Abstract
Context
Experimental data support the hypothesis that oxidized low-density lipoprotein (LDL) is associated with the metabolic syndrome. However, this hypothesis has not been tested in humans.
Objective
To establish the relation of oxidized LDL with metabolic syndrome in the general community.
Design, Setting, and Participants
The Coronary Artery Risk Development in Young Adults (CARDIA) study is a population-based, prospective, observational study. We studied 1889 participants who were between the ages of 18 and 30 years at the time of recruitment in 1985 and 1986 and living in 1 of 4 US metropolitan areas (41% African American; 56% women) and were seen both at year 15 (2000–2001, ages 33–45 years) and year 20 examinations (2005–2006).
Main Outcome Measure
The longitudinal association of oxidized LDL and incident metabolic syndrome. Oxidized LDL was measured with a monoclonal antibody-based enzyme-linked immunosorbent assay. The metabolic syndrome was defined according to the Adult Treatment Panel III of the National Cholesterol Education Program.
Results
Incident metabolic syndrome was diagnosed at the year 20 follow-up in 12.9% (243 of 1889) of participants who did not have metabolic syndrome at the 15-year followup. The odds ratios (ORs) for incident metabolic syndrome after 5 years' follow-up and adjusted for age, sex, race, study center, cigarette smoking, body mass index, physical activity, and LDL cholesterol levels by quintiles of oxidized LDL were 2.1 (95% confidence interval [CI], 1.1–3.8) for the second quintile (55.4–69.1 U/L); 2.4 (95% CI, 1.3–4.3) for the third quintile (69.2–81.2 U/L); 2.8 (95% CI, 1.5–5.1) for the fourth quintile (81.3–97.3 U/L); and 3.5(95%CI, 1.9–6.6) for the fifth quintile (≥97.4 U/L). The adjusted ORs for incidence of dichotomous components of metabolic syndrome in the highest vs the lowest quintile of oxidized LDL were 2.1 (95% CI, 1.2–3.6) for abdominal obesity, 2.4 (95% CI, 1.5–3.8) for high fasting glucose, and 2.1 (95% CI, 1.1–4.0) for high triglycerides. Low-density lipoprotein cholesterol was not associated with incident metabolic syndrome or with any of its components in the fully adjusted model containing oxidized LDL.
Conclusion
Higher concentration of oxidized LDL was associated with increased incidence of metabolic syndrome overall, as well as its components of abdominal obesity, hyperglycemia, and hypertriglyceridemia.
Persons with the metabolic syndrome are at increased risk of developing type 2 diabetes and coronary heart disease (CHD) as well as increased mortality from CHD and other causes.1,2 Findings from the Third National Health and Nutrition Examination Survey showed that prevalence of metabolic syndrome increased with age from 6.7% among participants aged 20 to 29 years, to 43.5% for participants aged 60 to 69 years, and 42.0% for those aged 70 years or older.3
Several groups developed assays for oxidation-specific epitopes on plasma low-density lipoprotein (LDL). Two assays use antibodies against oxidized phospholipids4,5; our assay uses the monoclonal antibody (mAb) 4E6 directed against an oxidation-dependent epitope in the apolipoprotein B-100 moiety of LDL.6,7 It is generally believed that “fully oxidized LDL” does not exist in the circulation. Blood is rich in antioxidants. In addition, such highly oxidized particles would be rapidly cleared in the liver via scavenger receptors.8 In contrast, circulating minimally oxidized LDL in which oxidative modification has not been sufficient to cause changes recognized by scavenger receptors was demonstrated.9 Therefore, all assays for oxidized LDL presumably detect minimally oxidized LDL. This oxidized LDL is only a minor fraction of LDL ranging from 0.001% in healthy controls10 to approximately 5% in patients with acute coronary events.6 Because LDL is the substrate for oxidation, concentrations of oxidized LDL correlate with LDL concentrations, and in turn with the cholesterol within LDL. In addition, concentrations of oxidized LDL depend on the sensitivity of LDL particles to oxidation; small dense LDL contains smaller amounts of antioxidants and are therefore more prone to oxidation. Previously, metabolic syndrome was shown to be associated with a higher prevalence of small dense LDL.11
Using the mAb-4E6–based enzyme-linked immunosorbent assay (ELISA), we have shown that in the Health, Aging, and Body Composition cohort, a high coronary heart disease risk status (based on the Framingham score) before coronary heart disease events is associated with high concentrations of circulating oxidized LDL, even after adjustment for LDL cholesterol.12 Because individuals with the metabolic syndrome are at increased risk of macrovascular disease, death, or both,2,13 it was no surprise that metabolic syndrome was associated with high concentrations of oxidized LDL.14–17
No study, however, has examined the relation between oxidized LDL and the development of the metabolic syndrome. Because biological studies in cellular and animal models do suggest that oxidized LDL contributes to processes that lead to the incidence of metabolic syndrome,18,19 we hypothesized that oxidized LDL is associated with the incidence of metabolic syndrome. We tested this hypothesis by determining the association between the concentration of oxidized LDL and the incidence 5 years later of metabolic syndrome and its components.
METHODS
Study Population
The Cardiovascular Risk Development in Young Adults (CARDIA) Study is a longitudinal investigation of CHD risk factors in 5115 men and women between the ages of 18 and 30 years at study inception. Details of the study design, recruitment, and procedures have been published elsewhere.20,21 Participants were recruited between 1985 and 1986 at 4 US clinical sites: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. Participants were reexamined at 2, 5, 7, 10, 15, and 20 years after inception, with reexamination rates among surviving cohort members of 90% at 2, 86% at 5, 81% at 7, 79% at 10, 74% at 15, and 72% at 20 years. All participants signed extensive consent forms at each examination, with all aspects reviewed and approved by the institutional review board of each participating institution. The full CARDIA Study sample at baseline was balanced by age (45% aged 18–24 years; 55% aged 25–30 years), race (52% African American; 48% white), sex (46% men; 54%, women), and educational achievement (40% completed 12 years; 60% >12 years).
Oxidized LDL was assessed in 2823 participants at year 15 (2000–2001) as part of the Young Adult Longitudinal Trends in Antioxidants (YALTA) ancillary study to CARDIA. Among them, we excluded participants who were pregnant, did not fast at least 8 hours, or had missing data (FIGURE). Metabolic syndrome components were defined as detailed in the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) report22: (1) waist circumference of 102 cm or more in men and 88 cm or more in women; (2) fasting triglycerides of 150 mg/dL or more; (3) HDL cholesterol of less than 40 mg/dL in men and less than 50 mg/dL in women; (4) blood pressure of 130/85 mm Hg or higher or taking antihypertensive medication; and (5) fasting glucose 100 mg/dL or higher or taking antidiabetic medication. Persons with at least 3 of these characteristics were defined as having metabolic syndrome.23,24 At year 15, 372 participants (14.2%) were diagnosed with metabolic syndrome (Figure) and were excluded from this longitudinal study. We also excluded participants who were pregnant, did not fast at least 8 hours, and had missing data. Incident metabolic syndrome was diagnosed at year 20 (2005–2006) in 12.9% (243 of 1889) of participants. Among them, 71 were taking antihypertensive medication; 13, antidiabetic medication; and 13, cholesterol-lowering medication.
Figure. Study Flow Diagram.
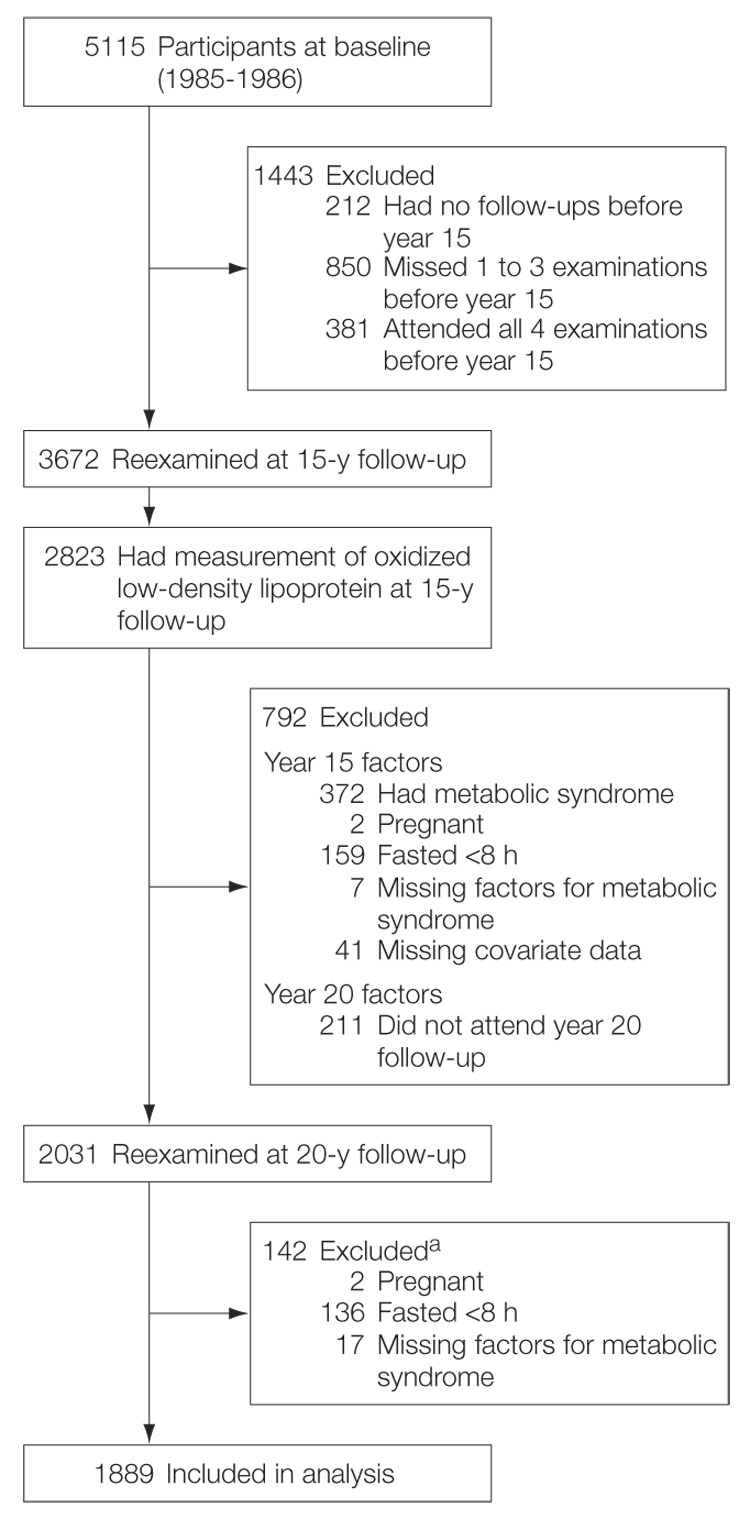
The Figure shows the selection of 1889 participants who did not have metabolic syndrome at year 15 (2000–2001) for whom fresh frozen blood samples were available at year 15 for measuring plasma oxidized low-density lipoprotein and for whom all data were available at years 15 and 20 (2005–2006).
aParticipants could be excluded for more than 1 reason.
To convert triglycerides to millimoles per liter, multiply by 0.0113; HDL, LDL, and total cholesterol to millimoles per liter, multiply by 0.0259; and glucose to millimoles per liter, multiply by 0.0555.
Measurements
Information on demographic characteristics (age, sex, and race) and educational achievement was self-reported on standardized questionnaires during each examination. The participant self-reported race of both natural mother and natural father as “white, not Hispanic” or “black, not Hispanic,” and was assigned parental race (mixed race excluded). During the examination, height and weight were measured. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.2 Waist circumference was measured as the abdominal girth midway between the iliac crest and the bottom of the ribcage. Hip circumference was determined at the maximum protrusion of the hips at the level of the symphysis pubis.
Smoking was measured as self-reported current smoking status (nonsmoker, ex-smoker,and current smoker). Habitual physical activity was measured by use of the CARDIA Physical Activity History, a simplified version of the Minnesota Leisure Time Physical Activity Questionnaire.24 Three seated blood pressure measurements were obtained with a random-zero sphygmomanometer; the mean of the second and third readings was used for this report.
Participants were asked to avoid smoking and heavy physical activity for at least 2 hours before each examination and those included herein were fasting at least 8 hours. Blood was taken from participants, and centrifuged, with aliquots stored at −70°C until shipped on dry ice to a central laboratory. All laboratory assessments were performed without knowledge of other CARDIA data. Plasma lipids were measured in samples of participants at year 15 and 20 at the University of Washington Northwest Lipid Research Clinic Laboratory (Seattle). Total cholesterol and triglycerides were measured by enzymatic methods. High-density lipoprotein(HDL)cholesterol was assayed after dextran sulfate-magnesium precipitation, and LDL cholesterol was estimated from the Friedewald equation.25 The 25 individuals with triglycerides higher than 400mg/dL were excluded from this calculation. Concentrations of oxidized LDL were measured in year 15 plasma samples at the Atherosclerosis and Metabolism Unit in Leuven by means of an mAb-4E6–based competition ELISA (Mercodia, Uppsala, Sweden).mAb-4E6 is directed against a conformational epitope in the apolipoprotein B-100 moiety of LDL that is generated as a consequence of the substitution of 60 lysine residues of apolipoprotein B-100 with aldehydes. Substituting aldehydes can be produced by peroxidation of lipids of LDL, which results in the generation of oxidized LDL. However, lipid peroxidation is not required. Indeed, aldehydes that are released by endothelial cells under oxidative stress or by activated platelets may also induce the oxidative modification of apolipoprotein B-100 in the absence of peroxidation of lipids of LDL.26 The analytical performance of this assay has been described elsewhere.27 Its coefficient of variation was 7.4%. Serum glucose was measured by means of hexokinase coupled to glucose-6-phosphate dehydrogenase, as was serum insulin with an immunoassay (Linco Research Inc, St Louis, Missouri). High-sensitivity ELISAs measured serum C-reactive protein at the Department of Pathology, University of Vermont, as described previously. 28 Adiponectin was measured in serum by radioimmunoassay at Linco Research, using a polyclonal antibody raised in a rabbit and purified recombinant adiponectin standards with an effective range of 0.2 to 40 mg/L.29
Statistical Analyses
Longitudinal associations were studied between year 15 plasma oxidized LDL as the independent variable of interest and year 20 metabolic syndrome as the dependent variable among individuals free of prevalent metabolic syndrome at year 15. Multiple logistic regression was used to examine the association of the quintiles of oxidized LDL and metabolic syndrome and its constituent risk factors, adjusted for age (years), sex, race, and study center. Further year 15 covariates in the fully adjusted model included cigarette smoking, BMI, physical activity, and LDL cholesterol. C-reactive protein, adiponectin, and antidiabetic, antihypertensive, and cholesterol-lowering medication were separately considered as possible mediators in the associations. We also established associations between LDL cholesterol and metabolic syndrome to compare with those of oxidized LDL, after adjusting for each other. All analyses were performed with SAS version 9.1 (SAS Institute Inc, Cary, North Carolina). A P value of less than .05 was considered statistically significant.
RESULTS
Among the 1889 participants included in the longitudinal analyses, oxidized LDL was positively associated with male sex, black race, BMI, and obesity (BMI >30), all metabolic syndrome components, and C-reactive protein (TABLE 1). Oxidized LDL had a correlation coefficient with LDL cholesterol of 0.6 and was inversely associated with HDL cholesterol and adiponectin. In contrast, LDL cholesterol was not related to race and C-reactive protein.
Table 1.
Cross-sectional Associations Between Year 15 Oxidized Low-Density Lipoprotein or Low-Density Lipoprotein Cholesterol and Demographic, Health Behaviors, and Clinical Variables (N = 1889 Without Metabolic Syndrome at Year 15)a
| Quintiles of Oxidized LDL (U/L) |
||||||
|---|---|---|---|---|---|---|
| 1(<55.4) | 2(55.4–69.1) | 3(69.2–81.2) | 4(81.3–97.3) | 5(≥97.4) | P Value for Trenda | |
| Participants, No. | 377 | 378 | 373 | 386 | 375 | |
| Age, y | 40.1 | 40.2 | 40.2 | 40.2 | 40.4 | .45 |
| Men, % | 34.8 | 38.3 | 40.5 | 51.5 | 56.5 | <.001 |
| African American % | 33.8 | 36.2 | 43.5 | 42.2 | 47.8 | <.001 |
| BMI | ||||||
| Mean | 25.8 | 26.4 | 27.7 | 27.9 | 28.7 | <.001 |
| >30, % | 15.4 | 17.9 | 27.4 | 25.7 | 32.9 | <.001 |
| Current smoker, % | 17.5 | 18.1 | 20.4 | 14.7 | 18.3 | .77 |
| Cholesterol, mean, mg/dL | ||||||
| LDL | 87.1 | 102.1 | 109.9 | 122.3 | 141.4 | <.001 |
| HDL | 57.4 | 54.0 | 53.6 | 50.9 | 48.6 | <.001 |
| Triglyceride, mean, mg/dL | 67.9 | 77.1 | 83.8 | 91.2 | 116.3 | <.001 |
| Glucose, mean, mg/dL | 81.9 | 83.1 | 83.0 | 84.1 | 84.9 | <.001 |
| Insulin, mean, µU/mL | 10.3 | 11.4 | 12.0 | 12.3 | 14.1 | <.001 |
| Blood pressure, mean, mm Hg | ||||||
| Systolic | 108.9 | 109.7 | 111.3 | 110.3 | 112.1 | <.001 |
| Diastolic | 70.7 | 72.6 | 73.5 | 72.8 | 73.7 | .001 |
| Waist circumference, mean, cm | 81.9 | 84.0 | 86.4 | 86.8 | 90.1 | <.001 |
| C-reactive protein, mean, mg/L | 1.6 | 1.7 | 1.9 | 1.7 | 2.0 | .04 |
| Adiponectin, mean, mg/L | 13.1 | 12.1 | 11.6 | 11.0 | 10.2 | <.001 |
|
Quintiles of LDL Cholesterol (mg/dL) |
||||||
| 1(<86) | 2(86–101) | 3(102–118) | 4(119–138) | 5(≥139) | ||
| Participants, No. | 367 | 381 | 396 | 364 | 381 | |
| Age, mean, y | 40.2 | 40.0 | 40.3 | 40.1 | 40.5 | .15 |
| Men, % | 34.2 | 36.0 | 42.3 | 50.5 | 58.7 | <.001 |
| African American, % | 41.9 | 35.4 | 41.1 | 43.2 | 42.1 | .30 |
| BMI | ||||||
| Mean | 25.6 | 27.2 | 27.6 | 27.6 | 28.5 | <.001 |
| >30, % | 16.0 | 23.0 | 24.9 | 25.1 | 29.8 | <.001 |
| Current smoker, % | 23.4 | 19.2 | 14.8 | 16.0 | 15.7 | .003 |
| Oxidized LDL, mean, U/L | 56.6 | 67.8 | 76.9 | 84.7 | 99.9 | <.001 |
| HDL cholesterol, mean, mg/dL | 57.0 | 53.7 | 52.4 | 51.1 | 50.4 | <.001 |
| Triglyceride, mean, mg/dL | 76.0 | 80.7 | 84.2 | 93.1 | 102.1 | <.001 |
| Glucose, mean, mg/dL | 81.2 | 82.8 | 83.5 | 84.2 | 85.3 | <.001 |
| Insulin, mean, µU/mL | 10.6 | 11.5 | 12.2 | 12.5 | 13.2 | <.001 |
| Blood pressure, mean, mm Hg | ||||||
| Systolic | 109.5 | 110.1 | 110.5 | 110.3 | 111.7 | .02 |
| Diastolic | 71.4 | 72.1 | 73.3 | 72.7 | 73.8 | <.001 |
| Waist circumference, mean, cm | 82.3 | 84.9 | 86.2 | 86.8 | 88.9 | <.001 |
| C-reactive protein, mean, mg/L | 1.8 | 1.6 | 2.0 | 1.7 | 1.8 | .60 |
| Adiponectin, mean, mg/L | 13.0 | 11.8 | 11.6 | 10.8 | 10.8 | <.001 |
Abbreviations: BMI, body mass index, which is calculated as weight in kilograms divided by height in meters squared; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
SI conversion factors: To convert glucose to millimoles per liter, multiply values by 0.0555; C-reactive protein to nanomoles per liter, multiply by 9.524; HDL cholesterol to millimoles per liter, multiply by 0.0259; and triglycerides to millimoles per liter, multiply by 0.0113.
Tabulated data are linear regression estimates of means that were adjusted for age, sex, race, and study center.
Oxidized LDL showed a graded relation to incident metabolic syndrome, amounting to an adjusted odds ratio (OR) of 3.5 in the top quintile of oxidized LDL compared with the lowest quintile in the fully adjusted model that includedage, sex, race, study center, cigarette smoking, BMI, physical activity, and LDL cholesterol (TABLE 2). Addition of C-reactive protein, adiponectin, and antihypertensive, antidiabetic, and cholesterol-lowering medication did not materially reduce the ORs. The adjusted ORs for oxidized LDL by quintiles of oxidized LDL were 2.1 (95% confidence interval [CI], 1.1–3.8) for the second quintile (55. 4–69.1 U/L); 2.4 (95% CI, 1.3–4.3) for the third quintile (69.2–81.3 U/L); 2.8(95%CI, 1.5–5.1) for the fourth quintile (81.3–97.3 U/L), and 3.5 (95% CI, 1.9–6.6) for the fifth quintile (97.4 U/L; P for trend<.001) compared with the lowest quintile of oxidized LDL.
Table 2.
Incidence of Year 20 Metabolic Syndrome by Year 15 Oxidized Low-Density Lipoprotein or Low-Density Lipoprotein Cholesterol Concentrations (N = 1889 Without Metabolic Syndrome at Year 15)a
| Quintiles of Oxidized LDL (U/L) |
||||||
|---|---|---|---|---|---|---|
| 1(<55.4) | 2(55.4–69.1) | 3(69.2–81.2) | 4(81.3–97.3) | 5(≥97.4) | P Value for Trend | |
| Cases/No. | 20/377 | 39/378 | 51/373 | 76/375 | ||
| Modelb | ||||||
| 1 | 1 [Reference] | 2.0 (1.2–3.6) | 2.7 (1.6–4.7) | 3.0 (1.7–5.1) | 4.1 (2.4–7.0) | <.001 |
| 2 | 1 [Reference] | 2.0 (1.1–3.6) | 2.2 (1.3–.4.0) | 2.5 (1.4–4.5) | 3.1 (1.8–5.4) | <.001 |
| 3 | 1 [Reference] | 2.1 (1.1–3.8) | 2.4 (1.3–4.3) | 2.8 (1.5–5.1) | 3.5 (1.9–6.6) | <.001 |
|
Quintiles of LDL Cholesterol (mg/dL) |
||||||
| 1(<86) | 2(86–101) | 3(102–118) | 4(119–138) | 5(≥139) | ||
| Cases/No. | 30/367 | 51/381 | 42/396 | 56/364 | 64/381 | |
| Modelb | ||||||
| 1 | 1 [Reference] | 1.8 (1.1–2.9) | 1.3 (0.8–2.2) | 2.0 (1.2–3.2) | 2.1 (1.3–3.4) | .002 |
| 2 | 1 [Reference] | 1.5 (0.9–2.5) | 1.0 (0.6–1.8) | 1.7 (1.1–2.9) | 1.5 (0.9–2.5) | .07 |
| 3 | 1 [Reference] | 1.2 (0.7–2.0) | 0.7 (0.4–1.3) | 1.1 (0.6–1.9) | 0.9 (0.5–1.5) | .55 |
SI conversion factors: To convert low-density lipoprotein (LDL) cholesterol to millimoles per liter, multiply values by 0.0259.
Data are presented as odds ratio (95% confidence interval).
Model 1 is adjusted for age, sex, race, and study center. Model 2 is adjusted for model 1 variables and for year 15 cigarette smoking status, physical activity, and body mass index (calculated as weight in kilograms divided by height in meters squared). Model 3 is adjusted for model 2 variables and for year 15 LDL cholesterol or oxidized LDL.
Low-density lipoprotein cholesterol was associated with the metabolic syndrome when adjusting for age, sex, race, and study center. This association was not significant when adjusting further for year 15 cigarette smoking, physical activity, and BMI. There was no association after adjustment for oxidized LDL (Table 2).
The ORs for incidence of dichotomous components of metabolic syndrome in the top quintile compared with the lowest quintile of oxidized LDL (adjusted as in model 3 of Table 2 including LDL cholesterol) were 2.4 (95% CI, 1.5–3.8; P for trend=.002) for high fasting glucose (≥100 mg/dL or taking antidiabetic medication), 2.1 (95% CI, 1.2–3.6; P for trend=.004) for abdominal obesity, and 2.1 (95% CI, 1.1–4.0; P for trend=.008) for triglycerides but was not significant for blood pressure or HDL cholesterol (TABLE 3). Numbers of study participants at risk at year 15 for incidence of each metabolic syndrome component at year 20 were 1635 for high blood pressure, 1851 for high fasting glucose, 1525 for abdominal obesity, 1344 for low HDL cholesterol, and 1741 for high triglycerides. Low-density lipoprotein cholesterol was not associated with incidence of any of the metabolic syndrome components (Table 3).
Table 3.
Incidence of Each Component of the Metabolic Syndrome at Year 20 by Year 15 Oxidized Low-Density Lipoprotein Concentrations or Low-Density Lipoprotein Concentrations (N = 1889 Without Metabolic Syndrome at Year 15)a
| Quintiles of Oxidized LDL (U/L) |
||||||
|---|---|---|---|---|---|---|
| 1(<55.4) | 2(55.4–69.1) | 3(69.2–81.2) | 4(81.3–97.3) | 5(≥97.4) | P Value for Trend | |
| High blood pressure | ||||||
| No./total No. of participants | 38/338 | 44/330 | 41/318 | 37/328 | 50/307 | |
| OR (95% CI) | 1 [Reference] | 1.0 (0.6–1.7) | 0.8 (0.5–1.4) | 0.7 (0.4–1.2) | 0.9 (0.5–1.6) | .36 |
| High fasting glucose | ||||||
| No./total No. of participants | 47/371 | 71/363 | 73/364 | 83/372 | 118/363 | |
| OR (95% CI) | 1 [Reference] | 1.6 (1.1–2.5) | 1.5 (1.0–2.4) | 1.6 (1.0–2.5) | 2.4 (1.5–3.8) | .002 |
| Abdominal obesity | ||||||
| No./total No. of participants | 38/335 | 39/317 | 45/280 | 54/309 | 51/269 | |
| OR (95% CI) | 1.0 | 1.1 (0.7–1.9) | 1.5 (0.9–2.4) | 1.8 (1.1–3.1) | 2.1 (1.2–3.6) | .004 |
| Low HDL cholesterol | ||||||
| No./total No. of participants | 19/304 | 20/277 | 29/269 | 30/259 | 21/225 | |
| OR (95% CI) | 1 [Reference] | 0.9 (0.5–1.7) | 1.1 (0.6–2.1) | 1.1 (0.6–2.2) | 0.8 (0.4–1.8) | .84 |
| High triglyceride | ||||||
| No./total No. of participants | 23/372 | 27/363 | 32/345 | 43/353 | 49/299 | |
| OR (95% CI) | 1 [Reference] | 1.0 (0.6–1.8) | 1.2 (0.6–2.1) | 1.5 (0.8–2.7) | 2.1 (1.1–4.0) | .008 |
|
Quintiles of LDL Cholesterol (mg/dL) |
||||||
| 1(<86) | 2(86–101) | 3(102–118) | 4(119–138) | 5(≥139) | ||
| High blood pressure | ||||||
| No./total No. of participants30/ | 39/321 | 43/338 | 37/339 | 44/315 | 47/308 | |
| OR (95% CI) | 1 [Reference] | 1.1 (0.7–1.8) | 0.9 (0.5–1.5) | 1.2 (0.7–2.0) | 1.2 (0.7–2.2) | .41 |
| High fasting glucose | ||||||
| No./total No. of participants | 49/358 | 84/371 | 70/384 | 81/352 | 108/368 | |
| OR (95% CI) | 1 [Reference] | 1.5 (1.0–2.3) | 0.9 (0.6–1.4) | 1.1 (0.7–1.7) | 0.8 (0.6–2.0) | .86 |
| Abdominal obesity | ||||||
| No./total No. of participants | 38/313 | 47/308 | 47/313 | 47/286 | 48/290 | |
| OR (95% CI) | 1 [Reference] | 1.3 (0.8–2.1) | 1.1 (0.7–1.9) | 1.3 (0.7–2.1) | 1.1 (0.6–2.0) | .76 |
| Low HDL cholesterol | ||||||
| No./total No. of participants | 12/282 | 25/281 | 26/268 | 32/240 | 24/263 | |
| OR (95% CI) | 1 [Reference] | 2.1 (1.0–4.4) | 2.1 (1.0–4.6) | 3.0 (1.4–6.4) | 2.0 (0.9–4.8) | .10 |
| High triglyceride | ||||||
| No./total No. of participants | 16/349 | 27/355 | 38/375 | 52/330 | 41/323 | |
| OR (95% CI) | 1 [Reference] | 1.3 (0.7–2.6) | 1.6 (0.8–3.1) | 2.5 (1.3–4.8) | 1.5 (0.7–3.0) | .13 |
Abbreviations: CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio.
SI conversion factors: To convert LDL cholesterol to millimoles per liter, multiply values by 0.0259.
Data are adjusted for age, sex, race, study center, cigarette smoking, physical activity, body mass index (calculated as weight in kilograms divided by height in meters squared), and LDL cholesterol or oxidized LDL (body mass index was not included in the model when abdominal obesity was the dependent variable). Each row is a separate logistic regression model in which prevalent cases of the dependent variable (that is, each component of metabolic syndrome) were excluded. The 5 metabolic syndrome components are defined in the “Methods” section.
COMMENT
This population-based study showed that oxidized LDL, a marker of oxidative stress specific to LDL particles, was significantly associated with the incidence of metabolic syndrome. In particular, oxidized LDL was associated with 2 of the 3 metabolic syndrome factors defined according to ATPIII: (1) the central metabolic factor comprising obesity and hypertriglycemia and (2) the glucose factor, as previously defined.30,31 Oxidized LDL was not associated with elevated blood pressure or HDL cholesterol. These associations remained significant after adjustment for age, sex, race, smoking, physical activity, and LDL cholesterol. These associations also remained significant after adjustment for C-reactive protein, adiponectin, and antihypertensive, and antidiabetic and cholesterol-lowering medication. In contrast, LDL cholesterol showed only a limited relation with metabolic syndrome, which tended to become flat in the fully adjusted model including oxidized LDL.
A novel finding of this study is that oxidized LDL was associated with incident metabolic syndrome. As yet, it is not possible to conclude whether oxidized LDL is a marker related to mechanistic underlying factors on the pathway to the development of metabolic syndrome, or whether it is by itself a functional intermediary in this pathway. However, the strong association of oxidized LDL with the incidence of metabolic syndrome is consistent with a causal role.
Our data are in agreement with the previous observation that circulating oxidized LDL is associated with obesity and that weight loss results in a decrease of oxidized LDL.32 This association may be explained by the occurrence of small dense LDL that is more prone to oxidation.11 Another possible explanation is that adipose tissue contributes to the oxidation of LDL by 2 biochemical actions. First, greater adipose tissue may increase production of arachidonate-5-lipoxygenase, which catalyzes LDL oxidation. Second, greater adipose tissue may decrease production of superoxide dismutase, which prevents LDL oxidation.33 Our longitudinal data suggest that oxidized LDL may be associated with the increase of adipose tissue in agreement with experimental findings that oxidized LDL contributes either directly by contributing to the hyperplasia and the hypertrophy of adipocytes18 or indirectly by increasing the infiltration of activated monocytes/ macrophages, which increase adipogenesis. 34 Interestingly, we observed a higher prevalence of obesity in the higher quintiles of oxidized LDL.
In addition, the observed association between glycemia and oxidized LDL is in agreement with the observation that even in healthy, nondiabetic persons, plasma glucose correlated with a higher susceptibility of in vitro oxidation of LDL,35 and glycemia was associated with increased in vivo LDL oxidation, reflected by higher prevalence of highly oxidized LDL.14 The association between oxidized LDL and glycemia could be due to reducing insulin-signaling36 and reducing glucose uptake by oxidized LDL.19
Finally, our data support the association between oxidized LDL and dyslipidemia in humans,15,37 which could be due to impairing of triglyceride storage and secretion by oxidized LDL.38
Study Strengths and Limitations
The strengths of the present study include the large community-based sample, the availability of many standardized clinical covariates, and the generalizability of our findings to men and women and whites and African Americans, even though the age range from 33 to 45 years at year 15 was narrow. The study had 85% power in a 2-sided z test for proportions with type 1 error of 5% to detect an OR of 1.95 for metabolic syndrome comparing the highest with the lowest quintile of oxidized LDL. Although our population-based data agree with molecular mechanistic data in cellular and animal models suggesting that oxidized LDL is biologically relevant for the development of metabolic syndrome, our data do not prove a causal role of metabolic syndrome or its constituent risk factors; indeed, oxidized LDL was cross-sectionally correlated with components of the metabolic syndrome within the 1889 people studied herein. Another limitation is the exclusion of a number, though limited, of participants because of pregnancy and missing samples or data. Previously, it was questioned whether a single reading adequately represents the oxidized LDL profiles of participants. However, we have recently shown that the between-person variability of oxidized LDL measured by ELISA assay was 74% (with the remaining 26% being within-person and analytical variance), and that this value was comparable with that of high-sensitivity C-reactive protein and cholesterol measured by means of automated tests.27 A limitation is however that only freshly frozen samples can be analyzed; several thawing and freezing cycles can lead to further in vitro oxidation.
CONCLUSION
Our population-based data show that higher concentrations of circulating oxidized LDL are associated with the incidence of metabolic syndrome and the accumulation of 3 of its risk factor constituents: obesity, hyperglycemia, and hypertriglyceridemia.
Acknowledgments
Funding/Support: This study was supported in part by National Heart, Lung, and Blood Institute contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, N01-HC-95095 (CARDIA Study), and 1RO1-HL53560 (YALTA Study). Additional funding was provided by the School of Public Health, University of Minnesota fund supporting Dr Jacobs' Mayo professorship, by the Interuniversitaire Attractiepolen Programma of the Belgian Federal Government (P06/30), and by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (G.0232.05).
Role of the Sponsor: The National Heart, Lung, and Blood Institute participates in the governance of CARDIA and reviewed the manuscript. Otherwise, none of the funding organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Additional Contributions: We thank M. Landeloos (laboratory technician employed by the Katholieke Universiteit Leuven) for excellent technical assistance, for which she did not receive additional compensation beyond her regular salary.
Footnotes
Financial Disclosures: Dr Holvoet reports being the inventor of the assay for oxidized LDL for which patents have been granted in Europe (EP 1110092) and the United States (US Patent 6.309.888 and US Patent 6.727.102). All rights on inventions made by members of the university are vested by KU Leuven Research & Development, the technology transfer unit of the Katholieke Universiteit Leuven, which also receives any royalties. KU Leuven Research & Development granted a license to National Screening Institute (NSI; Newport Beach, California) who gave a sublicense to Mercodia (Uppsala, Sweden). Neither NSI nor Mercodia was involved in this research in any way. None of the other authors reported disclosures.
REFERENCES
- 1.Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Trevisan M, Liu J, Bahsas FB, Menotti A Risk Factor and Life Expectancy Research Group. Syndrome X and mortality: a population-based study. Am J Epidemiol. 1998;148(10):958–966. doi: 10.1093/oxfordjournals.aje.a009572. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103(15):1955–1960. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 5.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41(3):360–370. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 6.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98(15):1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 7.Holvoet P, Mertens A, Verhamme P, et al. Circulating oxidized LDL is a useful marker for identifying patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2001;21(5):844–848. doi: 10.1161/01.atv.21.5.844. [DOI] [PubMed] [Google Scholar]
- 8.Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats: recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266(4):2282–2289. [PubMed] [Google Scholar]
- 9.Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis. 1988;8(1):79–87. [PubMed] [Google Scholar]
- 10.Shoji T, Nishizawa Y, Fukumoto M, et al. Inverse relationship between circulating oxidized low density lipoprotein (oxLDL) and anti-oxLDL antibody levels in healthy subjects. Atherosclerosis. 2000;148(1):171–177. doi: 10.1016/s0021-9150(99)00218-x. [DOI] [PubMed] [Google Scholar]
- 11.Lamarche B. Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron Artery Dis. 1998;9(8):473–481. doi: 10.1097/00019501-199809080-00002. [DOI] [PubMed] [Google Scholar]
- 12.Holvoet P, Harris TB, Tracy RP, et al. Association of high coronary heart disease risk status with circulating oxidized LDL in the well-functioning elderly: findings from the Health, Aging, and Body Composition study. Arterioscler Thromb Vasc Biol. 2003;23(8):1444–1448. doi: 10.1161/01.ATV.0000080379.05071.22. [DOI] [PubMed] [Google Scholar]
- 13.Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001;44(9):1148–1154. doi: 10.1007/s001250100615. [DOI] [PubMed] [Google Scholar]
- 14.Holvoet P, Kritchevsky SB, Tracy RP, et al. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53(4):1068–1073. doi: 10.2337/diabetes.53.4.1068. [DOI] [PubMed] [Google Scholar]
- 15.Lapointe A, Couillard C, Piche ME, et al. Circulating oxidized LDL is associated with parameters of the metabolic syndrome in postmenopausal women. Atherosclerosis. 2007;191(2):362–368. doi: 10.1016/j.atherosclerosis.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Sigurdardottir V, Fagerberg B, Hulthe J. Circulating oxidized low-density lipoprotein (LDL) is associated with risk factors of the metabolic syndrome and LDL size in clinically healthy 58-year old men (AIR study) J Intern Med. 2002;252(5):440–447. doi: 10.1046/j.1365-2796.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamagishi S, Matsuoka H, Kitano S, et al. Elevated circulating oxidized LDL levels in Japanese subjects with the metabolic syndrome. Int J Cardiol. 2007;118(2):270–272. doi: 10.1016/j.ijcard.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 18.Masella R, Vari R, D'Archivio M, et al. Oxidised LDL modulate adipogenesis in 3T3-L1 preadipocytes by affecting the balance between cell proliferation and differentiation. FEBS Lett. 2006;580(10):2421–2429. doi: 10.1016/j.febslet.2006.03.068. [DOI] [PubMed] [Google Scholar]
- 19.Maddux BA, See W, Lawrence JC, Jr, Goldfine AL, Goldfine ID, Evans JL. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by micromolar concentrations of alpha-lipoic acid. Diabetes. 2001;50(2):404–410. doi: 10.2337/diabetes.50.2.404. [DOI] [PubMed] [Google Scholar]
- 20.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults: The CARDIA baseline monograph. Control Clin Trials. 1991;12(1) (suppl):1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 21.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. Bethesda, MD: National Institutes of Health; Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002 NIH publication 01-3670.
- 23.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection. Evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs DR, Jr, Hahn L, Haskell W, et al. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.Holvoet P, Stassen JM, Van CJ, Collen D, Vanhaecke J. Oxidized low density lipoproteins in patients with transplant-associated coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(1):100–107. doi: 10.1161/01.atv.18.1.100. [DOI] [PubMed] [Google Scholar]
- 27.Holvoet P, Macy E, Landeloos M, et al. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin Chem. 2006;52(4):760–764. doi: 10.1373/clinchem.2005.064337. [DOI] [PubMed] [Google Scholar]
- 28.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43(1):52–58. [PubMed] [Google Scholar]
- 29.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 30.Lindblad U, Langer RD, Wingard DL, Thomas RG, Barrett-Connor EL. Metabolic syndrome and ischemic heart disease in elderly men and women. Am J Epidemiol. 2001;153(5):481–489. doi: 10.1093/aje/153.5.481. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd J. Issues surrounding age: vascular disease in the elderly. Curr Opin Lipidol. 2001;12(6):601–609. doi: 10.1097/00041433-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Weinbrenner T, Schroder H, Escurriol V, et al. Circulating oxidized LDL is associated with increased waist circumference independent of body mass index in men and women. Am J Clin Nutr. 2006;83(1):30–35. doi: 10.1093/ajcn/83.1.30. [DOI] [PubMed] [Google Scholar]
- 33.Verreth W, De Keyzer KD, Pelat M, et al. Weight-loss-associated induction of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma correlate with reduced atherosclerosis and improved cardiovascular function in obese insulin-resistant mice. Circulation. 2004;110(20):3259–3269. doi: 10.1161/01.CIR.0000147614.85888.7A. [DOI] [PubMed] [Google Scholar]
- 34.Nishimura S, Manabe I, Nagasaki M, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56(6):1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 35.Chen NG, Azhar S, Abbasi F, Carantoni M, Reaven GM. The relationship between plasma glucose and insulin responses to oral glucose, LDL oxidation, and soluble intercellular adhesion molecule-1 in healthy volunteers. Atherosclerosis. 2000;152(1):203–208. doi: 10.1016/s0021-9150(99)00460-8. [DOI] [PubMed] [Google Scholar]
- 36.Mazière C, Morliere P, Santus R, et al. Inhibition of insulin signaling by oxidized low density lipoprotein: protective effect of the antioxidant vitamin E. Atherosclerosis. 2004;175(1):23–30. doi: 10.1016/j.atherosclerosis.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;194(1):245–252. doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Koonen DP, Jacobs RL, Febbraio M, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56(12):2863–2871. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]


