithelial differentiation following naphthalene induced lung injury in Gata6Δ/Δ:CC10/cre mutants.
To determine whether the loss of Clara cell regeneration was associated with altered BASC development, SP-C/CC10 immunostaining was performed. Consistent with previous reports, a small expansion of BASCs was observed at four days after injury in both wild-type and Gata6Δ/Δ:CC10/cre mutants (data not shown and 9). However, at 14 days post-injury, a dramatic increase in BASCs was observed in Gata6Δ/Δ:CC10/cre mutants that was not observed in wild-type animals (Fig. 7O–R). These cells were associated with an increase in proliferation in both CC10 and SP-C/CC10 expressing cells in the terminal bronchiols (Fig. 7S–U and data not shown). Together, these data indicate that Gata6 plays an essential role in airway epithelial regeneration through regulation of BASC expansion and differentiation in the adult lung via modulation of Wnt signaling (Fig. 7V).
DISCUSSION
The molecular programs regulating endogenous tissue specific stem cell expansion and differentiation are of obvious importance in developing techniques to harness the ability of these cells to regenerate damaged and diseased organs. We show that the transcription factor Gata6, acting through Fzd2 mediated Wnt signaling, is required for proper development of BASCs, a recently identified stem/progenitor cell population found in the lung that plays a critical role in lung airway regeneration. Loss of Gata6 leads to unrestrained expansion of BASCs at the expense of lung epithelial differentiation during both lung development and in airway regeneration after injury which is similar to what occurs upon forced activation of canonical Wnt signaling. This finding underscores an important aspect of stem cell biology: the recapitulation of critical developmental pathways in the regulation of stem/progenitor cell expansion required for tissue regeneration in the adult.
The role of most tissue restricted transcription factors in stem/progenitor cell expansion and differentiation remains less well understood than their role in normal development. Although its commonly thought that these transcription factors and pathways play key roles in tissue repair by directing self-renewal and differentiation of endogenous stem/progenitor cells, this hypothesis has not been tested in many tissues including the lung. Gata6 is expressed throughout the developing foregut endoderm and plays an important role in regulating lung epithelial gene transcription and the regulatory regions of most lung epithelial specific genes contain evolutionarily conserved Gata DNA binding sites 5,10,27–30. Gata6 regulation of Fzd2 reveals an unexpected role for non-canonical Wnt antagonism of canonical Wnt signaling in controlling stem/progenitor cell expansion and differentiation. The role for Fzd2 in Wnt signaling has been somewhat controversial. Most studies have shown that Fzd2 regulates effectors of non-canonical Wnt signaling including G-protein signaling and activation of CamKII 31–33. Since increased canonical Wnt signaling leads to defective epithelial differentiation and tumor formation in the lung34,35, it is imperative that this pathway be controlled via multiple signals. Gata6 regulation of Fzd2 may play a key role in this through fine-tuning the activity of canonical Wnt signaling in airway epithelium.
Gata factors and Wnt signaling have each been implicated in stem/progenitor cell expansion and differentiation. Gata6 appears to appose Nanog in regulating primitive endoderm development with loss of Gata6 in ES cells leading to increased proliferation and decreased primitive endoderm differentiation 36–38. Wnt signaling has also recently been implicated in stem/progenitor self-renewal and tissue regeneration. In zebrafish, tail regeneration requires canonical Wnt signaling and inhibition of this pathway inhibits regeneration21. Zebrafish cardiac regeneration and expansion of anterior heart field progenitors in mammals also appears to require canonical Wnt signaling 19,21,39. Although hyperactive canonical Wnt signaling has been shown to disrupt lung development leading to a loss of epithelial differentiation and ectopic expression of intestinal marker genes, these studies have not addressed the role of this pathway in regulating stem/progenitor development in the lung34,35. We show that increased canonical Wnt signaling in lung epithelium not only results in defective epithelial differentiation but also increased stem/progenitor cell development including precocious appearance of BASCs. Our studies also highlight important regulatory cross-talk between canonical and non-canonical Wnt signaling pathways in stem/progenitor cell development by revealing that Fzd2 inhibits canonical Wnt signaling in lung epithelia which helps to balance epithelial differentiation with stem/progenitor cell expansion.
BASCs were originally defined by their expression of two lung epithelial specific marker proteins SP-C and CC10, in addition to expression of other general stem cell markers such as Sca1 9. The involvement of BASCs in lung cancer biology is supported by the observation that BASC numbers was significantly increased in a K-ras tumor model of lung adenocarcinoma9. The data on B ASC development and differentiation has remained scant with recent studies focusing on their expansion upon disruption of cell the cycle/proliferation genes p27kip1, MAPK p38, and PTEN 40–43. However, most of these studies did not address the lung epithelial specific role for these factors but rather addressed the global role of these genes in tumorgenesis and epithelial proliferation. Our data implicate BASC function in the normal regeneration process after lung injury and that Gata6 regulates a Wnt mediated pathway required for this regeneration. We show a dramatic increase in bronchiolar epithelial proliferation in response to increased canonical Wnt signaling in airway epithelium. Such an increase likely contributes to the precocious appearance and expansion of BASCs upon loss of Gata6 in the lung.
Our current study has revealed that Gata6 is required for the balance of BASC expansion and epithelial differentiation in the lung. Gata6 regulates Fzd2 gene expression, which acts as a negative regulator of canonical Wnt signaling in lung epithelium. Loss of Fzd2 correlates with increased canonical Wnt signaling, loss of lung epithelial differentiation, and the precocious appearance of BASCs in the embryonic lung as well as adult lung during regeneration. Moreover, Wnt signaling is activated upon lung epithelial regeneration and increased Wnt signaling expands the numbers of BASCs in the lung. Given this ability of canonical Wnt signaling to expand stem/progenitor cell populations in the lung, it may be possible in the future to use agonists of this pathway to increase lung injury repair and regeneration. However, given the role of Wnts as oncogenes and the fact that BASCs are thought to be progenitors of lung adenocarcinomas, careful consideration will have to be given to modulating the Wnt pathway for this purpose.
EXPERIMENTAL PROCEDURES
Animals
Gata6Δ/Δ, SP-C/rtta, teto-cre, β-cateninΔ/Δ, β-cateninΔex3, CC10/cre, and BAT-GAL mice were previously generated and genotyped as described11,13,14,23,44,45 26. SP-C/rtta:teto-cre animals were treated with dox (1mg/Kg in food and 1mg/ml in water) starting at E0.5 for developmental deletion. All animal experiments were performed with the approval of the University of Pennsylvania Institutional Animal Care and Use Committee.
Histology
In situ hybridization was performed as previously described 46. Immunohistochemistry and immunofluorescent staining was performed using the following antibodies at the indicated dilutions: SP-C (Chemicon, 1:500), CC10 (Santa Cruz T-18, 1:50), SP-B (Chemicon, 1:250), rat anti-Ki-67 (Dako, clone TEC-3), Gata6 (R and D Systems, Inc. AF1700 1:50), Sca-1 (R and D Systems, Inc. AF1226 1:50), Tlalpha (Developmental Studies Hybridoma Bank mAb 8.1.1, University of Iowa 1:100), (β-catenin (BD Biosciences, 1:50), activated (β-catenin (Millipore, Inc. clone 8E7,1:100), and (β-tubulin IV (BioGenex, Inc. MU178-UC 1:100). (β-galactosidase histochemical staining was performed as previously described 47.
Quantitative assessment of BASCs
Immunostained cells were counted 200μm proximal and distal of the bronchioalveolar duct junction in relation to the branching airways similar to that previously described 9. A minimum of five sections from each sample was used for quantitative measurements and at least four animals of each described genotype were analyzed in all experiments. FACS analysis was performed using lung epithelial cells isolated from E18.5 or adult lungs as indicated using a previously described protocol9. Lung epithelial cells were permeabalized using the Fix and Perm Cell kit (Caltag Labs) and then stained for SP-C and CC10 expression using the antibodies described above followed by Alexa fluor 647 and 488 secondary antibodies, respectively 40,42.
Microarray studies
RNA was isolated from E18.5 lungs from wild-type and Gata6Δ/Δ:SP-C/cre mutant littermates (three samples of each genotype). Total RNA was transcribed to generate biotinylated cRNA to use as a probe for Affymetrix mouse 230A GeneChips. Three chips were hybridized for each genotype. Data from these arrays was normalized using Microarray Suite 5.0 (MAS5, Affymetrix) and Significance Analysis of Microarrays (SAM, http://wwwstat.stanford.edu/~tibs/SAM/). 2.0-fold and greater changes in gene expression were considered significant. Treeview software was used to generate the heatmap (http://rana.lbl.gov). Total RNA was isolated with Trizol and Q-PCR was performed using the oligonucleotides listed in Supplemental Table 1 and an Applied Biosystems 7900HT system with Syber green reaction mixture as previously described 48.
Cell culture transfection assays
MLE-15 cells were transfected with the TOPFLASH luciferase reporter plasmid along with either Fzd2 siRNA Smartpool or control siRNA (Dharmacon) using Lipofectamine 2000. A Renilla luciferase control expression plasmid was included in the transfections to control for transfection efficiency. Cells were harvested 48 hours after transfection and luciferase assays were performed as previously described 49.
The proximal 1.5 kb promoter region of mouse Fzd2 was amplified and cloned into the pGL3basic vector to generate pGL3Fzd2pro.luc. The oligonucleotide sequences used to generate the Fzd2 promoter plasmid are: forward 5′-ATGGTACCTCTGGTAATTCTCCTGTCTCAACG-3′, reverse 5′-ATCTCGAGGATCCCAATTAGTCGGTTTCAAGG-3′. This plasmid was transfected along with a Gata6 expression plasmid into NIH-3T3 cells and a control Renilla luciferase expression plasmid. 48 hours after transfection, cells were harvested and luciferase assays were performed as previously described 49.
Quantitative PCR and chromatin immunoprecipitation (ChIP) assays
Total RNA was isolated with Trizol and Q-PCR was performed using the primers listed in Supplemental Table 1. Chromatin was made from E18.5 mouse lung tissue using a commercially available kit (Upstate Biotechnology, Inc.). Lung tissue was minced, fixed with 1% formaldehyde and chromatin was sheared by sonication to an average length of 500–600 bp then immunoprecipitated with an anti-GATA6 specific antibody (Santa Cruz Biotech, C-10). Reverse cross-linked immunoprecipitated chromatin was subjected to PCR using the primers listed in Supplementary table 1.
Napthalene injury
Mice were injected interperitoneally with 300 mg/Kg of naphthalene dissolved in corn oil while control mice were injected with the same volume of corn oil. For immunohistochemistry, lungs were inflation fixed at 25 cm H20 pressure with 4% paraformaldehyde (PFA) at the indicated time points after naphthalene injection and further fixed by submersion in 4% PFA for 24 hours. For lacZ staining, lungs were fixed with 2% PFA and processed as previously described 47.
Lung explant studies
Lungs from El 1.5 wild-type or Gata6Δ/Δ :SP-C/cre mutants were isolated and the airways were microinjected with 1mg/ml of either a Fzd2 expression plasmid or a control expression plasmid, rinsed briefly in PBS to remove extraneous external DNA and electroporated in a BTX ECM 2001 electroporator at 300 volts for 1 millisecond. Explants were then cultured as previously described for 48 hours after which they were collected and fixed for histology 47.
Supplementary Material
Supplemental Figure 1. Expression of Gata6 in airway epithelium. Immunohistochemistry was performed on E18.5 mouse lung tissue. Gata6 expression (A and C, green) was observed in both proximal (asterisk) and more distal airway epithelium. CC10 expression (B and C, red) is observed only in proximal airway epithelium. An overlay of the expression of these two proteins reveals Gata6 expression in CC10 positive proximal airway epithelium (C).
Supplemental Figure 2. Expression of Wnt induced genes in Gata6Δ/Δ:SP-C/cre mutants. Expression of the previously identified Wnt lung targets FGFR2, N-myc, and BMP4 is increased in Gata6 Δ/Δ :SP-C/cre mutants as measured by Q-PCR (A). Genes induced by hyper-activation of (β-catenin signaling in the lung including cryptidin 6 and Tff3 were slightly down-regulated whereas Atohl was slightly up-regulated in Gata6Δ/Δ SP-C/cre mutants.
Supplemental Figure 3. Expression of activated β in and Wnt target genes FGFR2, N-myc, and BMP4 in lung explant rescue cultures. Levels of activated (βin are increased in Gata6 Δ/Δ SP-C/cre mutant (B) and control plasmid transfected explants (C) as compared to wild-type explants (A). However, expression of activated (βin returns to wild-type levels upon re-expression of Fzd2 (D). Expression of FGFR2, N-myc, and BMP4 is increased in Gata6 Δ/Δ SP-C/cre mutant (F, J, N) and control plasmid transfected explants (G, K, O) as compared to wild-type explants (E, I, M). However, expression of all three of these genes returns to wild-type levels upon re-expression of Fzd2 (H, L, P).
Supplemental Figure 4. Increase in double positive BASCs in of β-catenin Δex3 :CC10/cre mice after naphthalene injury. FACS analysis was performed on wild-type and β-catenin Δex3:CC10/cre mice one week after naphthalene injury showing a greater than three-fold increase in BASCs upon expression of activated (β-catenin (A). Graphic depiction of the fold change in BASC numbers (B).
Supplemental Table 1. Oligonucleotide sequences used for real-time quantitative PCR and ChIP.
Acknowledgments
The authors thank Jon Epstein and Celeste Simon for helpful suggestions and critical reading of the manuscript. We thank Stefano Piccolo for providing the BAT-GAL mice, Makoto Taketo for providing the β-cateninΔex3 mice, and Min Min Lu for excellent technical assistance with the histological studies. These studies were supported by funding from the NIH to E. E. M. (HL064632, HL075215, and HL087825) and to M. S. P. (HL075215). E. E. M. is an Established Investigator of the American Heart Association.
References
- 1.Cardoso WV. Molecular regulation of lung development. Annu Rev Physiol. 2001;63:471–94. doi: 10.1146/annurev.physiol.63.1.471. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 3.Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1 (−/−) mouse embryos. Dev. 1999:60–71. doi: 10.1006/dbio.1999.9234. 5/0/209. [DOI] [PubMed] [Google Scholar]
- 4.Wan H, et al. Compensatory roles of Foxa1 and Foxa2 during lungmorphogenesis. J Biol Chem. 2005;280:13809–16. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–46. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- 6.Blenkinsopp WK. Proliferation of respiratory tract epithelium in the rat. Exp Cell Res. 1967;46:144–54. doi: 10.1016/0014-4827(67)90416-8. [DOI] [PubMed] [Google Scholar]
- 7.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–82. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–65. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 9.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, et al. GATA and Nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development. 2007;134:189–98. doi: 10.1242/dev.02720. [DOI] [PubMed] [Google Scholar]
- 11.Lepore JJ, et al. GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J Clin Invest. 2006;116:929–39. doi: 10.1172/JCI27363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu W, et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–39. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–9. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- 14.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem. 2000;275:11858–64. doi: 10.1074/jbc.275.16.11858. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Malbon CC, Wang HY. Gene profiling of Frizzled-1 and Frizzled-2 signaling: expression of G-protein-coupled receptor chimeras in mouse F9 teratocarcinoma embryonal cells. Mol Pharmacol. 2004;65:45–55. doi: 10.1124/mol.65.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Ishitani T, et al. The TAK1-NLK mitogen-activated protein kinase cascade functions in the Wnt-5a/Ca(2+) pathway to antagonize Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23:131–9. doi: 10.1128/MCB.23.1.131-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topol L, et al. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. J Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres MA, et al. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–37. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen ED, et al. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokoyama H, Ogino H, Stoick-Cooper CL, Grainger RM, Moon RT. Wnt/beta-catenin signaling has an essential role in the initiation of limb regeneration. Dev Biol. 2007;306:170–8. doi: 10.1016/j.ydbio.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoick-Cooper CL, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–89. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 22.Fathke C, et al. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol. 2006;7:4. doi: 10.1186/1471-2121-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plopper CG, Suverkropp C, Morin D, Nishio S, Buckpitt A. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract of mice, rats and hamsters after parenteral administration of naphthalene. J Pharmacol Exp Ther. 1992;261:353–63. [PubMed] [Google Scholar]
- 25.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwanaga K, et al. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res. 2008;68:1119–27. doi: 10.1158/0008-5472.CAN-07-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Z, et al. Hop functions downstream of Nkx2.1 and GATA6 to mediate HDAC-dependent negative regulation of pulmonary gene expression. Am J Physiol Lung Cell Mol Physiol. 2006;291:L191–9. doi: 10.1152/ajplung.00385.2005. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Morrisey EE, Whitsett JA. GATA-6 is required for maturation of the lung in late gestation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L468–75. doi: 10.1152/ajplung.00044.2002. [DOI] [PubMed] [Google Scholar]
- 29.Weidenfeld J, Shu W, Zhang L, Millar SE, Morrisey EE. The WNT7b promoter is regulated by TTF-1, GATA6, and Foxa2 in lung epithelium. J Biol Chem. 2002;277:21061–70. doi: 10.1074/jbc.M111702200. [DOI] [PubMed] [Google Scholar]
- 30.Bruno MD, Korfhagen TR, Liu C, Morrisey EE, Whitsett JA. GATA-6 activates transcription of surfactant protein A. J Biol Chem. 2000;275:1043–9. doi: 10.1074/jbc.275.2.1043. [DOI] [PubMed] [Google Scholar]
- 31.Ahumada A, et al. Signaling of rat Frizzled-2 through phosphodiesterase andcyclic GMP. Science. 2002;298:2006–10. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- 32.Sheldahl LC, et al. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–77. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–11. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 34.Mucenski ML, et al. Beta-catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;289:L971–9. doi: 10.1152/ajplung.00172.2005. [DOI] [PubMed] [Google Scholar]
- 35.Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–24. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Morrisey EE, et al. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–90. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralston A, Rossant J. Genetic regulation of stem cell origins in the mouse embryo. Clin Genet. 2005;68:106–12. doi: 10.1111/j.1399-0004.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- 39.Lin L, et al. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:9313–8. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besson A, et al. Discovery of an oncogenic activity in p27Kipl that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–46. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dave V, et al. Conditional Deletion of Pten Causes Bronchiolar Hyperplasia. Am J Respir Cell Mol Biol. 2007 doi: 10.1165/rcmb.2007-0182OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ventura JJ, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–8. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 43.Yanagi S, et al. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–40. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brault V, et al. Inactivation of the beta-catenin gene by Wntl-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 45.Iwanaga K, et al. Pten Inactivation Accelerates Oncogenic K-ras-initiated Tumorigenesis in a Mouse Model of Lung Cancer. Cancer Research. 2007 doi: 10.1158/0008-5472.CAN-07-3117. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–22. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 47.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–42. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 48.Lepore JJ, Cappola TP, Mericko PA, Morrisey EE, Parmacek MS. GATA-6 regulates genes promoting synthetic functions in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:309–14. doi: 10.1161/01.ATV.0000152725.76020.3c. [DOI] [PubMed] [Google Scholar]
- 49.Shu W, Yang H, Zhang L, Lu MM, Morrisey EE. Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem. 2001;276:27488–97. doi: 10.1074/jbc.M100636200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Expression of Gata6 in airway epithelium. Immunohistochemistry was performed on E18.5 mouse lung tissue. Gata6 expression (A and C, green) was observed in both proximal (asterisk) and more distal airway epithelium. CC10 expression (B and C, red) is observed only in proximal airway epithelium. An overlay of the expression of these two proteins reveals Gata6 expression in CC10 positive proximal airway epithelium (C).
Supplemental Figure 2. Expression of Wnt induced genes in Gata6Δ/Δ:SP-C/cre mutants. Expression of the previously identified Wnt lung targets FGFR2, N-myc, and BMP4 is increased in Gata6 Δ/Δ :SP-C/cre mutants as measured by Q-PCR (A). Genes induced by hyper-activation of (β-catenin signaling in the lung including cryptidin 6 and Tff3 were slightly down-regulated whereas Atohl was slightly up-regulated in Gata6Δ/Δ SP-C/cre mutants.
Supplemental Figure 3. Expression of activated β in and Wnt target genes FGFR2, N-myc, and BMP4 in lung explant rescue cultures. Levels of activated (βin are increased in Gata6 Δ/Δ SP-C/cre mutant (B) and control plasmid transfected explants (C) as compared to wild-type explants (A). However, expression of activated (βin returns to wild-type levels upon re-expression of Fzd2 (D). Expression of FGFR2, N-myc, and BMP4 is increased in Gata6 Δ/Δ SP-C/cre mutant (F, J, N) and control plasmid transfected explants (G, K, O) as compared to wild-type explants (E, I, M). However, expression of all three of these genes returns to wild-type levels upon re-expression of Fzd2 (H, L, P).
Supplemental Figure 4. Increase in double positive BASCs in of β-catenin Δex3 :CC10/cre mice after naphthalene injury. FACS analysis was performed on wild-type and β-catenin Δex3:CC10/cre mice one week after naphthalene injury showing a greater than three-fold increase in BASCs upon expression of activated (β-catenin (A). Graphic depiction of the fold change in BASC numbers (B).
Supplemental Table 1. Oligonucleotide sequences used for real-time quantitative PCR and ChIP.