Abstract
Upon liver injury, quiescent hepatic stellate cells (HSC), the most relevant cell type for hepatic fibrogenesis, become active, characterized by enhanced cell growth and over-production of extracellular matrix (ECM). Oxidative stress facilitates HSC activation and the pathogenesis of hepatic fibrosis. Glutathione (GSH) is the most important intracellular antioxidant. We previously showed that curcumin, the yellow pigment in curry from turmeric, significantly inhibited HSC activation. The aim of this study is to elucidate the underlying mechanisms. It is hypothesized that curcumin might inhibit HSC activation mainly by its antioxidant capacity. Results from this study demonstrate that curcumin dose1-, and time-dependently attenuates oxidative stress in passaged HSC demonstrated by scavenging reactive oxygen species and reducing lipid peroxidation. Curcumin elevates the level of cellular GSH and induces de novo synthesis of GSH in HSC by stimulating the activity and gene expression of glutamatecysteine ligase (GCL), a key rate-limiting enzyme in GSH synthesis. Depletion of cellular GSH by the inhibition of GCL activity using L-buthionine-sulfoximine evidently eliminates the inhibitory effects of curcumin on HSC activation. Taken together, our results demonstrate, for the first time, that the antioxidant property of curcumin mainly results from increasing the level of cellular GSH by inducing the activity and gene expression of GCL in activated HSC in vitro. de novo synthesis of GSH is a prerequisite for curcumin to inhibit HSC activation. These results provide novel insights into the mechanisms of curcumin as an anti-fibrogenic candidate in the prevention and treatment of hepatic fibrosis.
Keywords: Antioxidant, hepatic fibrosis, hepatic stellate cell, polyphenol, oxidative stress
INTRODUCTION
Hepatic stellate cells (HSC), previously termed fat- or vitamin A-storing cells, or Ito cells, are the key cellular element involved in the development of hepatic fibrosis [1, 2]. Upon liver injury, quiescent HSC become activated and trans-differentiate into myofibroblast-like cells characterized by increased cell proliferation, excessive deposition of ECM and de novo expression of α-smooth muscle actin (α-SMA) [1, 2]. Culturing quiescent HSC on plastic plates causes spontaneous activation, mimicking the process seen in vivo, which provides a good model for elucidating the underlying mechanisms of HSC activation and for studying possible therapeutic intervention of the process [3]. In the last decade, advances in the understanding of genes promoting HSC activation are impressive. There are, however, few breakthroughs in the therapeutic intervention of hepatic fibrogenesis. Therefore, research identifying anti-fibrogenic agents that are innocuous is of high priority and is urgently needed. Most evolving anti-fibrogenic therapies will be aimed at inhibiting HSC activation.
Although the underlying mechanisms remain incompletely understood, accumulating evidence has indicated that oxidative stress plays critical roles in activation of HSC [4, 5]. Oxidative stress is a deleterious imbalance between the production and removal of free radicals, including reactive oxygen species (ROS). They are generated from aerobic cells by inflammation and aerobic metabolism [6, 7]. Reducing oxidative stress in mammalian cells is through several antioxidant systems, including enzymes and non-enzymatic molecules. Among them, glutathione (GSH) is the main non-protein thiol. It reacts with ROS or functions as a cofactor of antioxidant enzymes. GSH is converted to its oxidized form (GSSG), leading to the conversion of H2O2 and lipid peroxides to water and lipid alcohols catalyzed by GSH peroxidase. This process thereby prevents degradation to highly toxic free radicals. GSSG is ultimately reduced to GSH by GSH reductase to maintain cellular GSH at a proper level. The GSH/GSSG ratio is regarded as a sensitive indicator of oxidant stress [6, 7]. A higher ratio of GSH/GSSH indicates a lower level of oxidant stress. de novo synthesis of GSH requires glutamate-cysteine ligase (GCL), a key rate-limiting enzyme in GSH synthesis [8]. The GCL enzyme is a heterodimer with a large catalytic subunit (GCLc, ∼73 kDa) and a small modifier subunit (GCLm, ∼30 kDa), which are encoded by different genes and dissociated under reducing conditions [9, 10].
The polyphenol compound curcumin is the main yellow pigment in curry from turmeric. Besides its dietary use, turmeric has been used in Chinese herbal medicine for skin and guts diseases and wound healing. Curcumin is a potent antioxidant [11]. Recent studies have indicated that dietary administration of curcumin improves both acute and subacute rat liver injury caused by carbon tetrachloride [12]. We previously demonstrated that curcumin inhibited activation of HSC in vitro [13]. The underlying mechanisms remain largely to be defined. The purposes of the current study are to evaluate the role of the antioxidant property of curcumin in the inhibition of HSC activation and to elucidate the underlying mechanisms. We hypothesize that the inhibition of HSC activation by curcumin might mainly result from its antioxidant property by increasing the level of cellular GSH. Results in the current report support our hypothesis and provide novel insights into the mechanisms of curcumin in the inhibition of HSC activation.
MATERIALS AND METHODS
Isolation and culture of hepatic stellate cells
HSC were isolated from male Sprague-Dawley rats (200∼250 grams) as we previously described [13]. Cells were cultured in Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), unless specific indication. HSC aged at passage 4-8 were used for experiments. Curcumin (purity > 94%) was purchased from Sigma (St. Louis, MO).
Determination of the level of intracellular ROS
The accumulation of ROS in HSC was determined by analyzing dichlorofluorescein fluorescence (DCF), as described previously [14]. Briefly, 2,7-dichlorofluorescein diacetate at 5 μM was applied to the culture medium after HSC were treated with curcumin as indicated. After incubation of cells with DCF at 37°C for 30-45 min, the medium was removed and replaced with phenol red-free Hank's balanced salt solution. DCF fluorescence was quantified (excitation at 485 nm; emission at 530 nm) using a fluorescence multi-well plate reader. The DCF intensity was normalized to that of Hoechst 33342 (10μM), which stains nuclei of living cells.
LPO assays
LPO assays were performed by using the Lipid Hydroperoxide Assay Kit purchased from Cayman Chemical (Ann Arbor, MI). Passaged HSC were treated with curcumin at indicated concentrations for 24 hr. Immediately after sonication, lipid hydroperoxides were extracted from the samples into chloroform using the extraction buffer provided by the manufacturer. Chromogenic reaction was carried out at room temperature for 5 min. The absorbance was read at 500nm using a 96-well plate spectrometer (SpectraMax 190, Molecular Devices Corp., Sunnyvale, CA). 13-HpODE (13-hydroperoxy- octadecadienoic acid) was used as a standard.
GSH assays
The levels of cellular GSH and GSSG were, respectively, determined by using the enzyme immune assay kit GSH-400© (Cayman, Ann Arbor, MI), following the protocol provided by the manufacturer.
Analyses of GCL activity
GCL activity was determined at 25°C spectrophotometrically using a coupled assay with pyruvate kinase and lactate dehydrogenase as described previously [15]. Reaction mixture (0.21ml) contained Tris-HCl 100mM pH 8.0, KCl 150mM, MgCl2 20mM, Na2EDTA 2mM, Na2ATP 5mM, phosphoenolpyruvate 2mM, L-glutamate 10mM, L-α-aminobutyrate 10mM, NADH 0.27mM, type II rabbit muscle pyruvate (Sigma) 2ug and lactate dehydrogenase (Sigma) 2ug. The reaction was initiated by the addition of ATP to a final concentration for 5mM. The rate of decrease in absorbance at 340nm was followed at 25°C in SepectroMax 190 plate reader. Specific enzymatic activity of GCL was measured and expressed as arbitrary unit, i.e. mmol of NADH oxidized/min/mg protein.
Determination of cell growth (MTS assays)
Pre-confluent HSC were treated with curcumin, or N-acetyl-cysteine (NAC), a precursor of GSH by supplying cysteine, or buthionine sulfoximine (BSO), a specific inhibitor of GCL, or BSO for 1 hour prior to the addition of curcumin or NAC, at indicated concentrations for 24 h. Cell growth was determined by using the CellTiter 96 Aqueous Non-radioactive Cell Proliferation Assay kit following the protocol provided by the manufacturer (Promega). MTS assay is a colorimetric method for determining the number of viable cells.
Western blotting analyses
Whole cell extracts were prepared from pre-confluent passaged HSC. SDS-PAGE with 10% resolving gel was used to separate proteins (25μg/well). Protein concentrations were determined using the BCA™ Protein Assay Kit according to the protocol provided by the manufacturer (Pierce, Rockford, IL). Separated proteins were detected by using primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotech Inc, CA). Protein bands were visualized by utilizing chemiluminescence reagent (KirKegarrd & Perry Laboratories, Maryland).
Flow cytometric analyses of apoptosis
Pre-confluent HSC incubated in DMEM with 10% FBS were treated as described for the above MTS assays for 24 hr. Cells were harvested by brief trypsin /EDTA treatment and washed several times with cold PBS. HSC ( ≥ 1-106 cells/each sample) were suspended in 2ml of FACS buffer (1% FA buffer (Difco), 0.1% sodium azide and 1% FBS). Cells were fixed with ethanol, and then labeled with propidium iodide (Sigma). Cells that were positively labeled with propidium iodide were detected with a Coulter® EPICS® XL-MCL flow cytometer.
Plasmids and transient transfection assays
The Gclc promoter luciferase reporter plasmid pGCLc-Luc, a gift from Dr. Shelly C. Lu (Division of Gastrointestinal and Liver Diseases, Department of Medicine. Keck School of Medicine USC, Los Angeles, CA), contains a fragment of the Gclc gene promoter (∼1758bp nucleotides) subcloned into the SmaI site of promoterless pGL-3 enhancer vector. Semi-confluent HSC in 6-well cell culture plates were transiently transfected using the Lipofectamine reagent (Life Technologies, Grand Island, NY). Each sample (3μg DNA/well) had triplicate in every experiment. Luciferase assays were performed as previously described [16]. Transfection efficiency was determined by co-transfection of a β-galactosidase reporter, pSV-β-gal (0.5μg/well) (Promega). β-galactosidase activity was measured by using a chemiluminescence assay kit (Tropix, Bedford, MA), according to the manufacturer's instructions. Results were combined from three independent experiments.
RNA isolation and Real-time Polymerase Chain Reaction (Real-time PCR)
Total RNA was isolated by TRI-Reagent (Sigma), following the protocol provided by the manufacturer. Real-time PCR was carried out using SYBR green, as we previously described [17]. mRNA fold changes of target genes relative to the endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control were calculated as suggested by Schmittgen et. al [18]. mRNA levels were expressed as fold changes after normalization with GAPDH. Primers used for real-time PCR were in the following:
Gclc: (F) 5′-TGT GTG ATG AGC CCA AGG AC-3';
(R) 5′-AGT TGG CTC GCA TCA TAG TTG-3′;
Gclm: (F) 5'-CTG CTA AACTGT TCA TTG TAG G-3';
(R) 5'-CTA TGG GTT TTA CCT GTG-3';
GAPDH: (F) 5'-GGC AAA TTC AAC GGC ACA GT-3',
(R), 5'-AGA TGG TGA TGG GCT TCC C-3'.
Statistical Analysis
Differences between means were evaluated using an unpaired two-sided Student's t-test (p<0.05 considered as significant). Where appropriate, comparisons of multiple treatment conditions with controls were analyzed by ANOVA with the Dunnett's test for post hoc analysis.
RESULTS
Curcumin time- and dose-dependently reduces the level of ROS and the content of LPO in activated HSC in vitro
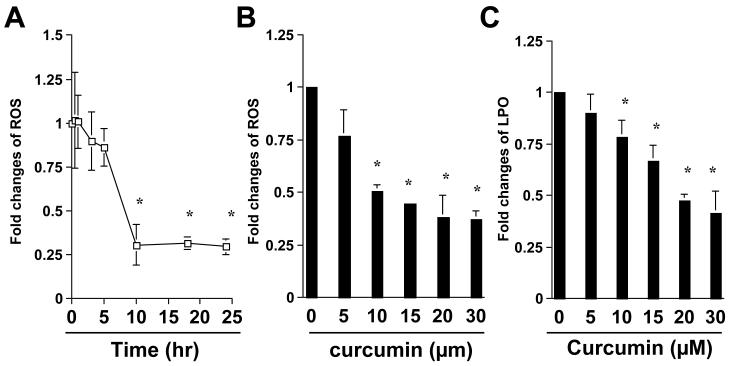
We previously showed that curcumin significantly suppressed expression of ECM genes, including αI(I) collagen and fibronectin in passaged HSC [13, 19]. We hypothesized that the antioxidant property of curcumin might play a pivotal role in the anti-fibrogenic effects. To demonstrate the antioxidant capability of curcumin in activated HSC, passaged HSC were treated with curcumin at 20μM for various hours, or at various concentrations for 24 hr. The level of cellular reactive oxygen species (ROS) was determined. As shown in Fig. 1A & B, curcumin significantly reduced the level of cellular ROS in a time- and dose-dependent manner, respectively. Further experiments indicated that curcumin dose-dependently decreased the content of cellular lipid hydroperoxides (LPO) (Fig. 1C). These results collectively demonstrated the antioxidant capability of curcumin in activated HSC. It bears notice that the result in Fig. 1A revealed that the level of cellular ROS was not significantly changed within the first 5 hours after the addition of curcumin, indicating a delayed response to the natural antioxidant.
Figure 1. Curcumin time-, and dose-dependently reduces the level of ROS and LPO in activated HSC in vitro.
Passaged HSC were either treated with curcumin at 20μM for various hours (A), or with curcumin at various concentrations (B & C) for 24 hr. The levels of intracellular ROS (A & B) and LPO (C) were assessed as described in Methods. The levels of cellular ROS (A & B), or LPO (C), were expressed as fold changes compared to that in the control either at the time 0 (A), or without curcumin treatment (B & C). Values were presented as means ± S.D. (n≥3). *p<0.05, versus the control (the 1st point, or column, on the left).
Curcumin causes a time- and dose-dependent increase in cellular GSH in activated HSC
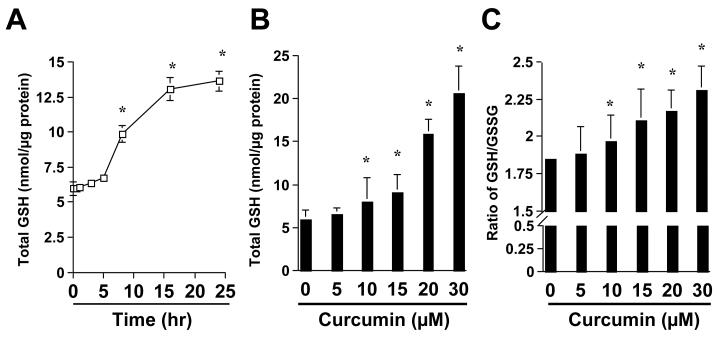
To understand the mechanisms of the antioxidant capability of curcumin, we assumed that curcumin might increase the level of cellular GSH in activated HSC, leading to the reduction in the levels of ROS and LPO. To test the assumption, HSC were treated with curcumin at various concentrations for 24 hr or with curcumin at 20μm for different hours. It was found that curcumin caused an increase in the content of total cellular GSH in a time- and dose-dependent manner (Fig. 2A & B). It bears notice that little increase in the level of total cellular GSH was observed in the first 5 hours after the addition of curcumin (Fig. 2A), suggesting a delayed response of the content of cellular GSH to curcumin. Further experiments revealed that curcumin significantly increased the ratio of reduced GSH verse oxidized GSH (i.e. GSSG) in activated HSC (Fig. 2c), indicating the reduction of the level of oxidative stress by curcumin in these cells.
Figure 2. Curcumin causes a time- and dose-dependent increase in the content of cellular GSH in activated HSC in vitro.
Passaged HSC were either treated with curcumin at 20μM for various hours (A), or with curcumin at various concentrations (B & C) for 24 hr. The levels of total cellular GSH (A & B) and the ratio of GSH/GSSG (C) were determined as described in Methods. The level of total cellular GSH was expressed as nmol/μg protein. Values were presented as means ± S.D. (n≥3). *p<0.05, versus the control (the 1st point, or column, on the left).
The antioxidant capability of curcumin mainly results from de novo synthesis of GSH
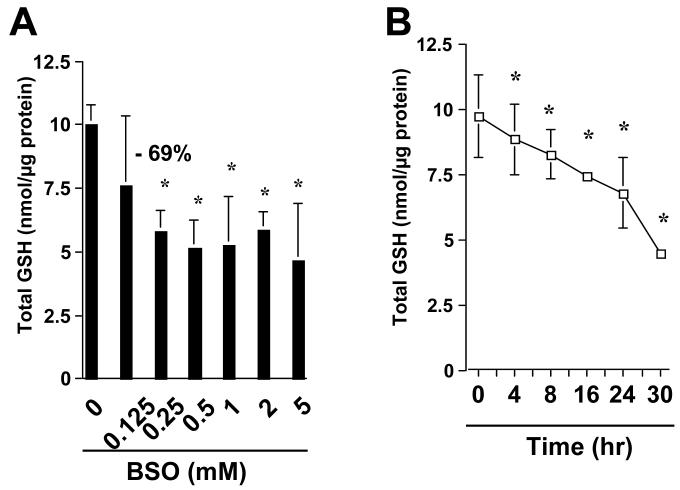
To elucidate the role of the elevated level of cellular GSH stimulated by curcumin in the attenuation of oxidative stress, it was hypothesized that the antioxidant capability of curcumin might mainly result from de novo synthesis of GSH. To begin to test the hypothesis, pilot experiments were conducted to determine the optimal conditions to deplete cellular GSH in activated HSC using L-buthionine sulfoximine (BSO), a specific inhibitor of glutamate-cysteine ligase (GCL). The latter is the key rate-limiting enzyme in GSH synthesis [20]. As shown in Fig. 3A, the treatment of HSC with BSO at 0.25mM for 24 hr caused a dramatic decrease in the level of total GSH by 69%. Higher doses of BSO resulted in no further apparent decrease in the level of GSH. Additional experiments demonstrated that BSO caused a time-dependent decrease in the content of total cellular GSH in HSC treated with BSO at 0.25mM for various hours (Fig. 3B).
Figure 3. The pretreatment with BSO reduces cellular GSH content in activated HSC.
Passaged HSC were either treated with BSO at various concentrations for 24 hr (A), or with BSO at 0.25 mM for various hours (B). The level of total cellular GSH was expressed as nmol/μg protein. The number of percentage indicated the reduction in the level of cellular GSH in cells treated with BSO at 0.25 mM, compared to that in the control cells with no treatment (the 1st column on the left). Values were presented as means ± S.D. (n≥3). *p<0.05, versus the control (the 1st column, or point, on the left).
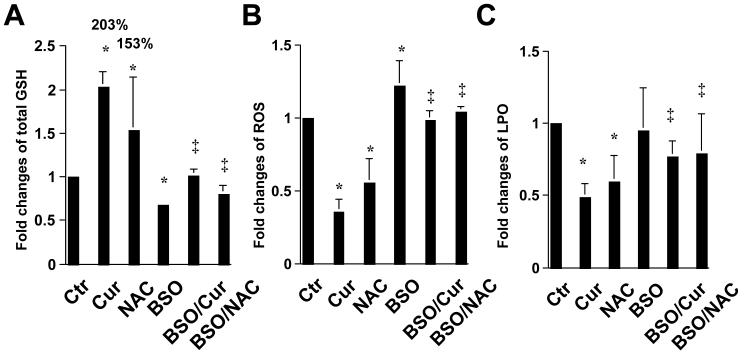
To examine the role of cellular GSH in the attenuation of oxidative stress by curcumin, the level of cellular GSH was altered by either N-acetyl-cysteine (NAC), a precursor of GSH by supplying cysteine [21], or by curcumin, or by BSO. HSC were treated for 24 hr with BSO (0.25mM), or curcumin (20μM), or NAC (5mM), with or without the pre-exposure to BSO (0.25mM) for 1 hr. The levels of total cellular GSH, cellular ROS as well as LPO were determined. As demonstrated in Fig. 4A, compared to the untreated control, curcumin as well as NAC significantly increased the level of cellular GSH by 203% and 153%, respectively. The pre-treatment of cells with BSO, which inhibited GCL activity and depleted cellular GSH (Fig. 3), dramatically diminished the stimulatory effect of curcumin or NAC on cellular GSH content. Further analyses indicated that NAC, mimicking curcumin, scavenged cellular ROS (Fig. 4B) and reduced the level of LPO (Fig. 4C) in HSC, suggesting the role of GSH in the attenuation of oxidative stress. The pretreatment of cells with BSO dramatically eliminated the ability of curcumin and NAC in scavenging cellular ROS (Fig. 4B) and in reducing cellular LPO (Fig. 4C). Taken together, these results demonstrated that the attenuation of oxidative stress by curcumin in activated HSC required de novo synthesis of GSH.
Figure 4. The antioxidant capability of curcumin mainly results from de novo synthesis of GSH.
Passaged HSC were either maintained in DMEM with 10% of FBS, or treated for 24 hr with BSO (0.25mM), or curcumin (20μM), or NAC (5mM) with or without the pre-exposure to BSO (0.25mM) for 1 hr. The levels of total cellular GSH (A), ROS (B) and LPO (C) were, respectively, determined and expressed as fold changes compared to that in the control with no treatment. The number of percentage in (A) indicated the increase in the level of cellular GSH in cells treated with curcumin or NAC compared to that in the control cells with no treatment. Values were means ± S.D. (n≥3). *p<0.05, versus the control with no treatment (the 1st column on the left); ‡ p<0.05, versus cells treated with curcumin or NAC (the 2nd, or 3rd column respectively, on the left).
Curcumin dose-dependently increases the activity of GCL in passaged HSC
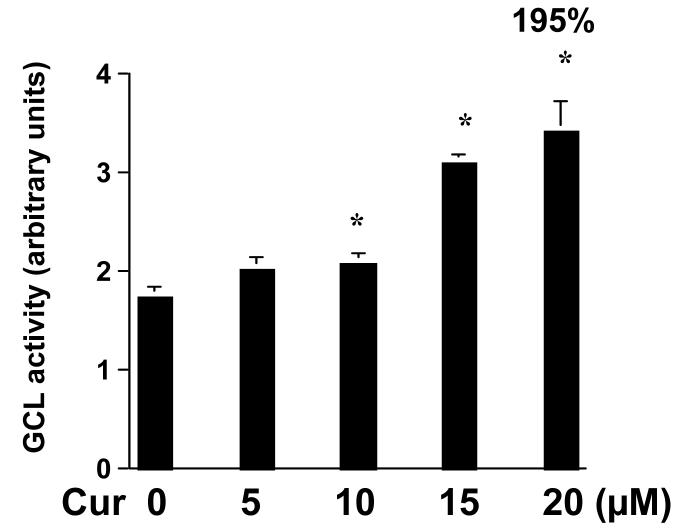
To answer the question how curcumin could induce the de novo synthesis of GSH and increase the level of cellular GSH in activated HSC, we hypothesized that curcumin might stimulate the activity of GCL, the rate-limiting enzyme in GSH synthesis, in HSC. To test the hypothesis, HSC were treated with curcumin at indicated concentrations for 24 hr. As demonstrated in Fig. 5, compared with the untreated control (the 1st column on the left), curcumin dramatically increased the activity of GCL in a dose-dependent manner in HSC. However, curcumin at the concentrations higher than 30μM showed no such a dose-dependent manner (data not shown). This result indicated that the induction of de novo synthesis of GSH and the increase in the level of cellular GSH by curcumin might mainly result from the stimulation of the activity of GCL.
Figure 5. Curcumin dose-dependently increases the activity of GCL in passaged HSC.
HSC were treated with curcumin at indicated concentrations for 24 hr. The activity of GCL was determined as described in Methods. Values were expressed as means ± S.D. (n≥6). The number of percentage indicated the increase in GCL activity in cells treated with curcumin at 20 μM, compared to that in control cells with no treatment (0). *p<0.05, versus control cells with no treatment (the 1st column on the left).
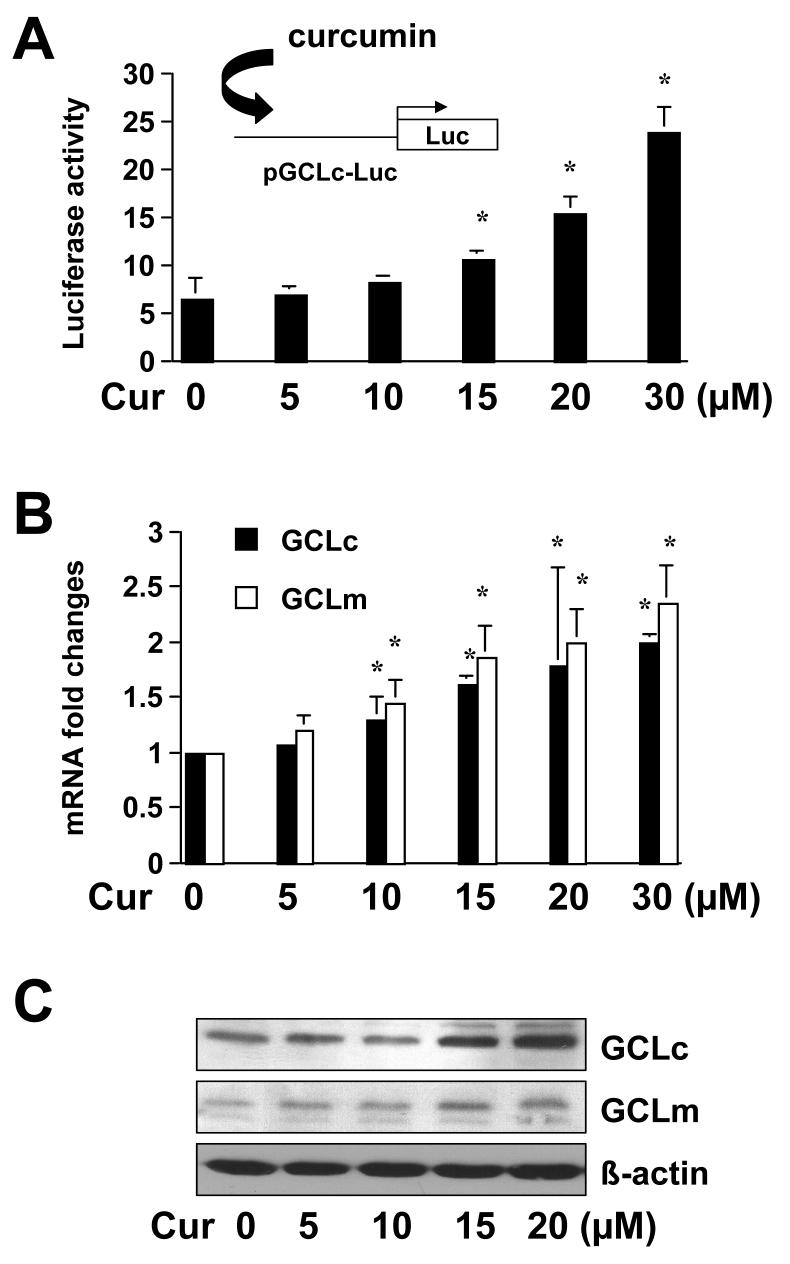
Curcumin induces gene expression of both subunits of GCL in activated HSC
To elucidate the molecular mechanisms underlying the stimulation of GCL activity by curcumin, we hypothesized that the phyto-chemical might induce gene expression of GCL in activated HSC. As indicated earlier, the enzyme of GCL is composed of a large catalytic subunit (GCLc, ∼73 kDa) and a small modulatory subunit (GCLm, ∼30 kDa). To test the hypothesis, HSC were transfected with the Gclc promoter luciferase reporter plasmid pGCLc-Luc, containing a fragment of the Gclc gene promoter (∼1758bp nucleotides) in the pGL3. Cells were subsequently treated with curcumin at indicated concentrations for 24 hr. As shown in Fig. 6A, curcumin increased luciferase activity in the cells in a dose-dependent manner, suggesting that curcumin might stimulate the promoter activity of Gclc gene in activated HSC in vitro. Further experiments were performed to verify the effect of curcumin on the regulation of gene expression of GCL subunits. HSC were treated with curcumin at indicated concentrations for 24 hr. The steady state level of Gclc and Gclm mRNA and the abundance of GCLc and GCLm were analyzed by real-time PCR and Western blotting analyses, respectively. As shown in Fig. 6B and 6C, curcumin significantly increased the steady state levels of Gclc and Gclm mRNA and the abundance of their proteins in a dose-dependent pattern. Taken together, these results demonstrated that curcumin induced gene expression of both subunits of GCL in activated HSC in vitro.
Figure 6. Curcumin induces gene expression of the two GCL subunits in passaged HSC.
HSC were treated with curcumin at indicated concentrations for 24 hr. (A). Luciferase assays of cells transfected with the plasmid pGCLc-Luc, containing −1758bp of Gclc gene promoter. Luciferase activities were normalized with ß-galactosidase activities (n≥6). *p<0.05, versus control cells with no treatment (the 1st column on the left). The floating schema denotes the pGCLc-Luc luciferase reporter construct in use and the application of curcumin to the system. (B). Real-time PCR assays of Gclc and Gclm. GAPDH was used as an invariant control for calculating mRNA fold changes (n=3). Values were means ± S.D. *p<0.05, versus cells with no treatment (the 1st columns on the left). (C). Western blotting analyses of GCLc and GCLm. ß-actin was used as an internal control. Representatives were shown from three independent experiments.
de novo synthesis of GSH plays a critical role in the inhibitory effects of curcumin on HSC activation
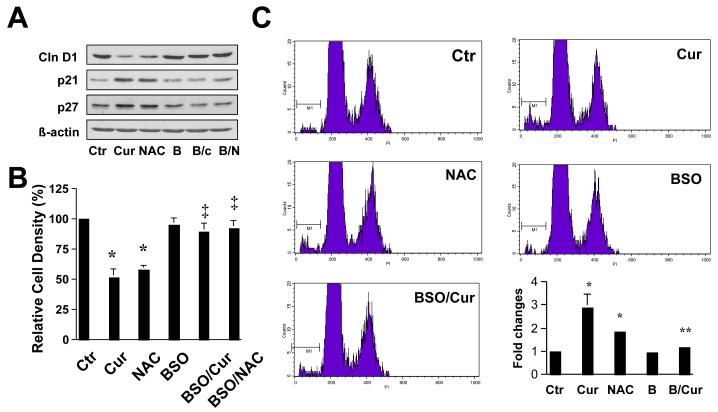
Additional experiments were performed to test the general hypothesis of this study that the inhibition of HSC activation by curcumin might mainly result from its antioxidant capacity by increasing the level of cellular GSH. To evaluate the role of the elevated GSH content in the inhibition of HSC proliferation and in the induction of apoptosis, passaged HSC were treated with curcumin at 20μM, NAC (5mM), or pretreated with BSO for 1 hr prior to the addition of curcumin, for an additional 24 hr. As shown in Fig. 7A by Western blotting analyses, curcumin as well as NAC significantly altered the abundance of proteins relevant to cell growth, including reducing the level of cell cycle-stimulating protein cyclin D1 and enhanced the content of cell cycle inhibitory proteins p21WAF1/Cip1 and p27Kip1. Pretreatment of cells with BSO dramatically eliminated the regulatory effects of curcumin and NAC on the expression of the genes in activated HSC. Further MTS assays (Fig. 7B) and flow cytometric assays (Fig. 7C) demonstrated that NAC, mimicking curcumin, reduced cell proliferation and induced apoptosis. The depletion of intracellular GSH by BSO dramatically eliminated their effects on reducing cell proliferation and inducing apoptosis.
Figure 7. de novo synthesis of GSH plays a critical role in the inhibitory effect of curcumin on HSC growth.
Passaged HSC were either maintained in DMEM with 10% of FBS, or treated for 24 hr with BSO (0.25mM), or curcumin (20μM), or NAC (5mM) with or without the pre-exposure to BSO (0.25mM) for 1 hr. (A). Western blotting analyses of proteins relevant to cell growth. ß-actin was used as an invariant control for equal loading. Representatives of three independent experiments were shown; (B). Cell growth was determined by MTS assays. Values were expressed as means ± S.D (n=3). *p<0.05, versus control cells with no treatment (the 1st column on the left); ‡ p<0.05, versus cells treated with curcumin, or NAC (the 2nd or 3rd column, respectively, on the left). (C). Flow cytometric analyses of apoptosis (n=3). *p<0.05, versus control cells with no treatment (the 1st column on the left); ** p<0.05, versus cells treated with curcumin (the 2nd column on the left).
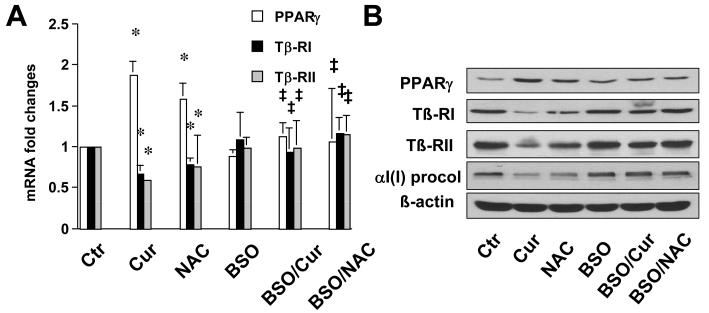
Additional experiments were conducted to evaluate the role of elevated GSH in regulating expression of genes relevant to pro-fibrogenesis. We previously demonstrated that curcumin induced gene expression of the peroxisome proliferator-activated receptor-gamma (PPARγ), leading to the activation of PPARγ signaling in activated HSC in vitro [13]. The latter was required for curcumin to suppress gene expression of transforming growth factor-beta (TGF-ß) receptors, leading to the interruption of pro-fibrogenic TGF-ß signaling pathways [16, 19]. HSC were similarly treated with curcumin at 20μM, NAC (5mM), or pretreated with BSO for 1 hr prior to the addition of curcumin or NAC for an additional 24 hr. Real-time PCR (Fig. 8A) and Western blotting analyses (Fig. 8B) demonstrated that NAC, like curcumin, induced gene expression of PPARγ, and suppressed gene expression of type I and II TGF-β receptors (Tß-RI & Tß-RII), as well as reduced the abundance of αI(I) pre-collagen in activated HSC. The pretreatment of cells with BSO abolished the impacts of curcumin or NAC on regulating expression of these genes (Fig. 8A & B), suggesting that the increased level of GSH played a pivotal role in the regulation of the expression of the genes. Taken together, these results supported our general hypothesis for this study and suggested that curcumin might inhibit HSC activation mainly by increasing the level of cellular GSH.
Figure 8. de novo synthesis of GSH is a necessity for curcumin to regulate expression of genes relevant to fibrogenesis in activated HSC in vitro.
Passaged HSC were either maintained in DMEM with 10% of FBS, or treated for 24 hr with BSO (0.25mM), or curcumin (20μM), or NAC (5mM) with or without the pre-exposure to BSO (0.25mM) for 1 hr. (A). Real-time PCR analyses. GAPDH was used as an invariant control for calculating mRNA fold changes. Values were expressed as means ± S.D (n=3). *p<0.05, versus control cells (the 1st column on the left); ‡ p<0.05, versus cells treated with curcumin or NAC (the 2nd or 3rd column on the left). (B). Western blotting analyses. ß-actin was used as an invariant control for equal loading. Representatives of three independent experiments were shown.
DISCUSSION
Oxidative stress represents an important and novel class of “the 3rd messenger”, leading to activation of several signal pathways associated with inflammation. Oxidative stress is a major player in activation of HSC and in hepatic fibrogenesis, regardless of etiology [2, 5, 22, 23]. Although the antioxidant vitamin E inhibits HSC activation [5] and hepatic fibrogenesis [24], currently known antioxidants in protecting the liver from fibrogenesis are not very effective [25]. In recent years, the beneficial effects of some commonly used food-derived products have attracted attention for preventing various pathologic conditions. In this respect, flavonoids and other poly-phenolic compounds have received a great deal of attention. Curcumin is a potent antioxidant [26]. The antioxidant capability of curcumin might mainly result from two sources, i.e. its innate poly-phenolic chemical structure and the ability of this compound to affect activities of enzymes involved in the defense against oxidative stress [27-29]. These unique features might allow curcumin to succeed where other “regular” antioxidants have failed to inhibit hepatic fibrogenesis. In this report, we demonstrated that curcumin attenuated oxidative stress in activated HSC in vitro (Fig. 1). The antioxidant capability of curcumin mainly resulted from de novo synthesis of GSH (Fig. 2-4). Further experiments indicated that curcumin stimulated the activity of GCL by inducing gene expression of GCL subunits, leading to the de novo synthesis of GSH (Fig. 5-6). The latter played a critical role in the inhibitory effects of curcumin on HSC activation, including reducing cell proliferation, inducing apoptosis and suppressing production of αI(I) collagen (Fig. 7-8).
We previously demonstrated that curcumin inhibited HSC activation in vitro, including reducing cell proliferation, inducing apoptosis and suppressing gene expression of ECM [13, 16, 19]. The underlying mechanisms are largely to be defined. Oxidative stress reflects the imbalance of pro-oxidants and antioxidants and has long been implicated in activation of HSC and hepatic fibrogenesis [2, 5, 22, 23]. GSH, the most abundant thiol antioxidant in cells, reacts with ROS or functions as a cofactor of antioxidant enzymes, leading to the protection of functions of redox-sensitive molecules [30]. Results in the present report showed that curcumin, like NAC, a precursor of GSH, acted as an antioxidant by elevating the level of cellular GSH, leading to the attenuation of ROS and the reduction of LPO in activated HSC. These results are consistent with prior observations that curcumin enhanced the level of cellular GSH in other cell types [31, 32]. It bears, however, notice that in terms of the elimination of ROS (Fig. 1A) and in the elevation of the GSH content (Fig. 2A), HSC showed no significant responses to curcumin within the first 5 hours. It is a typical delayed response [33], suggesting requirement of a rate-limiting enzyme in the process of the de novo synthesis of GSH and the attenuation of oxidative stress. Results from additional experiments supported this suggestion and demonstrated that curcumin induced gene expression of GCL, the key rate-limiting enzyme in GSH synthesis, in activated HSC (Fig. 5-6).
Suppression of GSH synthesis by the pretreatment of cells with the GCL inhibitor BSO evidently diminished the ability of curcumin and NAC to attenuate oxidative stress (Fig. 4) and eliminated their roles in the regulation of expression of the genes (Fig. 7A and 8B), suggesting the pre-requisite of the de novo synthesis of GSH in these processes. NAC increases GSH contents by supplying cysteine in a ratio of one molecule of NAC to one molecule of GSH [21]. It is intriguing to elucidate the mechanisms of curcumin in the elevation of the level of cellular GSH in activated HSC in vitro. It is worth notice that, compared to the effects of curcumin at 20μM, NAC at a much higher concentration (5mM) showed similar, if not weaker, impacts on the attenuation of oxidative stress (Fig. 4) and on the regulation of gene expression (Fig. 7 and 8). These observations suggested that curcumin, unlike NAC as a GSH precursor, might act in a more efficient and effective mechanism to elevate the level of cellular GSH. This suggestion was supported by results from additional experiments in this report. It was demonstrated that curcumin stimulated the enzymatic activity of GCL (Fig. 5) and induced gene expression of GCL (Fig. 6), which might result in the de novo synthesis of GSH and the increase in the level of cellular GSH in activated HSC. The induction of GCL gene expression by curcumin was recently reported in HBE1 cells, an immortalized human bronchial epithelial cell line [28]. It was suggested that curcumin induced gene expression of the GCL subunits by altering the binding complexes of EpRE and AP-1, both of which played an important role in the regulation of gene expression of both Gclc and Gclm [34, 35]. It bears emphasis that the antioxidant capability of curcumin might not be restricted in the induction of GCL gene expression. Curcumin also shows its impacts on other enzyme systems involved in the defense against oxidative stress. For instance, curcumin induces a general xenobiotic response by activating multiple defense genes, such as phase II enzymes and heme oxygenase-1 [36, 37].
Results in this report demonstrated that curcumin at concentrations of 10 to 30μM caused a dose-dependent increase in the level of cellular GSH (Fig. 2B). Curcumin at 10 to 30μM also caused a dose-dependent increase in the activity of GCL (Fig. 5) by inducing gene expression of GCL in HSC (Fig. 6). However, the capability of curcumin in increasing the level of cellular GSH was, in fact, limited within concentrations at 10 to 30μM. Higher concentrations of curcumin showed no such dose-dependent effects (data not shown). It could be explained by the theory of the feedback control. Curcumin increases the activity of GCL, leading to the de novo synthesis of GSH and the elevation of the level of cellular GSH. The elevated GSH content might suppress, in turn, the activity of GCL in a mechanism of the feedback inhibition, resulting in elimination of the role of curcumin in elevating the level of GSH. The feedback inhibition restricts these abilities of curcumin within a limited range of concentrations. Curcumin at 20μM was, therefore, chosen for most of the experiments in this report. Our explanation is supported by other prior studies. Compelling evidence has shown that GSH synthesis is regulated primarily by GCL activity, cysteine availability, and GSH feedback inhibition [9]. GSH is known to be a feedback inhibitor of GCL. GSH is an abundant cellular antioxidant, being maintained at a certain level in cells [38]. The level of cellular GSH is mainly determined by GSH synthesis (GSH supply) and GSH-consuming (GSH demand). This current report focused on the effect of curcumin on GSH synthesis. Additional experiments are ongoing to evaluate the role of curcumin in regulating gene expression and activity of enzymes involved in consuming GSH, including GSH Stransferase and GSH peroxidase.
In many studies, the Gclc and Gclm genes have been demonstrated to be transcriptionally responsive to extracellular stimuli, including reactive oxygen species [39]. In this report, the expression of both Gclc and Gclm genes was induced by curcumin (Fig. 6), which might increase the activity of GCL and stimulate the de novo synthesis of GSH, ultimately leading to the attenuation of oxidative stress. Studies have shown the simultaneous up-regulation of both GCL subunits [40]. However, the regulation of Gclc and Gclm is not always coordinated. In manycases, induction of one gene is favored over the other [41-43]. In fact, in some tissues, including the heart and liver, the ratio of GCLc/GCLm is more than 1.0 [44]. Our results from Western blotting analyses in Fig. 6C suggested that the induced gene expression of Gclc might surpass that of Gclm. The subunit of GCLc shows GCL activity and catalyzes the formation of γ-glutamylcysteine in the absence of GCLm, but its activity is increased substantially by covalent interactions with GCLm [45]. Transfection of cells with Gclc cDNAs elevates GSH levels aswell as resistance to oxidative stress [46]. Additional studies are required to understand the significance of the differential regulation of gene expression of the two subunits in activated HSC.
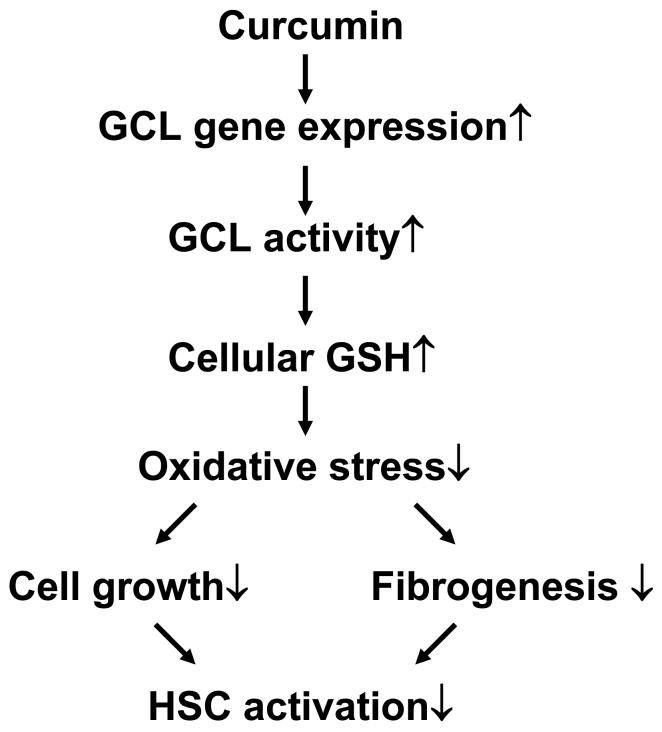
In summary, our results demonstrated that curcumin acted as an antioxidant in the inhibition of HSC activation by inducing gene expression of GCL subunits and elevating the level of cellular GSH. A model is proposed to explain the sequential events in the process (Fig. 9). Curcumin inhibits HSC activation by reducing cell growth and ECM production through the attenuation of oxidative stress. The latter might result from increasing the level of cellular GSH, at least partially, by inducing gene expression of GCL. It bears emphasis that our results and the proposed model do not exclude the involvement of any other antioxidant mechanisms in the inhibition of HSC activation by curcumin. These results, generated from the in vitro system, might not necessarily and comprehensively reflect effects of curcumin on activated HSC in vivo. However, our preliminary studies in an animal model with CCL4-caused liver injury supported these above observations and demonstrated that dietary administration of curcumin (200 or 400 mg/kg body weight/day) increased the level of cellular GSH and attenuated cellular ROS and LPO in the rat liver (unpublished data). The present report provides novel insights into mechanisms of curcumin, as a potential anti-fibrogenic agent, in the prevention and treatment of hepatic fibrosis.
Figure 9. A Schema of the role of the antioxidant capability of curcumin in the inhibition of HSC activation.
Curcumin increases the level of intracellular GSH by inducing expression of GCL genes and stimulating the activity of GCL, leading to the attenuation of oxidative stress in activated HSC. These actions, together with others, reduce cell growth and fibrogenesis, resulting in the inhibition of HSC activation.
ACKNOWLEDGMENT
The work was supported by the grant RO1 DK 047995 from NIH/NIDDK to A. Chen
ABBREVIATIONS
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HSC
hepatic stellate cells
- ECM
extracellular matrix
- GSH
glutathione
- ROS
reactive oxygen species
- LPO
Lipid hydroperoxides
- GCL
glutamate-cysteine ligase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Bissell DM. Hepatic fibrosis as wound repair: a progress report. J Gastroenterol. 1998;33:295–302. doi: 10.1007/s005350050087. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 3.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S84–87. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- 5.Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461–2468. doi: 10.1172/JCI118304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 7.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 8.Fraser JA, Kansagra P, Kotecki C, Saunders RD, McLellan LI. The modifier subunit of Drosophila glutamate-cysteine ligase regulates catalytic activity by covalent and noncovalent interactions and influences glutathione homeostasis in vivo. J Biol Chem. 2003;278:46369–46377. doi: 10.1074/jbc.M308035200. [DOI] [PubMed] [Google Scholar]
- 9.Seelig GF, Simondsen RP, Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem. 1984;259:9345–9347. [PubMed] [Google Scholar]
- 10.Sekura R, Meister A. gamma-Glutamylcysteine synthetase. Further purification, “half of the sites” reactivity, subunits, and specificity. J Biol Chem. 1977;252:2599–2605. [PubMed] [Google Scholar]
- 11.Ruby AJ, Kuttan G, Babu KD, Rajasekharan KN, Kuttan R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett. 1995;94:79–83. doi: 10.1016/0304-3835(95)03827-j. [DOI] [PubMed] [Google Scholar]
- 12.Park EJ, Jeon CH, Ko G, Kim J, Sohn DH. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J Pharm Pharmacol. 2000;52:437–440. doi: 10.1211/0022357001774048. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20–30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 14.Casado JA, Merino J, Cid J, Subira ML, Sanchez-Ibarrola A. Simultaneous evaluation of phagocytosis and Fc gamma R-mediated oxidative burst in human monocytes by a simple flow cytometry method. J Immunol Methods. 1993;159:173–176. doi: 10.1016/0022-1759(93)90155-z. [DOI] [PubMed] [Google Scholar]
- 15.Yumei F, Zhou Y, Zheng S, Chen A. The antifibrogenic effect of (−)-epigallocatechin gallate results from the induction of de novo synthesis of glutathione in passaged rat hepatic stellate cells. Lab Invest. 2006;86:697–709. doi: 10.1038/labinvest.3700425. [DOI] [PubMed] [Google Scholar]
- 16.Zheng S, Chen A. Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am J Physiol Gastrointest Liver Physiol. 2006;290:G883–893. doi: 10.1152/ajpgi.00450.2005. [DOI] [PubMed] [Google Scholar]
- 17.Chen A, Zhang L, Xu J, Tang J. The antioxidant (−)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. Biochem J. 2002;368:695–704. doi: 10.1042/BJ20020894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 19.Zheng S, Chen A. Activation of PPARgamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J. 2004;384:149–157. doi: 10.1042/BJ20040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson ME, Luo JL. Glutathione therapy: from prodrugs to genes. Semin Liver Dis. 1998;18:415–424. doi: 10.1055/s-2007-1007174. [DOI] [PubMed] [Google Scholar]
- 21.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 22.Tsukamoto H. Oxidative stress, antioxidants, and alcoholic liver fibrogenesis. Alcohol. 1993;10:465–467. doi: 10.1016/0741-8329(93)90066-w. [DOI] [PubMed] [Google Scholar]
- 23.Greenwel P, Dominguez-Rosales JA, Mavi G, Rivas-Estilla AM, Rojkind M. Hydrogen peroxide: a link between acetaldehyde-elicited alpha1(I) collagen gene up-regulation and oxidative stress in mouse hepatic stellate cells. Hepatology. 2000;31:109–116. doi: 10.1002/hep.510310118. [DOI] [PubMed] [Google Scholar]
- 24.Pietrangelo A, Gualdi R, Casalgrandi G, Montosi G, Ventura E. Molecular and cellular aspects of iron-induced hepatic cirrhosis in rodents. J Clin Invest. 1995;95:1824–1831. doi: 10.1172/JCI117861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halliwell B. Drug antioxidant effects. A basis for drug selection? Drugs. 1991;42:569–605. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sreejayan, Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 27.Araujo CC, Leon LL. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–728. doi: 10.1590/s0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. Faseb J. 2003;17:473–475. doi: 10.1096/fj.02-0566fje. [DOI] [PubMed] [Google Scholar]
- 29.Manikandan P, Sumitra M, Aishwarya S, Manohar BM, Lokanadam B, Puvanakrishnan R. Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int J Biochem Cell Biol. 2004;36:1967–1980. doi: 10.1016/j.biocel.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 31.Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46:273–279. doi: 10.1016/s1043-6618(02)00123-8. [DOI] [PubMed] [Google Scholar]
- 32.Kenny DJ, Barrett EJ, Johnston DH, Sigal MJ, Tenenbaum HC. Clinical management of avulsed permanent incisors using Emdogain: initial report of an investigation. J Can Dent Assoc. 2000;66:21. [PubMed] [Google Scholar]
- 33.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 34.Moinova HR, Mulcahy RT. An electrophile responsive element (EpRE) regulates beta-naphthoflavone induction of the human gamma-glutamylcysteine synthetase regulatory subunit gene. Constitutive expression is mediated by an adjacent AP-1 site. J Biol Chem. 1998;273:14683–14689. doi: 10.1074/jbc.273.24.14683. [DOI] [PubMed] [Google Scholar]
- 35.Mulcahy RT, Wartman MA, Bailey HH, Gipp JJ. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J Biol Chem. 1997;272:7445–7454. doi: 10.1074/jbc.272.11.7445. [DOI] [PubMed] [Google Scholar]
- 36.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 37.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 38.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 39.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. Faseb J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 40.Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox Rep. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai J, Huang ZZ, Lu SC. Differential regulation of gamma-glutamylcysteine synthetase heavy and light subunit gene expression. Biochem J. 1997;326(Pt 1):167–172. doi: 10.1042/bj3260167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai J, Sun WM, Lu SC. Hormonal and cell density regulation of hepatic gamma-glutamylcysteine synthetase gene expression. Mol Pharmacol. 1995;48:212–218. [PubMed] [Google Scholar]
- 43.Moellering D, McAndrew J, Patel RP, Cornwell T, Lincoln T, Cao X, Messina JL, Forman HJ, Jo H, Darley-Usmar VM. Nitric oxide-dependent induction of glutathione synthesis through increased expression of gamma-glutamylcysteine synthetase. Arch Biochem Biophys. 1998;358:74–82. doi: 10.1006/abbi.1998.0854. [DOI] [PubMed] [Google Scholar]
- 44.Krzywanski DM, Dickinson DA, Iles KE, Wigley AF, Franklin CC, Liu RM, Kavanagh TJ, Forman HJ. Variable regulation of glutamate cysteine ligase subunit proteins affects glutathione biosynthesis in response to oxidative stress. Arch Biochem Biophys. 2004;423:116–125. doi: 10.1016/j.abb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Orr WC, Radyuk SN, Prabhudesai L, Toroser D, Benes JJ, Luchak JM, Mockett RJ, Rebrin I, Hubbard JG, Sohal RS. Overexpression of glutamate-cysteine ligase extends life span in Drosophila melanogaster. J Biol Chem. 2005;280:37331–37338. doi: 10.1074/jbc.M508272200. [DOI] [PubMed] [Google Scholar]
- 46.Moore WR, Anderson ME, Meister A, Murata K, Kimura A. Increased capacity for glutathione synthesis enhances resistance to radiation in Escherichia coli: a possible model for mammalian cell protection. Proc Natl Acad Sci U S A. 1989;86:1461–1464. doi: 10.1073/pnas.86.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]