Abstract
The interaction between tongue size/volume and craniofacial skeletal growth is essential for understanding the mechanism of specific types of malocclusion and objectively measuring outcomes of various surgical and/or orthodontic treatments. Currently available information on this interaction is limited. This study was designed to examine how tongue body volume reduction affects craniofacial skeleton and dental arch formation during the rapid growth period in five 12-week-old Yucatan minipig sibling pairs. One of each pair received a standardized reduction glossectomy to reduce tongue volume by 15-17% (reduction group), and the other had the reduction glossectomy incisions without tissue removal (sham group). Before surgery, five stainless steel screws were implanted into standardized craniofacial skeletal locations. A series of cephalograms, lateral and axial, were obtained longitudinally at 1 week preoperative, and 2 and 4 weeks postoperative. These images were traced using superimposition, and linear and angular variables were measured digitally. Upon euthanasia, direct osteometric measurements were obtained from harvested skulls. Five en-bloc bone pieces were further cut for bone mineral examination by dual photon/energy X-ray absorptiometry (DEXA). The results indicate that: (1) while daily food consumption and weekly body weight were not significantly affected, tongue volume reduction showed an overall negative effect on the linear expansion of craniofacial skeletons; (2) premaxilla and mandibular symphysis lengths, and anterior dental arch width were significantly less in reduction than sham animals at 2 and/or 4 weeks after the surgery; (3) both premaxilla/maxilla and mandible bone mineral density and content were lower in reduction than sham animals, significantly lower in anterior mandible; (4) craniofacial skeletal and dental arch size were significantly smaller in reduction than sham animals, being most significant in the mandibular anterior length and ramus height, the anterior dental arch and midface width. These results suggest that reducing tongue body volume in young animals slows craniofacial skeletal growth and anterior dental arch expansion during rapid growth. The mandible, in particular its symphysis portion, and the anterior dental arch width are most affected. These effects may in part contribute to the decrease of functional loads in the anterior mouth by a volume-reduced tongue.
Keywords: Tongue volume reduction, Craniofacial growth, Dental arch, Cephalometrics, Pig
1. Introduction
The interaction between tongue size/volume and craniofacial skeletal growth is essential for understanding the mechanism of specific types of malocclusion and objectively measuring outcomes of various surgical and/or orthodontic treatments. Currently available information on this interaction is limited. Controversy as to whether the tongue adapts to existing oral morphology, or actively molds its surrounding tissues, is longstanding.1-3 Although smaller than the variation of body mass,4 the tongue size differences range from 15% to 29% in the normal population.5-8 Unfortunately, no study has described a “normal tongue size or volume” or used direct measures to define a pathologically enlarged tongue.9,10 Despite this, numerous clinical studies have claimed that tongue volume is correlated with multiple factors including: dentition position,11-14 mandibular arch size and posture,15,16 maxillary expansion,17 vertical facial height18 and combined horizontal and vertical location of chin and symphysis.8 Others have rejected a role for tongue volume in mandibular prognathism and cranial size.8,19 On the other hand, tongue volume is integrated functionally with tongue position.12 Prolonged low tongue position from oral breathing during critical growth period in children may initiate a sequence of events resulting in excessive molar eruption, causing a clockwise rotation of the growing mandible, a disproportional increase in anterior lower vertical face height, retrognathia, and open bite. A low tongue position may also impede lateral expansion and anterior maxillary development as the mandible rotates to a more downward position.20-22
There is limited information about the effects of altering tongue volume/position on craniofacial growth and dental arch formation. Using rhesus monkeys, Harvold and his colleagues demonstrated that reducing tongue volume by partial glossectomy caused the dental arch to collapse lingually (crowding).20,23 Artificially lowering the tongue and mandible by inserting an acrylic block into the palatal vault or by obstructing the nostrils resulted in an anterior open bite, posterior crossbite (spacing) and increased tooth extrusion and facial height.21,24,25 Another experiment on the effect of tongue volume on craniofacial morphology was performed in miniature pigs. They concluded that partial glossectomy in young animals caused reduction in mandibular length and width, but had no significant effect on mandibular vertical growth.26-29 However, only cross-sectional osteometric data of mandibular growth after anterior regional glossectomy were obtained. No serial longitudinal cephalometric follow-ups, midfacial and cranial morphology, and bone mineralization measures were available and no functional consequences were reported in these studies. Therefore, using the well-established miniature pig model and widely used clinical approach of uniform tongue body volume reduction,30 the present study was designed to examine how the volume-reduced tongue affects the growth of craniofacial skeletons including bone mass and mineralization, and dental arch formation. We hypothesized that a volume-reduced tongue would lead to negative effects on skeletal growth in young animals.
2. Materials and methods
2.1. Animal care
Five same-gender 12-week Yucatan miniature pigs (Sinclair Research Center, Columbia, MO) sibling pairs (three male and two female pairs), were used. Under aseptic conditions, five stainless steel screws (0.8 mm in diameter and 4 mm in length) were implanted into the following alveolar sites: one each between the upper and lower central incisors, one each near the roots of the left upper and lower second molars, and one above the same tooth of the right maxilla. Maxillary markers served as reference landmarks to ensure accurate superimposition of serial cephalometrics. The right and left molar alveolar bone markers served as a reference line for axial cephalograms. Each pair of sibling had the screw implantation at the same day. The tongue surgery was performed 9-10 days after the implantation. This procedure has been reported elsewhere.31,32 In brief, the bilateral incisions first diverged to meet the lateral margin anteriorly. Cutting diathermy was used to undermine and create lateral muco-muscular flaps and to excise a conical wedge (above the tongue neurovascular bundles) from the central tongue. The removed tongue muscular tissue uniformly reduced tongue volume in three dimensions (length, width and thickness). After hemostasis, the incision was closed in layers with absorbable sutures (Vicryl 4.0). The removed tongue tissue was preserved in a 50% alcohol solution. For the sham surgery, identical incisions were made and sutures placed, but without tissue removal (Fig. 1). Surgery was performed on each pair of sibling on the same day, one to reduce the tongue volume by about 15% (reduction group), and the other, with incision only, without removal of tongue tissue (sham group). The actual changes of the tongue mass (volume and weight) after the surgery were measured postmortem, and the linear changes of the tongue dimensions (length, width and thickness) were measured using longitudinal tongue impressions (Table 1). Slurry food was offered for 2-3 days postoperatively, followed by regular pig chow diet. Antibiotics (Clavamox suspension, 50 mg, Bid, Pfizer Animal Health, New York, NY) was given before and after surgery for 10 days. Each pair of siblings received tongue surgery at the same day. Animals were raised for 4 weeks postoperatively with weekly weighing, and were euthanized through cardiac injection of pentobarbital sodium (Beuthanasia D, Schering-Plough Animal Health Corp. Union, NJ). All procedures were approved by the IACUC of University of Washington.
Fig. 1.
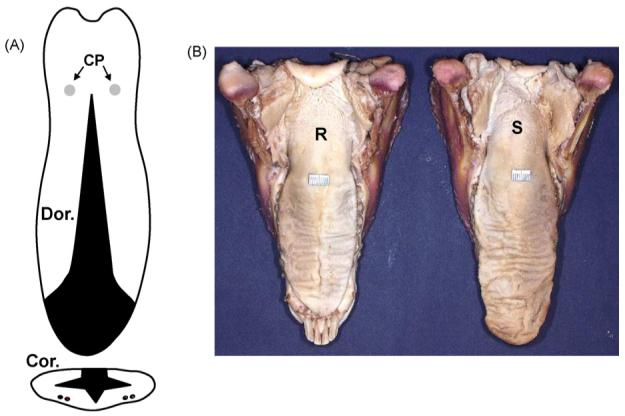
(A) Schema of the tongue reduction surgery on dorsal (Dor.) and coronal (Cor.) views. Black areas indicate removed tongue tissue. Two dots in each side indicate the locations of neurovascular bundles in the ventral surface. CP: circumvallate papillae. (B) Postmortem tongue specimens 4 weeks after surgery. R: reduction tongue; S: sham tongue.
Table 1.
Changes of tongue dimensions and mass after surgery
| Dimensions |
Mass |
|||||||
|---|---|---|---|---|---|---|---|---|
| Length | Width | Thickness | Volume(ml) | Loss(%) | Weight(g) | Loss(%) | ||
| Sham | Before | 103.45±4.90 | 60.15±2.24 | 7.91±0.33 | n/a | n/a | n/a | n/a |
| After | 113.05±5.21* | 67.76±0.61* | 9.16±0.58* | 71.45±2.20 | n/a | 71.43±1.52 | n/a | |
| Reduction | Before | 104.52±3.68 | 57.88±2.26 | 8.03±0.33 | n/a | n/a | n/a | n/a |
| After | 82.99±4.69* | 50.66±2.41* | 10.49±0.27* | 60.62±0.91# | 15.21±0.78 | 59.42±1.33# | 15.18±0.19 | |
Note: Dimension measures on tongue casts (body only, anterior 2/3), and mass measures on postmortem tongue specimens. % of loss calculated by specimen volume (weight)/removed part volume (weight) × 100%.
p < 0.05 before and 4 weeks after surgery in each group.
p < 0.05 between the two groups 4 weeks after surgery.
2.2. Serial cephalometric radiographs and skull harvests
The initial caphalometric radiograph (baseline) was taken preoperatively and 2-3 days after metallic marker implantation (Fig. 2). This procedure was repeated 2 and 4 weeks after tongue surgery. Pigs were mask-anaesthetized with isoflurane and placed on the X-ray table (Summit LX125V, Summit Industries Inc., Chicago, IL). The head of the pig was oriented by adjusting the occlusal plane parallel to the X-ray cassette margin and to the X-ray table surface for lateral and axial projections, respectively. The cross of X-ray central beam was always located at the medial canthus for lateral and the intersection between the line connecting bilateral medial canthus and the mid-sagittal lines for axial projections. A 10 × 12 inch X-ray film (Les Wilkins and Associate, Seattle, WA) was used. Radiation settings were as follows: Kvp: 61 (lateral) and 65 (axial); Ams: 15; Exposure: 3/20 s.
Fig. 2.
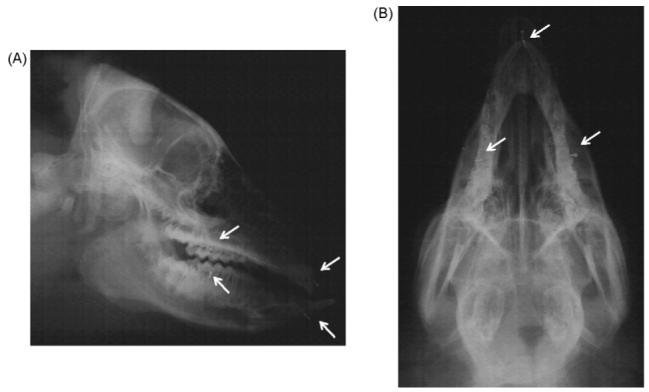
Lateral (A) and axial (B) radiocephalograms. Arrows indicate implanted stainless screws for superimposition.
After euthanasia, the heads were dissected and cleaned of all soft tissues to exposure craniofacial sutures. The harvested skulls were stored in a freezer for further osteometric measurements.
3. Cephalometric, osteometric and dental measurements
The superimposition of longitudinal tracings using implanted screws was applied to corroborate accuracy. After film tracing, reference points were identified by one investigator (GGM) and landmark identification accuracy was confirmed by two secondary investigators (ZJL and VS). These tracings with marked reference points (Fig. 3) were digitized, and 16 linear and 14 angular variables on the lateral and 13 linear variables on the axial cephalometric radiographs were calculated automatically using a Macro program written in MS Excel (Microsoft Co. Redmond, WA, Table 1).
Fig. 3.
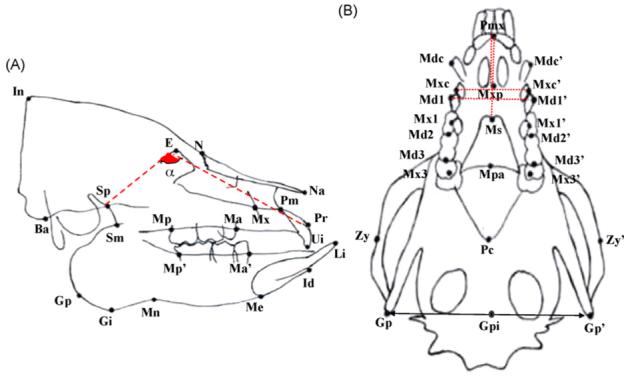
Reference points of lateral and axial cephalometric measurements.
(A) Lateral cephalogram. Cranial—In: the most posterior point of external occipital protuberance; Ba: the most antero-inferior point of occipital condyle; E: intersection between the frontal bone and the most supero-anterior point of posterior limit of ethmoid bone; Sp: intersection between the body and posterior border of pterygoid process of sphenoid; N: front-nasal suture point; Na: the most anterior point of the nasal bone. Maxillary—Pr: the most infero-anterior point of the labial alveolar process of the maxillary incisor; Ui: the incisal edge of the maxillary incisor; Mx: the most convex point of the maxillary anterior limit; Pm: the most concave point of the anterior border of premaxilla; Ma: intersection between maxillary alveolar process and the mesial surface of the maxillary first molar; Mp: intersection between maxillary alveolar process and distal surface of maxillary third molar. Mandible—Sm: intersection between the pterygoid process of sphenoid and the anterior border of mandibular ramus; Gp: the most posterior point of the angular process of the mandible; Gi: the most inferior point of the angular process of mandible; Mn: the deepest point of the antegonial notch; Me: the most inferior point of the mandibular symphysis; Id: the most supero-anterior point of the labial alveolar process of mandibular incisor; Li: the incisal edge of the mandibular incisor; Ma’: intersection between the mandibular alveolar process and the mesial surface of the mandibular first molar; Mp’: intersection between the mandibular alveolar process and the distal surface of mandibular third molar. (B) Axial cephalogram. Pmx: the most anterior point of the mandible; Mxp: intersection of premaxilla and maxilla; Ms: the most prominent point of mandibular symphysis; Mpa: intersection point of maxilla and palatine; Pc: intersection point of pterygoid and occipital bones; Zy/Zy’: intersection between the line through Pc and bilateral zygomatic arches; Gp: intersection between the mid-sagittal line and the line connecting Gp and Gp’; Mdc/Mdc’: the most posteror point of the mandibular canine cusp; Mxc/Mxc’: the most posteror point of the maxillary canine cusp; Md1/Md1′: the most prominent point of the mandibular 1st molar; Mx1/Mx1′: the most prominent point of the maxillary 1st molar; Md2/Md2′: contact point of mandibular 2nd and 3rd molars; Md3/Md3′: the most posterior point of the mandibular 3rd molar; Mx3/Mx3′: central fossa of maxillary 3rd molar. Dotted and dashed lines indicate the significant decrease of linear distances and angulation (α) at 2 and/or 4 weeks after the surgery respectively in reduction as compared to sham animals.
Osteometry of harvested skulls were performed using a digital calliper and a needle compass. The 21 osteologic landmarks for craniofacial skeletons and 6 dental landmarks for dental arches were defined which resulted in 33 linear variables (Fig. 4).
Fig. 4.
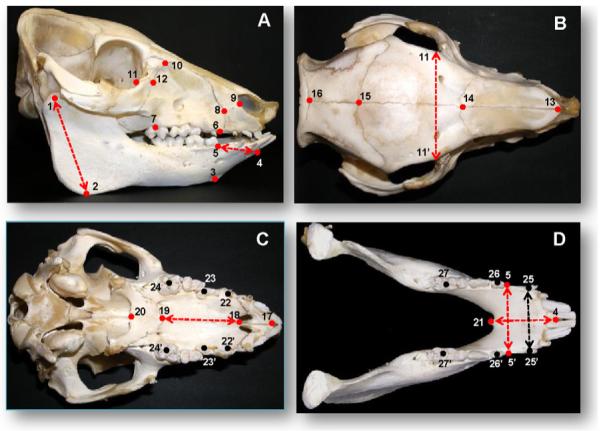
Osteometric and dental landmarks for the measurements of postmortem skulls. Red and black dots indicate landmarks on the skeletons and teeth, and red and black dashed lines represent significantly changed variables between the two groups in skeletons and dental arches, respectively. (A) Lateral view; (B) parietal view; (C) palatal view; (D) occlusal view, mandible. (#1-2) Mandibular ramus height; (#1-4) mandibular total length; (#2-3) mandibular border length; (#3-5) mandibular body height; (#4-5) mandibular anterior length; (#4-21:) mandibular symphysis length; (#6-7) maxillary buccal length; (#7-10) maxillary height; (#8-9) mid-premaxillary length; (#11-12) zygomatico-lacrimal suture length; (#13-14) nasal bone length; (#14-15) frontal bone length; (#15-16) bregma-lambda length; (#17-18) premaxillary palatal length; (#18-19) maxillary palatal length; (#19-20) palatine bone length; (#1-1′ and #2-2′) mandibular widths at condyle and angle; (#3-3′ and #5-5′) ventral and dorsal widths of anterior mandible; (#6-6′) premaxillary width; (#7-7′) maxillary posterior width; (#8-8′ and #9-9′) anterior and posterior nasal widths; (10-10′) front width of brain case; (#11-11′ and #12-12′) posterior and anterior midfacial widths; (#22-22′, #23-23′ and #24-24′) maxillary dental arch widths at canine, the 1st and 3rd deciduous molars; (#25-25′, #26-26′ and #27-27′) mandibular dental arch widths at canine, the 1st and 3rd molars. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
The reliability of the above measurements was examined by the following approaches. First, of 10 harvested skulls and 60 cephalometric films, 4 skulls and 10 films were randomly selected and re-measured by a second investigator (ZJL), who was also responsible for identifying the reference points. A paired t-test showed that there were no significant differences between these two measurements. Second, the skulls and traced cephalometric radiographs were re-measured and re-digitized after 2-3-week interval by the two investigators (VS and ZJL) who performed the primary measurements and digitization. The error was calculated as 0.050-0.065 mm for the skulls, 0.05 mm and 0.30° for linear and angular variables of cephalometric radiographs using Dahlberg formula ().33
4. Measurements of bone mineral components
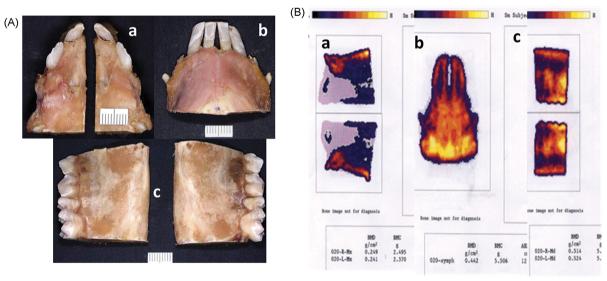
After the completion of skull measurements, five en-bloc bone pieces were cut for dual photon/energy X-ray absorptiometry (DEXA) examinations: (1) left and right premaxillary/maxillary blocs cut at the medial side of the 1st molar (Fig. 5Aa); (2) mandibular symphysis bloc cut at the medial side of the 1st molar (Fig. 5Ab); (3) left and right mandibular corpus blocs with three molars (Fig. 5Ac).
Fig. 5.
En-bloc bone pieces (A) and bone mineral density measurement by DEXA (B). a, b and c indicate premaxillary/maxillary bone blocs (left and right), mandibular symphysis bloc, and mandibular corpus blocs (left and right). Asterisk indicates statistical significance by non-paired t-test.
The DEXA scan was performed with a Norland XR-26 Mark II (Norland Inc, Fort Atkinson, WI). This instrument was calibrated daily and the setup for scanning resolution (0.5 mm × 0.5 mm), scan speed (35 mm/s) and scan width (300 mm) was confirmed at each session. Each bone piece was placed on the scan table, and the measurement was performed using small-subject software (Host Software revision, 2.5.3a) and gathered as bone mineral density (BMD, g/cm2) and bone mineral content (BMC, g) (Fig. 4B), similar to the use of DEXA for the whole body, head and other regions of the pig.34
5. Statistics
Paired t-tests were first performed to examine the difference of measurements between left and right sides in each group. One-way ANOVA and Bonferroni post-hoc tests were used to examine body weight changes over time, and non-paired t-tests were used to detect the differences of body weight at each time points and DEXA values between the two groups. Probability levels of 0.05 or less were considered to indicate statistical significance for paired t and ANOVA tests.
Since the body weight was a covariance for cephalometric and osteometric measures, a Generalized Estimating Equation (GEE) model with independent working correlation clustering on each individual animal ID to the data for each measurement was applied in all variables of these two sources. The GEE was used to correct variance for repeated measurements on each animal. The model for each measurement was adjusted linearly for body weight and for time and intervention group and their interactions as factors. Each hypothesis was then tested through a contrast of the estimated parameters. The time trend in each group was examined separately, for differences between groups at each time point, and for any evidence of a difference between groups at any time point. A Bonferroni correction was used to account for multiple testing and the probability levels of 0.013 or less were considered to indicate statistical significance. For the measurements that were not repeated at successive times a linear regression of the parameter on bodyweight and intervention group with robust standard errors was adopted to do a t-test of difference in groups.
6. Results
6.1. Feeding behaviours and body weight
No noticeable feeding behaviour changes were identified in sham animals. Reduction animals were able to eat softened pig chow after the surgery and had almost the same daily food consumption as sham animals. However several feeding behaviour changes were noticed. These changes included using the mandible, rather than the anterior tongue, to ingest food into the oral cavity; a slightly distorted chewing rhythm, longer feeding sessions, food leaking from the mouth during chewing, and an “inertial” chewing/swallowing pattern (i.e. head moving and shaking while chewing and swallowing, a way of taking the advantage of gravity effect).
Over the 6-week experimental period, body weight increased progressively and significantly in both groups. A slight drop in body weight (∼3%) in the first postoperative week was seen in reduction animals. However, the catch-up followed from the second week (Fig. 6). There was no significant difference of body weight between the two groups at any time point. This fact indicated that the reduction surgery might not affect general health.
Fig. 6.
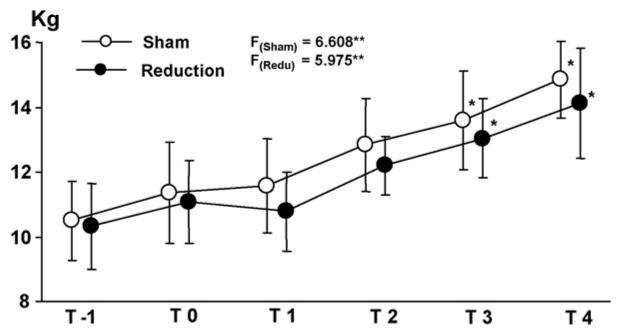
Body weight curve over the 6-week experimental period. T-1: 1 week before the surgery; T0: day of surgery; T1, T2, T3 and T4: 1, 2, 3 and 4 weeks after the surgery. Asterisks indicate statistical significance by ANOVA (superscripted at F values) and Bonferroni post-hoc (above the dots) tests. *p < 0.05; **p < 0.01.
6.2. Craniofacial skeleton changes over time
The longitudinal distances between the implanted screws remained stable and demonstrated no evidence of interstitial growth within the bones. As summarized in Table 3, over the period of 5 weeks (T0-T4), increases of all linear variables were identified. Of 16 and 13 linear variables from lateral and axial cephalometric radiographs respectively in both groups (Table 2), significant increases over time (p < 0.013) were found in mandibular dorsal (Sm-Id) and ventral (Gp-Id) lengths, inter-maxillary canine (Mxc-Mxc’), bi-zygomatic (Zy-Zy’) and bi-gonial (Gm-Gm’) widths. Significant increases in premaxillary palatal (Pmx-Mxp) and mandibular symphysis (Pmx-Ms) lengths were only found in sham animals, and these numbers even became smaller over time in reduction animals. Of 13 angular variables, both groups showed significant increases in the angles of premaxilla to cranial vault (Pr-E-In), maxillary incisor to cranial vault (Ui-E-In), and mandibular antegonial notch (Gi-Mn-Me). Significant changes in gonial angle (Sm-Gp/Me-Gi) were in an opposite direction for each group, decreased in sham and increased in reduction groups over time.
Table 3.
Comparisons of linear (mm) and angular (°) measurements over time between the two groups
| LA Linear | Sham |
Reduction |
LA Angular | Sham |
Reduction |
AX Linear | Sham |
Reduction |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Na-In | 0 | 145.4 | 4.73 | 143.9 | 1.56 | Ba-In-E | 62.3 | 1.67 | 61.8 | 2.44 | Mdc-Mdc’ | 36.1 | 2.39 | 35.6 | 1.64 |
| 2 | 144.0 | 2.51 | 143.7 | 1.46 | 60.6 | 2.52 | 61.5 | 2.04 | 36.8 | 2.86 | 35.6 | 1.54 | |||
| 4 | 148.0 | 2.56 | 146.5 | 1.94 | 60.3 | 2.29 | 60.4 | 2.75 | 38.1 | 1.88 | 37.1 | 2.06 | |||
| Pr-Ba | 0 | 133.4 | 3.60 | 126.0 | 1.28 | In-Ba-Sp | 78.6 | 3.69 | 80.2 | 3.87 | Mxc-Mxc’S.R | 29.0 | 3.27 | 30.1 | 1.46 |
| 2 | 132.0 | 4.53 | 128.1 | 4.53 | 79.1 | 2.67 | 81.2 | 2.67 | 31.8 | 2.43 * | 30.1 | 1.05 | |||
| 4 | 136.7 | 6.17 | 133.1 | 6.17 | 79.6 | 3.78 | 81.1 | 2.79 | 32.7 | 3.30 | 30.9 | 0.49 | |||
| Sp-E | 0 | 45.9 | 1.92 | 46.2 | 1.92 | In-E-Sp | 65.6 | 2.58 | 64.7 | 2.11 | Md1-Md1′ | 36.7 | 2.15 | 36.1 | 1.84 |
| 2 | 45.5 | 2.38 | 46.5 | 2.38 | 66.1 | 2.15 | 66.4 | 2.60 | 37.4 | 2.37 | 35.9 | 1.34 | |||
| 4 | 46.0 | 3.62 | 47.5 | 3.62 | 67.2 | 2.15 | 67.4 | 2.87 | 38.6 | 1.94 * | 35.8 | 1.05 | |||
| Sp-Ba | 0 | 30.9 | 1.47 | 29.4 | 1.47 | Na-N/Sp-E | 117.1 | 1.86 | 112.8 | 3.38 | Mx1-Mx1′ | 31.8 | 0.96 | 33.3 | 1.88 |
| 2 | 30.3 | 1.57 | 30.2 | 1.57 | 117.0 | 2.10 | 113.2 | 3.41 | 32.0 | 1.09 | 32.5 | 1.83 | |||
| 4 | 31.4 | 3.06 | 30.2 | 3.06 | 117.9 | 2.15 | 114.4 | 4.04 | 33.4 | 0.36 | 33.1 | 2.62 | |||
| E-In | 0 | 75.1 | 3.83 | 75.0 | 3.83 | Pr-E-InS.R | 173.0 | 3.16 | 169.7 | 1.31 | Md2-Md2′ | 35.4 | 1.38 | 35.3 | 1.06 |
| 2 | 75.9 | 2.39 | 75.8 | 2.39 | 174.8 | 3.11 | 171.0 | 3.35 | 35.7 | 1.02 | 35.4 | 1.61 | |||
| 4 | 77.5 | 2.90 | 77.2 | 2.90 | 174.9 | 2.01 | 173.4 | 1.26 | 37.5 | 0.96 | 35.1 | 2.51 | |||
| Ba-In | 0 | 69.2 | 1.58 | 68.0 | 1.58 | Pr-E-Sp | 153.5 | 3.94 | 153.3 | 4.75 | Md3-Md3′ | 36.7 | 1.20 | 36.4 | 2.44 |
| 2 | 70.2 | 1.90 | 69.5 | 1.90 | 154.3 | 1.75 * | 150.9 | 1.74 | 37.4 | 1.51 | 37.1 | 2.38 | |||
| 4 | 72.2 | 2.96 | 71.4 | 2.56 | 152.9 | 4.13 | 150.9 | 2.31 | 39.4 | 1.71 | 36.4 | 2.60 | |||
| Pr-E | 0 | 80.6 | 3.26 | 75.0 | 1.30 | Ui-E-InS.R | 167.2 | 2.60 | 163.7 | 0.43 | Mx3-Mx3′ | 35.5 | 1.30 | 34.3 | 3.03 |
| 2 | 79.4 | 3.14 | 76.7 | 3.14 | 168.9 | 2.43 | 164.8 | 3.29 | 36.1 | 1.47 | 35.3 | 1.38 | |||
| 4 | 82.4 | 2.73 | 79.3 | 2.73 | 170.5 | 2.71 | 168.5 | 4.39 | 36.9 | 0.64 | 35.3 | 1.83 | |||
| Ma-E | 0 | 52.5 | 2.42 | 48.8 | 2.42 | Ui-E-Sp | 101.6 | 2.08 | 98.9 | 2.43 | Zy-Zy’S.R | 94.8 | 1.69 | 93.2 | 3.40 |
| 2 | 51.3 | 3.42 | 51.0 | 3.42 | 102.8 | 2.29 | 98.4 | 3.27 | 97.3 | 2.65 | 95.4 | 3.10 | |||
| 4 | 52.7 | 3.23 | 52.8 | 3.23 | 103.3 | 2.99 | 100.8 | 4.82 | 101.6 | 2.09 | 98.2 | 4.33 | |||
| Mp-E | 0 | 42.4 | 2.10 | 43.6 | 2.10 | Ui-Pr-Ma | 94.1 | 3.62 | 93.9 | 6.22 | Gp-Gp’S.R | 84.3 | 1.50 | 82.2 | 4.07 |
| 2 | 42.3 | 1.71 | 44.3 | 1.71 | 95.3 | 6.17 | 92.5 | 3.40 | 86.6 | 5.56 | 85.8 | 3.29 | |||
| 4 | 43.8 | 2.32 | 44.9 | 2.32 | 93.0 | 5.97 | 90.0 | 4.69 | 91.3 | 4.17 | 88.0 | 3.41 | |||
| Na-N | 0 | 53.6 | 2.77 | 51.4 | 2.77 | Ma-Mp/E-Sp | 40.2 | 2.19 | 38.0 | 3.68 | Pmx-MxpS | 23.0 | 2.14 | 22.7 | 1.81 |
| 2 | 53.4 | 3.05 | 53.8 | 3.05 | 38.4 | 2.12 | 39.9 | 6.26 | 26.0 | 2.36 * | 21.4 | 1.26 | |||
| 4 | 52.4 | 5.21 | 55.9 | 5.21 | 37.4 | 2.87 | 38.3 | 7.29 | 26.3 | 1.30 * | 23.5 | 1.97 | |||
| Mx-Pm | 0 | 13.5 | 1.06 | 12.3 | 1.06 | Li-Id-Ma’ | 118.6 | 4.44 | 112.6 | 6.34 | Mxp-Mpa | 34.0 | 4.17 | 31.2 | 4.63 |
| 2 | 14.2 | 1.69 | 12.7 | 1.69 | 115.5 | 8.39 | 111.9 | 4.87 | 33.7 | 5.00 | 32.8 | 3.27 | |||
| 4 | 14.6 | 2.30 | 13.6 | 2.30 | 116.0 | 6.32 | 114.3 | 7.32 | 35.3 | 4.48 | 32.3 | 5.71 | |||
| Pr-Ma | 0 | 37.2 | 4.10 | 34.5 | 4.10 | Gi-Mn-MeS.R | 168.8 | 4.54 | 167.4 | 6.67 | Pmx-MsS | 35.0 | 2.89 | 32.6 | 2.88 |
| 2 | 37.6 | 3.51 | 36.2 | 3.51 | 171.9 | 5.64 | 171.2 | 7.88 | 35.9 | 2.76 * | 31.4 | 2.03 | |||
| 4 | 39.5 | 3.14 | 37.1 | 3.14 | 173.4 | 2.71 | 172.1 | 3.47 | 36.5 | 2.42 * | 32.0 | 3.10 | |||
| Id-Ma’ | 0 | 29.4 | 2.21 | 26.3 | 2.21 | Me-Gi/E-Sp | 35.0 | 3.87 | 35.2 | 3.35 | Pmx-Gpi | 80.6 | 4.85 | 78.4 | 3.30 |
| 2 | 28.2 | 1.88 | 27.3 | 1.88 | 34.0 | 3.76 | 35.8 | 6.25 | 82.8 | 4.36 | 83.4 | 2.56 | |||
| 4 | 31.6 | 2.28 | 29.1 | 2.28 | 32.0 | 2.89 | 35.6 | 6.42 | 87.1 | 5.62 | 85.8 | 1.92 | |||
| Sm-IdR | 0 | 99.5 | 3.75 | 97.2 | 1.54 | Sm-Gp/Me-GiS.R | 48.3 | 5.95 | 46.3 | 7.90 | |||||
| 2 | 98.8 | 2.87 | 95.0 | 2.87 | 50.5 | 6.33 | 49.4 | 7.80 | |||||||
| 4 | 101.1 | 0.92 | 102.4 | 0.92 | 42.7 | 5.34 | 47.5 | 7.85 | |||||||
| Gp-IdS.R | 0 | 122.6 | 3.53 | 117.5 | 3.12 | ||||||||||
| 2 | 121.9 | 4.96 | 117.5 | 4.96 | |||||||||||
| 4 | 130.8 | 4.65 | 126.7 | 4.65 | |||||||||||
| Gp-Sm | 0 | 43.7 | 2.30 | 42.3 | 2.30 | ||||||||||
| 2 | 47.1 | 3.01 | 44.3 | 3.01 | |||||||||||
| 4 | 50.2 | 3.90 | 44.1 | 3.90 | |||||||||||
Note: LA: lateral; AX: axial; T: week after the surgery; S.D.: standard deviation. See Table 2 for variables. Bolded/underlined variables: p < 0.05 over three time points. The subscribed letters indicate the group, S: sham; R: reduction. Bolded/italic numbers and asterisks: p < 0.013 between sham and reduction animals at each time point.
Table 2.
Linear and angular variables of craniofacial skeletons and dental arches from cephalograms
| Lateral-linear | Lateral-angular | Axial-linear |
|---|---|---|
| Na-In: superior cranial length | Ba-In-E: neurocranial height to cranial vault | Mdc-Mdc’: inter-mandibular canine width |
| Pr-Ba: inferior cranial length | In-Ba-Sp: neurocranial height to posterior cranial base | Mxc-Mxc’: inter-maxillary canine width |
| Sp-E: anterior cranial base length | In-E-Sp: cranial vault angle | Md1-Md1′: inter-mandibular 1st molar width |
| Sp-Ba: posterior cranial base length | Na-N/Sp-E: nasal bone angle | Mx1-Mx1′: inter-maxillary 1st molar width |
| E-In: neurocranial length | Pr-E-In: premaxilla to cranial vault | Md2-Md2′: inter-mandibular 2nd molar width |
| Ba-In: neurocranial height | Pr-E-Sp: premaxillary angle | Md3-Md3′: inter-mandibular 3rd molar width |
| Pr-E: exterior viscerocranial length | Ui-E-In: maxillary incisor to cranial vault | Mx3-Mx3′: inter-maxillary 3rd molar width |
| Ma-E: anterior viscerocranial height | Ui-E-Sp: maxillary incisor angle | Zy-Zy’: bi-zygomatic width |
| Mp-E: posterior viscerocranial height | Ui-Pr-Ma: maxillary incisor inclination | Gp-Gp’: bi-gonial width |
| Na-N: nasal bone length | Ma-Mp/E-Sp: palatal angle | Pmx-Mxp: premaxillary palatal length |
| Mx-Pm: mid-premaxillary length | Li-Id-Ma’: mandibular incisor inclination | Mxp-Mpa: maxillary palatal length |
| Pr-Ma: maxillary incisor-molar distance | Gi-Mn-Me: ante gonial notch angle | Pmx-Ms: mandibular symphysis length |
| Id-Ma’: mandibular incisor-molar distance | Me-Gi/E-Sp: mandibular plane angle | Pmx-Gpi: Mandibular total length |
| Sm-Id: mandibular dorsal length | Gp-Sm/Me-Gi: gonial angle | |
| Gp-Id: mandibular ventral length | ||
| Gp-Sm: height of mandibular angle |
No statistical difference in any variables was identified between sham and reduction groups at the initial time point (T0). Reduction animals exhibited significantly smaller values in premaxillary angle (Pr-E-Sp) and inter-maxillary canine width (Mxc-Mxc”) at 2 weeks, and inter-mandibular 1st molar width (Md1-Md1’) at 4 weeks. Consistent with the overall growth trend, premaxillary palatal (Pmx-Mxp) and mandibular symphysis lengths (Pmx-Ms) were significantly smaller in reduction animals at both 2 and 4 weeks (Table 3).
6.3. DEXA examinations
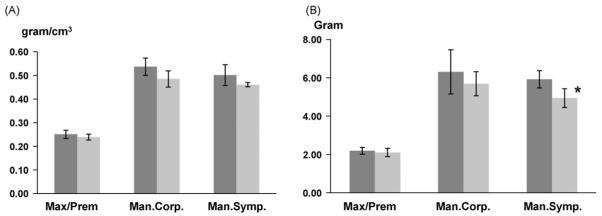
As compared to sham animals, both BMD and BMC values of all bone blocs dropped about 4.8-16.5% in reduction animals. A significant decrease was found in the BMC of mandibular symphysis bloc (p < 0.05, Fig. 7).
Fig. 7.
The comparisons of bone mineral density (BMD, A) and bone mineral content (BMC, B) between sham and reduction groups. Max/Prem: maxillary/premaxillary bloc; Man.corp: mandibular corpus bloc; Man.symp: mandibular symphysis bloc. Measurements from left and right maxillary/premaxillary and mandibular corpus blocs were combined in each group. Asterisk indicates statistical significance between the two groups.
6.4. Morphology of skull and dental arches
Postmortem skull measurements further revealed that, compared to sham animals, skeletal sizes and dental arches were significantly smaller in reduction animals in the following variables (Fig. 4 and Table 4): mandibular ramus height (#1-2), anterior mandibular length (#4-5), the maxillary palatal length (#18-19), the posterior midfacial width (#11-11), the mandibular symphysis length (#4-21), the dorsal width of anterior mandible (#5-5), and the mandibular dental arch at canine (#25-25′). While these seven variables involved all three dimensions of craniofacial skeletons (height, width and length), five of them were related to the mandible alone (Fig. 4).
Table 4.
Comparisons of measurements from skeletons and dental arches
| Sham |
Reduction |
Sham |
Reduction |
Sham |
Reduction |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LA | Mean | SD | Mean | SD | P-P | Mean | SD | Mean | SD | O-M | Mean | SD | Mean | SD |
| 1-2 | 69.70 | 2.80 * | 65.80 | 1.35 | 13-14 | 55.04 | 7.86 | 51.15 | 9.41 | 4-21 | 35.81 | 1.65 * | 33.43 | 1.70 |
| 1-4 | 131.11 | 5.39 | 129.32 | 2.03 | 14-15 | 49.45 | 7.63 | 50.07 | 8.31 | 1-1′ | 77.93 | 2.16 | 77.08 | 3.26 |
| 2-3 | 73.73 | 3.83 | 74.30 | 2.64 | 15-16 | 26.73 | 1.46 | 26.88 | 2.68 | 2-2′ | 82.93 | 6.43 | 80.90 | 4.93 |
| 3-5 | 28.47 | 1.33 | 27.75 | 2.66 | 17-18 | 21.91 | 1.24 | 20.56 | 1.23 | 3-3′ | 17.03 | 2.06 | 16.30 | 1.67 |
| 4-5 | 33.91 | 1.20 * | 31.66 | 1.28 | 18-19 | 43.39 | 2.86 * | 40.33 | 2.45 | 5-5′ | 32.66 | 2.39 * | 30.10 | 1.10 |
| 6-7 | 38.44 | 0.61 | 39.04 | 2.59 | 19-20 | 22.68 | 1.55 | 22.70 | 2.61 | 25-25′ | 32.69 | 2.78 * | 30.14 | 0.99 |
| 7-10 | 40.73 | 2.62 | 40.43 | 2.07 | 6-6′ | 31.09 | 1.92 | 30.51 | 0.74 | 26-26′ | 34.53 | 2.83 | 31.57 | 2.65 |
| 8-9 | 12.77 | 1.47 | 12.49 | 1.29 | 7-7′ | 45.40 | 0.40 | 44.26 | 2.02 | 27-27′ | 37.38 | 1.66 | 36.73 | 1.78 |
| 11-12 | 8.92 | 1.46 | 8.40 | 0.24 | 8-8′ | 24.61 | 1.07 | 23.53 | 0.53 | |||||
| 9-9′ | 23.76 | 1.45 | 22.97 | 0.98 | ||||||||||
| 10-10′ | 36.03 | 1.40 | 36.92 | 1.27 | ||||||||||
| 11-11′ | 70.14 | 1.62 * | 66.27 | 0.54 | ||||||||||
| 12-12′ | 57.03 | 2.74 | 54.34 | 2.36 | ||||||||||
| 22-22′ | 34.13 | 2.66 | 32.93 | 3.19 | ||||||||||
| 23-23′ | 32.22 | 1.18 | 33.79 | 1.57 | ||||||||||
| 24-24′ | 37.50 | 0.77 | 36.40 | 1.23 | ||||||||||
Note: LA: lateral; P-P: parietal-palatal; O-M: occlusal-mandible; S.D.: standard deviation. Bolded/italic variables and asterisks indicate statistical significance between the two groups (p < 0.05). Refer to the legend of Fig. 2 for the abbreviations of variables.
7. Discussion
As a large muscular organ, the tongue fills the majority of the oral cavity in most mammals. The argument for tongue volume reduction is that decreasing oral cavity volume through skeletal correction, such as mandibular set-back or maxillary Le Fort osteotomy, encroaches on the space for the tongue, thus causing relapse of the prognathism or forward positioning of the teeth.1 Clinically, this type of relative macroglossia (insufficient space in the oral cavity) could be responsible for speech disorders, obstructive sleeping apnoea syndrome, and dysphagia.35 Even though the diagnostic criteria for relative macroglossia and the deciding factors for tongue volume reduction are highly subjective and its application for different types of macroglossia30,36-38 are debated, tongue volume reduction is a relatively common part of treatment for Class III skeletal malocclusion, severe open bite and bimaxillary dentoalveolar protrusion.19,39
It has been generally accepted that volume increase in soft tissue induces osteogenesis at the growth site of the bone.40 It has been claimed that the tongue growth and size influence midfacial control mechanism, which determines the growth of surrounding orofacial elements.41 Studies also demonstrate that tongue volume has a measurable effect on jaw growth at certain time points.27,42 Therefore, reducing tongue volume during the period of fast growth should lead to the serial alterations in craniofacial skeletal growth and dental arch formation. Available data on growth consequences following tongue volume reduction is extremely limited. Only a Germany team reported that partial glossectomy in young minipigs significantly reduced mandibular growth in length including overall and tooth-bearing portion, and in width of the region of the first deciduous molar and canine, but mandibular vertical growth was less affected.26,28,29 These negative effects on the growth were identified in 12-week-old but not in 6-week-old minipigs 23 weeks after partial glossoectomy.27 Unfortunately, these findings were determined one time at the end of experiment entirely from osteometric measurements on harvested mandibles. Longitudinal cephalometry and other changes in craniofacial structures were not obtained and assessed.
The pig grows very rapidly in a relatively short period of time, and the age of 12 weeks fits in this rapid growing period when the first permanent molar is erupting.43,44 It has been demonstrated that, during rapid growth period, the pig’s mandible increases in total length at the posterior and anterior borders, in ramus height at the condyle and inferior border, and in body at the alveolar and inferior borders.43 Interestingly, although the overall increases in linear distances and angular alterations over time were considerably similar between the two groups, a volume-reduced tongue could slow down the overall lengthening of the premaxilla and mandibular symphysis region, the two osseous components composing of anterior mouth. Increasing in the gonial angle (Sm-Gp/Me-Gi, Table 3) over time in reduction animals as compared to decreasing in sham animals further suggested that a flatten gonial angle could be induced by a volume-reduced tongue. In addition to these findings, the present study also demonstrated that a volume-reduced tongue could lead to dental arch narrowing, typically in anterior dentition (Mxc-Mxc’ and Md1-Md1′, Fig. 4 and Table 3). Furthermore, a significantly decreased premaxillary angle (Pr-E-Sp) in reduction animals might also attribute to the decrease of premaxillary length as discussed above.
The osteometric measures at the ending-time point (T4) are consistent with the cephalometric results in the widths of anterior mandible and dental arches (#5-5′ and #25-25′), and the length of anterior mandible (#4-5 and #4-21). Thus, narrowing and shortening in the anterior mandible and dental arch after reduction surgery were confirmed by both measures. However, significant decreases in the height of mandibular ramus (#1-2), the length of maxillary palatal process (#18-19), and the breadth of midface (#11-11′) in reduction animals were not identified in cephalometric measures. These inconsistencies might be due to the following reasons. First, because the landmark for condyle could not be defined, the measure for the ramus height was not available in cephalometrics. Given the fact that the condyle produces 80% of total ramus height,43 this significant difference between the two groups in osteometric measures may suggest that a volume-reduced tongue has an negative effect on lengthening, and vertical growth of mandible. Second, premaxilla and the palatal process of maxilla are active growth/remodelling fronts during midfacial development,45 thus both skeletal components should be affected by a volume-reduced tongue during growth. However, significant changes were only found in cephalometrics for the premaxilla (Pmx-Mxp) and in osteometrics for the palatal process of maxilla (#17-18). It should be noted that despite not significant, measurements of premaxilla and maxilla showed smaller values in reduction than sham animals in both cephalometrics and osteometrics (Tables 3 and 4). Considering a small sample size of the present study, these differences may reflect the true trend of negative effects of a volume-reduced tongue on the premaxilla/maxilla complex. Third, while measurement of the midfacial breadth was not available in cephalometrics as measured in osteometrics (#11-11″), cephalometric measurements did demonstrate significant increases of bi-zygomatic (Zy-Zy’) and bigonial (Gp-Gp’) over time in both groups. Again, although not significant, smaller values of these measures were seen in reduction animals as compared to sham animals at the 4-week time point. Therefore, a volume-reduced tongue may also have a negative effect on the transverse expansion of the midface during growth.
Interestingly, although the above-mentioned growth effects by a volume-reduced tongue cover all three dimensions (width, height and length) in both the mandible and facial bones, the majority of these effects occurred around the anterior mouth or anterior dental arch, particularly in the mandibular symphysis and premaxilla (Figs. 3 and 4). The volume reduction was performed on the anterior 2/3 of the tongue in the present study. During function, this portion of the tongue is thought to produce greater forces than does the tongue base.46 Our previous study of in vivo functional loads of the tongue on surrounding skeletal surfaces revealed that the tongue produces more load in mandibular lingual surfaces than the premaxillary and maxillary palatal surfaces. These loads decrease in the anterior mouth (symphysis and premaxilla) after the volume reduction, whereas loads in the posterior mouth (mandibular corpus and posterior maxillary palatal surface) were less affected.32 Therefore, the observed slow growth in the skeletal components of the anterior mouth might be related to the change in the local mechanical environment produced by a volume-reduced tongue, i.e., decrease of functional loads.
The present study not only reveals that the skeletal components of anterior mouth are mostly involved by a volume-reduced tongue, but also demonstrates that the mandible was affected more than the nasomaxillary skeletons in all length, width and height. This striking difference between upper and lower jaws was also confirmed by DEXA examinations in which the only significant decrease in bone mineral content was found in the mandibular symphysis bloc of reduction animals (Fig. 7). Anatomically, the tongue is directly attached to the mandible through its musculature. Functionally, there is an inherent linkage between the tongue and mandible.47 Furthermore, the mechanism of cranial/nasomaxillary postnatal growth is mostly attributed to sutures, distinctively different from that of mandible which mainly depends on appositional deposition through intra-membranous ossification at the borders and alveolar ridges, and on secondary cartilage through endochondrous ossification at the condyle.48,49 Based on these, it is not surprising that the postnatal mandibular growth would be suppressed more than the other portion of the craniofacial skeletons by tongue volume reduction.
Taking the present results and previous loading study32 together, on one hand it is suggested that the tongue volume reduction could lessen the chance of relapse after the surgical mandibular setback for correcting mandibular prognathism through the mechanism of reducing the functional loads, on the other hand it should be cautious that this procedure may slow the growth of craniofacial skeletons, both in size and mineralization, particularly in anterior mandible (symphysis region). Therefore, a long-term growth tracking is necessary for young subjects receiving this procedure alone for a true macroglossia or adjunctively along with other orthopaedic procedures (mandible setback, Le Fort osteotomy, etc.) for a relative macroglossia.
Acknowledgements
The authors would like to thank Dr. Sue Herring for her critical comments and discussion, Ms. Xian-Qin Bai for her assistance with all animal experiments and recordings, and Liz Thomas for statistical analysis. This study was supported by the grant R01DE15659 from NIDCR to ZJL.
REFERENCES
- 1.Ingervall B, Schmoker R. Effect of surgical reduction of the tongue on oral stereognosis, oral motor ability, and the rest position of the tongue and mandible. Am J Orthod Dentofacial Orthop. 1990;97(1):58–65. doi: 10.1016/S0889-5406(05)81710-X. [DOI] [PubMed] [Google Scholar]
- 2.Frohlich K, Ingervall B, Schmoker R. Influence of surgical tongue reduction on pressure from the tongue on the teeth. Angle Orthod. 1993;63(3):191–8. doi: 10.1043/0003-3219(1993)063<0191:IOSTRO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Frohlich K, Thuer U, Ingervall B. Pressure from the tongue on the teeth in young adults. Angle Orthod. 1991;61(1):17–24. doi: 10.1043/0003-3219(1991)061<0017:PFTTOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C. Human variation in body mass: evidence for a role of the genes. Nutr Rev. 1997;55(1 Pt 2):S21–7. doi: 10.1111/j.1753-4887.1997.tb06094.x. discussion S27-S30. [DOI] [PubMed] [Google Scholar]
- 5.Bandy HE, Hunter WS. Tongue volume and the mandibular dentition. Am J Orthod. 1969;56(2):134–42. doi: 10.1016/0002-9416(69)90230-9. [DOI] [PubMed] [Google Scholar]
- 6.Oliver RG, Evans SP. Tongue size, oral cavity size and speech. Angle Orthod. 1986;56(3):234–43. doi: 10.1043/0003-3219(1986)056<0234:TSOCSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Lauder R, Muhl ZF. Estimation of tongue volume from magnetic resonance imaging. Angle Orthod. 1991;61(3):175–84. doi: 10.1043/0003-3219(1991)061<0175:EOTVFM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Yoo E, Murakami S, Takada K, Fuchihata H, Sakuda M. Tongue volume in human female adults with mandibular prognathism. J Dent Res. 1996;75(12):1957–62. doi: 10.1177/00220345960750120701. [DOI] [PubMed] [Google Scholar]
- 9.Vogel JE, Mulliken JB, Kaban LB. Macroglossia: a review of the condition and a new classification. Plast Reconstr Surg. 1986;78(6):715–23. [PubMed] [Google Scholar]
- 10.Ueyama Y, Mano T, Nishiyama A, Tsukamoto G, Shintani S, Matsumura T. Effects of surgical reduction of the tongue. Br J Oral Maxillofac Surg. 1999;37(6):490–5. doi: 10.1054/bjom.1999.0196. [DOI] [PubMed] [Google Scholar]
- 11.Lowe AA, Takada K, Yamagata Y, Sakuda M. Dentoskeletal and tongue soft-tissue correlates: a cephalometric analysis of rest position. Am J Orthod. 1985;88(4):333–41. doi: 10.1016/0002-9416(85)90133-2. [DOI] [PubMed] [Google Scholar]
- 12.Cohen AM, Vig PS. A serial growth study of the tongue and intermaxillary space. Angle Orthod. 1976;46(4):332–7. doi: 10.1043/0003-3219(1976)046<0332:ASGSOT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Vig PS, Cohen AM. The size of the tongue and the intermaxillary space. Angle Orthod. 1974;44(1):25–8. doi: 10.1043/0003-3219(1974)044<0025:TSOTTA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Turner S, Nattrass C, Sandy JR. The role of soft tissues in the aetiology of malocclusion. Dent Update. 1997;24(5):209–14. [PubMed] [Google Scholar]
- 15.Vig PS, Cohen AM. The size of the human tongue shadow in different mandibular postures. Br J Orthod. 1974;1(2):41–3. doi: 10.1179/bjo.1.2.41. [DOI] [PubMed] [Google Scholar]
- 16.Tamari K, Shimizu K, Ichinose M, Nakata S, Takahama Y. Relationship between tongue volume and lower dental arch sizes. Am J Orthod Dentofacial Orthop. 1991;100(5):453–8. doi: 10.1016/0889-5406(91)70085-B. [DOI] [PubMed] [Google Scholar]
- 17.Enlow DH. Handbook of facial growth. 2nd edition WB Saunders Co.; Philadelphia: 1982. [Google Scholar]
- 18.Doual-Bisser A, Doual JM, Crocquet M. Hyoglossal balance and vertical morphogenesis of the face. Orthod Fr. 1989;60(Pt 2):527–32. [PubMed] [Google Scholar]
- 19.Ruff RM. Orthodontic treatment and tongue surgery in a class III open-bite malocclusion. A case report. Angle Orthod. 1985;55(2):155–66. doi: 10.1043/0003-3219(1985)055<0155:OTATSI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Harvold EP. The role of function in the etiology and treatment of malocclusion. Am J Orthod. 1968;54(12):883–98. doi: 10.1016/0002-9416(68)90241-8. [DOI] [PubMed] [Google Scholar]
- 21.Harvold EP, Vargervik K, Chierici G. Primate experiments on oral sensation and dental malocclusions. Am J Orthod. 1973;63(5):494–508. doi: 10.1016/0002-9416(73)90162-0. [DOI] [PubMed] [Google Scholar]
- 22.Principato JJ. Upper airway obstruction and craniofacial morphology. Otolaryngol Head Neck Surg. 1991;104(6):881–90. doi: 10.1177/019459989110400621. [DOI] [PubMed] [Google Scholar]
- 23.Harvold EP, Chierici G, Vargervik K. Experiments on the development of dental malocclusions. Am J Orthod. 1972;61(1):38–44. doi: 10.1016/0002-9416(72)90174-1. [DOI] [PubMed] [Google Scholar]
- 24.Harvold EP. Altering craniofacial growth: force application and neuromuscular-bone interaction. In: McNamara JM, Ribbens KA, Howe RP, editors. Clinical alteration of the growing face. Center for Human Growth and Development, The University of Michigan; Ann Arbor: 1983. pp. 35–45. [Google Scholar]
- 25.Harvold EP, Tomer BS, Vargervik K, Chierici G. Primate experiments on oral respiration. Am J Orthod. 1981;79(4):359–72. doi: 10.1016/0002-9416(81)90379-1. [DOI] [PubMed] [Google Scholar]
- 26.Schumacher GH, Becker R, Hubner A, Pommerenke F. The tongue as a factor in craniofacial growth. 1. Modification of the linear dimensions of the lower jaw. Anat Anz. 1988;166(15):309–15. [PubMed] [Google Scholar]
- 27.Pommerenke F, Schumacher GH, Becker R, Hubner A. The tongue as a factor in craniofacial growth. 4. Results of animal experiments. Anat Anz. 1988;167(4):281–7. [PubMed] [Google Scholar]
- 28.Hubner A, Pommerenke F, Schumacher GH, Becker R. The tongue as a factor in craniofacial growth. 3. The influence of the height and angle of the lower jaw. Anat Anz. 1988;167(3):191–7. [PubMed] [Google Scholar]
- 29.Becker R, Hubner A, Pommerenke F, Schumacher GH. The tongue as a factor in craniofacial growth. 2. The influence of the width dimension of the lower jaw. Anat Anz. 1988;167(2):81–6. [PubMed] [Google Scholar]
- 30.Davalbhakta A, Lamberty BG. Technique for uniform reduction of macroglossia. Br J Plast Surg. 2000;53(4):294–7. doi: 10.1054/bjps.1999.3311. [DOI] [PubMed] [Google Scholar]
- 31.Shcherbatyy V, Perkins JA, Liu ZJ. Functional deformations of the tongue following volume reduction. Anat Rec. doi: 10.1002/ar.20699. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu ZJ, Shcherbatyy V, Perkins JA. Functional loads of the tongue and consequence of the volume reduction. J Oral Maxillofac Surg. doi: 10.1016/j.joms.2007.11.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahlberg G. Statistical method for medical and biological students. Interscience Publications; New York: 1940. pp. 37–40. [Google Scholar]
- 34.Mitchell AD, Scholz AM, Pursel VG. Total body and regional measurements of bone mineral content and bone mineral density in pigs by dual energy X-ray absorptiometry. J Anim Sci. 2001;79(10):2594–604. doi: 10.2527/2001.79102594x. [DOI] [PubMed] [Google Scholar]
- 35.Bell WH, Proffit WR, White RP. Adjunctive surgical procedures: reduction of the tongue. In: Bell WH, Proffit WR, White RP, editors. Surgical correction of dentofacial deformities. Saunders; Philadelphia: 1980. p. 1113. [Google Scholar]
- 36.Herren P, Muller-Boschung P, Stutz G. Macroglossia and partial resection of the tongue out of orthodontic indication. Proc Finn Dent Soc. 1981;77(13):45–55. [PubMed] [Google Scholar]
- 37.Wolford LM, Cottrell DA. Diagnosis of macroglossia and indications for reduction glossectomy. Am J Orthod Dentofacial Orthop. 1996;110(2):170–7. doi: 10.1016/s0889-5406(96)70105-1. [DOI] [PubMed] [Google Scholar]
- 38.Gasparini G, Saltarel A, Carboni A, Maggiulli F, Becelli R. Surgical management of macroglossia: discussion of 7 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(5):566–71. doi: 10.1067/moe.2002.127583. [DOI] [PubMed] [Google Scholar]
- 39.Deguchi T. Case report: three typical cases of glossectomy. Angle Orthod. 1993;63(3):199–207. doi: 10.1043/0003-3219(1993)063<0199:CRTTCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Fränkel F, Fränkel C. Orofacial orthopedics with the functional regulator. Karger; Basel: 1989. [Google Scholar]
- 41.Moss ML. Neurotrophic processes in orofacial growth. J Dent Res. 1971;50(6):1492–4. doi: 10.1177/00220345710500062301. [DOI] [PubMed] [Google Scholar]
- 42.Schumacher G-H. Principals of skeletal growth. In: Dixon AD, Hoyte DAN, Ronning O, editors. Fundamentals of craniofacial growth. CRC Press LLC; Boca Raton, New York: 1997. pp. 1–21. [Google Scholar]
- 43.Robinson IB, Sarnat BG. Growth pattern of the pig mandible. A serial roentgenographic study using metallic implants. Am J Anat. 1955;96:37–64. doi: 10.1002/aja.1000960103. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Zhang G, Herring SW. Age changes in mastication in the pig. Comp Biochem Physiol Comp Physiol. 1994;107(4):647–54. doi: 10.1016/0300-9629(94)90364-6. [DOI] [PubMed] [Google Scholar]
- 45.Barteczko K, Jacob M. A re-evaluation of the premaxillary bone in humans. Anat Embryol (Berl) 2004;207(6):417–37. doi: 10.1007/s00429-003-0366-x. [DOI] [PubMed] [Google Scholar]
- 46.Pouderoux P, Kahrilas PJ. Deglutitive tongue force modulation by volition, volume, and viscosity in humans. Gastroenterology. 1995;108(5):1418–26. doi: 10.1016/0016-5085(95)90690-8. [DOI] [PubMed] [Google Scholar]
- 47.Palmer JB, Hiiemae KM, Liu J. Tongue-jaw linkages in human feeding: a preliminary videofluorographic study. Arch Oral Biol. 1997;42(6):429–41. doi: 10.1016/s0003-9969(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 48.Sarnat BG. Postnatal growth of the nasomaxillary complex. In: Dixon AD, Hoyte DAN, Ronning O, editors. Fundamentals of craniofacial growth. CRC Press LLC; Boca Raton, New York: 1997. pp. 205–24. [Google Scholar]
- 49.Kantomaa T, Ronning O. Growth mechanism of the mandible. In: Dixon AD, Hoyte DAN, Ronning O, editors. Fundamentals of craniofacial growth. CRC Press LLC; Boca Raton, New York: 1997. pp. 204–89. [Google Scholar]