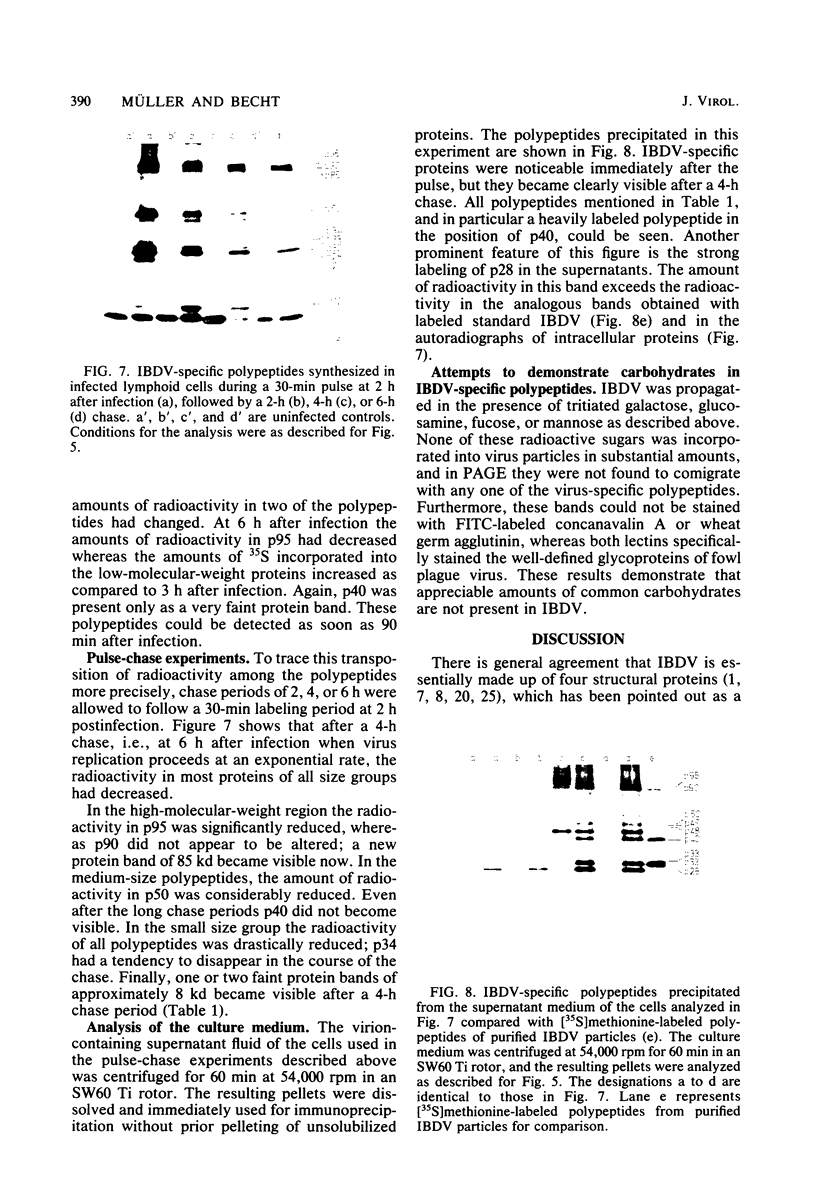
Abstract
It has previously been shown that infectious bursal disease virus is a naked icosahedral particle with a diameter of about 60 nm and a genome consisting of two segments of double-stranded RNA (Müller et al., J. Virol. 31:584-589, 1979). One of the two major structural polypeptides (molecular weight, 40,000) of this virus could not be found in lysates of infected cells; it is derived from a precursor polypeptide demonstrable inside the cells in relatively large quantities and seems to be processed during virus assembly or later. The precursor molecule is regularly present in the infectious virus particle (buoyant density, 1.33 g/ml) in minor proportions, but it represents an outstanding structural element of incomplete noninfectious particles ("top components"; buoyant density, 1.29 g/ml) which contain viral RNA. This type of incomplete particles is mainly produced by chicken embryo fibroblasts in contrast to lymphoid cells from the bursa of Fabricius. Precursor-product relationships also seem to exist in the biosynthesis of the other viral polypeptides. In contrast to some other viruses with a segmented double-stranded RNA genome, none of the structural proteins of infectious bursal disease virus is appreciably glycosylated.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becht H. Infectious bursal disease virus. Curr Top Microbiol Immunol. 1980;90:107–121. doi: 10.1007/978-3-642-67717-5_5. [DOI] [PubMed] [Google Scholar]
- Becht H., Rott R., Klenk H. D. Effect of Concanavalin A on cells infected with enveloped RNA viruses. J Gen Virol. 1972 Jan;14(1):1–8. doi: 10.1099/0022-1317-14-1-1. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cohen J., Maget-Dana R., Roche A. C., Monsigny M. Calf rotavirus: detection of outer capsid glycoproteins by lectins. FEBS Lett. 1978 Mar 1;87(1):26–30. doi: 10.1016/0014-5793(78)80125-2. [DOI] [PubMed] [Google Scholar]
- Cursiefen D., Käufer I., Becht H. Loss of virulence in a small plaque mutant of the infectious bursal disease virus. Arch Virol. 1979;59(1-2):39–46. doi: 10.1007/BF01317893. [DOI] [PubMed] [Google Scholar]
- Dobos P., Hill B. J., Hallett R., Kells D. T., Becht H., Teninges D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J Virol. 1979 Nov;32(2):593–605. doi: 10.1128/jvi.32.2.593-605.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Peptide map comparison of the proteins of infectious bursal disease virus. J Virol. 1979 Dec;32(3):1047–1050. doi: 10.1128/jvi.32.3.1047-1050.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P., Rowe D. Peptide map comparison of infectious pancreatic necrosis virus-specific polypeptides. J Virol. 1977 Dec;24(3):805–820. doi: 10.1128/jvi.24.3.805-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobos P. Virus-specific protein synthesis in cells infected by infectious pancreatic necrosis virus. J Virol. 1977 Jan;21(1):242–258. doi: 10.1128/jvi.21.1.242-258.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Gielkens A. L., Van Zaane D., Bloemers H. P., Bloemendal H. Synthesis of Rauscher murine leukemia virus-specific polypeptides in vitro. Proc Natl Acad Sci U S A. 1976 Feb;73(2):356–360. doi: 10.1073/pnas.73.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilead Z., Jeng Y. H., Wold W. S., Sugawara K., Rho H. M., Harter M. L., Green M. Immunological identification of two adenovirus 2-induced early proteins possibly involved in cell transformation. Nature. 1976 Nov 18;264(5583):263–266. doi: 10.1038/264263a0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Krystal G., Perrault J., Graham A. F. Evidence for a glycoprotein in reovirus. Virology. 1976 Jul 15;72(2):308–321. doi: 10.1016/0042-6822(76)90160-4. [DOI] [PubMed] [Google Scholar]
- Käufer I., Weiss E. Significance of bursa of Fabricius as target organ in infectious bursal disease of chickens. Infect Immun. 1980 Feb;27(2):364–367. doi: 10.1128/iai.27.2.364-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundgren G., Zukoski C. F., Möller G. Differential effects of human granulocytes and lymphocytes on human fibroblasts in vitro. Clin Exp Immunol. 1968 Oct;3(8):817–836. [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. D., Dobos P. Identification of the proteins encoded by each genome segment of infectious pancreatic necrosis virus. Virology. 1981 Oct 30;114(2):414–422. doi: 10.1016/0042-6822(81)90222-1. [DOI] [PubMed] [Google Scholar]
- Maher P., Molday R. S. Binding of concanavalin A to Ricinus communis agglutinin and its implication in cell-surface labeling studies. FEBS Lett. 1977 Dec 15;84(2):391–394. doi: 10.1016/0014-5793(77)80732-1. [DOI] [PubMed] [Google Scholar]
- Müller H., Scholtissek C., Becht H. The genome of infectious bursal disease virus consists of two segments of double-stranded RNA. J Virol. 1979 Sep;31(3):584–589. doi: 10.1128/jvi.31.3.584-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick H., Cursiefen D., Becht H. Structural and growth characteristics of infectious bursal disease virus. J Virol. 1976 Apr;18(1):227–234. doi: 10.1128/jvi.18.1.227-234.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Porath J., Sundberg L. High capacity chemisorbents for protein immobilization. Nat New Biol. 1972 Aug 30;238(87):261–262. doi: 10.1038/newbio238261a0. [DOI] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G., Müller H., Riesner D. Helix-coil transitions in double-stranded viral RNA. Fine resolution melting and ionic strength dependence. Biochim Biophys Acta. 1980 Feb 29;606(2):274–284. doi: 10.1016/0005-2787(80)90037-4. [DOI] [PubMed] [Google Scholar]
- Todd D., McNulty M. S. Biochemical studies with infectious bursal disease virus: comparison of some of its properties with infectious pancreatic necrosis virus. Arch Virol. 1979;60(3-4):265–277. doi: 10.1007/BF01317498. [DOI] [PubMed] [Google Scholar]