Abstract
The effects of ethanol at physiological concentrations on neutrophil membrane tether pulling, adhesion lifetime, rolling, and firm arrest behavior were studied in parallel-plate flow chamber assays with adherent 1-micron diameter P-selectin-coated beads, P-selectin-coated surfaces, or interleukin-1 stimulated human endothelium. Ethanol (0.3% by vol.) had no effect on PSGL-1, CD62L, or CD11b levels, but caused PSGL-1 redistribution. Also, ethanol prevented fMLP-induced CD11b upregulation. During neutrophil collisions with P-selectin-coated beads at venous wall shear rates of 25 to 100 s-1, ethanol increased membrane tether length and membrane growth rate by 2- to 3-fold, but reduced the adhesion efficiency (detectable bonding/total collisions) by 2- to 3-fold, compared to untreated neutrophils. Without ethanol treatment, adhesion efficiency and adhesion lifetime declined as wall shear rate was increased, while ethanol caused the adhesion lifetime over all events to increase from 0.1 sec to 0.5 sec as wall shear rate was increased, an example of pharmacologically induced “hydrodynamic thresholding.” Consistent with this increased membrane fluidity and reduced capture, ethanol reduced rolling velocity by 37 % and rolling flux by 55 % on P-selectin surfaces at 100 s-1, compared to untreated neutrophils. On IL-1 stimulated endothelium, rolling velocity was unchanged by ethanol treatment, but the fraction of cells converting to firm arrest was reduced from 35 % to 24 % with ethanol. Overall, ethanol caused competing biophysical and biochemical effects that: (i) reduced capture due to PSGL-1 redistribution, (ii) reduced rolling velocity due to increased membrane tether growth, and (iii) reduced conversion to firm arrest.
Keywords: neutrophils, adhesion molecules, cell surface molecules, inflammation
Introduction
Selectins facilitate the recruitment of neutrophils to sites of inflammation. L-selectin is constitutively expressed on the tips of neutrophil microvilli and binds cutaneous lymphocyte antigen (CLA) on the endothelial cell surface. P-selectin glycoprotein ligand-1 (PSGL-1) is also presented on microvilli and can bind L- or P-selectin. P-selectin is stored in the membranes of platelet α-granules and endothelial cell Weibel-Palade bodies and becomes rapidly mobilized to the plasma membrane of these cells in response to various stimuli (1). PSGL-1 presents the tetrasaccharide sialyl Lewisx (sLex) that is recognized by the lectin domain of selectins. The binding and unbinding of selectins and sLex allows for the capture of flowing neutrophils and subsequent stable rolling on P-selectin (2, 3). In response to stimuli, PSGL-1 is redistributed from microvilli tips to the uropod (4). PSGL-1 redistribution is considered a transition state from adhesion via P-selectin/PSGL-1 to integrin-mediated adhesion (5), leading to firm arrest and transendothelial migration.
Under flow conditions and hemodynamic force loading, neutrophil tether formation and whole cell deformation during rolling can alter force transmission and selectin bond dynamics (6, 7) after the initial capture event. Membrane tethers are pulled when a force is exerted at a point on the cell surface and play an important role in stabilizing rolling interactions between neutrophils and the endothelium or activated platelets. During membrane tether growth, the lipid bilayer is pulled from the microvilli to form ∼100 to 200 nm diameter membrane tubes, presumably devoid of cytoskeleton. Because lipid membrane can only expand in area by about 4% or less (8), the lipid for the tether must come from the cell body. The total surface area of the tether plus the abundantly excess lipid bilayer of the neutrophil remains constant. Prior work has shown direct visualization of membrane tethers pulled during neutrophil adhesion to P-selectin (7). Increases and decreases in tether growth rate resulted in increases and decreases in P-selectin/PSGL1 lifetime, respectively (9).
Since membrane fluidity may control membrane tether growth rate and adhesion dynamics, the effect of ethanol at physiological levels may have effects on neutrophil function. Epidemiologic studies indicate that moderate consumptions of alcohol (1–2 alcohol beverages, about 30 g ethanol per day) may decrease incidence of cardiovascular disease (10). A 12-year study of over 38,000 healthy males found that small amounts of alcohol consumed 3-4 days per week significantly reduced risk of myocardial infarction, regardless of the type of alcohol consumed (11). For reference, a BAC (Blood Alcohol Content) of 0.20 % by volume (i.e. 2 mL ethanol per L, corresponding to 1.58 mg/mL or 0.034 M) represents serious intoxication and 0.45% represents potentially fatal alcohol poisoning (one drink corresponds to BAC ≈ 0.03% and the legal driving limit in most states is 0.08%). The ethanol clearance rate in the body is about 0.015-0.025% per hour (12). It remains to be elucidated how ethanol affects human neutrophil mechanics, particularly at relevant concentrations achieved in humans by alcohol consumption.
Many studies have demonstrated profound anti-platelet effects of ethanol. Owens et al. have shown that ethanol inhibited platelet accumulation on collagen-coated glass at concentrations as low as 0.02% by vol, and 0.2% ethanol inhibited mural thrombus formation (13). McKenzie et al. found that 25 mmol/L ethanol (0.14% by vol) significantly reduced P-selectin levels on platelets (14). In contrast to platelets, fewer studies address effects of ethanol on neutrophils. Some have found that acute ethanol exposure inhibits neutrophil chemotaxis (15-17) and attenuates expression of surface adhesion molecules (18-20), while other studies failed to show these effects under similar concentrations of exposure (21, 22). Ethanol has been shown to block leukocyte recruitment and endothelial cell activation both in vivo and in vitro (23, 24), yet others have observed that ethanol enhances leukocyte-endothelial cell interactions (25). From these studies, ethanol may have competing effects on neutrophil function and specific assay conditions may impact conclusions about whether ethanol has no effect, attenuating effect, or potentiating effect on inflammatory processes.
At unphysiological ethanol levels, Patel M et al. (23) found that ethanol inhibits fMLP-induced CD11b upregulation and neutrophil elongation at 500 mg/dl (0.62% by vol). Nilsson E et al. (20) also reported that fMLP- or PMA-stimulated up-regulation of CD18 expression was reduced by 1.0% ethanol. Others (19) found that ethanol at concentrations higher than 500 mg/dL (0.62 % by vol) inhibits CD18 expression in fMLP-induced neutrophils in a dose-dependent manner, although serum-free neutrophil suspensions showed normal resting adherence to endothelial monolayers even in very high ethanol concentrations (1000 mg/dL, or 1.2 % by vol). They found that ethanol (0.3-1.3%) inhibits stimulated neutrophil adhesion to endothelial cells under no flow conditions, which was attributed to changes in neutrophils since no change in endothelial cells were detected.
Ethanol-induced changes in membrane fluidity have been previously observed using electronic paramagnetic resonance spectroscopy and fluorescence spectroscopy, although the effect of ethanol may be underestimated because of its nonuniform distribution and partitioning into the core of the lipid bilayer (26). Due in part to this partitioning, ethanol affects cholesterol homeostasis in biological membranes and can modify membrane fluidity in a nonhomogeneous or asymmetric fashion (27). Fourier transform infrared, fluorescence, and spectroscopic as well as computational chemistry models have established that ethanol favors interaction or binding at the lipid-water interface, in the heterogeneous region between the phosphate and carbonyl groups near the lipid glycerol backbone and upper methylene segments of the lipid hydrocarbon chains (28). While ethanol interacts with membrane bilayers primarily via hydrophilic interactions, it can also penetrate into the region of upper chain segments, facilitated by hydrogen bonding to lipid phosphate groups and carbonyls as well as weak hydrophobic van der Waals attraction between the short ethyl group and upper chain segments (29). Using micropipette aspiration on unilamellar vesicles, Ly et al. (30) have shown that area compressibility moduli, bending moduli, lysis tensions, and lysis area strains all decreased with ethanol, and the area/molecule of lipids increased while the thickness of the membrane decreased. They confirmed the lateral expansion by flow experiments as well, although the ethanol concentrations used in the study (5-20% by vol) were not in a physiological regime.
Since the effects of ethanol on neutrophil membrane fluidity, membrane-cytoskeleton function, and the adhesion strength are poorly studied, we employed the bead-collision assay to examine neutrophil tether mechanics (31) during PSGL-1/P-selectin bonding. Anti-PSGL-1, calcium-free buffer, or P-selectin-free beads all result in zero detectable adhesion events in this assay, confirming that interactions between the selectin and ligand of interest can be singled out. The assay allowed simultaneous determination of adhesion life time dynamics and membrane dynamics under flow conditions using real-time high resolution DIC videomicroscopy in the presence of ethanol.
Material and Methods
Materials and cell culture
Human serum albumin (HSA; Golden West Biologicals, Temecula, CA) and Hank's Balanced Salt Solution (HBSS; Invitrogen) without phenol red and calcium chloride were stored according to manufacturers' instructions. Polystyrene 1.05-μm-diamter microspheres coated with Protein A (Bangs Labs) were labeled with recombinant human CD62P/Fc chimera P-selectin with IgG1 Fc region (R&D Systems). N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP; Calbiochem) and Interleukin-1 (IL-1; BioLegend) were used to activate neutrophils and endothelial cells, respectively.
Human aortic endothelial cells (HAECs) were cultured (passage<10) in EGM-2 medium (Lonza), supplemented with hydrocortisone, hEGF, FBS, VEGF, hFGF-B, R3-IGF-1, ascorbic acid, heparin and gentamicin/ amphotericin-B according to manufacturer's instructions (final serum concentration: 2%). Glass slides (38 × 75-mm) were coated with type 1 collagen (BD Biosciences) at 50 μg/mL in 0.02 N acetic acid. For flow chamber experiments, cells were seeded on at a density of 106 cells per slide and cultured to confluency.
Isolation of neutrophils
Human blood samples were obtained via venipuncture from healthy adult donors who had not taken any medications or consumed any alcohol in the prior 72 hours. Neutrophils were isolated by centrifugation with Lympholyte®-poly separation medium (Cedarlane Laboratories) as previously described (31). This procedure was performed in accordance with a protocol approved by the Institutional Review Board. Neutrophils were counted and diluted with a 2% solution of human serum albumin (HSA) in Hank's Balanced Salt Solution (HBSS) with Ca2+ to a final concentration of 1–2 × 106 cells/mL.
Neutrophils were incubated with physiological concentrations of ethanol (0.1-0.5 vol%) and perfused in neutrophil-bead collision assay chambers or over HAEC-coated glass slides as follows.
Neutrophil-bead collision assay
To measure membrane tether formation dynamics and bond mechanics, the neutrophil-bead collision assay was used to probe interactions between neutrophil under flow and a small point source of ligand. Protein A-coated microspheres were labeled with P-selectin as previously described (32). The P-selectin-coated beads were washed and incubated for attachment to glass capillary flow chambers to a final concentration of 5400 P-selectin/μm2. Bead-coated flow chamber surfaces were washed and blocked with HBSS with 2% HSA before perfusion studies. Neutrophils were perfused into the chambers using a syringe pump at various wall shear rates. To measure any direct effect of ethanol on the protein A beads coated with P-selectin-IgG, beads were exposed to 0.3% ethanol for 30 min following P-selectin labeling and washed before untreated neutrophils were perfused over them. In these fully matched experiments, neutrophils from a single donor and a single donor session were run side-by-side against beads that were unmodified or pretreated with 0.3 % ethanol so that the only variable in the control study was ethanol pretreatment.
P-selectin-coated surface perfusion assay
To prepare P-selectin–coated surfaces, microcapillary flow chambers were incubated with affinity-purified human platelet P-selectin (∼1 μg/ml final concentration) in Ca2+- and Mg2+-free HBSS for at least 3 h at room temperature. Excess or unbound protein were removed by washing the flow chamber by perfusing Ca2+- and Mg2+-free HBSS with 2% HSA through the chamber for 30 min. The final P-selectin site density was previously determined as ∼10 sites/μm2 (7) at these coating conditions.
Endothelial Cell Parallel Plate Flow Chamber
Confluent monolayers of HAECs on glass slides were exposed to steady laminar shear stress in parallel plate flow chambers attached to flow loops for media recirculation (15 mL) in a 37°C incubator as previously described (33). HAECs were exposed to 1 ng/mL IL-1 for 5 hours for activation under continuous flow conditions (34).
Shear rates for capillary neutrophil-bead assay and parallel-plate flow chamber
In both the glass capillary neutrophil-bead collision assay and the HAEC flow chambers, shear rates were determined by the Navier-Stokes equation for laminar flow of a Newtonian fluid: τw = 6Qμ/B2W. In this equation, Q represents the flow rate (cm3/s), μ is the viscosity (0.01 Poise at room temperature), B is the total plate separation of the rectangular flow chamber, and W represents the width of the chamber. The wall shear rate, γw (sec-1), can be calculated as γw = 6Q/B2W. A flow rate of 20 μL/min corresponds to a shear stress of 0.25 dyne/cm2 and a wall shear rate of 25 s-1. For the capillary assay, B=0.02 cm, W=0.2cm; for the HAEC flow chamber, B=0.025 cm and W=2.5 cm.
Imaging and video analysis
Flow chambers were imaged by Zeiss Axiovert 135 microscopy: for capillary flow chambers with P-selectin beads, differential interference contrast (DIC) microscopy was used with the 63X objective, while HAEC-coated flow chambers were imaged by phase contrast microscopy (20X Plan Apochromat). Data were recorded using a JVC Professional Series VCR Super VHS and Sony Trinitron connected to the CPU/microscope/image processor system (34). Images were captured at 28 frames per second (fps) and analyzed frame-by-frame using the JVC VHS and Adobe Premier Elements ® software. For high-speed imaging, images were captured using a Motion-Corder Analyzer high-speed digital camera (Eastman Kodak, Rochester, NY) at an imaging rate of 240 fps and analyzed frame-by-frame. ImageTool (UTHSCSA, Version 2.00) software was used to transfer images from the videotape onto the CPU. For HAEC flow chambers, each field of view (FOV, 0.1 mm2) of neutrophils flowing over HAEC was recorded in 10-sec video segments. Scion Image and ImageJ (NIH) software was used to analyze trajectories of neutrophils and membrane tether lengths. ParticleTracker and MultiTracker plugins in ImageJ were used to obtain rolling length, velocity, and firm arrest data.
The following definitions were employed for image analysis of neutrophils interacting with P-selectin beads (adapted from Edmondson et al. (31)). Adhesive interactions refer to collisions that have a visible pause in neutrophil motion lasting for at least one frame during frame-by-frame analysis, with velocities below the hydrodynamic velocity. Adhesive tether-forming neutrophils are neutrophils that translate in the direction of flow at a velocity below the hydrodynamic stream velocity, form a tether, and are rapidly released. The following parameters were obtained from the neutrophil-bead collision assay: adhesion efficiency (ε), which is the number of collisions resulting in an adhesive interaction divided by the total number of collisions observed; membrane tethering fraction (f), which refers to the ratio of tether-forming events to the total number of adhesive interactions; lifetimes are the duration of adhesive and tether-forming interactions; tether lengths are the distance from the center of the adhesive bead to the lagging edge of the neutrophil as measured by the ImageJ software. The mean tether growth velocity (vt) was calculated by dividing the tether length by the tether lifetime. For the neutrophil-HAEC assay on flow chambers, the following definitions were used: firmly arrested neutrophils refer to cells that remain motionless during time of observation in FOV (10 sec); rolling distance is the distance between the point of first adhesive contact between a neutrophil and the endothelial cells and the point at which the neutrophil stops rolling and remains stationary.
Flow Cytometry
Isolated neutrophils at 106 cells/mL in HBSS/Ca/Mg/HSA were incubated with FITC-anti-CD11b (Ancell), PE-anti-CD162 (BD Pharmingen), and AlexaFluor647-CD62L (BioLegend). Appropriate mouse isotype control for unspecific binding to neutrophils was carried out in parallel. For compensation control, BD CompBeads (BD Pharmingen) were used: BD CompBeads Anti-Mouse Ig-k for positive control, and BD CompBeads Negative Control (FBS) for negative control. Sample were incubated for 30 min at room temperature in the dark, washed and diluted in 1% formaldehyde solution in PBS, and immediately run on the cytometer (FACSCalibur, Becton-Dickinson). The acquisition process was stopped after 10,000 neutrophils were collected for each sample. Neutrophils were identified by their characteristic forward scatter and side scatter properties as previously described (35).
To obtain relative cellular sizes from FSC (forward scatter) distribution, FSC parameters were collected using linear amplification mode and FSC analysis was carried out using Cellquest® software FSC histogram overlays and histogram statistics. Mean FSC values were then obtained by using CELLQUEST histogram statistic tools. Cell sizes were verified with the Coulter Counter Z2 Particle Analyzer (Beckman Coulter, Fullerton, CA).
Immunofluorescence Confocal Microscopy
Isolated neutrophils (106 cells/mL, 0.5 ml) were incubated with buffer alone or with ethanol, and fixed with 4% paraformaldehyde at room temperature for 10 min. After washing with PBS, the cells were stained with PE-anti-CD162 (BD Pharmingen), washed with PBS, and allowed to adhere to poly-l-lysine-coated slide glass at 4°C for 45 min. The cells were again fixed with 4% formaldehyde at room temperature for 10 min, mixed with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing DAPI (4′,6-diamidino-2-phenylindole) followed by mounting coverslips. The samples were then sealed with nail polish and observed under a confocal laser scanning microscope (Olympus IX81, Plan Apochromat APO 60x/1.4). A z-series scan (collection of multiple images displaced along the z axis) of 0.5 μm step size was taken, yielding a stack of 20 images acquired from the top of the cell to the bottom. The intensity correlation between each slice and the next was analyzed using the ImageJ plugin (NIH), a modified method of intensity correlation analysis as previously described (56,57), in which the pixel intensity in each image is compared to the corresponding pixel of the next image in the sequence, and the PDM (product of the differences from the mean) for each pixel is calculated. The resulting intensity correlation coefficient, R, ranges between zero and 1 with zero being low-correlation, 1 being high. The average correlation coefficients for 5 FOV (fields of view) with 3-10 cells per view of untreated cells were compared to that of ethanol-treated cells.
Statistical Analysis
Statistical analysis of the data was performed using a standard two-sample Student's t-test assuming unequal variances of the two data sets. Statistical significance was determined using a two-tail distribution. For control experiments with ethanol treatment on beads only, the paired Student's t-test was used. A p value <0.05 was considered significant.
Results
Effect of ethanol on neutrophil adhesion to P-selectin coated beads
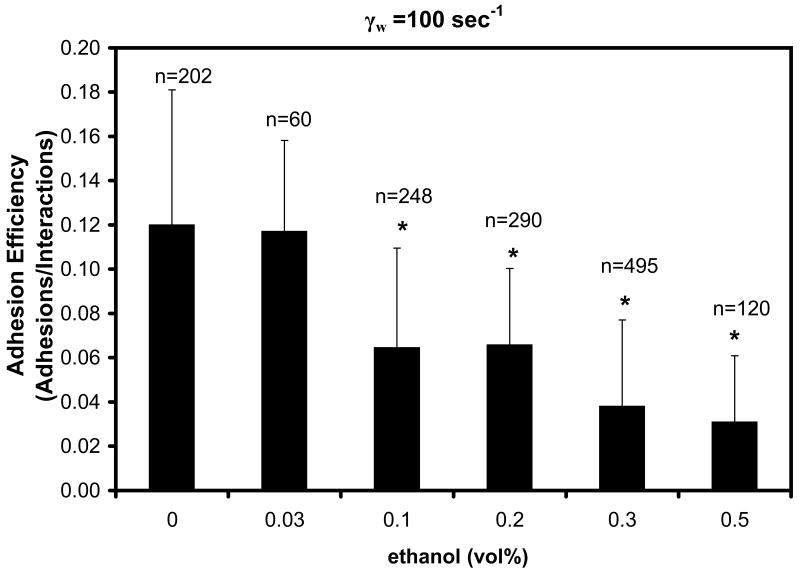
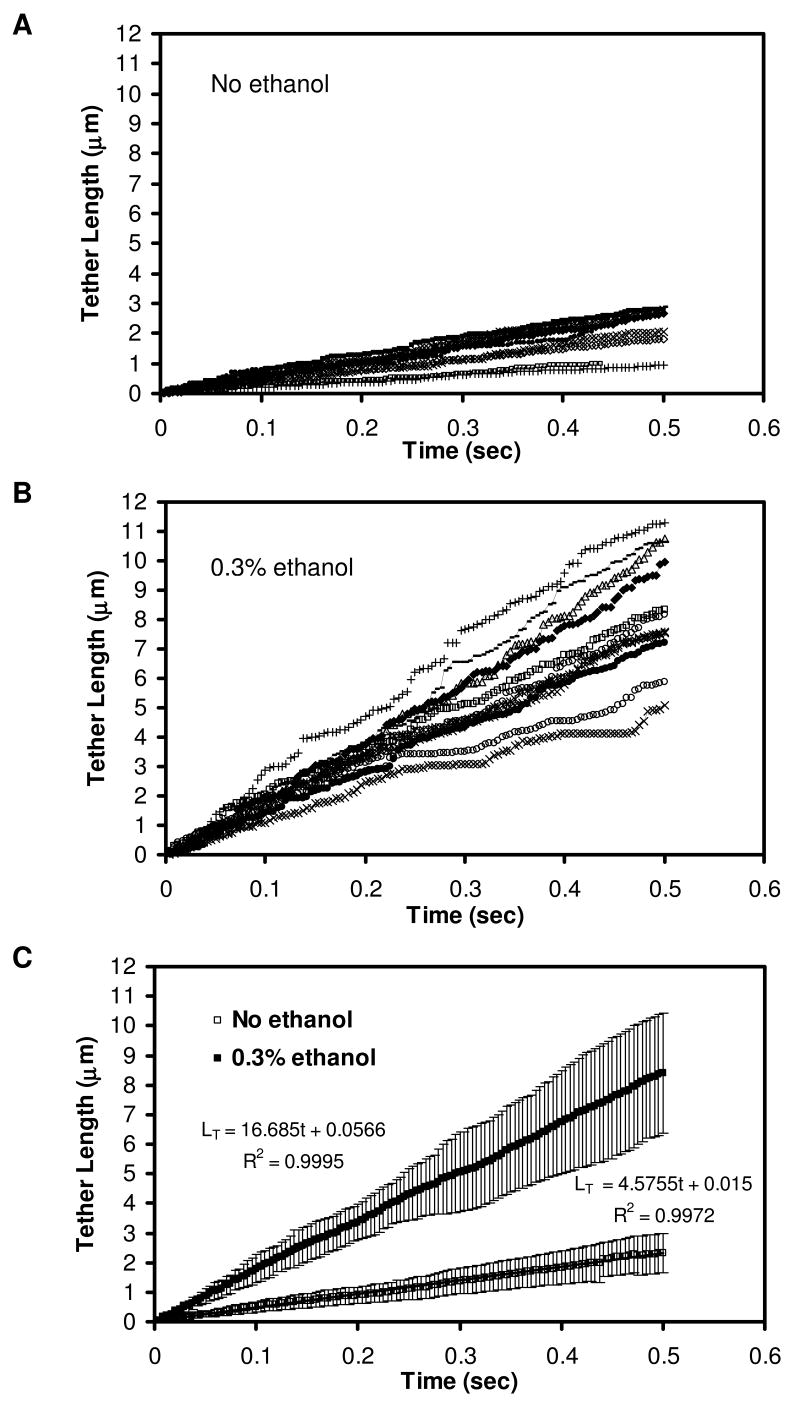
During collisions of flowing neutrophils with P-selectin coated beads, ethanol promoted the formation of longer membrane tethers compared to untreated controls (Fig. 1). However, ethanol also reduced the capture of flowing neutrophils. At a post-capillary venule wall shear rate of 100 sec-1, the adhesion efficiency (ε) displayed a dose-dependent reduction from ε = 0.12 ± 0.06 to 0.03 ± 0.03 (p < 0.001) as ethanol was increased from 0 to 0.3 % by vol (Fig. 2). In further studies conducted at 0.3 % ethanol, a reduction in the adhesion efficiency was observed at all wall shear rates tested between 25 and 125 sec-1 (Fig. 3A). At 125 sec-1, 0.3 % ethanol completely prevented successful adhesion with the P-selectin coated beads.
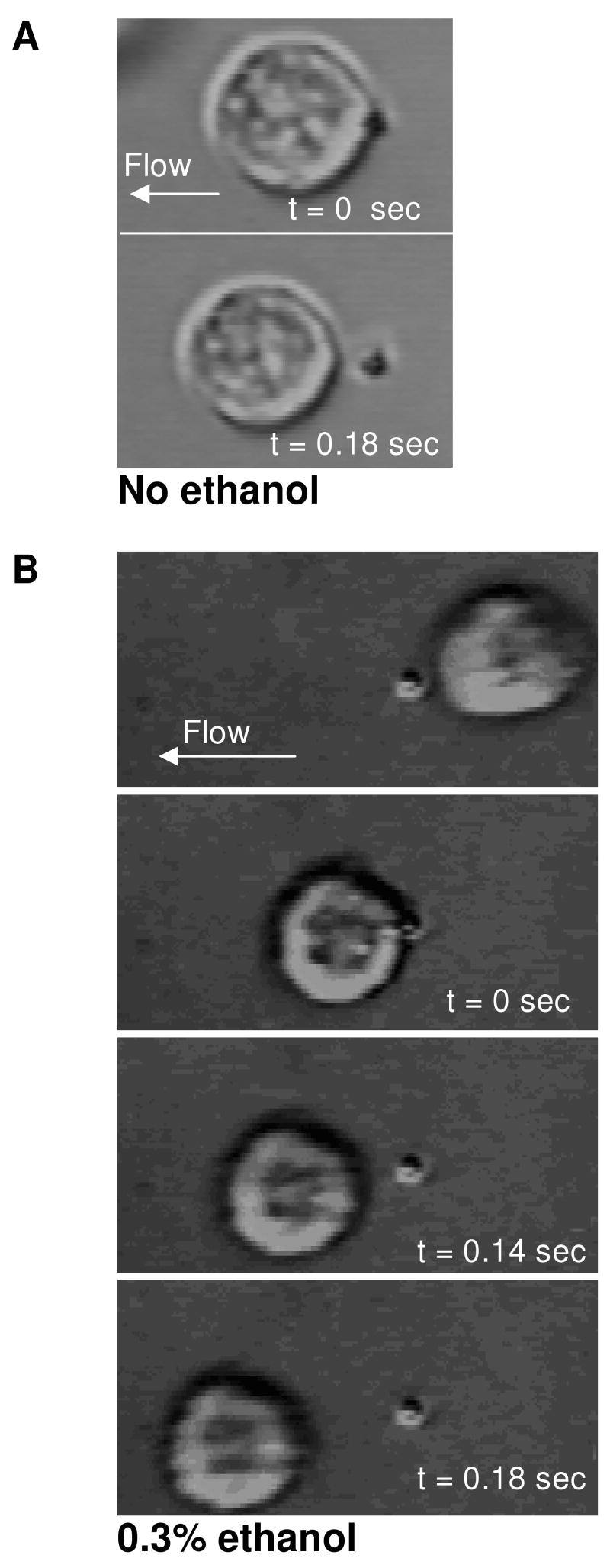
Figure 1. Images of neutrophils tethering to P-selectin-coated beads under flow at γw =100 sec-1.
Typical tether length with no ethanol treatment is 1.9±2.6 μm (A), compared to 7.7±3.1 μm for neutrophils with 0.3% ethanol treatment (B). Digitized images taken from a video sequence of a neutrophil at a video capture rate of 240 fps. (C) A single membrane tether was observed between a flowing neutrophil and P-selectin-coated bead (flow from right to left). Bar, 10 μm.
Figure 2. Dose-dependent effect of ethanol on neutrophil adhesion to P-selectin–coated beads.
Adhesion efficiency decreases at higher ethanol concentrations. N>3 donors and n=interactions observed at each concentration. *p<0.001 comparing untreated vs ethanol-treated samples.
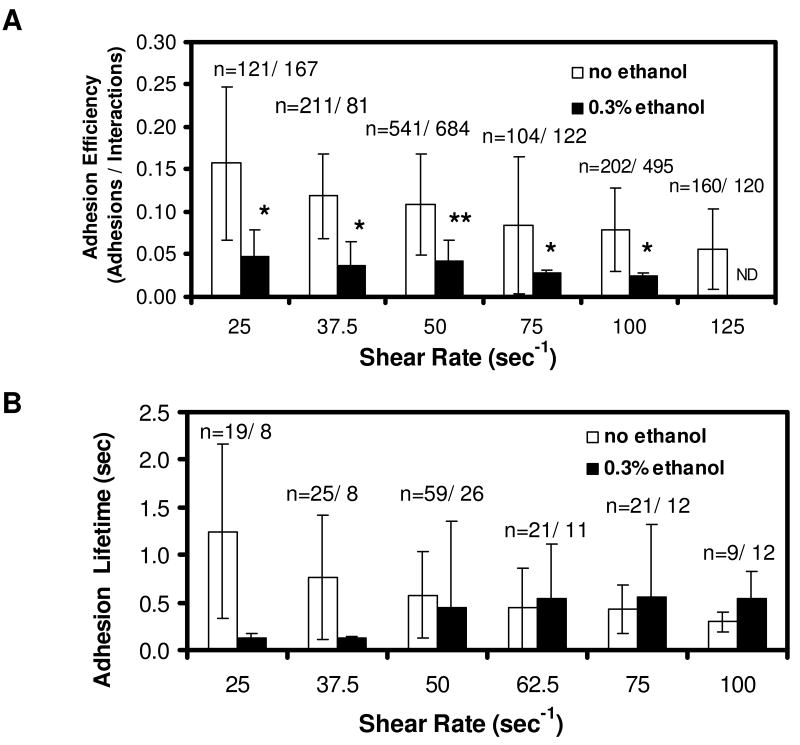
Figure 3. Adhesion lifetime (A) and adhesion efficiency (B) of neutrophils treated with 0.3% ethanol.
Data points are the average ± SD of n measurements at each shear rate with N=4 donors. ND = Not Detected. *, p<0.001 comparing untreated vs. ethanol-treated samples.
As previously reported for untreated neutrophils (31), both the adhesion efficiency and the adhesion lifetime declined as wall shear rate was increased from 25 to 100 s-1. Interestingly, 0.3 % ethanol caused the adhesion lifetime over all events (with and without membrane tethers) to increase from 0.1 sec to 0.5 sec as shear rate was increased from 25 to 100 s-1 (Fig. 3B), an example of pharmacological induction of “hydrodynamic thresholding” (shear enhancement of apparent adhesion strength) consistent with the membrane tether shielding the bond from loading (7, 36).
The adhesion efficiency is a measurement over all collisional events, with and without detectable adhesion, while the adhesion lifetime is a measurement over all adhesive events, with and without detectable membrane tether formation. The ethanol-dependent reduction of adhesion efficiency is an indication of reduced PSGL-1 interaction with P-selectin in ethanol-treated neutrophils, possibly due to PSGL-1 redistribution. In contrast, the enhancing effect of ethanol on adhesion lifetime (Fig. 3B) may result from an increase in membrane tether growth rate as shown in Fig. 1.
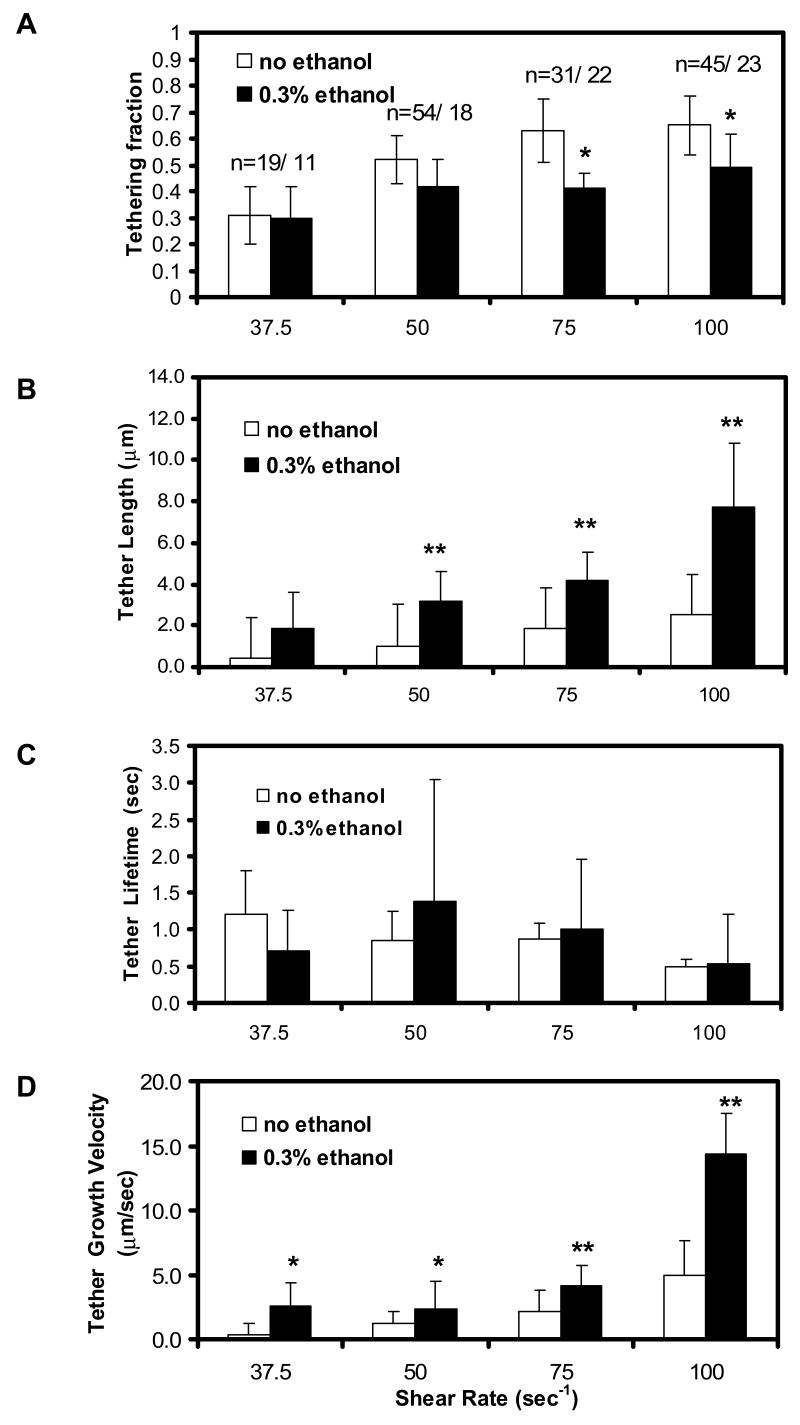
At 0.1% ethanol, tethering fraction (0.63± 0.17) and tether length (2.83± 2.09 μm) were not statistically different from the control values of tethering fraction (0.65±0.15) and tether length (2.50±2.61) for 3 donors (p= 0.62 and 0.56, respectively). However, 0.3 % ethanol reduced the fraction of adhesive collisions that resulted in detectable membrane tethers (Fig. 4A). By image analysis of the adhesive collision events that formed tethers, we found that 0.3 % ethanol resulted in a ∼2- to 3-fold increase in final tether length (Fig. 4B) compared to untreated neutrophils, while the tether lifetime was essentially unaffected (Fig. 4C) by ethanol treatment. At a venous shear rate of 100 sec-1, neutrophils without ethanol treatment had an average tether length of 2.5 ± 2.6 μm, while neutrophils incubated with 0.3% ethanol formed tethers that were 7.7 ± 3.1 μm long (Fig. 4B). Overall, 0.3 % ethanol increased (p < 0.001) the calculated average tether growth velocity at all shear rates tested (Fig. 4D), indicating a direct effect on membrane fluidity. The most notable increase in tether growth rate occurred at 100 sec-1, in which the velocity of ethanol-treated samples was 14.3 ± 3.2 μm/s, a 3.8-fold increase from the control value of 3.8 ± 2.6 μm/sec (Fig. 4D). Frame-by-frame analysis of a set of individual tethers formed at 100 s-1 (± 0.3 % ethanol) showed that the tether length increased linearly with time, typically with small microfluctuations in the growth rate (Fig. 5). To verify that the microfluctuations of tether length are not an artifact of image processing, we compared instantaneous tether length measurements from high-speed imaging (240 fps image acquisition) to those obtained from 28 fps. Microfluctuations were present in both, even at 240 fps which yields very high temporal resolution. We also performed manual particle tracking to compare the result to those obtained from the ImageJ plugin (NIH), in which we found the microfluctuations as well. While overall tether growth is linear in time (Fig. 5c), the growth in small time steps appear to be fluctuating.
Figure 4. Effect of 0.3% ethanol on tether length (A), tether lifetime (B), tether growth rate (C), and tethering fraction (D) at various shear rates, γw.
(N=4 donors for 50 s-1; N=3 donors for all other shear rates)
Data points are the average ± SD of n measurements at each shear rate. The force on the PSGL-1/P-selectin bond was calculated from Goldman's equations as described in the text. *, p<0.002; **p<0.0001, comparing untreated vs ethanol-treated samples.
Figure 5. Instantaneous tether length of ten representative untreated neutrophils (A), ten ethanol-treated neutrophils (B), and the average comparing untreated and ethanol-treated neutrophils (C).
Tether length was measured in a frame-by-frame analysis of tethers formed during neutrophil-bead adhesion for ten representative tethers each for untreated neutrophils (A) and neutrophils incubated with 0.3% ethanol (B). The averages of all ten tethers and the linear fit are compared (C), in which the slope is the average tether growth velocity. Error bars are for standard deviation.
We performed a control experiment to verify that the effect of ethanol was neutrophil-based and not due to an unexpected solvent effect on protein-A/IgG linkages or P-selectin-IgG chimeric function. Adherent P-selectin coated beads were either untreated or pre-exposed to 0.3 % ethanol for 30 min, the chambers were rinsed, and untreated neutrophils were perfused into the chambers from which we measured the adhesion efficiency, tethering fraction, tether length, and tether lifetime (Table 1). In these fully matched experiments where ethanol pretreatment on beads was the only variable, we found that neutrophil interactions with ethanol-treated beads were not different from those with untreated beads (p>0.50 for all parameters, based on paired Student's t-test). We conclude that the reduced adhesion efficiency seen in Fig. 3 and the changes in tethering parameters reported in Figures 4 and 5 are due to the direct action of ethanol on neutrophils, not ethanol elution of the P-selectin from the beads or ethanol mediated denaturation of P-selectin IgG chimeric.
Table I. Effect of ethanol pretreatment of P-selectin-coated Protein A beads.
All measurements at 100 s-1 with untreated neutrophils.
| P-sel beads only
|
P-sel beads +
0.3% ethanol |
|
|---|---|---|
| Total Interactions | n=123 | n=96 |
| Adhesion Efficiency | 0.070 ± 0.031 | 0.072 ± 0.011
(NS: p=0.87) |
| Tethering Fraction | 0.59 ± 0.11 | 0.53 ± 0.06
(NS; p=0.54) |
| Tether Length | 2.29 ± 1.91 μm | 2.32 ± 0.56 μm
(NS; p=0.95) |
| Tether Lifetime | 0.55 ± 0.10 sec | 0.51 ± 0.12 sec
(NS; p=0.77) |
| Average Tether Growth Velocity | 4.09 ± 1.91 μm/sec | 4.54 ± 0.57 μm/sec
(NS; p=0.96) |
Effect of ethanol on neutrophil rolling to uniformly coated P-selectin surfaces
Stable neutrophil rolling on P-selectin represents a dynamic balance between bond creation at the leading edge of the contact area and bond rupture at the lagging edge of the neutrophil where membrane tethers are pulled. In prior work using the neutrophil-bead collision assay, pharmacological treatments that enhanced the membrane tether growth velocity were shown to enhance the PSGL-1/P-selectin adhesion lifetime (SeeFig. 7Bof (31)). Using ethanol-treated neutrophils, we tested the expectation that enhanced membrane tether growth rate would result in force shielding of bonds at the lagging edge of the contact area, thus causing a consequent slower rolling on P-selectin coated surfaces. We observed that 0.3 % ethanol caused a 37 % reduction (p < 0.001) in the rolling velocity (Table 2). Consistent with the detection of reduced adhesion efficiency in the bead assay, we observed that 0.3 % ethanol caused a 55 % reduction (p < 0.001) in the rolling flux.
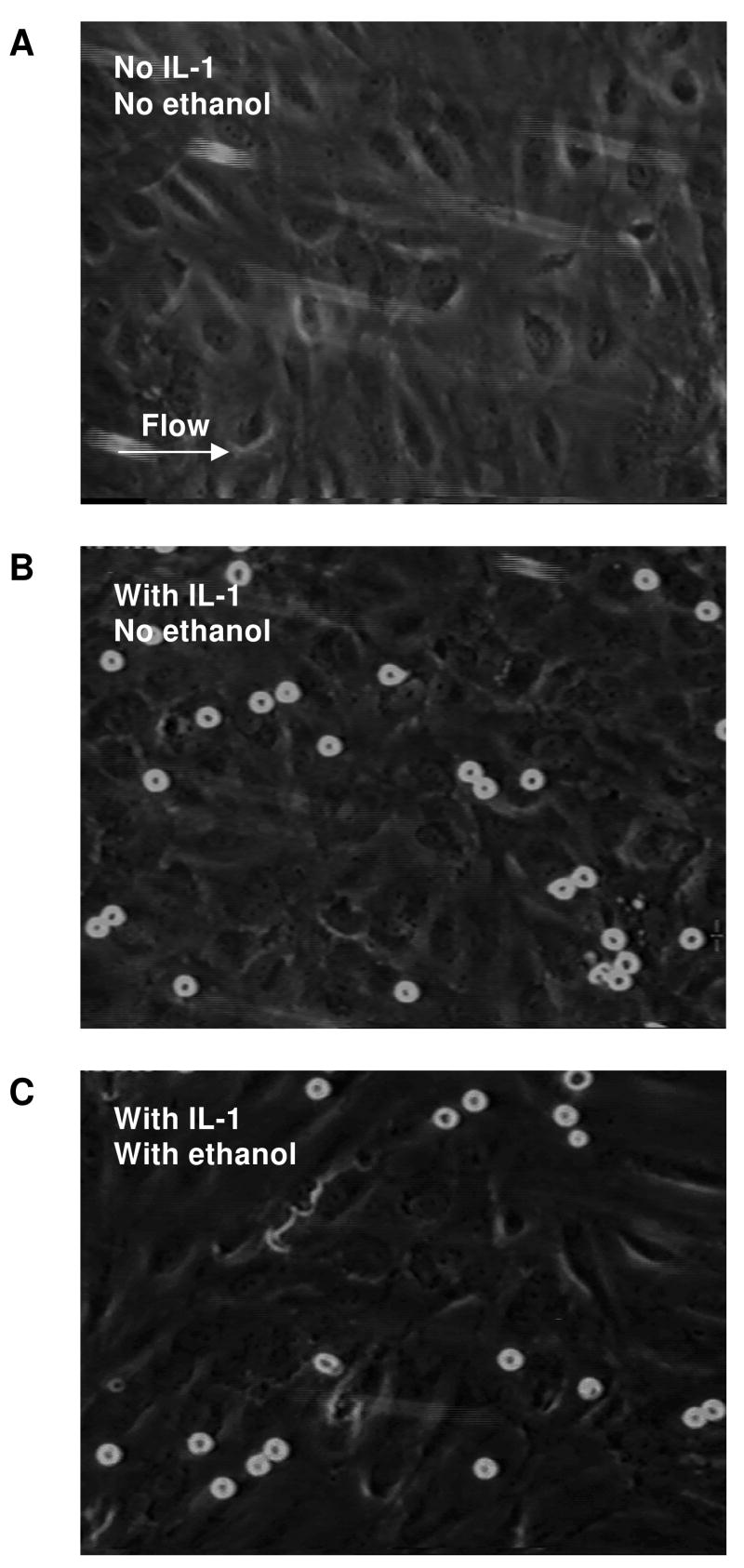
Figure 7. Neutrophil rolling and firm arrest on endothelial cells activated with IL-1.
Without IL-1 treatment, none of the neutrophils roll or adhere to the endothelial cells (A), which indicates no activation. Neutrophils roll and convert to firm arrest on IL-1 activated endothelial cells (B), but fewer ethanol-treated neutrophils (C) convert to firm arrest.
Table II. Neutrophil rolling on P-selectin-coated surface. (N=3 donors).
| No ethanol
|
With 0.3% ethanol
|
|
|---|---|---|
| Average rolling velocity
(all rolling events) |
8.9 ± 1.3 μm/sec
(n=37) |
5.6 ± 1.0 μm/sec *
(n=31) |
| Flux of rolling cells
(# cells/FOV, FOV = 0.01 mm2) |
12.8 ± 4.2 | 5.8 ± 2.1 * |
p<0.001 comparing untreated vs ethanol-treated samples.
Thus, the membrane microphysics revealed in the bead assay using ethanol (reduced ε, but enhanced vt) were consistent with observations made with uniformly coated surfaces (lower rolling flux, but slower rolling velocity). These observations led to the hypothesis that ethanol caused PSGL-1 redistribution from the microvilli thus reducing adhesion efficiency, while those events that do result in bond formation are stabilized by the more rapid growth of membrane tethers in ethanol treated neutrophils. For both untreated and ethanol-treated neutrophils, PSGL-1 was easily detected on the plasma membrane, as observed with confocal laser-scanning microscopy. Untreated neutrophils displayed punctuate surface staining consistent with microvilli presentation, while on neutrophils incubated with 0.3% ethanol the PSGL-1 was more likely to be found in a more evenly distributed, ring-shaped form on the periphery of the cell, with some cell presenting accumulated larger clumps instead of the smaller punctuate clusters scattered across the surface of untreated neutrophils (Fig. 6B).
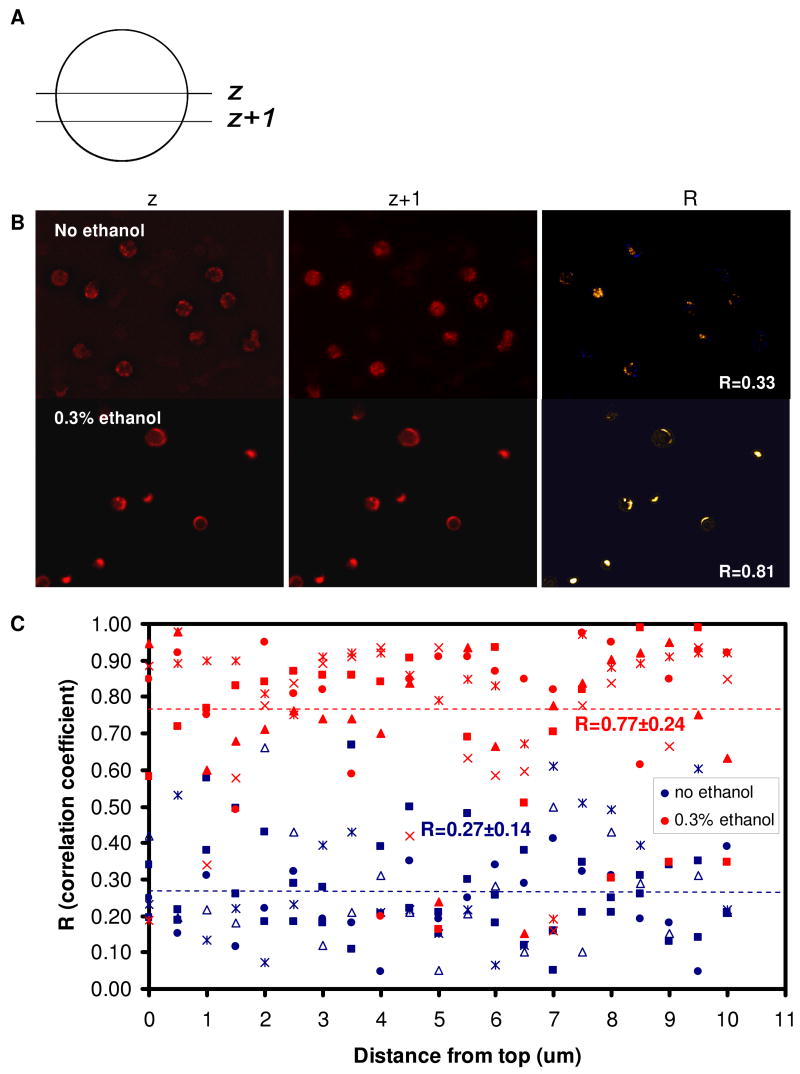
Figure 6. Surface PSGL-1 distribution on untreated neutrophils and ethanol-treated neutrophils.
Neutrophils were stained for PSGL-1, fixed, and observed with a spinning disk confocal microscope. A z-series scan was performed with 0.5 μm step size (A), yielding a stack of 20 images acquired from the top of the cell to the bottom. The intensity correlation between each slice and the next was analyzed using the ImageJ plugin (NIH) and the intensity correlation coefficient, R, was computed (B). The average correlation coefficients for 5 FOV (fields of view) with 3-10 cells per FOV of untreated cells were compared to that of ethanol-treated cells (C). Bar, 10 μm.
To further quantify the PSGL-1 distribution in control vs ethanol-treated cells, we performed a z-series scan in which the individual slices are in the X-Y plane and the sequence of slices represents a sequential change in the location of the cutting plane along the z axis (Fig. 6A). The spacing between the slices was 0.5 μm and resulted in a stack of 20 images acquired from the top of the cell to the bottom. An intensity correlation analysis between each slice and the next in the stack was performed with ImageJ (NIH), in which a intensity correlation map was generated (Fig. 6B) to compute the average correlation factor, R. Correlation was low (0.27± 0.14) for untreated cells, compared to 0.77±0.24 for ethanol-treated cells, which confirms that PSGL-1 is unevenly distributed on control cells but are localized into clusters on ethanol-treated cells. Together with the significant reduction in neutrophil adhesion efficiency to P-selectin and no apparent change in the overall expression of PSGL-1 on the surface (which removes the possibility of PSGL-1 shedding or internalization), the confocal images and correlation coefficient analysis show that PSGL-1 is redistributed on neutrophils treated with 0.3% ethanol.
Effect of ethanol on neutrophil rolling and arrest on activated endothelium
In parallel-plate flow chambers, neutrophils (± 0.3 % ethanol) were perfused at a shear rate of 100 sec-1 over HAEC (± pretreatment of 1 ng/mL IL-1 for 5 hrs). As expected, without IL-1 stimulation, neutrophils displayed little rolling or firm arrest (Fig. 7A). With IL-1 stimulation, substantially more neutrophils rolled and arrested on the endothelium. Treatment of neutrophils with 0.3 % ethanol had no significant effect on rolling velocity, but reduced the fraction of rolling cells that arrested. Table 3 compares neutrophil rolling and firmly arresting on HAEC with or without ethanol (0.3%) treatment.
Table III. Neutrophil rolling on IL-1 stimulated endothelial cells. (N=3 donors).
| No ethanol
|
With 0.3% ethanol
|
|
|---|---|---|
| Average rolling velocity
(all rolling events) |
12.3 ± 5.6 μm/sec
(n=215) |
14.6 ± 6.7 μm/sec
(n=190) |
| Average velocity (firm arrest) | 2.5±0.3 μm/sec
(n=120) |
3.4±0.4 μm/sec *
(n=90) |
| Fraction arrested / FOV | 35.3 ± 4.7 % | 24.2 ± 5.1 % * |
| Total cells/FOV
(FOV = ∼0.08 mm2) |
55.4 ± 13.2 | 48.7 ± 15.2 |
p<0.001 comparing untreated vs ethanol-treated samples.
Cell size and surface antigens on ethanol treated neutrophils
The Coulter Counter Z2 Particle Analyzer was used to analyze cell size for neutrophils treated with ethanol. Compared to control, 0.1% and 0.3% ethanol treatment caused an increase in size (4.0% and 2.7%, respectively), but the increase was statistically insignificant (p>0.50 comparing ethanol-treated and control samples). This result was verified with flow cytometry analysis of cell size: relative cellular size distribution from FSC (forward scatter) histogram overlays showed no change in cell size normalized to control (100.0), which was 105.2 ± 6.2 for 0.1% ethanol-treated cells and 103.5 ± 6.0 for 0.3% ethanol-treated cells (p=0.65).
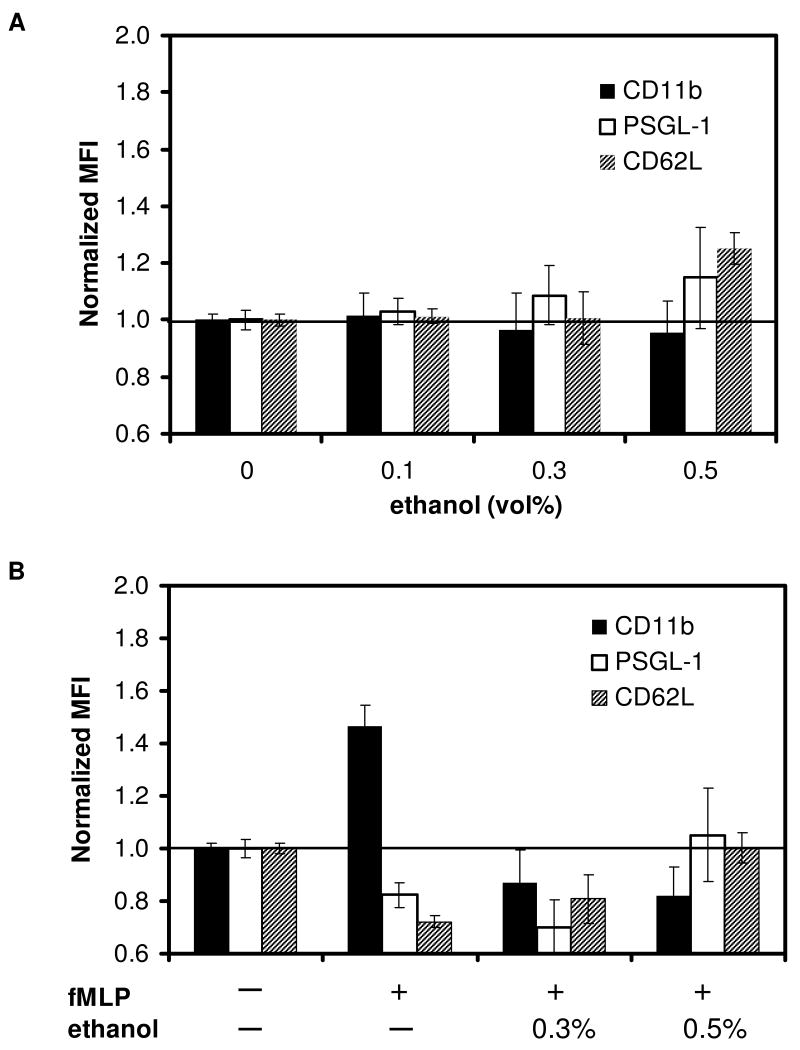
Since ethanol reduced the adhesion efficiency to P-selectin beads and reduced the conversion to firm arrest on IL-1 stimulated endothelium, we also evaluated surface antigens by flow cytometry. Fig. 8A shows the effect of increasing ethanol doses on CD11b, PSGL-1 and L-selectin expression in neutrophils in their quiescent state. The flat level of CD11b and retention of round shape of neutrophils confirmed that neutrophils were not activated by ethanol.
Figure 8. Effect of ethanol treatment on surface adhesion molecule expression of unstimulated or fMLP-activated neutrophils.
Normalized fluorescence intensity on unstimulated neutrophils was measured at increasing ethanol concentrations (A). Neutrophils were treated with 0.1%, 0.3%, or 0.5% ethanol for 15 minutes. Expression levels on neutrophils activated with fMLP (B) with or without ethanol treatment were also measured. N>4 donors.
At the supraphysiological level of 0.5% ethanol, PSGL-1 and L-selectin antigen levels increased slightly by 15% and 25%, respectively. We also assessed the effect of ethanol on neutrophils activated by 1 μM fMLP and found that ethanol had a greater influence on surface molecule expression for activated neutrophils (Fig. 8B) compared to nonactivated cells in Fig. 8A. A 30-min incubation with fMLP alone caused a ∼50% increase in CD11b expression and resulted in visible neutrophil shape change (not shown). Ethanol treatment, however, inhibited this upregulation (p<0.01 for fMLP only vs. ethanol/fMLP-treated cells). Ethanol treatment did not influence PSGL-1 expression on fMLP-activated neutrophils, whereas ethanol inhibited both CD11b upreguation and L-selectin downregulation at both 0.3% and 0.5% ethanol concentrations (Fig. 8B). This observation agrees with previous studies that showed CD11b level increases while L-selectin (CD62L) is shed from the surface upon neutrophil activation (37, 38). The effect of activation on PSGL-1 levels is less well established, although studies have shown that PSGL-1 is preferentially localized on the tips of microvilli on quiescent neutrophils but becomes redistributed to one pole of fMLP-activated, polarized neutrophils (4). Others found that PSGL-1 is shed from the surface of neutrophils stimulated with platelet activating factor (PAF) and PMA, reducing the ability to bind to P-selectin (39).
Discussion
Microvillus and tether formation occurs during capture and subsequent neutrophil rolling on P-selectin presenting surfaces. These adhesion and tethering dynamics depend on forces generated by the membrane receptor-ligand interaction, force imposed by venous blood flow, and alterations in the viscosity of the cell membrane. Ethanol generally increases bilayer fluidity, but may alter membrane-cytoskeletal interactions so ethanol action on neutrophils is difficult to predict without direct measurement. The objective of this study was to examine the effect of ethanol at physiological concentrations on both macroscopic (adhesion properties) and microscopic (bond formation) levels; both whole cell mechanics and local plasmalemma dynamics must be examined since a change in one does not predict the other. Using in vitro flow chamber assays, we have found that ethanol significantly alters neutrophil interaction with P-selectin and stimulated human aortic endothelial cells (HAEC).
When neutrophil PSGL-1/P-selectin interactions were probed using a bead-collision assay, ethanol significantly reduced the ability of neutrophils to adhere. For those that did adhere and form tethers, however, tether lengths and growth velocity were 3- to 4-fold higher for neutrophils incubated with ethanol compared to control, while bond lifetimes remained unaffected. Neutrophils also retained their characteristic spherical shape and β2-integrin expression level, indicating ethanol alone did not activate neutrophils at concentrations used in this study.
Neutrophils treated with ethanol were less likely to convert to firm arrest on IL-1-activated HAEC, possibly due to the decrease in β2-integrin expression. Upon fMLP stimulation, neutrophils responded with a change in morphology as well as increased expression of CD11b and loss of PSGL-1 and L-selectin, in agreement with previous findings on neutrophil activation (38, 40). Treatment with ethanol at 0.3%, however, inhibited both CD11b upregulation and L-selectin downregulation. While fMLP incubation yielded a 46% increase in mean fluorescence intensity (MFI) for CD11b and a 18% decrease in L-selectin compared to untreated control, ethanol incubation reduced these values to 18% below control value for CD11b and restored L-selectin expression to the same level as control.
The reduction in the rolling velocity of neutrophils treated with ethanol is consistent with the expectation from the increase in membrane tether growth rate, which elicits force shielding of bonds at the lagging edge of the neutrophil in contact with P-selectin surface. We observed that ethanol reduced the rolling flux as well, which agrees with the reduction in adhesion efficiency in the bead collision assay. Slower rolling of ethanol-treated cells on P-selectin is consistent with the increase in tether deformability, since reduction in rolling velocity has been attributed to more compliant microvilli both experimentally and in simulations (41). That rolling velocity was reduced on P-selectin but not on the IL-1 stimulated endothelium demonstrates the complex and dynamic nature of the neutrophil-endothelial cell interaction, especially on an inflamed endothelium. The interaction depends on not only the P-selectin/PSGL-1 bond but also on shear rate, adhesion receptor density, and the presence of pro- and anti-inflammatory cytokines. Cytokine activity also varies with the timing of release and the local chemokine concentration gradient. The reduced rolling velocity on the P-selectin-coated surface suggests ethanol-induced change in the P-selectin/PSGL-1 linkage and tether deformability in nonactivated neutrophils; neutrophil rolling on stimulated endothelium, however, is a far more complex process that can be mediated by all three selectins (44), their ligands, and other inflammatory agents so the ethanol treatment alone may not be able to alter significantly.
From these observations, we hypothesized that ethanol affects membrane fluidity and adhesive behavior by changing PSGL-1 distribution. Since overall expression levels on the surface of ethanol-treated cells remained unchanged in flow cytometry, the ligand was likely redistributed rather than shed from the surface or internalized. Previous studies have found PSGL-1 in low-density lipid microdomains, and an intact actin cytoskeleton is a requirement for its redistribution since the cytoplasmic tail of PSGL-1 interacts with linking proteins between the plasma membrane and the actin cytoskeleton (5). This surface redistribution may promote bond breakage, preventing neutrophils from adhering to P-selectin. Neutrophils with PSGL-1 scattered throughout the surface may be stimulated efficiently by P-selectin binding, although the polarized distribution (as found in ethanol-incubated cells) may weaken the adhesion of neutrophils to P-selectin, as shown previously on P-selectin-expressing monolayer (42). Ethanol also interacts directly with lipid groups in the bilayer through both hydrophilic and hydrophobic forces. The weak hydrophobic van der Waals attraction between the short ethyl group and upper chain segments are unable to compete with the strong desire of ethanol to form hydrogen bonds with lipid phosphate groups, thus preventing high concentrations of ethanol within the hydrophobic core (29). Since hydrogen bonding is intimately coupled to the ordering of hydrocarbon chains, it is likely that ethanol disrupts the order of the chains. Ethanol may interact with interface region of proteins in a similar mechanism as in the lipid-water interface.
Tether growth affects the magnitude of the force applied on the bond by changing the moment arm of the force on the bond. Assuming negligible inertia (due to the low Reynolds number) and a quasi-steady state, the net force and torque on the cell is approximately zero in any position of the cell. Under these assumptions, we performed a force and torque balance on the tethering neutrophil as described by Shao, Ting-Beall et al. (6):
| (1) |
| (2) |
FS is the shear force on the cell, FB is the force on the bond, and θ is the angle between the top of the bead and the tether attachment point on the neutrophil. The length of the lever arm, l, between the neutrophil and the bead was directly measured as the sum of the measured tether length, Lmtether, plus the hydrodynamic radius of the neutrophil, R. For the purpose of comparison, we assumed a radius of R=4.25 μm for the neutrophil. Ts refers to the torque imposed by the shear flow on the cell. To solve for Fs and Ts, Goldman's equations (43) give the relationship between shear stress and the forces experienced by a flowing sphere as it flows parallel to a plane wall:
| (3) |
| (4) |
In these equations, μ represents viscosity, which is 0.01 poise. γw represents the wall shear rate, and h refers to the distance from the center of the cell to the chamber surface, or h = R + δ, where δ is the gap distance between the neutrophil and the chamber surface. C and D are constants that are determined from the ratio of h/R based on extrapolation of numerical data given in Goldman, which was 1.65 and 0.95 for our system.
At each shear rate and concentrations tested, the calculated force on the bond was above 45 pN, the average minimum force to pull a tether from a neutrophil (36). The force on untreated neutrophils are in the tether formation range (>61 pN) in which a tether forms at a constant velocity that depends linearly on the force (6). We found that calculated forces on ethanol-treated neutrophils were in the “transition zone” (between 34 pN and 61 pN) in which tether formation depends on the membrane-cytoskeleton association strength.
The ability of ethanol to significantly enhance passive neutrophil deformability has been reported previously (19). Blood flow can deform rolling neutrophils into a more hydrodynamic profile and increase the contact area with the vascular endothelium (45, 46). In vitro flow chamber studies indicate that an increase in wall shear stress (40 sec-1 to 200 sec-1) can compress the height of a neutrophil-like HL-60 cell to double the contact area with the substrate (47). While both ethanol-treated and untreated cells were sensitive to shear, increase in shear rate tended to have a larger effect on neutrophils treated with ethanol.
At the same shear rate, the cells experience the same force on the cell surface, so the increased tether lengths of ethanol-treated cells compared to untreated cells at each shear rate indicates a weaker association between membrane and cytoskeleton for ethanol-treated cells. The significant increase in tether length and tether growth velocity with ethanol treatment is similar to the effects of actin-depolymerizing agents such as cytochalasin (31) and suggests increased lipid flow from the neutrophil and separation of the lipid bilayer from the underlying cytoskeleton, which decreases the necessary force for tether formation since the membrane-cytoskeletal adhesion term is removed (48). The calculated force on the bonds obtained from our measurements are much lower for ethanol-treated cells (∼66 pN) than untreated cells (∼115 pN), which further supports this argument. Lower force implies lower adhesion energy likely due to weaker linkage of adhesion molecules to the cytoskeleton.
The average forces required to extract L-selectin and β2 integrin from neutrophils have been estimated as 25–45 pN and 60–130 pN, respectively (49), for slow processes lasting longer than 1 sec. For tethering events with shorter lifetimes, bond forces were greater than 60 pN (6, 7, 50). These findings suggested that the cytoplasmic domains of selectins and their counterreceptors help anchor these molecules to the cytoskeleton so that they can resist extraction. Our results indicate that by reducing these forces and adhesion energy, ethanol inhibits the ability of P-selectin and its counterreceptor, PSGL-1, to resist tether extraction in quiescent neutrophils. Since an increase in adhesion energy is a possible mechanism to slow down a cell once it becomes activated to promote firm adhesion, the ability of ethanol to decrease this adhesion energy in unstimulated cells may help lower the probability of undesirable aggregation of neutrophils in the absence of stimuli.
Previous studies have shown significant effects of ethanol on inflammatory responses including anion channel blocking properties, aggregation, hyperadherence inhibition and superoxide inhibition (20, 23, 54), but have remained largely inconclusive. Many were observing concentrations too high to be relevant to human biology. Our results indicate that ethanol has significant effects on bond chemistry, membrane mechanics, and molecule expression, which impacts the tethering, rolling, and adhesive properties of neutrophils, both with and without fMLP stimulus.
In summary, we found that ethanol decreased neutrophil capture and caused redistribution of PSGL-1, suggesting that ethanol may lower the likelihood that neutrophils will adhere to activated platelets or endothelial cells. These reductions may be counter-balanced by the increased rate of tether growth and possibility for slower rolling. Additionally, for fMLP-activated neutrophils, ethanol inhibited both the upregulation of CD11b and L-selectin shedding. Ethanol caused competing biophysical and biochemical effects that: (i) reduced capture due to PSGL-1 redistribution, (ii) reduced rolling velocity due to increased membrane tether growth, and (iii) reduced conversion to firm arrest. These findings have important implications in physiologically relevant ethanol dosage for in vivo studies as well as solvent effects of ethanol during in vitro experimentation.
Acknowledgments
This study was supported by NIH HL 56621 and NIH HL 66565.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- 1.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: Molecules, functions and pathophysiological aspects. Laboratory Investigation. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic Signals for Lymphocyte Recirculation and Leukocyte Emigration - the Multistep Paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Konstantopoulos K, Neelamegham S, Burns AR, Hentzen E, Kansas GS, Snapp KR, Berg EL, Hellums JD, Smith CW, McIntire LV, Simon SI. Venous levels of shear support neutrophil-platelet adhesion and neutrophil aggregation in blood via P-selectin and beta(2)-integrin. Circulation. 1998;98:873–882. doi: 10.1161/01.cir.98.9.873. [DOI] [PubMed] [Google Scholar]
- 4.Bruehl RE, Moore KL, Lorant DE, Borregaard N, Zimmerman GA, McEver RP, Bainton DF. Leukocyte activation induces surface redistribution of P-selectin glycoprotein ligand-1. J Leukoc Biol. 1997;61:489–499. doi: 10.1002/jlb.61.4.489. [DOI] [PubMed] [Google Scholar]
- 5.Itoh S, Susuki C, Takeshita K, Nagata K, Tsuji T. Redistribution of P-selectin glycoprotein ligand-1 (PSGL-1) in chemokine-treated neutrophils: a role of lipid microdomains. J Leukoc Biol. 2007;81:1414–1421. doi: 10.1189/jlb.0606398. [DOI] [PubMed] [Google Scholar]
- 6.Shao JY, Ting-Beall HP, Hochmuth RM. Static and dynamic lengths of neutrophil microvilli. Proc Natl Acad Sci U S A. 1998;95:6797–6802. doi: 10.1073/pnas.95.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidtke DW, Diamond SL. Direct observation of membrane tethers formed during neutrophil attachment to platelets or P-selectin under physiological flow. J Cell Biol. 2000;149:719–730. doi: 10.1083/jcb.149.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Needham D, Nunn RS. Elastic-Deformation and Failure of Lipid Bilayer-Membranes Containing Cholesterol. Biophys J. 1990;58:997–1009. doi: 10.1016/S0006-3495(90)82444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadash KE, Lawrence MB, Diamond SL. Neutrophil string formation: hydrodynamic thresholding and cellular deformation during cell collisions. Biophys J. 2004;86:4030–4039. doi: 10.1529/biophysj.103.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renaud S, Delorgeril M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart-Disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 11.Mukamal KJ, Conigrave KM, Mittleman MA, Camargo CAJ, Stampfer MJ, Willett WC, Rimm EB. Roles of drinking pattern and type of alcohol consumed in coronary heart disease in men. N Engl J Med. 2003;348:109–118. doi: 10.1056/NEJMoa022095. [DOI] [PubMed] [Google Scholar]
- 12.Taylor L. Drunk Driving Defense. 5th. Aspen Law and Business Publishers; New York: 1999. p. 1193. [Google Scholar]
- 13.Owens CK, Mcintire LV, Lasslo A. Ethanol Inhibition of Thrombus Formation on Collagen-Coated Glass. Thromb Haemost. 1990;63:510–516. [PubMed] [Google Scholar]
- 14.McKenzie ME, Bell CR, Horowitz ED, Oshrine BR, Atar D, Serebruany VL. Effects of in vitro exposure of alcohol on surface receptor expression of human platelets. Clinical Physiology and Functional Imaging. 2002;22:153–156. doi: 10.1046/j.1365-2281.2002.00411.x. [DOI] [PubMed] [Google Scholar]
- 15.Spagnuolo PJ, Macgregor RR. Acute Ethanol Effect on Chemotaxis and Other Components of Host Defense. J Lab Clin Med. 1975;86:24–31. [PubMed] [Google Scholar]
- 16.Astry CL, Warr GA, Jakab GJ. Impairment of Polymorphonuclear Leukocyte Immigration as a Mechanism of Alcohol-Induced Suppression of Pulmonary Anti-Bacterial Defenses. Am Rev Respir Dis. 1983;128:113–117. doi: 10.1164/arrd.1983.128.1.113. [DOI] [PubMed] [Google Scholar]
- 17.Zhang P, Nelson S, Summer WR, Spitzer JA. Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcoholism-Clinical and Experimental Research. 1997;21:773–778. [PubMed] [Google Scholar]
- 18.Patarroyo M, Clark EA, Prieto J, Kantor C, Gahmberg CG. Identification of a Novel Adhesion Molecule in Human-Leukocytes by Monoclonal-Antibody Lb-2. FEBS Lett. 1987;210:127–131. doi: 10.1016/0014-5793(87)81321-2. [DOI] [PubMed] [Google Scholar]
- 19.Macgregor RR, Safford M, Shalit M. Effect of Ethanol on Functions Required for the Delivery of Neutrophils to Sites of Inflammation. J Infect Dis. 1988;157:682–689. doi: 10.1093/infdis/157.4.682. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson E, Lindstrom P, Patarroyo M, Ringertz B, Lerner R, Rincon J, Palmblad J. Ethanol Impairs Certain Aspects of Neutrophil Adhesion Invitro - Comparisons with Inhibition of Expression of the CD18 Antigen. J Infect Dis. 1991;163:591–597. doi: 10.1093/infdis/163.3.591. [DOI] [PubMed] [Google Scholar]
- 21.Corberand JX, Laharrague PF, Fillola G. Human-Neutrophils are Not Severely Injured in Conditions Mimicking Social Drinking. Alcoholism-Clinical and Experimental Research. 1989;13:542–546. doi: 10.1111/j.1530-0277.1989.tb00374.x. [DOI] [PubMed] [Google Scholar]
- 22.Borghese C, Storustovu S, Ebert B, Herd M, Belelli D, Lambert J, Marshall G, Wafford K, Harris RA. The {delta} Subunit of {gamma}-Aminobutyric Acid Type A Receptors Does Not Confer Sensitivity to Low Concentrations of Ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- 23.Patel M, Keshavarzian A, Kottapalli V, Badie B, Winship D, Fields JZ. Human neutrophil functions are inhibited in vitro by clinically relevant ethanol concentrations. Alcohol Clin Exp Res. 1996;20:275–283. doi: 10.1111/j.1530-0277.1996.tb01640.x. [DOI] [PubMed] [Google Scholar]
- 24.Saeed RW, Varma S, Peng T, Tracey KJ, Sherry B, Metz CN. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. Journal of Immunology. 2004;173:6376–6383. doi: 10.4049/jimmunol.173.10.6376. [DOI] [PubMed] [Google Scholar]
- 25.Kvietys PR, Perry MA, Gaginella TS, Granger DN. Ethanol Enhances Leukocyte-Endothelial Cell-Interactions in Mesenteric Venules. Am J Physiol. 1990;259:G578–G583. doi: 10.1152/ajpgi.1990.259.4.G578. [DOI] [PubMed] [Google Scholar]
- 26.Colles S, Wood WG, Myerspayne SC, Igbavboa U, Avdulov NA, Joseph J, Schroeder F. Structure and Polarity of Mouse-Brain Synaptic Plasma-Membrane - Effects of Ethanol In-Vitro and In-Vivo. Biochemistry (N Y) 1995;34:5945–5959. doi: 10.1021/bi00017a024. [DOI] [PubMed] [Google Scholar]
- 27.Daragan VA, Voloshin AM, Chochina SV, Khazanovich TN, Wood WG, Avdulov NA, Mayo KH. Specific binding of ethanol to cholesterol in organic solvents. Biophys J. 2000;79:406–415. doi: 10.1016/S0006-3495(00)76302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holte LL, Gawrisch K. Determining ethanol distribution in phospholipid multilayers with MAS-NOESY spectra. Biochemistry (N Y) 1997;36:4669–4674. doi: 10.1021/bi9626416. [DOI] [PubMed] [Google Scholar]
- 29.Feller SE, Brown CA, Nizza DT, Gawrisch K. Nuclear overhauser enhancement spectroscopy cross-relaxation rates and ethanol distribution across membranes. Biophys J. 2002;82:1396–1404. doi: 10.1016/S0006-3495(02)75494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ly HV, Block DE, Longo ML. Interfacial tension effect of ethanol on lipid bilayer rigidity, stability, and area. Langmuir. 2002;18:8988–8995. [Google Scholar]
- 31.Edmondson KE, Denney WS, Diamond SL. Neutrophil-Bead Collision Assay: Pharmacologically Induced Changes in Membrane Mechanics Regulate the PSGL-1/P-Selectin Adhesion Lifetime. Biophys J. 2005;89:3603–3614. doi: 10.1529/biophysj.105.066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers SD, Camphausen RT, Hammer DA. Sialyl Lewis(x)-Mediated, PSGL-1-independent rolling adhesion on P-selectin. Biophys J. 2000;79:694–706. doi: 10.1016/S0006-3495(00)76328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diamond SL, Eskin SG, Mcintire LV. Fluid-Flow Stimulates Tissue Plasminogen-Activator Secretion by Cultured Human-Endothelial Cells. Science. 1989;243:1483–1485. doi: 10.1126/science.2467379. [DOI] [PubMed] [Google Scholar]
- 34.Ji JY, Jing HY, Diamond SL. Hemodynamic Regulation of Inflammation at the Endothelial–Neutrophil Interface. Annals of Biomedical Engineering. 2008;36:586–595. doi: 10.1007/s10439-008-9465-4. [DOI] [PubMed] [Google Scholar]
- 35.van Eeden SF, Klut ME, Walker BAM, Hogg JC. The use of flow cytometry to measure neutrophil function. J Immunol Methods. 1999;232:23–43. doi: 10.1016/s0022-1759(99)00148-9. [DOI] [PubMed] [Google Scholar]
- 36.Shao JY, Hochmuth RM. Micropipette suction for measuring piconewton forces of adhesion and tether formation from neutrophil membranes. Biophys J. 1996;71:2892–2901. doi: 10.1016/S0006-3495(96)79486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett TA, Lynam EB, Sklar LA, Rogelj S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes - Functional consequences for neutrophil aggregation. Journal of Immunology. 1996;156:3093–3097. [PubMed] [Google Scholar]
- 38.Goel MS, Diamond SL. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood. 2002;100:3797–3803. doi: 10.1182/blood-2002-03-0712. [DOI] [PubMed] [Google Scholar]
- 39.Davenpeck KL, Brummet ME, Hudson SA, Mayer RJ, Bochner BS. Activation of human leukocytes reduces surface P-selectin glycoprotein ligand-1 (PSGL-1, CD162) and adhesion to P-selectin in vitro. Journal of Immunology. 2000;165:2764–2772. doi: 10.4049/jimmunol.165.5.2764. [DOI] [PubMed] [Google Scholar]
- 40.Choi KS, Garyu J, Park J, Dumler JS. Diminished adhesion of Anaplasma phagocytophilum-infected neutrophils to endothelial cells is associated with reduced expression of leukocyte surface selectin. Infect Immun. 2003;71:4586–4594. doi: 10.1128/IAI.71.8.4586-4594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caputo KE, Lee D, King MR, Hammer DA. Adhesive dynamics simulations of the shear threshold effect for leukocytes. Biophys J. 2007;92:787–797. doi: 10.1529/biophysj.106.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorant DE, Mcever RP, Mcintyre TM, Moore KL, Prescott SM, Zimmerman GA. Activation of Polymorphonuclear Leukocytes Reduces their Adhesion to P-Selectin and Causes Redistribution of Ligands for P-Selectin on their Surfaces. J Clin Invest. 1995;96:171–182. doi: 10.1172/JCI118018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman AJ, Cox RG, Brenner H. Slow Viscous Motion of a Sphere Parallel to a Plane Wall .2. Couette Flow. Chemical Engineering Science. 1967;22:653–&. [Google Scholar]
- 44.Lawrence MB, Kansas GS, Kunkel EJ, Ley K. Threshold levels of fluid shear promote leukocyte adhesion through selectins (CD62L,P,E) J Cell Biol. 1997;136:717–727. doi: 10.1083/jcb.136.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong C, Cao J, Struble EJ, Lipowsky HW. Mechanics of leukocyte deformation and adhesion to endothelium in shear flow. Ann Biomed Eng. 1999;27:298–312. doi: 10.1114/1.143. [DOI] [PubMed] [Google Scholar]
- 46.Firrell JC, Lipowsky HH. Leukocyte Margination and Deformation in Mesenteric Venules of Rat. Am J Physiol. 1989;256:H1667–H1674. doi: 10.1152/ajpheart.1989.256.6.H1667. [DOI] [PubMed] [Google Scholar]
- 47.Lei X, Lawrence MR, Dong C. Influence of cell deformation on leukocyte rolling adhesion in shear flow. Journal of Biomechanical Engineering-Transactions of the Asme. 1999;121:636–643. doi: 10.1115/1.2800866. [DOI] [PubMed] [Google Scholar]
- 48.Dai JW, Sheetz MP. Membrane tether formation from blebbing cells. Biophys J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shao JY, Hochmuth RM. Mechanical anchoring strength of L-selectin, beta(2) integrins, and CD45 to neutrophil cytoskeleton and membrane. Biophys J. 1999;77:587–596. doi: 10.1016/S0006-3495(99)76915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alon R, Hammer DA, Springer TA. Lifetime of the P-Selectin-Carbohydrate Bond and its Response to Tensile Force in Hydrodynamic Flow. Nature. 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 51.Heinrich V, Leung A, Evans E. Nano-to-microscale mechanical switches and fuses mediate adhesive contacts between leukocytes and the endothelium. Journal of Chemical Information and Modeling. 2005;45:1482–1490. doi: 10.1021/ci0501903. [DOI] [PubMed] [Google Scholar]
- 52.Evans E, Leung A, Heinrich V, Zhu C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proc Natl Acad Sci U S A. 2004;101:11281–11286. doi: 10.1073/pnas.0401870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 2003;423:190–193. doi: 10.1038/nature01605. [DOI] [PubMed] [Google Scholar]
- 54.Vedder NB, Harlan JM. Increased Surface Expression of Cd11b. J Clin Invest. 1988;81:676–682. doi: 10.1172/JCI113372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcus WD, Hochmuth RM. Experimental studies of membrane tethers formed from human neutrophils. Ann Biomed Eng. 2002;30:1273–1280. doi: 10.1114/1.1528614. [DOI] [PubMed] [Google Scholar]
- 56.Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, G alpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: Analysis by quantitative immunocolocalization. J Neurosci. 2004;24:4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chinga G, Syverud K. Quantification of paper mass distributions within local picking areas. Nordic Pulp and Paper Res J. 2007;22(4):441–446. [Google Scholar]