Abstract
Background
Amorphous calcium phosphate (ACP) polymeric composites release calcium and phosphate ions in aqueous environments, which may lead to the deposition of apatitic mineral in tooth structures. This study evaluates the strength of the composite/adhesive/dentin bond (SBS) for ACP basing-composites after various periods of water-aging.
Methods
Experimental composites were made using two resin matrices, with various ACPs or a commercial strontium ion-leachable glass. They applied successive coats of a dentin adhesive and basing composite to an acid-etched dentin surface and photopolymerized them. They added a commercial resin-based composite and light cured it. They determined the specimens’ SBS after they were aged in water for various periods at 37°C.
Results
The SBS of ACP composites was (18.3 ± 3.5) MPa, regardless of filler type, resin composition and water aging interval. After 24 hours of water aging, 92.6 percent of surfaces showed the adhesive failure. After two weeks of water aging, adhesive/cohesive failures were predominant in unmilled and milled ACP composites.
Conclusions
The SBS of ACP composites appears to be unaffected by filler type or immersion time for up to six months. The type of adhesive failure occurring with prolonged aqueous exposure is affected by filler type.
Clinical Implications
These materials may be effective remineralizing/antidemineralizing agents and may be clinically applicable as adhesives, protective liners and bases, orthodontic cements and pit-and-fissure sealants.
Keywords: Amorphous calcium phosphate, composites, remineralization, dentin
INTRODUCTION
During the last decade, we have been developing biologically active restorative materials that may stimulate the repair of tooth structure through the release of cavity fighting components including calcium and phosphate. These materials often are referred to as “smart composites.” They contain amorphous calcium phosphate (ACP) as a bioactive filler encapsulated in a polymer binder.1–4 Calcium and phosphate ions released from ACP composites, especially in response to changes in the oral environment caused by bacterial plaque or acidic foods, can be deposited into tooth structures as apatitic mineral, which is similar to the hydroxyapatite found naturally in teeth and bone.5 The ACP composites could be used as restorations in small carious lesions and to seal pits and fissures in teeth where plaque can accumulate and lead to caries. They also could be used as adhesives in orthodontics and help prevent the demineralization of tooth enamel that commonly occurs around bands and brackets. Furthermore, these remineralizing/antidemineralizing ACP composites can be used as basing materials under amalgam or resin-based composites or as temporary restorations in patients with salivary dysfunction who are prone to caries.3
Dental resin-based composites with silanated glass or ceramic filler generally are brittle restorative materials that are incapable of resisting crack initiation and growth under large masticatory stress, owing to their low strength and toughness. In ACP composites, the filler is weaker and of a lower modulus than in the usual glass or ceramic filler. The uncontrolled aggregation of ACP particulates has been identified, along with poor interfacial interaction with the resin matrices,4 as being a primary cause of their mechanical inferiority when compared with glass-reinforced composites. We have focused on strategies for improving the ACP filler and polymer matrix interfacial interaction (and, in turn, composite properties) by better controlling the particle size distribution (PSD)6 and surface properties of ACP fillers, in addition to fine-tuning the resin system5 to improve the overall mechanical properties of this material.
We conducted an investigation to evaluate the adhesive properties of experimental ACP composites formulated for base and lining applications to a dentin adhesive. Our working hypothesis was that with both composites based on the same resin systems, the bioactive, hybridized ACP fillers would not affect adversely the strength of the composites/adhesive/dentin bond when compared with a control group of strontium glass- (Sr-glass-) filled composites after various periods of aqueous immersion. In the same manner, we evaluated the effect of zirconia-hybridized amorphous calcium phosphate’s (Zr-ACP’s) particle size on the shear bond strength (SBS) of the composites.
MATERIALS AND METHODS
Synthesis and characterization of ACP filler
We synthesized ACP fillers using a a modified preparation protocol proposed by Eanes and colleagues.7 We used the hybridizing agents zirconyl chloride and tetraethoxysilane to introduce zirconia and silica elements, respectively, into precipitating ACP at a mole fraction of 5, 10 or 20 percent based on the calcium reactant. We assigned the resulting hybridized fillers Zr-ACP and silica-hybridized amorphous calcium phosphate (Si-ACP), respectively. In this article, we include the percentage of hybridizing agent we initially used during synthesis in parentheses after the fillers’ acronyms; for example, Zr-ACP(5 or 10 or 20) and Si-ACP(5 or 10 or 20). To avoid exposing the fillers to moisture that may have led to their premature conversion into crystalline apatite, we stored the fillers over anhydrous calcium sulfate under moderate vacuum pressure (2.7 kilopascals) until we used them to form composites.
We verified the amorphous state of fillers by using powder X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy. We measured the PSD of fillers dispersed in isopropanol by using gravitational and centrifugal sedimentation analysis from a particle size analyzer. We ultrasonicated the fillers for 10 minutes at room temperature before conducting analysis. We took the measurements three times. We obtained the median particle diameter of the samples from the PSD. We considered the median particle diameter to be an indicator of the aggregation of the ACP particulates (the higher the median diameter, the more aggregated the ACP). We compared the PSD data with morphological data we obtained by scanning electron microscopy (SEM). We determined the surface morphology/topology of the fillers by using SEM, after we sputter-coated the specimens with gold.
We performed wet ball-milling of the Zr-ACP filler by sealing 25 grams of ACP, 500 g of high-density 2-millimeter-diameter zirconium dioxide grinding balls and 150 milliliters of analytical grade isopropanol in a grinding jar and milled it by using a programmable planetary mill for two hours at 400 rotations per minute, with rotation direction being reversed every 15 mintes. We separated the milled Zr-ACP from the grinding balls by sieving and evaporated the remaining isopropanol in a vacuum oven at 70°C over a 24-hour period. We then screened the dry milled filler by using XRD and FTIR to verify that none of the filler had been converted to apatite during the milling process, examined it by using SEM and evaluated it to determine its PSD. We used the milled Sr-glass as we received it from the manufacturer.
Formulation of the Resins
We formulated two resin matrices from commercially available monomers and photo-activated for visible light photo-polymerization by using photoinitiator systems (Table 1). We obtained the resin’s acronyms by combining the initial letter of the acronym for each monomeric constituent of the matrix. The compositions of the resins are provided in Table 2.
TABLE 1.
COMPONENTS OF MONOMERS AND PHOTOINITIATOR SYSTEMS USED IN RESIN FORMULATIONS.
| COMPONENT | NAME (ACRONYM) |
|---|---|
| Monomers | Bisphenol glycidyldimethacrylate_(Bis-GMA) |
| triethyleneglycol dimethacrylate (TEGDMA) | |
| 2-hydroxyethyl methacrylate (HEMA) | |
| zirconyl dimethacrylate (ZrDMA) | |
| pyromellitic glycerol dimethacrylate (PMGDMA) | |
| Photoinitiator Systems | camphorquinone (CQ) |
| ethyl-4-N,N-dimethylaminobenzoate (4EDMAB) | |
| diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide & 2-hydroxy-2-methyl-1-phenyl-1-propanone | |
| 2-benzyl-2-(dimethylamino)-1-(4-[4-morpholinyl]phenyl-1-butanone |
TABLE 2.
COMPOSITION OF THE RESINS* EVALUATED IN THE STUDY.
| COMPONENT (ACRONYM) | BTHZ† RESIN (MASS FRACTION PERCENTAGE) | TP‡ RESIN (MASS FRACTION PERCENTAGE) |
|---|---|---|
| Bisphenol glycidyldimethacrylate (Bis-GMA) | 35.50 | - |
| Triethyleneglycol dimethacrylate (TEGDMA) | 35.44 | 48.65 |
| 2-hydroxyethyl methacrylate (HEMA) | 27.00 | - |
| Zirconyl dimethacrylate (ZrDMA) | 1.00 | - |
| Pyromellitic glycerol dimethacrylate (PMGDMA) | - | 48.65 |
| Camphorquinone (CQ) | 0.20 | 0.40 |
| Ethyl-4-N,N-dimethylaminobenzoate (4EDMAB) | 0.80 | - |
| 4265 DAROCUR | - | 0.80 |
| 369 IRGACURE | - | 1.50 |
Resin acronyms are obtained by combining the initial letter of the acronym for each monomeric constituent of the matrix.
BTHZ: Bisphenol glycidyldimethacrylate, tetraethyleneglycol dimethacrylate, 2 hydroxyethylmethacrylate, zirconyl dimethacrylate.
TP: Tetraethyleneglycol dimethacrylate, pyromellitic glycerol dimethacrylate.
BTHZ resin included a 1:1 bisphenol glycidyldimethacrylate (bis-GMA)/triethyleneglycol dimethacrylate (TEGDMA) mixture by mass, the hydrophilic and adhesion-promoting monomer 2-hydroxyethyl methacrylate (HEMA) and the surfactant monomer zirconyl dimethacrylate (ZrDMA). We added camphorquinone (CQ) and ethyl-4-N,N-dimethylaminobenzoate (4EDMAB) to BTHZ resins to act as photo-oxidant and photo-reductant components of the photo-initiator system, respectively.
The TP resin contained only the low-viscosity TEGDMA monomer (traditionally utilized as a diluent monomer in Bis-GMA matrices) and an equal amount (by mass) of the multifunctional, surface-active, adhesion-promoting pyromellitic glycerol dimethacrylate (PMGDMA) monomer.8,9 The photo-initiator system in TP resins comprised CQ, diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide and 2-hydroxy-2-methyl-1-phenyl-1-propanone, and 2-benzyl-2-(dimethylamino)-1-(4-[4-morpholinyl]phenyl)-1-butanone, which we introduced to enhance both the photopolymerization and the storage stability of the composites.
Preparation of Composite Specimens
We made the photopolymerizable composite pastes by mixing the appropriate resin (mass fraction, 60 percent) and the ACP filler or Sr-glass filler (mass fraction, 40 percent) by hand using a spatula. We stored the homogenized pastes under a moderate vacuum (2.7 kPa) overnight to eliminate the air entrained during the mixing process. Optical images of the typical ACP filler and the typical homogenized composite paste are shown in Figure 1.
Figure 1.

Optical photograph of amorphous calcium phosphate filler (A) and the homogenized composite paste (B).
Shear Bond Strength Measurements
We tested the ACP composite’s SBS to dentin through an adhesive substrate on 53 extracted human molars whose roots we embedded in polycarbonate holders with a chemical curing polymethylmethacrylate tray resin. We removed the occlusal surfaces of the molars and ground the exposed dentin surfaces flat by using 320 grit silicon carbide paper so that they were perpendicular to the longitudinal axis of the tooth and the polycarbonate holder. Figure 2 shows the equipment and materials used in the bonding process, which included the following steps:
Figure 2.
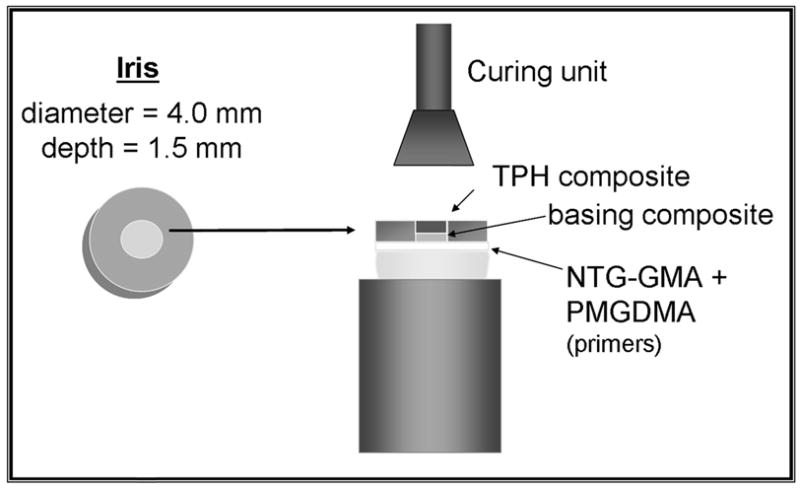
Schematic presentation of bonding protocol. TPH is manufactured by Dentsply Caulk, Milford, Del. mm: Millimeters. NTG-GMA: N-p-tolyglycine glycidyl methacrylate. PMGDMA: Pyromellitic glycerol dimethacrylate.
ground dentin surfaces were conditioned with aqueous phosphoric acid (mass fraction, 30 percent) for 15 seconds;
surfaces were rinsed with distilled water for 10 seconds;
dentin was blotted to a near-dry condition with a moistened paper towel;
moist dentin surfaces were sequentially primed with N-phenylglycine glycine (mass fraction, 5 percent) in acetone for 30 seconds (the approximate time needed for acetone to evaporate) and then a PMGDMA (mass fraction, 20 percent)/acetone solution photoactivated with camphorquinone (CQ) (mass fraction, 0.028 percent), which we applied in five consecutive coats;
the PMGDMA/acetone adhesive was dried gently with a stream of air to evaporate the solvent and was visible-light cured for 10 seconds;
experimental basing composites were then applied through a polytetrafluoroethylene-coated iris (4.0 mm in diameter, 1.5 mm in depth) that defined the bonding area and were light cured for 20 seconds;
specimens were completed by applying a resin-based composite (TPH, Dentsply Caulk, Milford, Del.), which was light-cured for 60 seconds.
We aged the specimens in distilled water at 37°C by using the following protocols: 24 hours, two weeks, one month, three months and six months for the unmilled and milled Zr-ACP-filled BTHZ composites.
We performed debonding (Figure 3) by using the shear test method with a computer-controlled universal testing machine at a crosshead speed of 0.5 mm per minute as described by Venz and Dickens.10 We examined the debonded specimens under an optical microscope to assess if the mode of failure was adhesive, cohesive or mixed.
Figure 3.
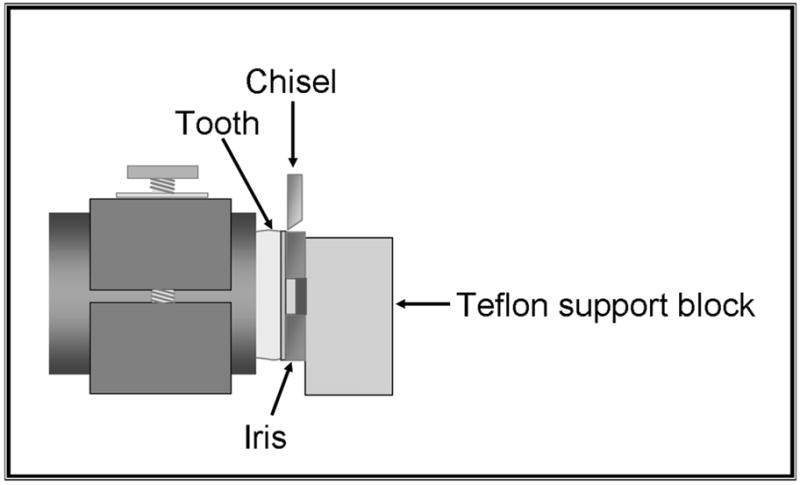
Shear bond strength testing assembly. Teflon is a polytetrafluoroethylene manufactured by DuPont, Wilmington, Del.
Statistical analyses
We analyzed the experimental data by using analysis of variance (α = .05). We determined significant differences between specific groups by using all pair-wise multiple comparisons (two-tailed t-test; unequal variances). We give one standard deviation (SD) in this article as the estimated standard uncertainty of the measurements for comparative purposes.
RESULTS
XRD patterns (Figure 4) of all of the investigated ACP fillers consisted of the two diffuse broad bands in 2-Theta in the 4- to 60-degree range. Their corresponding FTIR spectra (Figure 5) revealed wide absorbance bands at 1,200 to 900 wavenumber (absorbance per centimeter [cm−1]) and 630 to 500 wavenumber (cm−1) regions that are typical for noncrystalline phosphates. The PSD data for the unmilled Si-ACP fillers and the unmilled Zr-ACP fillers (Figure 6A) disclosed heterogeneous sizes, ranging from submicrometer to approximately 80 μm, with the median particle size diameter being 8.1 ± 2.1 μm. The PSD of milled Zr-ACP (Figure 6B) revealed particles ranging from 0.2 μm to 3.0 μm, with the mean particle size diameter being 0.7 ± 0.2 μm. The PSD data correlated well with our SEM observations.6
Figure 4.
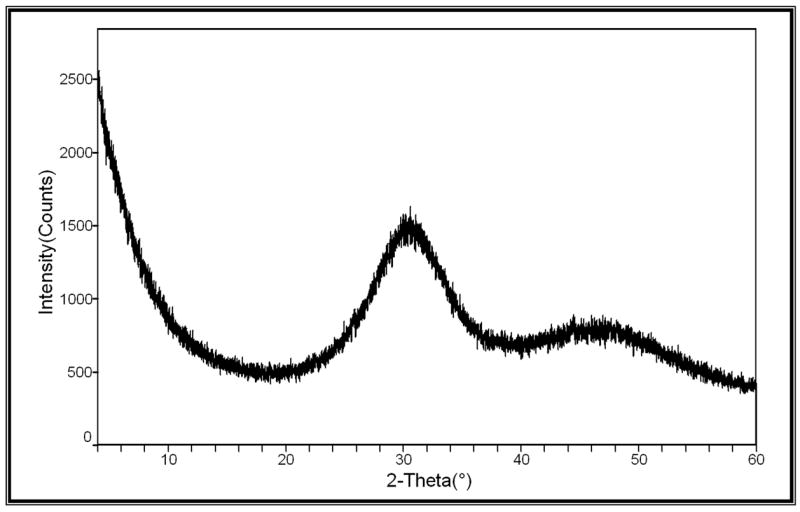
X-ray diffraction pattern.
Figure 5.
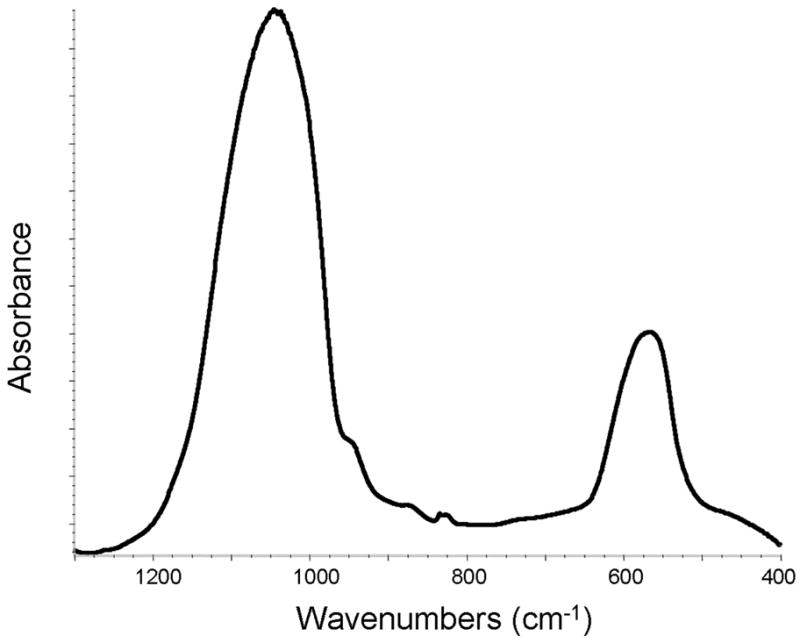
Fourier transformed infrared spectrum. cm−1: Inverse centimeters.
Figure 6.
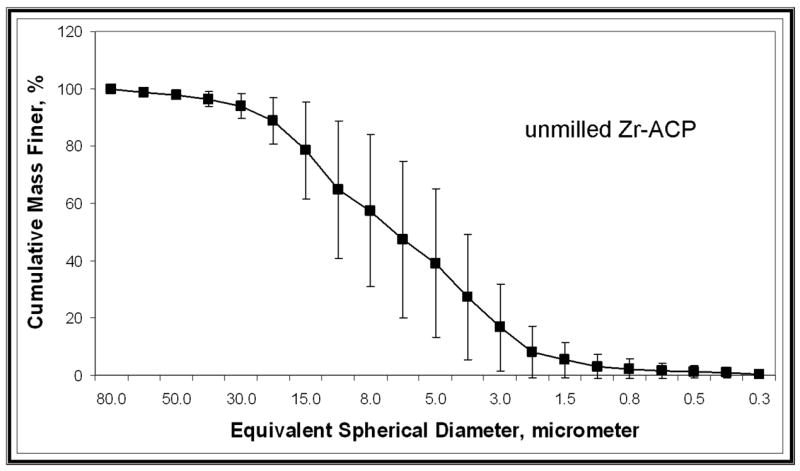
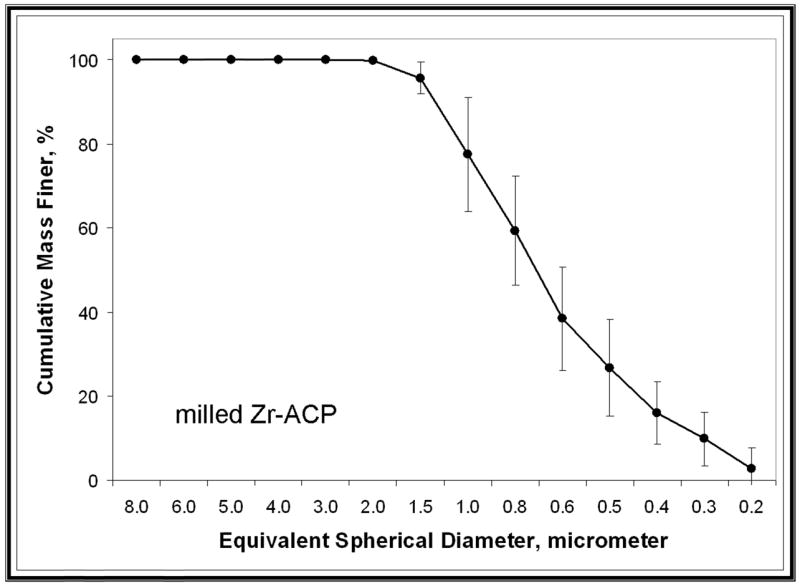
Representative particle size distributions of unmilled zirconia-hybridized amorphous calcium phosphate (Zr-ACP) (A) and milled Zr-ACP (B).
The 24-hour bond strength data obtained with the Si-ACP and Zr-ACP fillers in comparison with the unfilled copolymer specimens and Sr-glass control specimens are shown in Figures 7A and 7B (page 1482) for the TP and BTHZ resin matrices, respectively. The indicated SBS values for the hybridized ACP composites are the mean group SBS values for the combined Si-ACP(5), Si-ACP(10) and Si-ACP(20) specimens, since we found no significant differences in the Si-filler and Zr-filler series (two-tailed t test probability values were between 0.11 and 0.86 and between 0.24 and 0.51, respectively). The overall SBS of ACP composites was 18.3 ± 3.5 megapascals. Applying the same statistical criterion, we noticed the following trend of decreasing SBS for TP matrices: the SBS of Sr-glass composite, copolymer, Zr-ACP composite was greater than Si-ACP composite. However, in the BTHZ resin-based composites, all four groups were statistically indistinguishable.
Figure 7.
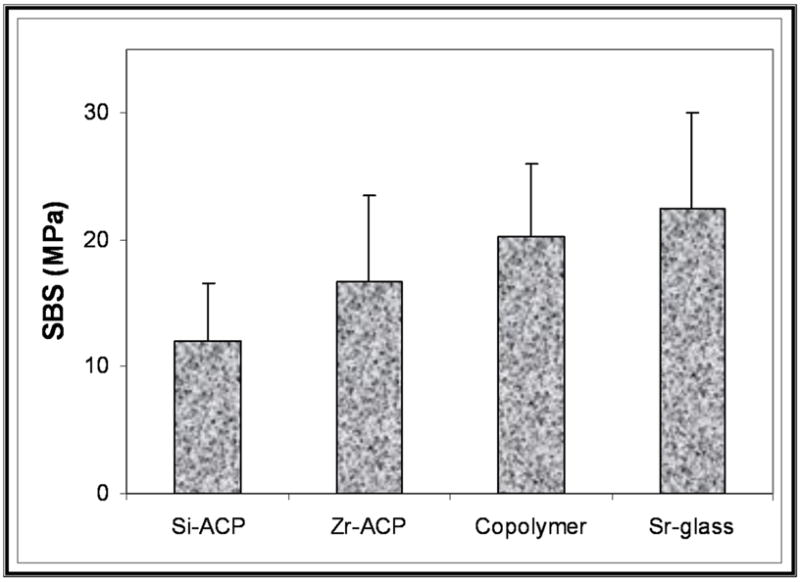
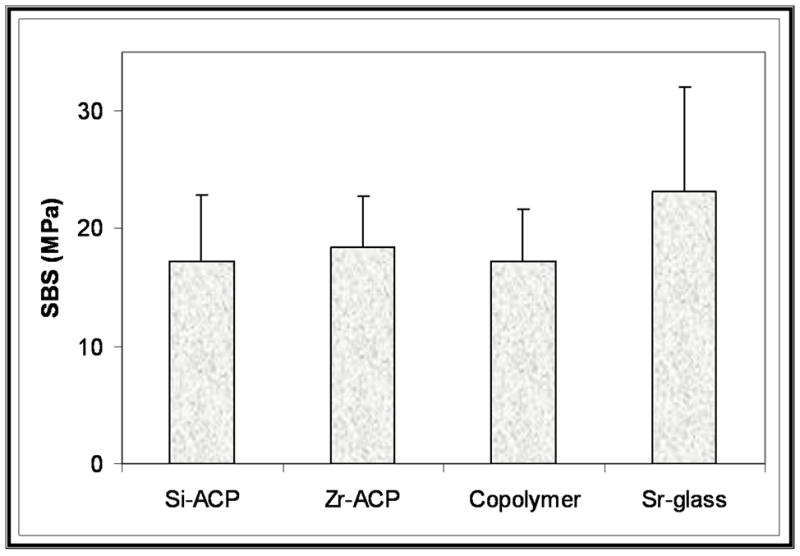
Mean group 24-hour shear bond strength data obtained with triethyleneglycol dimethacrylate, pyromellitic glycerol dimethacrylate resin (A) and bisphenol glycidyldimethacrylate triethyleneglycol dimethacrylate, 2 hydroxyethylmethacrylate, zirconyl dimethacrylate resin (B) basing-composites filled with silica-hybridized amorphous calcium phosphate (Si-ACP) and zirconia-hybridized amorphous calcium phosphate (Zr-ACP) compared with unfilled copolymer and strontium glass- (Sr-glass-) filled composites. The number of specimens per group was eight or more.
It appears that SBS was affected minimally by the type of the ACP filler used and practically unaffected by the compositions of the two experimental resins. Compared with the unfilled copolymer specimens, introducing hybrid ACP filler into copolymers had no adverse effect on the early bonding properties. The majority (92.6 percent) of debonded surfaces (N = 53) showed adhesive failures, 5.6 percent showed cohesive failures in the adhesive resin, and 1.8 percent showed partially cohesive failure in adhesive resin and cohesive failure in ACP composite. These observations indicate that the test measured the bond strength of the adhesive resin in shear mode.
We conducted a second set of evaluations to establish the possible correlation between the particle size of the filler and water aging time on the bonding ability of the BTHZ matrix composites. Kinetic changes in the SBS values are shown in Figure 8 (page 1483). All-pair comparisons revealed stronger bonds with Sr-glass composites compared with both types of ACP composites only for the 24-hour SBS data. For all other intervals, the apparent differences between the filler groups were not statistically significant. For the intervals of one month or more, we noticed the following order of apparently decreasing SBS values: Sr-glass composite (19.0 ± 4.9 MPa) had a greater value than did unmilled ACP composite (16.4 ± 4.6 MPa), which had a greater value than did milled ACP composite (14.2 ± 5.6 MPa). The differences between the mean values, however, were not statistically significant, most likely due to the wide range of SBS values; SDs were up to 40 percent of the reported mean SBS values.
Figure 8.
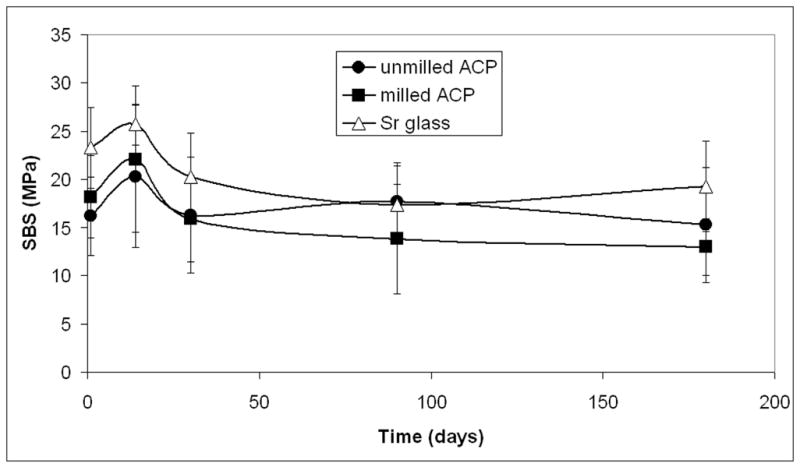
Effect of filler type on the shear bond strength of bisphenol glycidyldimethacrylate, triethyleneglycol dimethacrylate, 2 hydroxyethylmethacrylate, zirconyl dimethacrylate-based composites aged in distilled water at 37°C. The values are mean ± standard deviation of the minimum of seven specimens in each experimental group. ACP: Amorphous calcium phosphate. Sr-glass: Strontium glass.
The failure analysis of debonded two-week specimens (eight specimens/group) revealed that, in the unmilled ACP/BTHZ specimens, adhesive/cohesive failures were distributed equally between adhesive layer and the ACP base layer (37.5 percent each), while the remaining 25 percent of failures were adhesive. Milled ACP/BTHZ and ACP/TP specimens showed predominantly adhesive/cohesive failure in adhesive layer (62.5 percent), while the remaining 37.5 percent of the failures were distributed equally (12.5 percent each) as adhesive failures, partially cohesive failures or both in the adhesive layer and adhesive/cohesive failures in the ACP base layer.
In the Sr-glass specimens, however, we observed only adhesive (62.5 percent) and adhesive/cohesive failures in the adhesive layer (37.5 percent). Therefore, we conclude that after two weeks of water aging, adhesive failure mode between the dentin and adhesive resin was predominant in all groups.
DISCUSSION
Bis-GMA is the most frequently used base monomer in dental resins. To obtain dental resins with tractable viscosities, we included considerable amounts of the more flexible TEGDMA; the hydroxyl group-containing surface active HEMA, which is an adhesion-to-dentin-promoting diluent comonomer; and ZrDMA into the BTHZ resin formulation to improve inorganic/organic coupling. We expected the TP resin-based composites to adhere to tooth surfaces better, owing to a high level of the multifunctional, adhesion-promoting PMGDMA in the matrix. All of the comparisons of other physicochemical properties, such as polymerization shrinkage, water sorption and the mineral ion release, that may affect the clinical performance of these remineralizing/antidemineralizing basing materials significantly were in favor of BTHZ matrices compared with TP systems.2 Intensified hydrogen bonding that occurs in matrices with relatively high HEMA content such as our BTHZ resin, which had a mass fraction of 27 percent HEMA, leading to the densification of polymerization11 may play an important role in controlling the bonding properties of such matrices.
We have shown that highly agglomerated, unmilled Zr-ACP particulates disperse poorly throughout the matrix regardless of the compositional makeup of the resin.3,4,6 The inclusion of large agglomerates in the resin matrix often leads to insufficient wetting of the filler, which can introduce irregular-shaped flaws and surface irregularities into the composite (Figure 9 and Figure 10 [page 1484]). The resulting filler-matrix interfaces become susceptible to random spatial changes during water sorption and the subsequent mineral ion release from composites. Consequently, course ACP filler-based composites have lower mechanical strength than do the corresponding unfilled copolymers, and they are affected more by water exposure than are the unfilled copolymers.2 Water sorption of ACP based composites also is affected by the water-ACP filler interactions. The kinetics of the mineral ion release from anticariogenic ACP composites is governed by the nature of the polymer network structure, its permeability to water, its internal pH and the rate of intracomposite ACP conversion to thermodynamically stable apatite.
Figure 9.
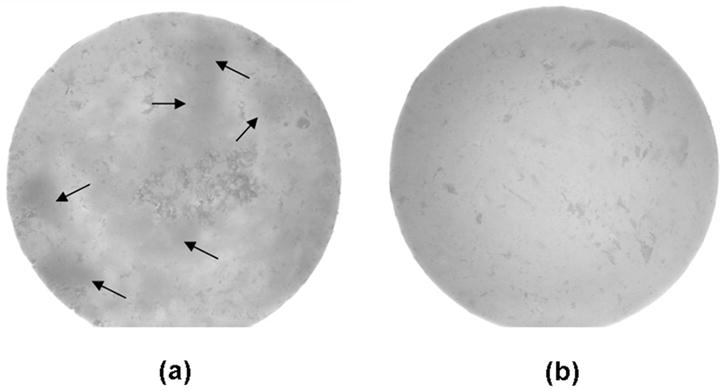
Pictures of unmilled amorphous calcium phosphate (ACP) (A) and milled ACP (B) composite disks taken using a stereomicroscope. Arrows indicate examples of filler-rich areas within the composite.
Figure 10.
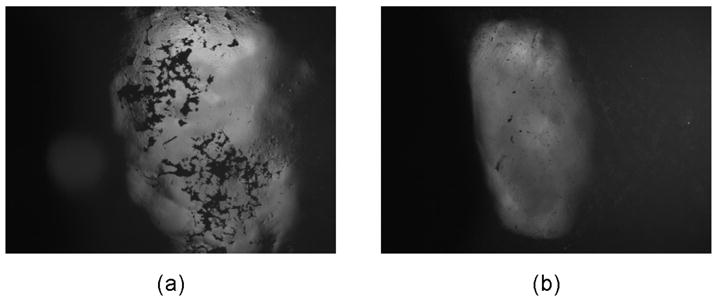
Unmilled amorphous calcium phosphate (ACP) (A) and milled ACP (B) composite disks showing differences in surface irregularities between the two groups.
In a recent study12 of other resin matrices based on the relatively flexible, low-viscosity, hydrophobic monomer ethoxylated bisphenol A dimethacrylate (EBPADMA) instead of the rigid, high-viscosity, somewhat more hydrophilic bis-GMA and milled ACP, improved mechanical stability of the composite specimens on aqueous soaking was achieved. However, the SBS results reported in our study showed no improvement with the use of milled ACP filler.
As gradients in fracture toughness and elastic modulus across the bonded interface are likely the governing factors in the adhesive strength of the bond, the chisel-on-iris shear method we used may not be sensitive enough to the effects of either. Using a sloppier method (such as wire-loop-on-composite) involving much higher stress concentrations might have been a better choice for discriminating the differences between the composites. While other factors (for example, the adhesive used and how well it penetrates, the flaws inherent to the tooth, etc.) might play critical roles in the perceived strength of the composite/adhesive/dentin bond, the part played by filler should not be ignored. Surface irregularities introduce by large, porous agglomerates (Figure 10) can lead to weak or unbonded areas between the composite and adhesive. However, the results of our study have shown that unmilled and milled ACPs, despite the flaws they introduce into the microstructure of composites, do not adversely affect the short- and long-term bonding behavior of the composite to an adhesive resin. Rather, they perform at least as well as glass-reinforced composites, while providing an additional bioactive component that may promote remineralization in dentin, enamel or both.
It appears that adjustments in the resin formulation also may be necessary to improve the bonding properties of bioactive ACP composites. SBS studies that we are conducting that use EBPADMA-based matrices may provide evidence on whether it is feasible to formulate ACP composites with improved bonding performance without compromising their biocompatibility, remineralizing/antidemineralizing potential or both.
Because of the unique ability of ACP resin-based composite to release calcium and phosphate ions, especially during an acid challenge, it has a number of potentially valuable uses in dental practice. We envision its use as a pit-and-fissure sealant that would act as a conventional sealant and help remineralize early carious lesions in fissures. As a cement, it could be used to cement crowns and inlays. It also may be well-suited as a cement for orthodontic brackets, potentially helping minimize enamel demineralization often seen adjacent to and under brackets. As our study implies, it may be a suitable lining or base material that has the viscosity of a flowable composite and sufficient strength to serve as a base material.13,14 Likewise, its formulations might make it suitable for use in atraumatic restorative treatment, and, as research continues to improve its mechanical properties, ACP-filled resins might be able to successfully function as composite restorative materials.
CONCLUSIONS
The type of bioactive ACP filler and its PSD did not significantly affect the strength of the composite/adhesive/dentin bond for these experimental, remineralizing/antidemineralizing resin-based composites. New formulations may be needed to improve the clinical appeal of these experimental basing materials with respect to their long-term dentin-bonding performance.
Supplemental Material
Acknowledgments
The National Institute of Dental and Craniofacial Research supported this study through research grant R01 DE13169-08.
The authors gratefully acknowledge the American Dental Association Foundation and the National Institute of Standards and Technology for their support. They would also like to thank Esstech (Essington, Pa.) for their contribution of bisphenol glycidyldimethacrylate, triethyleneglycol dimethacrylate and 2-hydroxyethyl methacrylate monomers; and Dentsply Caulk (Milford, Del.) for the donation of TPH resin-based composite and Sr-glass.
A figure showing the experimental steps involved in amorphous calcium phosphate synthesis and characterization is available with this article as posted on JADA Online (“http://jada.ada.org”). Interested readers may link to this article online, then click on the link in the Supplemental Databox.
Footnotes
“Official contribution of the National Institute of Standards and Technology; not subject to copyright in the United States.”
Publisher's Disclaimer: Certain commercial materials and equipment are identified in this work for adequate definition of the experimental procedures. In no instance does such identification imply recommendation or endorsement by the American Dental Association Foundation or the National Institute of Standards and Technology, or that the material and the equipment identified is necessarily the best available for the purpose.
Contributor Information
Gary E. Schumacher, Dr. Schumacher is a Chief Scientist/Clinical Program and an Associate Director at the Paffenbarger Research Center, American Dental Association Foundation, Gaithersburg, Maryland.
Joseph M. Antonucci, Dr. Antonucci is a Research Chemist in the Biomaterials Group of the Polymers Division, Materials Science and Engineering Laboratory of the National Institute of Standards and Technology, Gaithersburg, Maryland.
Justin N.R. O’Donnell, Mr. O’Donnell is a Research Assistant at the Paffenbarger Research Center, American Dental Association Foundation, Gaithersburg, Maryland.
Drago Skrtic, Dr. Skrtic is a Senior Project Leader at the Paffenbarger Research Center, American Dental Association Foundation, Gaithersburg, Maryland.
References
- 1.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physicochemical evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mat Res (Appl Biomater) 2000;53:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Skrtic D, Antonucci JM, Eanes ED. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J Res Natl Inst Stands Technol. 2003;108(3):167–182. doi: 10.6028/jres.108.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skrtic D, Antonucci JM, Eanes ED, Eidelman N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates – A FTIR microspectroscopic study. Biomaterials. 2004;25:1141–1150. doi: 10.1016/j.biomaterials.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Antonucci JM, Skrtic D. Matrix resin effects on selected physicochemical properties of amorphous calcium phosphate composites. J Bioact Comp Polym. 2005;20(1):29–49. [Google Scholar]
- 5.Skrtic D, Hailer AW, Takagi S, Antonucci JM, Eanes ED. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J Dent Res. 1996;75(9):1679–1686. doi: 10.1177/00220345960750091001. [DOI] [PubMed] [Google Scholar]
- 6.Lee SY, Regnault WF, Antonucci JM, Skrtic D. Effect of particle size of an amorphous calcium phosphate filler on the mechanical strength and ion release of polymeric composites. J Biomed Mater Res. 2007;80B:11–17. doi: 10.1002/jbm.b.30561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eanes ED, Gillessen IH, Posner AS. Intermediate states in the precipitation of apatite. Nature. 1965;208:265–267. doi: 10.1038/208365a0. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher GE, Eichmiller FC, Antonucci JM. Effects of surface-active resins on dentin/composite bonds. Dent Mater. 1992;8:278–282. doi: 10.1016/0109-5641(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 9.Khatri CA, Antonucci JM, Schumacher GE. Self-etching, polymerization-initiating primers for dental adhesion. In: Ottenbrite RM, Kim SW, editors. Polymeric drugs and drug delivery systems. Technomics Publ. Co., Inc; Lancaster, PA: 2000. pp. 289–300. [Google Scholar]
- 10.Venz S, Dickens B. Modified surface-active monomers for adhesive bonding to dentin. J Dent Res. 1993;72(3):582–586. doi: 10.1177/00220345930720030501. [DOI] [PubMed] [Google Scholar]
- 11.Skrtic D, Stansbury JW, Antonucci JM. Volumetric contraction and methacrylate conversion in photo-polymerized amorphous calcium phosphate/methacrylate composites. Biomaterials. 2003;24:2443–2449. doi: 10.1016/s0142-9612(02)00574-4. [DOI] [PubMed] [Google Scholar]
- 12.Skrtic D, Antonucci JM. Dental composites based on amorphous calcium phosphate – resin composition/physicochemical properties study. J Biomat Appl. 2007;21:375–393. doi: 10.1177/0885328206064823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ban S, Anusavice KJ. Influence of test method on failure stress of brittle dental materials. J Dent Res. 1990;69(12):1791–1799. doi: 10.1177/00220345900690120201. [DOI] [PubMed] [Google Scholar]
- 14.Antonucci JM, Skrtic D. Physicochemical properties of bioactive polymeric composites: Effects of resin matrix and the type of amorphous calcium phosphate filler. In: Shalaby SW, Salz U, editors. Polymers for Dental and Orthopedic Applications. CRC Press; Boca Raton, FL: 2007. pp. 217–242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


