Abstract
Mitosis is a crucial part of the cell cycle. A successful mitosis requires the proper execution of many complex cellular behaviors. Thus, there are many points at which mitosis may be disrupted. In cancer cells, chronic disruption of mitosis can lead to unequal segregation of chromosomes, a phenomenon known as chromosomal instability. A majority of colorectal tumors suffer from this instability, and recent studies have begun to reveal the specific ways in which mitotic defects promote chromosomal instability in colorectal cancer.
Introduction
A lot of things have to go right in order for a cell to succeed in mitosis. The nuclear membrane must disassemble, centrosomes must move to opposite poles of the cell, chromosomes must decatenate and condense, the mitotic spindle must find and attach to chromosomes, chromosomes must congress to the equator of the cell, sister chromatid cohesions must then be severed, newly separated chromatids must egress to their respective spindle poles, chromatin must decondense, nuclear membranes must reassemble, and the now-binucleate cell must undergo cytokinesis. With so much that must go right during mitosis, it follows that many things can also go wrong. In the century since von Hansemann [1] first described the variety of mitotic aberrations present in cancer cells, significant progress has been made in identifying the causes—and consequences—of things going wrong in mitosis. As seen in this review, these studies bear important implications for the understanding of the pathogenesis of colorectal cancer.
Microsatellite and Chromosomal Instability
Genetically speaking, there are two broad classes of colorectal cancer: those with microsatellite instability (MIN) and those with chromosomal instability (CIN) [2]. Comprising approximately 15% of sporadic cases of colorectal cancer, MIN tumors are characterized by an increased mutation rate at the nucleotide level due to inactivation of genes in the mismatch repair (MMR) system [2]. Importantly, these tumors have a diploid or near-diploid genome [2]. In contrast, CIN tumors, which comprise 85% of sporadic colorectal cancer, contain highly aneuploid genomes which arise by an increased rate of whole chromosome changes independently of MMR deficiencies [2]. In fact, although research has begun to unravel the mechanisms of CIN, its causes remain largely mysterious [3•]. Moreover, there is substantial debate over whether, and to what extent, CIN is a cause or consequence of cancer [4•]. Although the jury is still out on these questions [4•], the extent of this debate only underscores the importance of better understanding CIN. Additionally, there are many reasons to believe that greater knowledge of CIN might improve the diagnosis, prognostication, and even treatment of colorectal cancer [5••,6]. As it turns out, many of the causes of CIN which have so far been uncovered involve disruption of the process of mitosis (Fig. 1). In this review, we will discuss current knowledge of how mitotic defects may contribute to CIN. Although our focus will be on colorectal cancer, both mitotic defects and CIN are common in other malignancies [5••], and relevant lessons learned from other cancers will thus be included where appropriate.
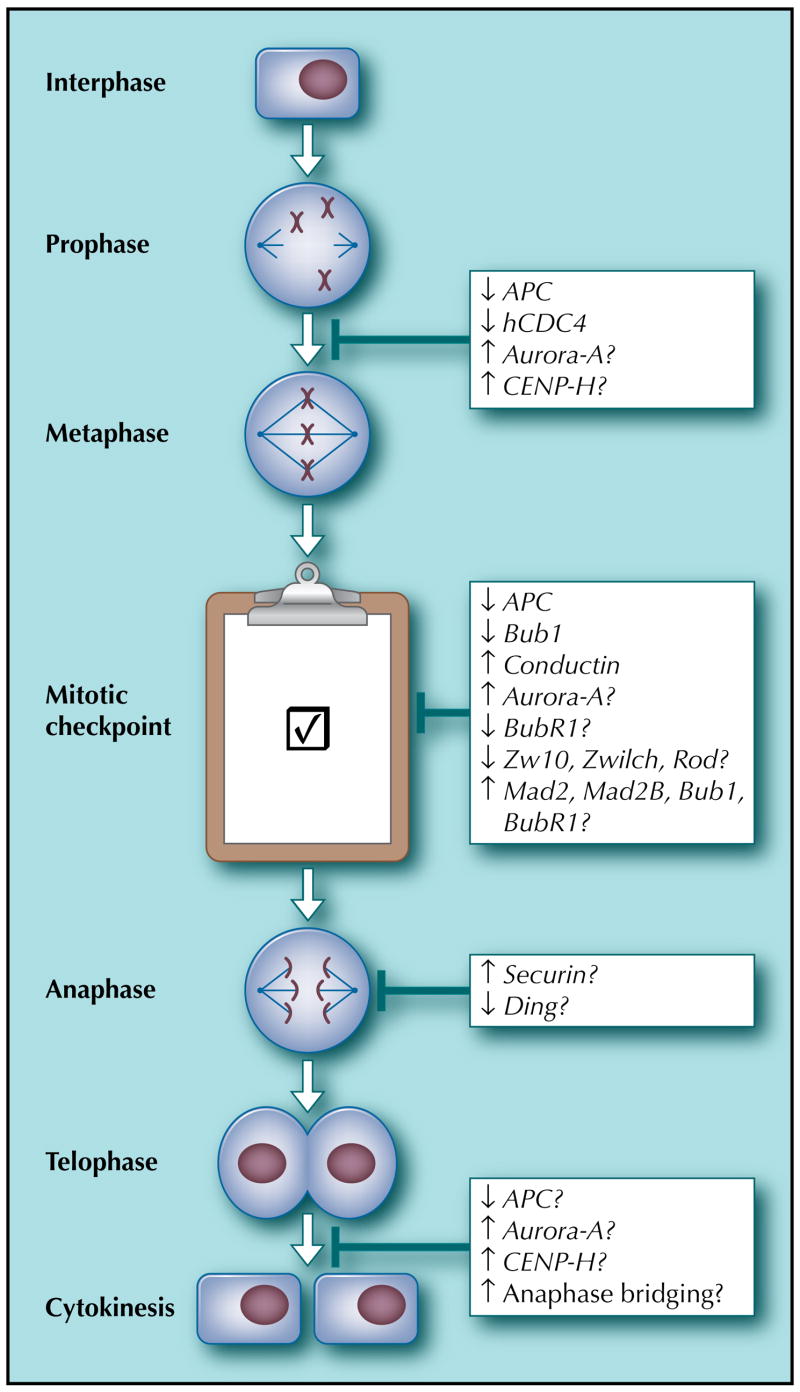
Figure 1.
Mitotic origins of chromosomal instability in colorectal cancer. This figure contains the reported genetic changes in colorectal cancer that are associated with chromosomal instability. The various phases of mitosis are shown. The genes are described in the text. Down arrows indicate either mutational inactivation or reduction in expression. Up arrows indicate overexpression. APC—adenomatous polyposis coli; CENP-H—centromere protein H.
Going Wrong in Lining Up: Chromosome Attachment and Congression Defects
After casting loose its chromosomes into the cytoplasm, a mitotic cell must first attach these chromosomes to mitotic spindles and “congress” them to the equator of the cell. As chromosomes which fail to properly attach or congress increase their risk of then being missegregated, defects in these mitotic behaviors can, in principle, promote CIN. In fact, Green and Kaplan [7] showed that, as compared with MIN colorectal cancer cells, CIN cells display a higher incidence of defective chromosome attachment and congression. Interestingly, one of the events which may be implicated in these defects is mutation of the adenomatous polyposis coli (APC) gene.
Although first described as a gene whose mutation causes oncogenic upregulation of the Wnt signaling pathway in 85% of sporadic colorectal tumors [2], APC has also been found to function during mitosis. Cultured mouse embryonic stem cells expressing mutant APC exhibit abnormal mitotic spindle architecture, an increased incidence of lagging chromosomes, and aneuploidy [8,9]. In human cells, the defects reported in APC-compromised cells include irregular spindle architecture, abnormal dynamics of spindle microtubules, reduced stretching between sister chromatids, abnormal positioning and segregation of chromosomes, an excess of spindle microtubules which remain unattached to chromosomes, and CIN [7,10,11•,12•,13,14]. Although the mechanism underlying these defects is not fully understood, it appears to result in part from disruption of the interaction between APC and the microtubule-binding protein EB-1 [10,12•]. Additionally, Hadjihannas et al. [11•] showed that CIN induced by APC inactivation can depend on the activity of β-catenin and conductin, genes which are themselves upregulated in CIN cells. Interestingly, whereas failed chromosome spindle attachments are precisely the stimuli which otherwise activate the mitotic checkpoint, these defects appear to inefficiently activate this checkpoint in APC-mutant cells [10,12•,13]. Taken together, these studies suggest that mutation of APC could promote CIN in colorectal cancer by disrupting attachment, positioning, and segregation of mitotic chromosomes. However, not all APC-mutated colon cancers exhibit CIN, so APC mutation alone may not be sufficient to promote CIN in every genetic background [9]. Moreover, it has been noted that the type of aneuploidy seen in APC-mutated mouse embryonic stem cells, namely an increase in polyploid cells, differs from the shuffling of a small number of chromosomes which characterizes the CIN of colorectal cancer cell lines [2]. Thus, it may be that by increasing polyploidy, APC mutation promotes CIN indirectly by stacking the genetic deck with more chromosomes which can then be shuffled [2]. At the same time, Hadjihannas et al. [11•] report small-number-shuffling CIN when APC is suppressed by RNA interference in MIN cells, so the type of instability produced by APC inactivation may depend on its genetic context. Finally, these data demonstrate how a single lesion, like APC mutation, can cause pleiotropic mitotic defects, a phenomenon that is not uncommon.
Other genetic aberrations have also been implicated in chromosome attachment and congression defects in colorectal cancer. Rajagopalan et al. [15•] showed that targeted inactivation of the hCDC4 gene in MIN colorectal cancer cell lines impairs proper chromosome congression. A ubiquitin ligase which regulates the G1-S transition of the cell cycle, hCDC4 is also mutated in 12% and 7% of primary colorectal carcinoma and adenoma tissue, respectively [15•]. How inactivation of a G1-S regulator leads to chromosome congression defects—and what aspect of congression is impaired—is unknown, but this study demonstrates that the consequences of such a defect can be severe, as many hCDC4−/− cells fail to segregate their chromosomes normally and thus develop CIN. It has also been reported that centromere protein H (CENP-H) is upregulated in CIN cell lines, and over-expression of CENP-H in MIN cells induces polyploidy [16]. Given that the CENP family of proteins regulates chromosome-spindle attachments, it is possible that dysregulation of this protein family contributes to CIN through disruption of these attachments [16]. Finally, both increased expression [17] and an oncogenic allele [18] of the Aurora-A gene have been associated with aneuploidy in colorectal tumors, and among the reported effects of overexpression of this gene in cultured cells are impaired chromosome attachment, impaired congression, and increased polyploidy [19]. Finally, it is important to point out that, like in the case of APC mutation, attachment and congression defects may not be the only mitotic disruptions caused by these aberrations. In fact, further disruptions in downstream processes, such as the mitotic checkpoint, might help to explain why attachment and chromosome errors introduced by these aberrations are able to go uncorrected on the path to CIN.
Going Wrong in Checking Up: Mitotic Checkpoint Defects
The mitotic checkpoint is the biochemical machinery which decides when a metaphase cell is ready to separate its chromosomes in anaphase. Since the initial discovery of the Bub and Mad family of genes in yeast genetic screens, nearly a dozen genes involved in mitotic checkpoint function have been identified in a range of different organisms [5••]. By operating at various points along a pathway which only allows anaphase once all chromosomes have achieved proper attachment to mitotic spindles, these checkpoint genes prevent the CIN which would result if anaphase were allowed to proceed before this achievement [5••]. It is precisely this sort of CIN which has been observed in mitotic checkpoint mutants in model organisms such as Saccharomyces cerevisiae and Drosophila melanogaster [3•].
To date, animal models have provided some of the best proof of principle that mitotic checkpoint deficiencies can contribute to CIN and tumor formation in mammals [5••]. Mice expressing reduced levels of the checkpoint proteins Mad2, BubR1, and Bub3 exhibit CIN, and some of these mice have increased susceptibility to cancer [20–23]. Furthermore, BubR1+/− mice exhibit an increased incidence of colon adenocarcinomas when exposed to azoxymethane [20]. When BubR1+/− mice are then crossed to APCMin/+ mice, the double heterozygote progeny exhibit a 10-fold higher incidence of spontaneous colonic tumors than APCMin/+ mice, as well as an augmentation of the mitotic checkpoint defect and CIN observed in BubR1+/− mice [24•]. As mentioned, APC mutation not only introduces chromosome attachment and positioning errors, but it also appears to dampen the ability of the mitotic checkpoint to address these errors [10,12•,13]. If this ability is dampened even further by BubR1 deficiency, perhaps the deadly synergism observed in BubR1+/−/APCMin/+ mice results both from an increase in the frequency of mitotic errors and a decrease in the ability to correct them. Whatever the mechanism, these data demonstrate that mitotic checkpoint deficiencies can, in principle, directly contribute to colorectal tumorigenesis.
The first evidence that mitotic checkpoint defects may occur in human colorectal cancer came from a pioneering report by Cahill et al. [25]. This study found that, compared with MIN colorectal cancer cell lines, CIN cell lines exhibit a marked defect in their ability to execute mitotic arrest when challenged with spindle-disrupting agents such as nocodazole and colcemid. The authors also found that out of 19 CIN cell lines studied, two contain mutations in the mitotic checkpoint gene BubR1 and two in the checkpoint gene Bub1, the latter of which, when expressed in checkpoint-competent MIN cells, elicits a dominant-negative inactivation of checkpoint activity. Thus, these authors conclude that reduced mitotic checkpoint activity may result from direct mutation of mitotic checkpoint genes. Consistent with this, additional studies in colorectal tumors have found mutations in established or putative mitotic checkpoint genes, including Bub1, Zw10, Zwilch, and Rod [3•,26,27].
However, such mutations in colorectal cancer are rare, and mutations in many checkpoint genes have failed to be detected at all [5••,28]. This had led some to investigate whether nonmutational disruption of mitotic checkpoint genes may occur in colorectal cancer. Shichiri et al. [26] report a nonmutational reduction in expression of the checkpoint proteins Bub1 and BubR1 in a small percentage of colorectal tumors. Interestingly, the majority of tumors examined in this study actually exhibit increased expression of these genes. Similarly, other studies have found increased expression of the checkpoint genes Mad2 and Mad2B in colorectal tumors [29–31]. As Hernando et al. [32] showed that increased expression of Mad2 will prolong mitosis and generate CIN in cultured cells, it is possible that too much, as well as too little, expression of mitotic checkpoint genes may impair mitotic function and genome stability. Along these lines, in addition to inducing chromosome attachment and congression defects, overexpression of Aurora-A has been reported to interfere with mitotic checkpoint signaling [19,33]. Likewise, overexpression of conductin, which is observed in CIN colorectal tumors, disrupts the mitotic checkpoint [11•]. Both reduced and increased expression of mitotic checkpoint genes have been reported to correlate with measures of disease severity in colorectal cancer [26,29–31]. How such expression changes play a causative role in driving CIN and colorectal tumorigenesis remains to be seen.
Amidst these studies, some of the original conclusions by Cahill et al. [25] have been subsequently challenged. Tighe et al. [14] conclude that CIN cell lines do possess a “robust” mitotic checkpoint after challenge with nocodazole, albeit one that, in at least some of the CIN lines, is weakened when compared with MIN cells. What accounts for the differences in these reports is unclear, and other studies are no more clarifying, as some data support the existence of mitotic checkpoint defects upon drug challenge in CIN cells [8,34,35] and some do not [8,36]. It is worth noting, however, that the assay used in all these studies—measurement of mitotic index over time in a population of cells treated with antimitotic drugs—does not, in itself, provide definitive evidence of a compromised mitotic checkpoint, as some cancer cell lines undergo G1 or G2 cell cycle arrest before ever reaching mitosis upon treatment with these drugs [37]. Thus, cells which exhibit such premitotic arrest may be falsely classified as possessing a defective mitotic checkpoint with this assay [37]. Furthermore, the nature of this premitotic arrest is dependent on the class and dose of antimitotic agent employed [37], so it is possible that some, although not all, of the differences between these studies might result from nonmitotic effects of the different drugs and concentrations used to challenge cells. To complicate these matters further, Grigorova et al. [38] have argued that the CIN cell lines in which Cahill et al. [25] found Bub1 and BubR1 mutations may not, in fact, exhibit CIN. Taken together, these studies raise some doubt over the extent to which mitotic checkpoint deficiencies accompany or govern CIN in colorectal cancer cell lines. Nevertheless, as we have seen, several lines of evidence do support a causative link. Perhaps with the increasing availability of time lapse videomicroscopy to observe individual cells in mitosis, future studies will help to clarify the role of mitotic checkpoint defects in CIN colorectal cancer cells.
Going Wrong in Pulling Apart: Chromosome Separation Defects
Once all metaphase chromosomes have properly attached to spindle microtubules, the arrest signal of the mitotic checkpoint is extinguished and the cell proceeds to separate its sister chromatids in anaphase. Although disruption of chromatid separation offers a potential path to CIN, whether, and how, such a path occurs in colorectal cancer remains largely unknown. One candidate for this pathway is dysregulation of the anaphase regulator securin, a gene upregulated in many cancers, including colorectal [39]. In fact, securin overexpression (or expression of its nondegradable form) can induce chromatid separation failure, aneuploidy, and transformation in cultured cells [40,41]. Another potential candidate for anaphase interruption is mutation of Ding, an uncharacterized gene recently found mutated in CIN colorectal tumors and homologous to the budding yeast anaphase inhibitor Pds1 [3•]. How dysregulation of these and other anaphase regulators contributes to CIN in colorectal cancer is worthy of further study.
Going Wrong in Splitting Up: Cytokinesis Defects
Because many human tumors, including colorectal, often exhibit aneuploidies which actually contain a near-tetraploid DNA content, some have proposed that polyploidization of the genome through cytokinesis failure may represent a common, even causative, phenomenon in tumorigenesis [4•,42]. As mentioned, it is possible that such whole genome duplication may indirectly promote CIN by providing a genetic “buffer” from which single chromosome gains and losses are more easily tolerated [2]. Moreover, due to the inheritance of an additional centrosome, as well as additional chromosomes, a tetraploid cell formed from cytokinesis failure can go on to produce a mitotic cell containing more than two spindle poles [42]. Such “multipolar” mitoses are indeed observed at increased frequency in cancer cells [5••], and these cells can, in principle, divide asymmetrically to produce aneuploid progeny. Thus, in addition to providing a genetic buffer for toleration of single chromosome changes, polyploidy could also increase the rate at which such changes arise.
At present, there are only limited data to inform ideas about the causes of spontaneous polyploidization in cancer, as many investigations of polyploidy focus on the tetraploidization which results from pharmacologic manipulations which have no clear physiologic equivalent [43]. Nonetheless, several of the aberrations already discussed, such as APC mutation, CENP-H overexpression, and Aurora-A overexpression, can induce polyploidization [8,9,16,19,44]. How this occurs is unknown. Interestingly, Shi and King [45] recently showed that in cultured human cells which spontaneously become binucleate through cytokinesis failure, there is an increased incidence of nondisjunction between the two nuclei. Although the authors conclude that a nondisjunction “sensor” triggers cytokinesis failure, others argue that such failure is more likely the result of chromatin trapped in the cytokinetic cleavage furrow [46]. Along these lines, Stewénius et al. [47•] have demonstrated that inhibition of telomerase in colorectal cancer cells produces not only an increase in anaphase bridging of dicentric chromosomes but also an increase in polyploidy and multipolar mitosis. Thus, one route to polyploidy might be cytokinesis failure caused by any process which leaves chromatin trapped in the cytokinetic cleavage furrow. Whether this mechanism, and others, is responsible for polyploidization in colorectal cancer is worthy of further study.
Data investigating the long-term consequences of spontaneous polyploidization are, likewise, limited. However, Stewénius et al. [47•] report that out of 29 spontaneous multipolar mitotic cells examined in a CIN colorectal cancer cell line, none had the ability to continue proliferating, and most did not even complete mitosis, providing some evidence against a role for multipolar mitosis in CIN. At the same time, Fujiwara et al. [48] have reported that p53−/ − mouse epithelial cells made tetraploid through pharmacologic inhibition of cytokinesis exhibit greater tumorigenicity and CIN than their diploid counterparts. However, the data provided as evidence for increased CIN in tetraploids are an approximately twofold increase in the accumulation rate of cells with nonmodal chromosome numbers and an approximately twofold increase in the total number of chromosomal rearrangements in the tetraploid population. Given that tetraploid cells have twice as many chromosomes, and thus twice as many opportunities to missegregate or rearrange one, a twofold increase in these rates would be expected even without increased CIN. Only an increased rate of missegregation or rearrangement per chromosome, rather than per cell or per population, would be evidence of increased CIN in tetraploids. As they stand, these data actually argue against the idea that tetraploidy is, in itself, sufficient to induce CIN, a conclusion consistent with the cell fusion experiments originally conducted by Lengauer et al. [49]. Nevertheless, the tetraploid cells in this study were indeed more tumorigenic than diploids, possibly providing evidence for the “genetic buffer” explanation for the utility of polyploidy in tumorigenesis. Perhaps future investigations will help clarify both the causes and consequences of cytokinesis failure and polyploidization in tumorigenesis.
Conclusions
As Storchova and Pellman [42] pointed out, aneuploidies in cancer are a bit like Tolstoy’s unhappy families: every aneuploidy is aneuploid in its own way. The same might be said for mitosis. All normal mitoses are alike; each abnormal mitosis is abnormal in its own way. As we have seen, some colorectal tumors go wrong in chromosome attachment and some in the mitotic checkpoint. Some go wrong in both. Some no doubt go wrong in ways not yet discovered. The diversity of means that a cancer cell has to disrupt mitosis offers one explanation for why defects in any one mitotic process may only occur in a minority of tumors. If disruption of genome stability, and by extension mitosis, truly is a goal of the silent hand of tumor evolution, then this hand has many options to choose from. Alternatively, tumor evolution may not care much about disrupting mitosis, and the variety of mitotic defects, in this scenario, merely represents the accumulation of evolutionary road kill. Like others, we favor the former explanation, and we thus eagerly anticipate future discoveries of other specific ways in which mitosis goes wrong in colorectal cancer.
Acknowledgments
This work was in part support by grants from the National Institutes of Health (DK52230 and CA84197). Dr. Yang was a recipient of a Georgian Cancer Coalition Distinguished Cancer Clinician Scientist Award.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.von Hansemann D. Ueber asymmetrische Zellheilteilung in epithelkrebsen und deren biologische bedeutung. Virschows Arch Pathol Anat. 1890;119:299–326. [Google Scholar]
- 2.Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C. The significance of unstable chromosomes in colorectal cancer. Nat Rev Cancer. 2003;3:695–701. doi: 10.1038/nrc1165. [DOI] [PubMed] [Google Scholar]
- 3•.Wang Z, Cummins JM, Shen D, et al. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res. 2004;64:2998–3001. doi: 10.1158/0008-5472.can-04-0587. This study identified three distinct pathways of CIN in colorectal cancer by determining whether mutations occur in genes that previously have been shown to cause CIN in model systems. [DOI] [PubMed] [Google Scholar]
- 4•.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. doi: 10.1016/j.ceb.2006.10.002. This review article discussed the relationship between aneuploidy and human cancer. [DOI] [PubMed] [Google Scholar]
- 5••.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. This is an excellent review article of the various defects observed in the mitotic checkpoint that are potential causes of aneuploidy. [DOI] [PubMed] [Google Scholar]
- 6.Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1:109–117. doi: 10.1038/35101065. [DOI] [PubMed] [Google Scholar]
- 7.Green RA, Kaplan KB. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J Cell Biol. 2003;163:949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaplan KB, Burds AA, Swedlow JR, et al. A role for the adenomatous polyposis coli protein in chromosome segregation. Nat Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- 9.Fodde R, Kuipers J, Rosenberg C, et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nat Cell Biol. 2001;3:433–438. doi: 10.1038/35070129. [DOI] [PubMed] [Google Scholar]
- 10.Draviam VM, Shapiro I, Aldridge B, Sorger PK. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. Embo J. 2006;25:2814–2827. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Hadjihannas MV, Bruckner M, Jerchow B, et al. Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci U S A. 2006;103:10747–10752. doi: 10.1073/pnas.0604206103. This study describes the possible mechanism by which Wnt signaling pathway may contribute to CIN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol Biol Cell. 2005;16:4609–4622. doi: 10.1091/mbc.E05-03-0259. This study describes how APC may regulate mitotic spindle function and chromosome segregation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tighe A, Johnson VL, Taylor SS. Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability. J Cell Sci. 2004;117:6339–6353. doi: 10.1242/jcs.01556. [DOI] [PubMed] [Google Scholar]
- 14.Tighe A, Johnson VL, Albertella M, Taylor SS. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2001;2:609–614. doi: 10.1093/embo-reports/kve127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Rajagopalan H, Jallepalli PV, Rago C, et al. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. This study demonstrated how inactivation of the hCDC4 gene, which is commonly mutated in colorectal cancer, induces CIN through defects in chromosome congression and segregation. [DOI] [PubMed] [Google Scholar]
- 16.Tomonaga T, Matsushita K, Ishibashi M, et al. Centromere protein H is up-regulated in primary human colorectal cancer and its overexpression induces aneuploidy. Cancer Res. 2005;65:4683–4689. doi: 10.1158/0008-5472.CAN-04-3613. [DOI] [PubMed] [Google Scholar]
- 17.Gerlach U, Kayser G, Walch A, et al. Centrosome-, chromosomal-passenger- and cell-cycle–associated mRNAs are differentially regulated in the development of sporadic colorectal cancer. J Pathol. 2006;208:462–472. doi: 10.1002/path.1914. [DOI] [PubMed] [Google Scholar]
- 18.Ewart-Toland A, Briassouli P, de Koning JP, et al. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nat Genet. 2003;34:403–412. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- 19.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3:51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]
- 20.Dai W, Wang Q, Liu T, et al. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004;64:440–445. doi: 10.1158/0008-5472.can-03-3119. [DOI] [PubMed] [Google Scholar]
- 21.Baker DJ, Jeganathan KB, Cameron JD, et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–749. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 22.Babu JR, Jeganathan KB, Baker DJ, et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160:341–353. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michel LS, Liberal V, Chatterjee A. MAD2 haploinsufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 24•.Rao CV, Yang YM, Swamy MV, et al. Colonic tumorigenesis in BubR1+/−ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc Natl Acad Sci U S A. 2005;102:4365–4370. doi: 10.1073/pnas.0407822102. This study demonstrated enhanced colonic tumor formation and genetic instability in mice that are compound heterozygous for the BubR1 and ApcMin mutations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill DP, Lengauer C, Yu J, et al. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 26.Shichiri M, Yoshinaga K, Hisatomi H, et al. Genetic and epigenetic inactivation of mitotic checkpoint genes hBUB1 and hBUBR1 and their relationship to survival. Cancer Res. 2002;62:13–17. [PubMed] [Google Scholar]
- 27.Imai Y, Shiratori Y, Kato N, et al. Mutational inactivation of mitotic checkpoint genes, hsMAD2 and hBUB1, is rare in sporadic digestive tract cancers. Jpn J Cancer Res. 1999;90:837–840. doi: 10.1111/j.1349-7006.1999.tb00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahill DP, da Costa LT, Carson-Walter EB, et al. Characterization of MAD2B and other mitotic spindle checkpoint genes. Genomics. 1999;58:181–187. doi: 10.1006/geno.1999.5831. [DOI] [PubMed] [Google Scholar]
- 29.Li GQ, Li H, Zhang HF. Mad2 and p53 expression profiles in colorectal cancer and its clinical significance. World J Gastroenterol. 2003;9:1972–1975. doi: 10.3748/wjg.v9.i9.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li GQ, Zhang HF. Mad2 and p27 expression profiles in colorectal cancer and its clinical significance. World J Gastroenterol. 2004;10:3218–3220. doi: 10.3748/wjg.v10.i21.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rimkus C, Friederichs J, Rosenberg R, et al. Expression of the mitotic checkpoint gene MAD2L2 has prognostic significance in colon cancer. Int J Cancer. 2007;120:207–211. doi: 10.1002/ijc.22155. [DOI] [PubMed] [Google Scholar]
- 32.Hernando E, Nahle Z, Juan G, et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature. 2004;430:797–802. doi: 10.1038/nature02820. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Zhang Y, Lees E, Seghezzi WL. AuroraA overexpression overrides the mitotic spindle checkpoint triggered by nocodazole, a microtubule destabilizer. Oncogene. 2003;22:8293–8301. doi: 10.1038/sj.onc.1206873. [DOI] [PubMed] [Google Scholar]
- 34.Tao W, South VJ, Zhang Y, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49–59. doi: 10.1016/j.ccr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Kasai T, Iwanaga Y, Iha H, Jeang KT. Prevalent loss of mitotic spindle checkpoint in adult T-cell leukemia confers resistance to microtubule inhibitors. J Biol Chem. 2002;277:5187–5193. doi: 10.1074/jbc.M110295200. [DOI] [PubMed] [Google Scholar]
- 36.Scolnick DM, Halazonetis TD. Chfr defines a mitotic stress checkpoint that delays entry into metaphase. Nature. 2000;406:430–435. doi: 10.1038/35019108. [DOI] [PubMed] [Google Scholar]
- 37.Blajeski AL, Phan VA, Kottke TJ, Kaufmann SH. G(1) and G(2) cell-cycle arrest following microtubule depolymerization in human breast cancer cells. J Clin Invest. 2002;110:91–99. doi: 10.1172/JCI13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigorova M, Staines JM, Ozdag H, et al. Possible causes of chromosome instability: comparison of chromosomal abnormalities in cancer cell lines with mutations in BRCA1, BRCA2, CHK2 and BUB1. Cytogenet Genome Res. 2004;104:333–340. doi: 10.1159/000077512. [DOI] [PubMed] [Google Scholar]
- 39.Heaney AP, Singson R, McCabe CJ, et al. Expression of pituitary-tumour transforming gene in colorectal tumours. Lancet. 2000;355:716–719. doi: 10.1016/S0140-6736(99)10238-1. [DOI] [PubMed] [Google Scholar]
- 40.Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister–chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999;285:418–422. doi: 10.1126/science.285.5426.418. [DOI] [PubMed] [Google Scholar]
- 41.Yu R, Lu W, Chen J, et al. Overexpressed pituitary tumor-transforming gene causes aneuploidy in live human cells. Endocrinology. 2003;144:4991–4998. doi: 10.1210/en.2003-0305. [DOI] [PubMed] [Google Scholar]
- 42.Storchova Z, Pellman D. From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol. 2004;5:45–54. doi: 10.1038/nrm1276. [DOI] [PubMed] [Google Scholar]
- 43.Stukenberg PT. Triggering p53 after cytokinesis failure. J Cell Biol. 2004;165:607–608. doi: 10.1083/jcb.200405089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. Embo J. 2002;21:483–492. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Q, King RW. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. doi: 10.1038/nature03958. [DOI] [PubMed] [Google Scholar]
- 46.Weaver BA, Silk AD, Cleveland DW. Cell biology: nondisjunction, aneuploidy and tetraploidy. Nature. 2006;442:E9–10. doi: 10.1038/nature05139. [DOI] [PubMed] [Google Scholar]
- 47•.Stewénius Y, Gorunova L, Jonson T, et al. Structural and numerical chromosome changes in colon cancer develop through telomere–mediated anaphase bridges, not through mitotic multipolarity. Proc Natl Acad Sci U S A. 2005;102:5541–5546. doi: 10.1073/pnas.0408454102. This study is the first to investigate the long-term consequences of multipolar mitosis in colorectal cancer cells, providing evidence that such mitoses may not contribute to CIN or tumorigenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara T, Bandi M, Nitta M, et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 49.Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]