Abstract
Consumption of the nutrients omega-3 fatty acids (ω-3 FA) during pregnancy and lactation is considered beneficial to fetal and infant development. It may also reduce the incidence and severity of preterm births by prolonging gestational length. However several recent human and animal studies have reported that over-supplementation with ω-3 FA, especially in the form of fish oil, can have adverse effects on fetal and infant development and the auditory brainstem response (ABR). Our goal was to assess further the effects of ω-3 FA excess and deficiency during pregnancy and lactation on the offspring’s auditory acuity as evidenced by their ABR thresholds. Female Wistar rats were given diets that were either deficient, adequate (control) or excess in ω-3 FA from day 1 of pregnancy through lactation. The offspring were ABR-tested at the postnatal age of 24 days. The rat pups in the Excess treatment condition had significantly elevated (worse) ABR thresholds, postnatal growth restriction, and a trend for increased postnatal mortality in comparison to the Control group. The Deficient group was intermediate. In conclusion, excess or deficient amounts of ω-3 FA during pregnancy and lactation in the laboratory rat adversely affected the offspring’s auditory acuity. Postnatal thriving was also adversely affected. Consuming or administering large or inadequate amounts of ω-3 FA during pregnancy and lactation seems inadvisable because of the potential for adverse effects on infant development.
Keywords: Auditory brainstem response (ABR), Auditory acuity, Fetal development, Omega-3 fatty acids (ω-3 FA), Lactation, Pregnancy
1. Introduction
Omega-3 fatty acids (ω-3 FA) are “essential fatty acids” because they are not manufactured in the human body. Instead they are acquired through the consumption of certain foods such as canola oil, soybean oil, linseed oil, oily fishes and from the milk, eggs and meat of animals that have consumed diets rich in ω-3 FAs [4]. These FAs, particularly docosahexanoic acid (DHA) and eicosapentanoic acid (EPA), are responsible for several crucial body functions including the development of the central nervous system through the formation of cell membrane components, immune function, and visual (retinal) development [6,8,22–23,29,48]. Numerous studies have touted the benefits on fetal and infant development from dietary ω-3 FA supplementation during pregnancy and lactation. These benefits include enhanced fetal and infant brain development [2,8,23,40,46–48] and a possible reduction in the incidence and severity of preterm births by prolonging gestational length [31,41,43].
Although moderate amounts of ω-3 FAs are beneficial to the developing fetus and infant, there is increasing evidence that too much can be harmful. Some recent human studies have reported various harmful effects due to excess ω-3 FA consumption in the form of fish and other marine oils. For example, several pregnancy studies have reported decreased gestational length and/or growth retardation when mothers consumed relatively high amounts of seafood [17–18, 30,32–33,38]. In a study by Thorsdottir et al [45], birth length and head circumference were lowest and morbidity rates were the highest in infants born to women who consumed the highest amounts of seafood during pregnancy. Some of these studies have ruled out confounding variables such as maternal size, age, parity, health, smoking and alcohol consumption [18,21] as well as methylmercury and other contaminants [17]. Adverse effects from excess ω-3 FA consumption by infants through infant formulas that are fortified with ω-3 FA include reduced body growth and head circumference [10,12], decreased blood arachidonic acid (AA) levels [7] and decreased verbal skills [26,42]. Two studies found prolonged ABR latencies in the children born to women who consumed large amounts of seafood [27–28]. Prolonged ABR latencies are indicative of delayed neural maturation or a permanent neurological deficit. The authors concluded that the negative ABR results were due to the methylmercury contaminants in the seafood. They did not consider the possibility that these adverse effects were caused by developmental exposure to excess amounts of ω-3 FA.
Animal studies have also reported adverse effects. Prenatal and/or postnatal dietary supplementation with excess ω-3 FA can reduce birth weight, impair postnatal growth, increase pre- and postnatal mortality, decrease brain sizes, decrease AA levels, decrease brain myelination and/or cause abnormal neurobehavioral function [20,34,36,49,51] and abnormal retinal function [25,52]. ABR studies investigating excess ω-3 FA supplementation in pregnant and lactating rats reported that the offspring had prolonged ABR wave latencies as well as a delay in the acoustic startle reflex and a decrease in brain AA concentrations when tested as postweanling pups [20,40,44]. The prolonged brainstem neural transmission times was related to impaired myelination [20].
Harmful effects can also be caused by pre- and postnatal ω-3 FA deficiency. Omega-3 FA deficiency in developing animals has resulted in reduced visual acuity [52], behavioral and learning disorders, decreased brain weight [8,23,48,50] and ABR findings suggesting that the central auditory nervous system ages faster or earlier [5].
Uncertainty still exists about whether the adverse ABR and other developmental effects found in human studies are caused by the exposure to methylmercury or excess ω-3 FA. In addition, a recent US Department of Health and Human Services report felt that research on the developmental benefits and safety of ω-3 FA were inconclusive and urged more research on the fetal and infant health consequences from high and prolonged marine oil exposure [1]. Our study’s primary goal was to further investigate the possibility that excess ω-3 FA consumption, in the absence of methyl mercury and other environmental contaminants, during pregnancy and lactation results in auditory and neural toxicity as evidenced by the ABR. The ABR is a sensitive measure of peripheral and central neurotoxicity, auditory function [13] and brain maturation [16]. We sought to expand the findings of Stockard and colleagues [20,40,44] by adding a deficient ω-3 FA group as a negative control group, using different tone pip frequencies instead of click stimuli to see at which tonal frequencies beneficial and/or adverse effects occur, controlling for the confounding variables of body temperature and within-litter effects, and looking at ABR thresholds instead of ABR latencies. ABR thresholds are a measure of a subject’s hearing acuity [13,15]. That is, it can tell us who has a hearing loss, how much of a hearing loss and at which tonal frequencies.
It was hypothesized that rat pups exposed to both excess and deficient ω-3 FA through maternal dietary consumption during pregnancy and lactation would show poorer auditory acuity as evidenced by elevated (worse) ABR thresholds over a broad range of hearing frequencies. It was also predicted that the excess and deficient ω-3 FA conditions would cause growth restriction and that the effects of these two dietary conditions on all outcome variables would be similar to each other.
2. Methods
Wayne State University’s animal investigation committee approved the procedures for this study. Institutional and NIH guidelines were followed.
2.1 Animals & Diets
Female Wistar rats, 10 weeks of age, were purchased from Harlan (Indianapolis, IN). They were mated individually with male Wistar rats in suspended cages. The presence of a sperm plug was designated as gestational day one. The females were then placed in separate polycarbonate cages (25 x 45 x 20 cm). The females were randomly assigned to one of the three diet conditions starting from day 1 of pregnancy through the entire period of pregnancy and lactation. The three diets were the Control ω-3 FA condition (ω-3/ω-6 ratio ~ 0.14), the Excess ω-3 FA condition (ω-3/ω-6 ratio ~ 14.5), and the Deficient ω-3 FA condition (ω-3/ω-6 ratio ~ 0% ratio). The Control diet was made with soybean oil (7% w/w), whereas the Deficient ω-3 FA diet contained safflower oil in place of soybean oil. The Excess ω-3 FA diet contained menhaden oil (a type of fish oil) in place of soybean oil. All diets were formulated according to AIN-93G standards and were prepared by Dyets Inc (Bethlehem, PA) as purified pelletted rodent diet. The AIN-93G control diet contains 7g soybean oil/100g diet and the ω-3/ω-6 ratio of ~ 0.14 as well as important vitamins, minerals and other nutrients to meet the nutritional needs of developing, pregnant and lactating rats [35]. Diets were stored at refrigeration temperatures and provided 3.96 kcal/g. The detailed composition of each diet is given in Table 1 and the fatty acid composition of the soybean, safflower and menhaden oils is given in Table 2.
Table 1.
Composition of the treatment diets (g/kg)
| Control | Deficient ω-3 | Excess ω-3 | |
|---|---|---|---|
| Casein | 200 | 200 | 200 |
| Cornstarch | 397.486 | 397.486 | 397.486 |
| Dextrinized Cornstarch | 132 | 132 | 132 |
| Sucrose | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 |
| Safflower Oil | 0 | 70 | 0 |
| Soybean Oil | 70 | 0 | 0 |
| Menhaden Oil | 0 | 0 | 70 |
| Vitamin Mix | 10 | 10 | 10 |
| Mineral Mix | 35 | 35 | 35 |
| L-Cystine | 3 | 3 | 3 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 |
| Tert-butylhydroquinone | 0.014 | 0.014 | 0.014 |
| ω-3/ω-6 ratio | 0.14 | 0 | 14.5 |
| Calories (kcal/g) | 3.96 | 3.96 | 3.96 |
Table 2.
Fatty acid composition of the 3 oils used in the study (% of total fatty acids)
| Fatty acids | Soybean oil | Safflower oil | Menhaden oil |
|---|---|---|---|
| 14:0 | -- | -- | 9.0 |
| 15:0 | -- | -- | 0.7 |
| 16:0 | 10.2 | 6.9 | 17.1 |
| 16:1 | -- | -- | 12.5 |
| 16:2 | -- | -- | 1.7 |
| 16:3 | -- | -- | 1.7 |
| 16:4 | -- | -- | 1.8 |
| 17:0 | -- | -- | 0.9 |
| 18:0 | 4.5 | 2.9 | 2.8 |
| 18:1 (ω-9) | 22.7 | 12.2 | 11.4 |
| 18:2 (ω-6) | 54.8 | 78.0 | 1.5 |
| 18:3 (ω-3) | 7.8 | -- | 1.6 |
| 18:4 (ω-3) | -- | -- | 3.5 |
| 20:0 | -- | -- | 0.2 |
| 20:1 (ω-9) | -- | -- | 1.6 |
| 20:4 (ω-6) | -- | -- | 2.3 |
| 20:5 (ω-3) | -- | -- | 15.5 |
| 21:5 | -- | -- | 0.8 |
| 22:1 (ω-9) | -- | -- | 0.5 |
| 22:5 (ω-3) | -- | -- | 2.4 |
| 22:6 (ω-3) | -- | -- | 9.1 |
| 24:1 | -- | -- | 0.1 |
| Unknown | -- | -- | 1.3 |
Dams had free access to food and water. Fresh diet was provided and weighed twice weekly. Dams were weighed on these days as well. All animals were housed at ~53% relative humidity and at ~22ºC. Within 24 hours after delivery, designated as postnatal day one (PND 1), litters were counted, weighed and reduced to 8 pups per litter, consisting of 4 male and 4 female offspring when possible. The remaining pups were euthanized by exposure to CO2 and decapitated to ensure death. The pups were weaned on PND 21. The numbers of pregnant dams in the Control, Excess, and Deficient groups are given in Table 3.
Table 3.
Maternal and birthing outcomes as a function of Diet Group (mean ± SD)
| Diet Group
|
||||
|---|---|---|---|---|
| Control | Deficient | Excess | Probability | |
| Pregnant dams (n) | 24 | 31 | 23 | |
| Gestation length (days) | 22.6 ± 0.9 | 22.6 ± 1.0 | 22.8 ± 1.1 | N.S. |
| Maternal weight gain | ||||
| GD1 to GD21 (g) | 86.3 ± 28.9 | 78.9 ± 25.9 | 83.8 ± 28.9 | N.S. |
| Maternal weekly food consumption | ||||
| GD1 to GD21 (g) | 169 ± 13 | 162 ± 13 | 166 ± 13 | N.S. |
| Maternal weekly food consumption | ||||
| PND1 to PND21 (g) | 257 ± 26 | 246 ± 25 | 233 ± 26 | N.S. |
| Litter size (# pups) | 14.6 ± 4.5 | 14.8 ± 2.6 | 14.6 ± 3.3 | N.S. |
| Live births (# pups) | 14.4 ± 5.0 | 13.8 ± 3.1 | 14.4 ± 3.7 | N.S. |
| Litter weight (g) | 93.7 ± 26.6 | 96.7 ± 19.6 | 89.1 ± 21.0 | N.S. |
| Pup birth weights (g) | 6.6 ± 0.9 | 6.7 ± 0.9 | 6.3 ± 0.7 | N.S. |
| Postnatal mortalities: | ||||
| Pups affected (%) | 9.1 | 9.4 | 15.2 * | 0.101 |
| Litters affects (%) | 29.2 | 40.0 | 52.2 ** | N.S. |
N.S. = not significant
Different from the Control group at p = 0.051 (Fisher’s Exact test, one-sided)
Different from the Control group at p = 0.095 (Fisher’s Exact test, one-sided)
2.2 ABR procedure
One male and one female pup per litter, when possible, were randomly selected for ABR testing. This allowed the testing of sex-dependent differences and controlled for within-litter effects by limiting the number of pups tested from any one litter. Rat pups were ABR-tested on PND 24 to simulate the studies by Stockard and her colleagues [20,40,44]. The rat ABR is well-developed by PND 24 [16].
Prior to ABR recording, each animal was given 100 mg/kg of the anesthetic ketamine (i.p.). Ketamine can influence the rodent ABR latencies and/or amplitudes, but the effects are minor and more importantly the thresholds are not altered and ABR quality is excellent [14]. Rectal temperature was monitored because temperature can influence the ABR [37]. A water-circulating heating pad maintained normothermia.
The ABR was differentially recorded between two subcutaneous platinum E-2 needle electrodes. The active (non-inverting) electrode was inserted at the vertex, the reference (inverting) electrode behind the left ear, and the ground electrode behind the right ear. Evoked potentials were collected by a Biologic Navigator and amplified by a factor of 300,000 times with a digital bandpass of 300–3000 Hz. Electrode impedances ranged from 0–9 k . At least 256 responses were averaged. The amplified signals were averaged with positivity displayed upwards and traces stored on computer disk for later analysis. ABR activity was sampled at a rate of 0.02 msec. The analysis epoch was 10.24 ms. An artifact rejection system eliminated individual responses if they contained voltages exceeding ± 8.2 μV. Recordings were made in an electrically shielded, double-walled sound attenuation chamber (Allotech, Inc.). Binaural, ‘open field’ tone pips of 16000 Hz, 8000 Hz, 4000 Hz and 2000 Hz were delivered through a TDH-39P headphone positioned directly in front of the animal (tone pip rise/fall time = 0.5 ms, plateau = 10.0 ms, polarity = alternating, repetition rate = 19.0/s).
ABR thresholds were determined by the method of limits [15]. Here, serial ABRs were gathered to a range of stimulus intensities starting at 100 dB peak-equivalent sound pressure level (peSPL), then descending to 80, 60, 50, 40, 35, 30, 25, 20 and 15 dB peSPL as the ABR threshold was reached and passed. To establish ABR threshold more precisely, 2 and 3 dB changes in stimulus intensity levels were tested around the ABR’s threshold (as determined by visual detection) and multiple ABR traces (2 to 5) were collected at each near-threshold intensity level. Threshold was defined as the lowest intensity to elicit a reliably scored ABR component. Stimulus intensity and frequency were measured at the animal’s pinnae with a Bruel & Kjaer sound level meter and an oscilloscope.
The rat ABR is composed of four vertex-positive waves (labeled P1 to P4) occurring within 6 ms of stimulus onset [13]. These ABR waves are generated by a series of action potentials and post-synaptic potentials ascending the lower portion of the auditory pathway. Although the neurogenerators of the rat’s ABRs have not been determined, in the mouse they probably reflect neural activity chiefly from the auditory nerve (P1), the cochlear nucleus (P2), the superior olivary complex (P3), and the lateral lemniscus and/or inferior colliculus (P4) [21]. The latency of each ABR component was measured as the time from the computer’s triggering of the earphone to a wave’s positive peak, including a 0.3 ms acoustic transit time between the earphone and the animal’s pinnae. An experimenter, who was ‘blind’ as to each animal’s treatment condition, scored the ABR thresholds and wave peaks. A second experimenter then checked the ABR scoring for reliability purposes.
2.3 Data analysis
Analyses of variances (ANOVA) were used to assess statistical significance. If an ANOVA indicated a group difference, a planned comparison test (Student-Newman-Keuls test) was used to make pair-wise comparisons between treatment groups. The criterion for statistical significance was p ≤ 0.05. One-sided tests were used when comparing the ω-3 FA Deficient and Excess groups to the Control group. Two-sided tests were used to compare the Deficient and Excess groups with each other. The Bonferroni correction for multiple tests was used.
3. Results
3.1 Maternal and birthing outcomes
There were no significant group differences in gestational length, maternal weight gain, food consumption during pregnancy or lactation, the number of pups per litter, litter or pup weights at birth. There was a trend for increased postnatal mortality between birth and weaning for pups in the Excess group (see Table 3).
3.2 ABR thresholds
The numbers of pups tested in each diet group and their ABR thresholds are given in Table 4. A 3-way ANOVA for Diet Group x Sex x Tone Pip Frequency was first used to analyze the ABR threshold data, wherein Tone Pip Frequency was a repeated measure. The 3-way ANOVA indicated no significant gender effects, so this factor was dropped from the ANOVA design. The subsequent Diet Group x Tone Pip ANOVA showed a significant main effect for the Tone Pip condition, indicating that ABR thresholds varied as a function of tone pip frequency (p < 0.001). Specifically, ABR thresholds were the highest (worst) for the 2 kHz tone pip condition and got progressively lower (better) as tone pip frequency increased to 4 kHz, 8 kHz and 16 kHz. These results reflect the fact that rats have relatively poor hearing at 2 kHz and good hearing at 16 kHz [13]. There were no significant interactions with the Tone Pip, Group or Sex conditions.
Table 4.
ABR thresholds (dB) as a function of Diet Group (mean ± S.D.)
| Diet Group
|
||||
|---|---|---|---|---|
| Control (n=45) | Deficient (n=62) | Excess (n=43) | Probability | |
| Tone Pip Condition | ||||
| All | 48.3 ± 1.1 | 49.1 ± 1.0* | 50.2 ± 1.1**, *** | 0.001 |
| 2 kHz | 41.4 ± 2.3 | 42.3 ± 3.3 | 44.3 ± 4.4** | 0.001 |
| 4 kHz | 25.8 ± 1.8 | 26.5 ± 2.5 | 28.3 ± 4.5**, *** | 0.001 |
| 8 kHz | 25.7 ± 1.7 | 26.0 ± 2.0 | 26.2 ± 3.1 | N.S. |
| 16 kHz | 20.5 ± 3.0 | 20.9 ± 3.0 | 21.9 ± 4.0* | 0.028 |
Different from Control group, p = 0.027 or better, one-sided test
Different from Control group, p < 0.001, one-sided test
Different from Deficient group, p = 0.015 or better, two-sided test
N.S. = not significant
n = number of pups, one male and one female per litter when possible
The 2-way ANOVA that used data from all the tone pip conditions collectively showed a significant main effect for the Diet Group condition (p < 0.001). Planned pair-wise comparisons indicated that the Excess group (p < 0.001) and the Deficient group (p = 0.027) had higher ABR thresholds than the Control group. The Excess group also had higher ABR thresholds than the Deficient group (p = 0.006). Next, univariate ANOVAs were performed for each individual Tone Pip condition to see if these group differences occurred at each tone pip frequency. The results of the univariate ANOVAs are presented in Table 4. Significant group differences were found during the 2 kHz, 4 kHz and 16 kHz conditions but not the 8 kHz condition. In the 2 kHz condition, planned comparisons indicated that only the Excess group had significantly higher ABR thresholds than the Control group. No other comparisons were significant, although the Deficient group was nearly significant in comparison to the Control group (p = 0.063). In the 4 kHz condition, the Excess group had significantly higher ABR thresholds than both the Control and Deficient groups. The Deficient and Control groups did not differ from each other. In the 16 kHz condition, the Excess group had significantly higher ABR thresholds than the Control group. No other pair-wise comparisons were significant.
Figure 1 shows ABRs collected from a typical rat pup in the Control group and from a rat pup in the Excess group during the 4 kHz Tone Pip condition. The Control animal had ABRs in response to stimulus intensities as low as 25 dB. In contrast, the Excess pup had an ABR in response to the stimulus intensity of 40 dB, but not in response to lower intensities. Thus, the Excess pup had an ABR threshold that was about 15 dB higher than its Control pup cohort. The ABRs from this Excess pup were also abnormal in morphology. Specifically, its ABRs had relatively normal wave P1 amplitudes but the P2, P3 and P4 amplitudes were greatly reduced in size, were spread out and less well defined. This suggests a neurological abnormality at or just before the neural generator of the P2 wave (the cochlear nucleus).
Fig. 1.
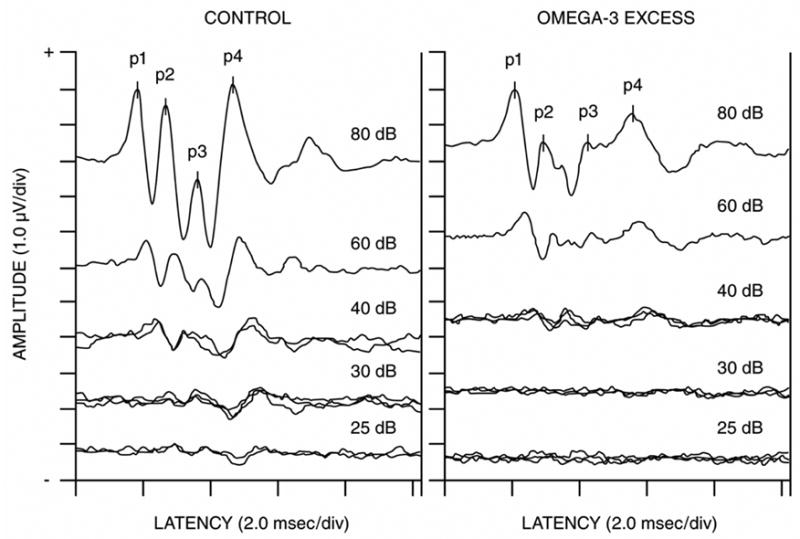
This figure shows serial ABRs in response to 4 kHz tone pips from a typical Control pup and from a pup in the ω-3 FA Excess groups. The Control pup had an ABR threshold of 25 dB, whereas the Excess pup had an ABR threshold of 40 dB. Thus, the pup from the Excess group had an ABR threshold elevation of approximately 15 dB. The ABRs from this Excess pup were also morphologically abnormal. Note the relatively normal amplitudes of its P1 waves in response to the 80 and 60 dB tone pip intensities, but the greatly reduced amplitudes of its P2, P3 and P4 waves.
3.3 Body temperatures
The respective mean ± SD body temperatures of 37.1 ± 0.1, 37.1 ± 0.1 and 37.0 ± 0.1 for the Control, Deficient and Excess diet groups did not differ significantly.
3.4 Pup weights on PND 24
The weights of the ABR-tested pups on PND 24 were analyzed by a 1-way ANOVA for Diet Group. There was a significant group difference (p <0.001). The mean (± SD) pup weights on PND 24 for the Control, Deficient and Excess groups were 63.3 ± 1.5g, 58.0 ± 1.3g and 49.7 ± 1.5g, respectively. Planned pair-wise comparisons indicated that the Excess group weighed less than the Control and the Deficient groups (p < 0.001) and that the Deficient group weighed less than the Control group (p = 0.01). These significant group differences in weights on PND 24 contrast with the lack of significant group differences in weights at birth (see Table 3).
4. Discussion
4.1 Effects on the ABR
Our animal study found that excess ω-3 FA (fish oil) consumption by mothers during pregnancy and lactation caused elevated ABR thresholds in their offspring. This effect was significant at the tone pip frequencies of 2, 4 and 16 kHz but not 8 kHz. Elevated ABR thresholds are indicative of poorer auditory acuity. Figure 1 shows a morphologically abnormal ABR in a rat pup from the Excess condition. The ABR had a normal wave P1 amplitude but the P2, P3 and P4 amplitudes were greatly reduced is size, were spread out and less well defined. These anomalies suggest a neurological abnormality at or just before the neural generator of the P2 wave (the cochlear nucleus). Sarda et al [39] found that DHA incorporation into the developing rat was especially high in the cochlear nucleus and other structures along the brainstem’s auditory pathway.
Our ABR threshold and morphology findings indicate abnormal auditory and neurological outcomes for pups in the Excess condition. These results support previous findings that excess maternal ω-3 FA during pregnancy and lactation had adverse ABR effects in rat pups [20,40,44]. Our results extend these previous studies in several ways. First, we used four different tone pip frequencies to elicit the ABRs whereas the previous studies used only click stimuli that had peak spectral energy at about 4 kHz. This allowed us to determine at which tonal frequencies the treatment effects occurred. Second, we examined ABR thresholds (hearing acuity) whereas the previous studies examined ABR latencies (neural transmission times). Third, we examined only one male and one female from each litter to avoid the confounding of within-litter effects and to assess gender-dependent effects (of which there were none). Fourth, we regulated body temperature to ensure that it was not a confounding factor in the ABR results. Finally, we included a treatment group that was deficient in ω-3 FA for comparison.
Two recent human studies found adverse ABR effects in children born to women who consumed high amounts of seafood [27–28]. These authors concluded that their results were caused by methylmercury and other seafood contaminants. Our fish oil-containing diets were devoid of such contaminants. Moreover, our results of adverse ABR effects from excess ω-3 FA are consistent with animal and human studies reporting harmful effects on nervous system growth and function (see Introduction). Excess ω-3 FA affects the myelination within the developing brain [20]. Thus, nutritional toxicity from excess amounts of ω-3 FA should be considered as an important factor in the adverse neurological and developmental outcomes in children born to women who consume large amounts of seafood during pregnancy and lactation.
One unanswered question is whether the adverse effects on the ABR thresholds are transient or permanent. Preliminary results from our animals when tested as young adults indicate that such effects persist into adulthood (study in progress). We are also evaluating the ABR latencies to assess delayed neural transmission times and sensorineural hearing loss (SNHL). This latter study is indicating that the Excess condition caused both delayed neural transmission times, occasional instances of SNHL, and more evidence of morphologically abnormal ABRs (study in progress).
A deficiency of ω-3 FA during pregnancy or lactation is considered harmful to the developing nervous system (see Introduction). It was surprising therefore that the Deficient condition had relatively modest and fewer significant effects than the Excess condition.
4.2 Effects on birthing and growth
Neither the Excess or Deficient conditions had significant effects on gestational length, maternal weights and food consumption, litter size or birth weight. These results contrast with Olsen et al [34] who reported that pregnant rats fed a diet high in fish oil had reduced maternal weights and food consumption and pups with lower birth weights. In contrast, there were significant group differences in pup weights on PND 24. The Excess group weighed less than the Deficient group which weighed less than the Control group. The Excess group also showed a trend for increased postnatal mortality. These outcomes suggest that the effects from the prenatal exposures were subtle but nonetheless “programmed” the fetus for later neurological abnormalities and a “failure to thrive”. It is well accepted that adverse prenatal conditions can program the fetus for subsequent childhood and adult-onset disorders [3]. On the other hand, the postnatal ABR abnormalities and the failure to thrive that were seen in the Excess and Deficient conditions could have been caused by the postnatal exposures during lactation. Researching the effects of prenatal and postnatal exposures separately could resolve this issue.
4.3 Mechanism of ω-3 FA’s harmful effects
The ω-3/ω-6 ratio is considered an important parameter because a balanced ratio ω-3 and ω-6 FA is required for optimal growth and development. Too much or too little of either can have adverse effects [1]. The mechanism of harm caused by excess ω-3 FA occurs through the displacement and lowering of blood concentrations of ω-6 FAs such as AA. Lowered concentrations of AA impair fetal and infant growth because AA promotes adipose tissue deposition and is a mediator of growth and metabolic hormones [8–9,11,19]. Excess ω-3 FA can also cause oxidative stress which can be harmful to the developing fetus and infant [17].
4.4 Comments on dosing
The Excess condition in our rats did not have significant effects on birth weights, gestational length or other birthing variables; whereas several human studies showed such effects in infants born to women who consumed the highest amounts of seafood [17–18,30,32–33,38,45]. Thus, our Excess condition in the rat was less toxic than the high dose condition in humans. It was also less toxic than the dose used in the rat study by Olsen et al [34] who was studying the effects of fish oil on gestational length and birth weight. It is recommended that rodent developmental toxicology studies use a high dose condition that is 10 to 100 times the human dose in order to assess the treatment’s safety factor and because rodents frequently need such high doses to achieve adverse physiological endpoints that are equivalent to the human condition, such as a 10% reduction in birth weight [24]. It has also been recommended that a broad and high range of ω-3/ω-6 FA ratios be explored in pregnancy and lactation studies in regards to clinical benefit and adverse events [1]. The current study was a preliminary study designed to demonstrate the principle that ω-3 FA deficiency and excess can have adverse effects on auditory and nervous system functions as evidenced by the ABR. Future studies in our laboratory hope to examine more moderate dose levels, to establish a dose-dependency curve, and to assess long-term fetal programming effects. Such studies will help determine how much ω-3 FA dietary supplementation is too much, too little or just right for an infant’s optimal health.
4.5 Conclusion
In summary, our results and those from other laboratories indicate that both excess and deficient amounts of dietary ω-3 FA during pregnancy and lactation can cause postnatal growth retardation as well as sensory and neurological abnormalities in the offspring. Our results suggest that these effects were more dramatic for the excess than the deficient ω-3 FA condition. Thus, consuming or administering large amounts of ω-3 FA to pregnant women or nursing infants for health or therapeutic reasons could have harmful consequences and seems inadvisable. More research is needed to determine how much perinatal ω-3 FA is too much, too little or just right for the developing human and to determine the long-term consequences in regards to the fetal programming of neurodevelopmental and sensory impairments and adult-onset diseases such as hypertension, diabetes, age-related neural degeneration and a shortened life span.
Acknowledgments
This project was supported by grants from the Gerber Foundation and the National Institutes of Health (GM58905-8). The Gerber Foundation did not contribute to the design, analysis or writing of this manuscript. We wish to thank Lindsay Dowhan, Aliyah Ali, Marjan Moghaddam, Sharon Stephens and Dr. Karen Rossman (DVM) for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agency for Healthcare Research and Quality. Effects of omega-3 fatty acids on child and maternal health, AHRQ Publication No. 05-E025-2. United States Department of Health and Human Services; Rockville, MD 20850: 2005. [Google Scholar]
- 2.Anderson JW, Johnstone BM, Remley DT. Breast-feeding and cognitive development: a meta-analysis. Am J Clin Nutr. 1999;4:525–35. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 4.Bourre JM. Where to find omega-3 fatty acids and how feeding animals with diet enriched in omega-3 fatty acids to increase nutritional value of derived products for human: what is actually useful? J Nutr Health Aging. 2005;9:232–42. [PubMed] [Google Scholar]
- 5.Bourre JM, Durand G, Erre JP, Aran JM. Changes in auditory brainstem responses in alpha-linolenic acid deficiency as a function of age in rats. Audiology. 1999;38:13–18. doi: 10.3109/00206099909072997. [DOI] [PubMed] [Google Scholar]
- 6.Bourre JM, Francois M, Youyou A, Dumont O, Piciotti M, Pascal G, Durand G. The effects of dietary alpha-linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons and performance of learning tasks in rats. J Nutr. 1989;119:1880–92. doi: 10.1093/jn/119.12.1880. [DOI] [PubMed] [Google Scholar]
- 7.Carlson SE. Arachidonic acid status of human infants: Influence of gestational age at birth and diets with very long chain n-3 and n-6 fatty acids. J Nutr. 1996;126:1092S–1098S. doi: 10.1093/jn/126.suppl_4.1092S. [DOI] [PubMed] [Google Scholar]
- 8.Carlson SE. Docosahexaenoic acid and arachidonic acid in infant development. Semin Neonatol. 2001;6:437–49. doi: 10.1053/siny.2001.0093. [DOI] [PubMed] [Google Scholar]
- 9.Carlson SE, Cooke RJ, Rhodes PG, Peeples JM, Werkman SH, Tolley EA. Long-term feeding of formulas high in linoleic acid and marine oil to very low birth weight infants: Phospholipid fatty acids. Pediatr Res. 1991;30:404–12. doi: 10.1203/00006450-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids. 1992;27:901–7. doi: 10.1007/BF02535870. [DOI] [PubMed] [Google Scholar]
- 11.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA. 1993;90:1073–77. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson SE, Werkman SH, Tolley EA. Effect of long-chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am J Clin Nutr. 1996;63:687–97. doi: 10.1093/ajcn/63.5.687. [DOI] [PubMed] [Google Scholar]
- 13.Church MW, Blakley BW, Burgio DI, Gupta AK. WR-2721 (Amifostine) ameliorates cisplatin-induced hearing loss but causes neurotoxicity in hamsters: Dose-dependent effects. J Assoc Res Otolaryngol. 2004;5:227–37. doi: 10.1007/s10162-004-4011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church MW, Gritzke R. Effects of ketamine anesthesia on the rat brain-stem auditory evoked potential as a function of dose and stimulus intensity. Electroencephalogr Clin Neurophysiol. 1987;67:570–83. doi: 10.1016/0013-4694(87)90060-5. [DOI] [PubMed] [Google Scholar]
- 15.Church MW, Shucard DW. Age-related hearing loss in BDF1 mice as evidenced by the brainstem auditory evoked potential. Audiology. 1986;25:363–72. doi: 10.3109/00206098609078400. [DOI] [PubMed] [Google Scholar]
- 16.Church MW, Williams HL, Holloway JA. Postnatal development of the brainstem auditory evoked potential and far-field cochlear microphonic in non-sedated rat pups. Brain Res. 1984;316:23–31. doi: 10.1016/0165-3806(84)90005-1. [DOI] [PubMed] [Google Scholar]
- 17.Grandjean P, Bjerve KS, Weihe P, Steuerwald U. Birth weight in a fishing community: significance of essential fatty acids and marine food contaminants. Int J Epidemiol. 2001;30:1272–78. doi: 10.1093/ije/30.6.1272. [DOI] [PubMed] [Google Scholar]
- 18.Grandjean P, Weihe P. Neurobehavioral effects of intrauterine mercury exposure: potential sources of bias. Environ Res. 1993;61:176–83. doi: 10.1006/enrs.1993.1062. [DOI] [PubMed] [Google Scholar]
- 19.Hardy SC, Kleinman RE. Fat and cholesterol in the diet of infants and young children: implications for growth, development, and long-term health. J Pediatric. 1994;125:69–77. doi: 10.1016/s0022-3476(06)80739-0. [DOI] [PubMed] [Google Scholar]
- 20.Haubner LY, Stockard JE, Saste MD, Benford VJ, Phelps CP, Chen LT, Barness L, Wiener D, Carver JD. Maternal dietary docosahexanoic acid content affects the rat pup auditory system. Brain Research Bulletin. 2002;55:1–5. doi: 10.1016/s0361-9230(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 21.Henry KR. Auditory brainstem volume-conducted responses: origins in the laboratory mouse. J Am Aud Soc. 1979;4:173–78. [PubMed] [Google Scholar]
- 22.Hoffman DR, Theuer RC, Castaneda YS, Wheaton DH, Bosworth RG, O'Connor AR, Morale SE, Wiedemann LE, Birch EE. Maturation of visual acuity is accelerated in breast-fed term infants fed baby food containing DHA-enriched egg yolk. J Nutr. 2004;134:2307–13. doi: 10.1093/jn/134.9.2307. [DOI] [PubMed] [Google Scholar]
- 23.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000;22:474–80. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 24.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for human use, Detection of toxicity to reproduction for medicinal purposes S5A. 1993 Retrieved from http://www.ich.org.
- 25.Koutz CA, Wiegand RD, Rapp LM, Anderson RE. Effect of dietary fat on the response of the rat retina to chronic and acute light stress. Exp Eye Res. 1995;60:307–16. doi: 10.1016/s0014-4835(05)80112-5. [DOI] [PubMed] [Google Scholar]
- 26.Lauritzen L, Jorgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: effect on developmental outcome in breast-fed infants. Reprod Nutr Dev. 2005;45:535–47. doi: 10.1051/rnd:2005044. [DOI] [PubMed] [Google Scholar]
- 27.Murata K, Weihe P, Araki S, Budtz-Jorgensen E, Grandjean P. Evoked potentials in Faroese children prenatally exposed to methylmercury. Neurotoxicol Teratol. 1999;21:471–72. doi: 10.1016/s0892-0362(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 28.Murata K, Weihe P, Renzoni A, Debes F, Vasconcelos R, Zino F, Araki S, Jorgensen J, White R, Grandjean P. Delayed evoked potentials in children exposed to methylmercury from seafood. Neurotoxicol Teratol. 1999;21:343–48. doi: 10.1016/s0892-0362(99)00011-2. [DOI] [PubMed] [Google Scholar]
- 29.Neuringer M, Jeffrey BG. Visual development: Neural basis and new assessment methods. J Pediatr. 2003;143(4 Suppl):S87–95. doi: 10.1067/s0022-3476(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 30.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: Results from a US pregnancy cohort. Am J Epidemiol. 2004;160:774–83. doi: 10.1093/aje/kwh282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen S, Hansen H, Sommer S, Jensen B, Sorensen T, Secher N, et al. Gestational age in relation to marine n-3 fatty acids in maternal erythrocytes: A study in the Faroe Islands and Denmark. Am J Obstet Gynecol. 1991;164:1203–09. doi: 10.1016/0002-9378(91)90683-i. [DOI] [PubMed] [Google Scholar]
- 32.Olsen S, Sorensen J, Secher N, Hedegaard M, Henriksen T, Hansen H, et al. Randomised controlled trial of effect of fish oil supplementation on pregnancy duration. Lancet. 1992;339:1003–07. doi: 10.1016/0140-6736(92)90533-9. [DOI] [PubMed] [Google Scholar]
- 33.Olsen SF, Grandjean P, Weihe P, Videro T. Frequency of seafood intake in pregnancy as a determinant of birth weight: evidence for a dose dependent relationship. J Epidemiol Community Health. 1993;47:436–40. doi: 10.1136/jech.47.6.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen SF, Hansen HS, Jensen B. Fish oil versus arachis oil food supplementation in relation to pregnancy duration in rats. Prostaglandins Leukot Essent Fatty Acids. 1990;40:255–60. doi: 10.1016/0952-3278(90)90046-n. [DOI] [PubMed] [Google Scholar]
- 35.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 36.Roegge CS, Widholm JJ, Engeseth NJ, Wang X, Brosch KO, Seegal RF, Schantz SL. Delayed spatial alternation impairments in adult rats following dietary n-6 deficiency during development. Neurotoxicol Teratol. 2005;27:485–95. doi: 10.1016/j.ntt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Rossi G, Britt R. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalogr Clin Neurophysiol. 1984;57:143–55. doi: 10.1016/0013-4694(84)90173-1. [DOI] [PubMed] [Google Scholar]
- 38.Rump P, Mensink RP, Kester AD, Hornstra G. Essential fatty acid composition of plasma phospholipids and birth weight: a study in term neonates. Am J Clin Nutr. 2001;73:797–806. doi: 10.1093/ajcn/73.4.797. [DOI] [PubMed] [Google Scholar]
- 39.Sarda N, Gharib A, Moliere P, Grange E, Bobillier P, Lagarde M. Docosahexaenoic acid (cervonic acid) incorporation into different brain regions in the awake rat. Neurosci Lett. 1991;123:57–60. doi: 10.1016/0304-3940(91)90157-o. [DOI] [PubMed] [Google Scholar]
- 40.Saste MD, Carver J, Stockard JE, Benford VJ, Chen LT, Phelps CP. Maternal diet fatty acid composition affects neurodevelopment in rat pups. J Nutri. 1998;128:740–43. doi: 10.1093/jn/128.4.740. [DOI] [PubMed] [Google Scholar]
- 41.Sattar N, Berry C, Greer IA. Essential fatty acids in relation to pregnancy complications and fetal development. Br J Obstet Gynaecol. 1998;105:1248–55. doi: 10.1111/j.1471-0528.1998.tb10002.x. [DOI] [PubMed] [Google Scholar]
- 42.Scott DT, Janowsky JS, Carroll RE, Taylor JA, Auestad N, Montalto MB. Formula supplementation with long-chain polyunsaturated fatty acids: Are there developmental benefits? Pediatrics. 1998;102:E59. doi: 10.1542/peds.102.5.e59. [DOI] [PubMed] [Google Scholar]
- 43.Smuts CM, Huang M, Mundy D, Plasse T, Major S, Carlson SE. A randomized trial of docosahexanoic acid supplementation during the third trimester of pregnancy. Obstet Gynecol. 2003;101:469–79. doi: 10.1016/s0029-7844(02)02585-1. [DOI] [PubMed] [Google Scholar]
- 44.Stockard JE, Saste MD, Benford VJ, Barness L, Auestad N, Carver JD. Effect of docosahexaenoic acid content of maternal diet on auditory brainstem conduction times in rat pups. Dev Neurosci. 2000;22:494–99. doi: 10.1159/000017481. [DOI] [PubMed] [Google Scholar]
- 45.Thorsdottir I, Birgisdottir BE, Hallsdottir S, Geirrson RT. Association of fish and fish liver oil intake in pregnancy with infant size at birth among women of normal weight before pregnancy in a fishing community. Amer J Epidemiol. 2004;160:460–65. doi: 10.1093/aje/kwh239. [DOI] [PubMed] [Google Scholar]
- 46.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006;64(5 Pt 2):S24–33. doi: 10.1301/nr.2006.may.s24-s33. discussion S72–91. [DOI] [PubMed] [Google Scholar]
- 47.Valenzuela A, Von Bernhardi R, Valenzuela V, Ramirez G, Alarcon R, Sanhueza J, Nieto S. Supplementation of female rats with alpha-linolenic acid or docosahexaenoic acid leads to the same omega-6/omega-3 LC-PUFA accretion in mother tissues and in fetal and newborn brains. Ann Nutr Metab. 2004;48:28–35. doi: 10.1159/000075082. [DOI] [PubMed] [Google Scholar]
- 48.Wainwright PE. Dietary essential fatty acids and brain function: a developmental perspective on mechanisms. Proc Nutr Soc. 2002;61:61–69. doi: 10.1079/pns2001130. [DOI] [PubMed] [Google Scholar]
- 49.Wainwright PE, Jalali E, Mutsaers LM, Bell R, Cvitkovic S. An imbalance of dietary essential fatty acids retards behavioral development in mice. Physiol Behav. 1999;66:833–839. doi: 10.1016/s0031-9384(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 50.Wainwright PE, Xing HC, Mutsaers L, McCutcheon D, Kyle D. Arachidonic acid offsets the effects on mouse brain and behavior of a diet with a low (n-6):(n3) ratio and very high levels maze performance is unaffected in artificially of docosahexanoic acid. J Nutr. 1997;127:184–193. doi: 10.1093/jn/127.1.184. [DOI] [PubMed] [Google Scholar]
- 51.Wainwright PE, Xing HC, Ward GR, Huang YS, Bobik E, Auestad N, Montalto M. Water reared rats fed diets supplemented with arachidonic acid and docosahexaenoic acid. J Nutr. 1999;129:1079–1089. doi: 10.1093/jn/129.5.1079. [DOI] [PubMed] [Google Scholar]
- 52.Weisinger HS, Vingrys AJ, Sinclair AJ. The effect of docosahexanoic acid on the electroretinogram of the guinea pig. Lipids. 1996;31:65–70. doi: 10.1007/BF02522413. [DOI] [PubMed] [Google Scholar]


