Abstract
Background
S-Adenosylmethionine (AdoMet) is a major methyl donor for transmethylation reactions and propylamine donor for the biosynthesis of polyamines in biological systems, and therefore may play a role in lung cancer development. We hypothesized that AdoMet levels were elevated in patients with lung cancer and may prove useful as a biomarker for early lung cancer.
Methods
High-performance liquid chromatography was used to analyze plasma AdoMet levels in triplicate samples from 68 patients. This included 13 patients with lung cancer, 33 smokers with benign lung disease, and 22 healthy nonsmokers. The three groups of subjects were compared with respect to the distribution of demographic and disease characteristics and AdoMet levels. Distributions were examined using summary statistics and box plots, and nonparametric analysis of variance procedures.
Results
Serum AdoMet levels were elevated in patients with lung cancer as compared to smokers with benign lung disorders and healthy nonsmokers. There were no significant correlations between AdoMet levels and tumor cell types, nodule size, or other demographic variables.
Conclusions
Our data demonstrate that plasma levels of AdoMet are significantly elevated in patients with lung cancer. Plasma AdoMet levels may prove to be a useful tool for the diagnosis of early lung cancer, in combination with chest CT.
Keywords: methyl donor, methylation, S-adenosylmethionine
The overall 5-year survival rate for patients with non-small cell lung cancer remains at only 15%; however, if the cancer is detected at stage IA, the 5-year survival rate often exceeds 90%.1–3 Chest CT scans have greater sensitivity than chest radiographs in detecting small noncalcified nodules that may represent early lung cancers, and publications1,4 have described the success of chest CT screening in detecting early stage cancer. However, this technique has poor specificity because of the high prevalence of nonspecific benign pulmonary nodules, often representing granulomatous diseases in varying degrees of latency.5–7 In addition, CT screening detects many slow-growing solid and ground-glass nodules, requiring repeated CT scans to determine growth rates over time, exposing patients to significant amounts of potentially harmful radiation.7–9 Plasma or serum biomarkers might be a minimally invasive, highly sensitive, and specific way to differentiate benign noncalcified nodules from early lung cancers.
Methylation of promoters in multiple different tumor-suppressor genes occurs early in the development of lung cancer. Extensive research is ongoing to identify patterns of DNA methylation that could serve as biomarkers for the early detection of lung cancer as well as other malignancies.10–12 One obstacle to this pursuit is that many different tumor-suppressor genes can be repressed by promoter hypermethylation in different cancers, and therefore the sensitivity of the methylation of any single, or even any panel of gene promoters remains low. We propose that an evaluation of the enzymatic pathway that serves as a substrate for DNA methylation could lead us to a useful biomarker for the early detection of cancer.
S-Adenosylmethionine (AdoMet) is a critical biochemical intermediate that serves both as the methyl donor for a myriad of biochemical events13 and as a precursor to the higher polyamines. Methylation is associated with expression of DNA, and polyamines are known to help condense and stabilize DNA and RNA. Defects in either methylation or polyamine metabolism could potentially interfere with normal control of cell function and proliferation and lead to (or be caused by) increased levels of AdoMet. Elevated levels of AdoMet as well as methionine adenosyltransferase (the enzyme responsible for the formation of S-adenosylmethionine from L-methionine and adenosine triphosphate) have been found in certain tumor tissues.14–16 Tumor AdoMet levels in patients with colorectal carcinomas were increased using immunoblots and immunohistochemistry compared to normal colon.17
We hypothesized that AdoMet levels would be elevated in the plasma of lung cancer patients compared to the levels in control subjects without malignancies, and that plasma AdoMet may be a useful clinical biomarker for the early detection of lung cancer, in combination with chest CT scans. To test this hypothesis, we measured plasma AdoMet levels in lung cancer patients, in high-risk smokers with benign pulmonary nodules, and in nonsmoking healthy control subjects.
Materials and Methods
Study Population
All of the lung cancer patients and all of the high-risk smokers were consecutive participants in the New York University (NYU) Lung Cancer Biomarker Center (LCBC) enrolled from February to August 2004. The NYU LCBC is a program focused on lung cancer biomarker discovery. The NYU LCBC enrolls participants in two cohorts: a screening cohort composed of both high-risk smokers and individuals at no increased risk of lung cancer, and a “rule-out lung cancer” cohort composed of patients with suspicion of lung cancer. All participants complete a medical and respiratory symptom questionnaire, undergo pulmonary function testing, and undergo chest CT. We studied 13 patients with lung cancer from the rule-out lung cancer cohort, 22 nonsmoking, normal volunteers, and 33 high-risk smokers with small noncalcified nodules on CT scan from the screening cohort. Lung cancer patients were evaluated prior to surgery, and 12 of 13 patients were stage I or II. Smoker control subjects met the criteria of a > 20–pack-year smoking history and underwent a CT scan to identify pulmonary nodules. Follow-up scans confirmed stability of these lesions, consistent with benign nodules.
Subjects with nodules were followed up for an average of 27 months. The nodules were followed up for at least 3 months in all cases. The majority (23 of 33 subjects) were followed up for 2 years or until resolution. Four subjects had nodules that resolved, all at the 3-month follow-up CT. All the other nodules, in all the high-risk subjects, remained stable for the follow-up period, which ranged from 3 to 42 months. Eleven of the 13 lung cancer patients were stage 1, 1 was stage 2, and 1 was stage 3. Four lung cancer patients had squamous cell carcinoma, 7 had adenocarcinoma, and 2 had bronchoalveolar cell carcinoma (BAC).
All participants were administered a questionnaire on medical history, occupational exposures, family history, and respiratory symptoms. Spirometry was performed according to American Thoracic Society criteria. We defined COPD as an FEV1/FVC < 70% of predicted and either emphysema or chronic bronchitic changes on chest CT, or patient-reported, physician-diagnosed COPD. Low-dose chest CT using a multidetector scanner (16 detectors) was performed as described.5 CT scans were reviewed by a radiologist (D.N.) and a pulmonologist (A.G.). All study subjects signed an informed consent form, and the protocol was approved by both the NYU School of Medicine institutional review board and Bellevue Human Subjects Review Committee.
High-Performance Liquid Chromatography
Blood samples (2 mL) were drawn into prechilled 5-mL ethylenediamine tetra-acetic acid-coated tubes (Vacutainer tubes; Becton Dickinson; Franklin Lakes, NJ). Laboratory personnel were blinded to cases and controls. Initial processing was done within 2 h of sampling. Plasma was separated by centrifugation at 1,000g for 15 min at 4°C, collected, and transferred to labeled 1.5-mL screw-top tubes. For every 80 μL of plasma, 20 μL of 10% perchloric acid was added to precipitate the proteins. The sample was clarified by centrifugation at 5,000g for 10 min, and the supernatant was stored for up to 7 days at − 20°C. High-performance liquid chromatography was performed using a derivitizing reagent (AccQ.Fluor; Waters Corporation; Milford, MA) as previously reported.16,18 For each patient, three aliquots of plasma were analyzed. AdoMet concentrations of the samples were determined using the high-performance liquid chromatography AdoMet peak area and interpolation from a standard curve obtained by analyzing solutions containing known amounts of AdoMet. For quality control, batches of samples included plasma with known amounts of AdoMet.
Statistical Methods
The three groups of subjects were compared with respect to the distribution of demographic and disease characteristics and AdoMet levels. Distributions were examined using summary statistics and box plots. The nonparametric Kruskal-Wallis test was used to compare the three groups where applicable and the Wilcoxon test was used to compare cancer patients with high-risk subjects or with nonsmoking control subjects with respect to the distributions of age, pack-years, FEV1/FVC, and nodule size. Similarly, for gender, presence of COPD, and solitary vs multiple nodules, χ2 tests were used to compare the difference in the frequencies of the binary categories in each of the three groups. All statistical tests were two sided. No adjustments were made for multiplicity.
To examine the associations between AdoMet levels and baseline and disease characteristics within each group of subjects, nonparametric Spearman rank correlation coefficients were examined. Logistic regression models were also used to identify other factors that might be used in addition to AdoMet levels to distinguish among the groups of subjects. Variables were examined individually and in combination using all subsets of variables in the logistic regression models to compare cancer and high-risk subjects. The AIC criterion was used to select the best model from all subset regressions. Sensitivity and false-positive rates along with corresponding 95% confidence intervals (CIs) were estimated for possible cutoff values to distinguish the cancer and high-risk smoker groups. Receiver operating characteristic (ROC) curves were also plotted for the cancer cases and high-risk smoking groups.
Results
Demographic and Baseline Characteristics
Lung cancer patients and high-risk smokers were significantly older than normal nonsmokers (Table 1). Lung cancer patients had smoked a median of 30 pack-years, and the high-risk smokers had smoked a median of 44 pack-years. Both lung cancer patients and high-risk smokers had a high incidence of COPD (54% and 76% respectively; Table 2). The nonsmoking normal volunteers, by definition, had normal lung function. All of the high-risk smokers were found to have one or more pulmonary nodules on chest CT. A larger proportion of lung cancer patients had solitary nodules than high-risk smokers (38% vs 18%), and nodule sizes were considerably smaller in the high-risk smokers than those seen on the chest CTs of lung cancer patients (median, 5 mm; vs median, 13 mm; p < 0.001).
Table 1. Demographics of Study Participants*.
| Demographics | Lung Cancer
(n = 13) |
High-Risk Smokers
(n = 33) |
Nonsmokers
(n = 22) |
Z score or χ2 (p Value)
|
|
|---|---|---|---|---|---|
| Cancer/HRS | Cancer/NS | ||||
| Age, yr | |||||
| Mean | 69 | 58 | 48 | 3.05 (0.001) | 3.55 (0.0004) |
| Median | 71 | 56 | 45 | ||
| Range | 31–83 | 44–81 | 25–74 | ||
| Female gender, % | 54 | 67 | 55 | 0.66 (0.42) | 0.002 (0.97) |
HRS = high-risk smokers; NS = nonsmokers.
Table 2. Baseline Characteristics of the Study Participants.
| Characteristics | Lung Cancer Patients
(n = 13) |
High-Risk Smokers
(n = 33) |
Z score or χ2 (p Value) |
|---|---|---|---|
| Median (range) pack-years, No. | 30 (0–120) | 44 (19–220) | − 1.31 (0.10) |
| Median nodule size (range), mm | 13 (8–40) | 5 (3–15) | 4.71 (<0.0001) |
| With COPD, % | 54 | 76 | 1.03 (0.31) |
| With solitary nodules, % | 38 | 18 | 2.11 (0.15) |
AdoMet Levels
Adomet levels were markedly increased in cancer patients compared with high-risk smokers and nonsmoker control subjects (Fig 1; χ2 with two degrees of freedom = 39.6; p < 0.0001). In particular, levels in cancer patients were all above levels in nonsmoker control subjects (p < 0.0001) and were significantly higher than levels in high-risk smokers (p < 0.0001).
Figure 1.
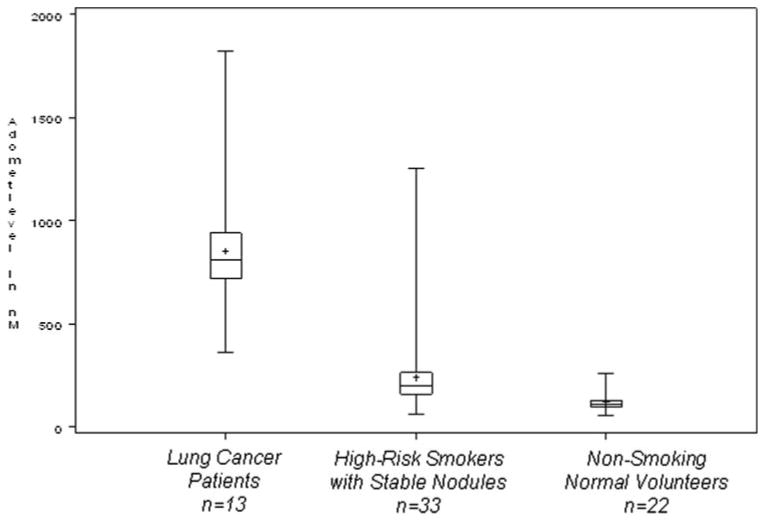
Box plot of AdoMet level by study group. The median for each data set is indicated by the center line, and the mean is represented by the plus sign. The first and third quartiles are the edges of the square area, which is known as the interquartile range. The extreme values (within 1.5 times the interquartile range from the upper or lower quartile) are the ends of the lines extending from the interquartile range.
We also examined the correlations within each group of subjects of AdoMet levels with age, pack-years of smoking, and nodule size. We note that among cancer patients, pack-years were highly correlated with AdoMet level (r = 0.70) as was age (r = 0.42) [Table 3]. This was not the case, however, among high-risk smokers. In fact, AdoMet levels and pack-years of smoking are negatively correlated in these high-risk smokers (r = − 0.27). The distributions of AdoMet levels were slightly elevated for subjects with solitary nodules compared with subjects with multiple nodules in both cancer patients and in high-risk smokers.
Table 3. Correlation Coefficients Between AdoMet Levels and Other Variables by Group*.
| Groups | Age | Pack-Years | Nodule Size | Solitary Nodule |
|---|---|---|---|---|
| Lung cancer | 0.42 (0.15) | 0.70 (0.01) | − 0.20 (0.51) | − 0.46 (0.11) |
| High-risk smokers | − 0.32 (0.07) | − 0.27 (0.12) | − 0.05 (0.80) | 0.61 (0.54) |
| Normal nonsmokers | − 0.01 (0.97) | NA | NA | NA |
Data are presented as nonparametric Spearman rank correlation coefficient (p value). NA = not available.
Among the cancer patients, the levels of AdoMet were somewhat higher in the four squamous cell carcinoma patients and lower in the two BAC patients than in the seven adenocarcinoma patients (p = 0.10). We also note that 6 of the 13 cancer patients smoked for ≤ 5 pack-years (5 of 7 adenocarcinoma patients and 1 of 2 BAC patients). The one lung cancer patient whose AdoMet level was not increased compared with high-risk smokers was one of the two patients with BAC.
When we compared cancer patients with high-risk patients using multiple logistic regression, we had a sensitivity of 100% and a false-positive rate of 6.1% based on AdoMet level and nodule size (with cut points of AdoMet level ≥ 731 and nodule size ≥ 8 mm, Table 4). The log odds ratio of cancer to high-risk control is estimated by − 12.4725 + 0.7526 × nodule size in millimeters + 0.00793 × AdoMet level (Akaike information criterion = 16.691). For each unit change in AdoMet level, the relative odds of cancer increase by 1.008 (95% CI, 1.002 to 1.014). For each millimeter change in nodule size, the odds of cancer increase by 2.123 (95% CI, 1.076 to 4.186). The ROC curve is shown in Figure 2, top, A. AdoMet level alone ≥ 536 had a sensitivity of 92.3% and false-positive rate of 3.0%, or sensitivity of 100% and false-positive rate of 9.1% for levels ≥ 362 (ROC curve in Fig 2, center, B). Nodule size alone ≥ 8 mm had a sensitivity of 84.6% and false-positive rate of 12.1% (ROC curve in Fig 2, bottom, C).
Table 4. Sensitivity and False-Positive Rates for Identification of Cancer vs High-Risk Patients*.
| Marker Cut Point | Sensitivity, % | Sensitivity, 95% CI | False-Positive Rate, % | False-Positive Rate, 95% CI |
|---|---|---|---|---|
| AdoMet ≥ 731 and nodule size ≥ 8 mm | 100 | 75.0–100 | 6.1 | 0.7–20.2 |
| AdoMet ≥ 536 | 92.3 | 64.0–100 | 3.0 | 0.1–15.8 |
| AdoMet ≥ 362 | 100 | 75.0–100 | 9.1 | 1.9–24.3 |
| Nodule size ≥ 8 mm | 84.6 | 55.0–98.0 | 12.1 | 3.4–28.2 |
Based on logistic models; Clopper-Pearson 95% binomial CIs.
Figure 2.

ROC curves for cancer compared with high-risk smokers. Top, A: based on AdoMet levels and nodule size jointly. Center, B: based on AdoMet levels alone. Bottom, C: based on nodule size alone.
Discussion
We found serum AdoMet levels were significantly higher in patients with lung cancer as compared to levels in healthy nonsmokers and in high-risk smokers with small noncalcified nodules. Many of the high-risk smokers had ground-glass nodules and/or multiple nodules. They also had mild-to-moderate obstructive impairment from COPD, emphysema, or bronchiectasis. Most (57%) of the noncalcified nodules remained stable on CT scan for > 2 years and therefore met the accepted radiographic criteria for benign lesions. All of the nodules either resolved or were stable for at least 3 months of follow-up. A major problem of chest CT scan screening for early detection of lung cancer is the finding of large numbers of nonspecific pulmonary nodules resulting in a 10-fold increase in interventions, mostly surgical resection.7 Our finding that AdoMet levels were significantly elevated in patients with early stage, small lung cancers, compared with both normal nonsmokers and high-risk individuals with benign nodules, indicates that this assay might be useful in conjunction with chest CT screening to distinguish small nodules that require biopsy or resection from those that can be managed more conservatively with serial CT scans. Using AdoMet level alone, we were able to distinguish patients with lung cancer from smokers with benign nodules with a sensitivity of 92 to 100% and a specificity of 97 to 91%, depending on the cut-off values used. When AdoMet level is combined with nodule size, we can achieve a sensitivity and specificity of 100% and 94%, respectively, in these subjects.
The mechanism behind the increased level of AdoMet in individuals with lung cancer and several other malignancies may be due to the tumor, a host response to the tumor, or an oncogenic stimulus. Biochemical reactions that require AdoMet for methyl group transfers include the formation of phosphatidyl choline, regeneration of methionine, methylation of phospholipids, and methylation of proteins, DNA, and RNA.19 In mammalian cells, the flux of AdoMet is overwhelmingly toward transmethylation reactions.
Because of data10 showing hypermethylation of the promoter regions of tumor suppressor genes in lung cancer (as well as other malignancies), we suggest that the increased levels of plasma AdoMet in lung cancer patients relate to the role of AdoMet in DNA methylation. Hypermethylation of gene promoter regions is associated with silencing of transcription. Methylation of pyrimidines in DNA is a postreplicative event leading to the formation of 6-methylcytosine from cytosine. A family of three enzymes (the cytosine-DNA methyltransferases) catalyzes methylation of the fifth position of the cytosine ring, using AdoMet as the donor molecule. When this occurs within a CpG island located in the promoter region of a gene, it is accompanied by changes in chromatin composition around the island. These changes may deny access to regulatory proteins needed for transcription, or methylation may serve as a recognition mechanism for DNA/protein interactions.11,13 For example, the expression of several tumor suppressor genes (including p16, FHIT, RASSF1, SEMA3B, E-cadherin, DAP kinase, and others) is turned off by promoter methylation.11,12,20,21
Cigarette smoke extract increases AdoMet in human lung epithelial A549 cells > 48 h of culture, and this increase may help restore intracellular glutathione to protect against oxidant stress.22 In contrast, infusion of nicotine into the rat depletes lung AdoMet levels by increasing lung polyamine catabolic/anabolic cycling and/or excretion.23 We noted that the highest (fourfold) levels of plasma AdoMet were in lung cancer patients who actually had significantly lower pack-years of smoking than our high-risk smokers (Figs 1, 2) consistent with the concept that lung cancer rather than smoking elevated AdoMet. Skelly and colleagues24 have published results of plasma AdoMet concentrations in a variety of human infectious diseases (bacterial pneumonia, tuberculosis, asymptomatic HIV, cryptococcosis), finding levels similar to our control subjects (100 to 150 nmol/L) and demonstrated a decline after treatment. Interestingly, Pneumocystis carinii scavenges this intermediate, and plasma levels in 15 patients were < 0.5 nmol/L, with levels increasing in 5 of the patients after treatment for pneumonia secondary to this organism.
Our study has shown that AdoMet may be a highly sensitive and specific marker to use in conjunction with chest CT scans for identifying individuals with early stage lung cancer. Our study involved limited sample size, and it is possible that some of the nodules considered benign could be misclassified because the follow-up was short (< 3 months) in 10 of 33 cases. Additional studies in a larger, independent population with longer follow-periods are required to confirm our findings, and to determine the source of the elevated AdoMet levels in lung cancer patients; follow-up studies would be needed to determine if this biomarker also has clinical utility for diagnosing recurrence.
Acknowledgments
We thank Lauren Hittson for assistance with manuscript preparation.
Grant support was provided by National Center for Cancer Resources M01 RR00096, National Cancer Institute U01 CA 86137, National Cancer Institute P30 CA 16087-24, and National Center for Cancer Resources RR16192.
Abbreviations
- AdoMet
S-adenosylmethionine
- BAC
bronchoalveolar cell carcinoma
- CI
confidence interval
- LCBC
Lung Cancer Biomarker Center
- NYU
New York University
- ROC
receiver operating characteristic
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: CHEST is the official journal of the American College of Chest Physicians. It has been published monthly since 1935. Copyright 2007 by the American College of Chest Physicians, 3300 Dundee Road, Northbrook IL 60062. All rights reserved. No part of this article or PDF may be reproduced or distributed without the prior written permission of the copyright holder
References
- 1.Henschke CI, Yankelevitz D, Libby DM, et al. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 2.Mulshine JL, Sullivan D. Lung cancer screening. N Engl J Med. 2005;352:2714–2720. doi: 10.1056/NEJMcp042630. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch FR, Franklin WA, Gazdar AF, et al. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res. 2001;7:5–22. [PubMed] [Google Scholar]
- 4.Bach PB, Jett JR, Pastorino U, et al. Computed tomography screening and lung cancer outcomes. JAMA. 2007;297:953–961. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 5.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet. 1999;35:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 6.Burns J, Haramati LB, Whitney K, et al. Consistency of reporting basic characteristics of lung nodules and masses on computed tomography. Acad Radiol. 2004;11:233–237. doi: 10.1016/s1076-6332(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 7.Bach PB, Jett JR, Pastorino U, et al. Computed tomography screening and lung cancer outcomes. JAMA. 2007;297:953–961. doi: 10.1001/jama.297.9.953. [DOI] [PubMed] [Google Scholar]
- 8.Patz EF, Jr, Goodman PC, Bepler G. Screening for lung cancer. N Engl J Med. 2000;343:1627–1633. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- 9.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231:440–445. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 10.Belinsky SA. Gene-promoter hypermethylation as a biomarker in lung cancer. Cancer. 2004;4:1–11. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- 11.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 12.Belinsky SA, Klinge DM, Dekker JD, et al. Gene promoter methylation in plasma and sputum increases with lung cancer risk. Clin Cancer Res. 2005;11:6505–6511. doi: 10.1158/1078-0432.CCR-05-0625. [DOI] [PubMed] [Google Scholar]
- 13.Chiang PK, Gordon RK, Tal J, et al. S-Adenosylmethionine and methylation. FASEB J. 1996;10:471–480. [PubMed] [Google Scholar]
- 14.Kreis W. Methionine dependency of malignant tumors. J Natl Cancer Inst. 1991;83:725. doi: 10.1093/jnci/83.10.725. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Castellano JM, Villanueva A, Healey JH, et al. Methylthioadenosine phosphorylase gene deletions are common in osteosarcoma. Clin Cancer Res. 2002;8:782–787. [PubMed] [Google Scholar]
- 16.Merali S, Clarkson AB., Jr Polyamine analysis using N-hydroxysuccinimidyl-6-aminoquinoyl carbamate for pre-column derivatization. J Chromatogr B Biomed Appl. 1996;675:321–326. doi: 10.1016/0378-4347(95)00363-0. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Ikeda S, Kojima N, et al. Correlation between the expression of methionine adenosyltransferase and the stages of human colorectal carcinoma. Surg Today. 2000;30:706–710. doi: 10.1007/s005950070081. [DOI] [PubMed] [Google Scholar]
- 18.Merali S, Vargas D, Franklin M, et al. S-Adenosylmethionine and Pneumocystis carinii. J Biol Chem. 2000;275:14958–14963. doi: 10.1074/jbc.275.20.14958. [DOI] [PubMed] [Google Scholar]
- 19.Kano Y, Sakamoto S, Kasahara T, et al. Methionine dependency of cell growth in normal and malignant hematopoietic cells. Cancer Res. 1982;42:3090–3092. [PubMed] [Google Scholar]
- 20.Robertson KD, Jones PA. DNA methylation: past, present and future directions. Carcinogenesis. 2000;21:461–467. doi: 10.1093/carcin/21.3.461. [DOI] [PubMed] [Google Scholar]
- 21.Kamb A, Gruis NA, Weaver-Feldhaus J, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 22.Panayiotidis MI, Stabler SP, Allen RH, et al. Cigarette smoke extract increases S-adenosylmethionine and cystathionine in human lung epithelial-like (A549) cells. Chem Biol Interact. 2004;147:87–97. doi: 10.1016/j.cbi.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Shivji M, Burger S, Moncada CA, et al. Effect of nicotine on lung S-adenosylmethionine and development of pneumocystic pneumonia. J Biol Chem. 2005;280:15219–15228. doi: 10.1074/jbc.M413946200. [DOI] [PubMed] [Google Scholar]
- 24.Skelly M, Hoffman J, Fabbri M, et al. S-adenosylmethionine concentrations in diagnosis of Pneumocytis carinii pneumonia. Lancet. 2003;361:1267–1268. doi: 10.1016/S0140-6736(03)12984-4. [DOI] [PubMed] [Google Scholar]


