Abstract
The pulmonary innate immune system responds to various airborne microbes. Although its specificity is broad and based on the recognition of pathogen-associated molecular patterns (PAMPs), it is uniquely regulated to limit inflammation and thereby prevent damage to the gas-exchanging alveoli. Macrophages, critical cell determinants of this system, recognize microbes through pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) which typically mediate pro-inflammatory responses. The lung collectin, surfactant protein-A (SP-A), has emerged as an important innate immune determinant that regulates microbe-macrophage interactions in this environment. Here we report the basal and SP-A-induced transcriptional and post-translational regulation of TLR2 and TLR4 expression during the differentiation of primary human monocytes into macrophages. Despite SP-A’s ability to up-regulate TLR2 expression on human macrophages, it dampens TLR2 and TLR4 signaling in these cells. SP-A decreases the phosphorylation of IκBα, a key regulator of NFκB activity, and nuclear translocation of p65 which result in diminished TNFα secretion in response to TLR ligands. SP-A also reduces the phosphorylation of TLR signaling proteins upstream of NFκB, including members of the MAP kinase family. Finally, we report for the first time that SP-A decreases the phosphorylation of Akt, a major cell regulator of NFκB and potentially MAP kinases. These data identify a critical role for SP-A in modulating the lung inflammatory response by regulating macrophage TLR activity.
Keywords: human monocytes/macrophages, cell trafficking, inflammation, lung
INTRODUCTION
While the majority of inhaled microbes and particulates are trapped and cleared by the upper airways of the lung, some organisms are able to travel further to the terminal bronchioles and alveoli. The degree of inflammation initiated in this site by a stimulus is tightly regulated by several elements of the innate immune system, as even moderate inflammation could be harmful to the gas-exchanging alveolar structures (1). Two key components involved in this regulation are phagocytes, mainly alveolar macrophages (AM), and pulmonary surfactant. AM, which are bathed in surfactant, are the first professional phagocytes to encounter, internalize, and degrade invading microbes (2).
AM are considered alternatively activated, based upon their unique biological attributes. These include a greater phagocytic potential compared to other macrophages (2,3) due to significant expression and activity of PRRs, such as the mannose receptor (MR) and scavenger receptor-A (SR-A) (4,5); and immunoregulation through production of pro-inflammatory cytokines (e.g.,such as tumor necrosis factor alpha (TNFα), and/or anti-inflammatory cytokines (e.g., transforming growth factor beta (TGFβ) (2,3). They also produce less Interleukin-1β (IL-1 β), have a reduced oxidative response to pathogens compared to blood monocytes (6-8), and serve as poor antigen presenting cells (APCs) (9), all of which are mechanisms that serve to control alveolar inflammation.
Toll-like receptors (TLRs) are important cell-associated and intracellular PRRs. There are currently twelve identified mammalian TLRs, of which TLR2 and TLR4 are among the most widely studied and are considered the major transmembrane TLRs (10). TLR2 and TLR4 play roles in initiating immune responses against pathogens. TLR2 forms a heterodimer with TLR6 or TLR1 to recognize diacyl and triacyl lipopeptides, respectively. TLR2 binds to zymosan, a particle composed of yeast cell wall components; peptidoglycan, a component of the Gram positive bacteria cell wall (11,12); and Pam3Cys-Ser-(Lys)4 hydrochloride (Pam3Cys), a lipohexapeptide analog of the immunologically active N-terminal portion of bacterial lipoprotein (13,14). TLR4 associates with MD2 and CD14, enabling recognition of lipopolysaccharide (LPS), a major component of the Gram negative cell wall, and heat shock proteins 60 and 70, which are ubiquitously expressed (15-18).
Since an unrestricted inflammatory response induced by TLR signaling can be potentially harmful to the host, this signaling is tightly regulated, detecting the presence of certain microbial determinants and responding differently depending on the stimulus. The signaling cascade typically produces pro-inflammatory cytokines; however, activation through TLRs can also generate negative regulators that inhibit a pro-inflammatory response (19).
Surfactant lipids and the surfactant-associated proteins SP-A, -B, -C, and -D are major components of pulmonary surfactant, which lines the lung alveolus and serves to decrease surface tension at the air-liquid interface of the lung (20-22). SP-A is a large, multimeric protein with trimers that are assembled into an 18-mer protein through interactions between the collagen-like domains (23-25). It is classified as a collectin since each monomer contains a linear collagen-like sequence, a short linking domain and a globular Ca2+-dependent carbohydrate recognition domain (CRD) (22).
SP-A has been implicated as a key component of the lung innate immune response because it mediates host interactions with a variety of microbial pathogens (26) and regulates the nature of the inflammatory response. In this regard, SP-A can serve both as a microbial opsonin and as a direct activator of macrophage function. SP-A has been shown to up-regulate certain PRRs, such as SR-A and the MR, on macrophages (4,5,27). SP-A also regulates TNFα production, either up or down, depending on which receptor it binds on the cell surface and the activation state of the cell (28). In human macrophages, SP-A down-regulates oxidant production to stimuli by decreasing NADPH oxidase activity (29).
Despite a large body of literature on the importance of TLR2 and TLR4 in pathogen recognition and host response, there is limited information about TLR2 and TLR4 expression on primary human mononuclear phagocytes and the response of these cells to TLR agonists, particularly during monocyte differentiation into macrophages. SP-A has emerged as a key regulator of the phagocyte response in the alveolar compartment and contributes to the characteristic alternative activation state of the macrophages in this environment. Here we report the discovery that SP-A differentially regulates the expression of TLR2 and TLR4 during primary human monocyte differentiation into macrophages. Despite up-regulation of TLR2 expression, SP-A markedly diminishes the macrophage pro-inflammatory response generated by both TLR2 and TLR4 agonists. The underlying mechanism is related to altered phosphorylation of a central regulator of cellular function, Akt, as well as downstream intermediates in the MAP kinase pathway; and the activation of NFκB. These studies underscore the important role of SP-A in shaping the biology of macrophages in the lung alveoli.
MATERIALS AND METHODS
Buffers, reagents, and media
Dulbecco’s PBS with and without Ca2+ and Mg2+ ions and RPMI 1640 medium with L-glutamine (RPMI) were purchased from Invitrogen (Carlsbad, CA). PBF buffer [PBS without Ca2+ and Mg2+ (Invitrogen), 5mg/ml bovine serum albumin (BSA) (Sigma; St. Louis, MO), and 10% heat-inactivated fetal bovine serum (FBS) (Hyclone; Logan, UT)] was used as a blocking agent for confocal microscopy experiments. RHH media [RPMI 1640 + 10mM HEPES + 0.4% human serum albumin (HSA)] was used for cell culture experiments. Pam3Cys (Calbiochem; San Diego, CA) and E. coli LPS (Sigma) were used for functional assays involving Western blotting and ELISAs.
Antibodies
APC-labeled anti-human TLR2 (clone T2.1), PE-labeled anti-human TLR4 (clone HTA125), and mouse IgG2a were purchased from eBioscience (San Diego, CA) for flow cytometry experiments. Unconjugated mouse anti-human TLR2 (clone TL2.1) (Novus Biologicals; Littleton, CO), mouse anti-human TLR4 (clone HTA125) (Gene Tex, Inc.; San Antonio, TX), mouse IgG2a (clone 20102) (R & D Systems; Minneapolis, MN), mouse anti-human MR (clone 19.2) (BD Biosciences; Franklin Lakes, NJ), and mouse IgG1 (R & D Systems) were used for confocal microscopy experiments in which Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes, Invitrogen detection technologies) was used as a secondary Ab. Phosphorylated Akt (phospho-Akt Ser 473), phosphorylated p38 (phospho-p38 MAP kinase thr180/tyr182), phosphorylated Erk (phospho-p44/42 map kinase thr202/tyr204), phosphorylated JNK (phospho-SAPK/JNK T183/Y185), phosphorylated IκB-α (Ser32), and IκB-α Abs were purchased from Cell Signaling Technology (Danvers, MA), while actin, total Akt, goat anti-rabbit IgG-HRP (secondary Abs for primary Abs to phosphorylated proteins), and donkey anti-goat IgG-HRP (actin and total Akt secondary Ab) Abs were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA) and used for Western blotting experiments. TNFα ELISA kits were purchased from R & D Systems.
SP-A proteins
The SP-A proteins used in this study were purified as previously described (4). In brief, bronchoalveolar lavage (BAL) from alveolar proteinosis patients (APP) was used to obtain APP-SP-A (4). Purity of the SP-A preparation was assessed by SDS-PAGE. Bacterial endotoxin levels were determined using the Limulus amebocyte lysate kit (BioWhittaker, Walkersville, MD). Endotoxin levels in SP-A preparations ranged from undetectable to 0.2 pg/μg protein. Two SP-A functional assays, oxidative burst and liposome aggregation (29,30), were performed as quality control experiments.
Human monocytes and macrophages
Blood was obtained from healthy adult volunteers using an approved protocol by The Ohio State University Institutional Review Board. Peripheral blood mononuclear cells (PBMC) from single donors were isolated from heparinized blood on Ficoll-Paque (Amersham, Piscataway, NJ) and cultured in Teflon wells (Savillex, Minnetonka, MN) for 1 (monocytes) through 5 [monocyte-derived macrophages (MDMs)] days in the presence of RPMI 1640 medium containing 20% autologous serum (2.0 × 106 PBMC/ml) at 37°C (4). On the day of each experiment, PBMCs were removed from Teflon wells and washed extensively. Monocytes and MDMs were further purified by adherence in tissue culture plates in some assays. Human AM were isolated from BALs of healthy human donors as described (31) using an approved protocol by The Ohio State University Institutional Review Board. Briefly, the BAL was centrifuged (200 × g, 4°C, 10 min), supernatant removed, and the pellet re-suspended in RPMI and washed two more times with RPMI.
Flow cytometry
PBMCs were incubated with APP-SP-A (10 μg/ml) or HSA (control) in Teflon wells for specific time periods. PBMCs were harvested from Teflon wells, centrifuged (200 × g, 4°C, 10 min), and re-suspended in RPMI. After the cells were counted, they were centrifuged, re-suspended in FACS buffer (2% BSA), and incubated with APC- or PE-conjugated Abs to TLR2 or TLR4 (20μl per million cells), respectively for 20 min. PBMCs incubated with the appropriate APC- or PE-conjugated subtypic control mAb served as negative controls.
For AM experiments, cells were counted, centrifuged, and re-suspended in FACS buffer. 2 × 105 AM were incubated with TLR2, TLR4, or subtype control Abs as described above for PBMCs.
Ab-stained cells were fixed in 2% paraformadehyde, and 1 × 104 MDM or AM were analyzed for mean fluorescence intensity (MFI) and percentage of positive cells (95/5% cutoff) using the BD FACS Calibur System (BD Biosciences, Franklin Lakes, NJ). Macrophages were distinguished by the side scatter vs forward scatter (4) and the MFI due to nonspecific binding (subtypic control Ab) was subtracted from each sample in order to obtain a specific MFI. The percent change in specific MFI in experiments with no treatment vs SP-A treatment were calculated as follows: (treated MFI - untreated MFI)/untreated MFI) × 100. Triplicate samples in each experiment were analyzed.
RNA isolation
One- to five-day-old PBMCs in Teflon wells were harvested and mononuclear phagocytes adhered to a 12-well tissue culture plate with 10% autologous serum (3 × 106 PBMC/ml). After washing away lymphocytes, the monocytes or MDMs (3 × 105 cells) were treated with SP-A (10μg/ml) or media (untreated) over a time course. Finally, cells were lysed in Trizol (Invitrogen) and total RNA was isolated by using the Qiagen RNeasy column method (Valencia, CA). The Experion (Bio-Rad; Hercules, CA) was used to determine the RNA quality and quantity of each sample.
Real time PCR
RNA (550 ng) was converted to cDNA by reverse transcriptase enzyme and real time PCR was performed with a human TLR2, TLR4, or MR Taqman gene expression kit (Applied Biosystems; Foster City, CA). TLR2, TLR4, and MR amplification were normalized to the β actin housekeeping gene (ΔCt). Relative copy number (RCN) and fold change were determined. RCN was calculated as follows: RCN = E-ΔCt × 100. E is the efficiency (2 = 100% efficiency) and ΔCt = Ct(target) – Ct(reference) (32). Fold change was calculated as 2-ΔΔCt. ΔΔCt = ΔCt (experimental cell group) – ΔCt (unstimulated cells). Duplicate samples were analyzed in each experiment.
Confocal microscopy
Two × 105 MDMs were adhered to a glass coverslip in each well of a 24-well tissue culture plate for 2 h at 37°C, washed to remove lymphocytes, and incubated with APP-SP-A (10 μg/ml) or RHH for 2 h at 37°C. The cells were washed, fixed with 2% paraformaldehyde (10 min at room temperature), and then incubated overnight in blocking buffer (PBF). The cells were then stained with TLR2 (8 μg/ml), TLR4 (8 μg/ml), MR (1 μg/ml), or the appropriate subtype (8 μg/ml or 1 μg/ml) control Ab for 1 h at room temperature, washed with PBF buffer and counter-stained with an Alexa 488-conjugated secondary antibody (1:500) for 1 h at room temperature. The cells were next washed with PBF buffer and coverslips were mounted on glass slides. Slides were viewed using a confocal, scanning laser microscope (Zeiss, Thornwood, NY) and fluorescence intensity of each cell slice was quantified using a pixel intensity measurement (Image J program, NIH). An analytic box was placed on the cell membrane of each cell at four points: 12, 3, 6, and 9 o’clock and the MFI was calculated as the mean of the MFI of all four points. The MFI was determined for 20 cells per coverslip, with duplicate slides per experiment, and a minimum of two donors used for each treatment condition.
Western blotting
Three × 105 day 5 MDMs were adhered to wells of a 12-well plate for 2 h at 37°C, washed to remove lymphocytes, and repleted with RPMI containing 10% autologous serum overnight at 37°C. APP-SP-A in RHH medium was added to appropriate wells for 10 min, and then cells were washed with RHH, and incubated for another 10 min at 37°C to allow for internalization of all bound SP-A, as previously published (33). Dose (5 μg/ml to 20 μg/ml) and time (5 min – 2 h) course experiments were conducted initially to determine the optimal concentration and time for SP-A incubation. Pam3Cys (100 or 500 ng/ml) or LPS (100 or 500 ng/ml) was added to appropriate wells for 5, 10, 15, or 30 min at 37°C, followed by the removal of media in preliminary experiments to determine the optimal time point for analysis. Lysis buffer [TN1 buffer: 50 mM Tris (pH 8.0), 10 mM EDTA, 10 mM Na4PO7, 10 mM NaF, 1% Triton-X 100, and 125 mM NaCl, 10 mM Na3VO4, 10μg/ml aprotinin, and 10μg/ml leupeptin (34) was added to each well, the resultant lysates were added to Eppendorf tubes and incubated on ice, and then centrifuged at 13,000 × g for 10 min to remove cell debris. The cleared lysates containing soluble proteins were collected and protein content was calculated using a BCA kit (Pierce; Rockford, IL). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Finally, the blots were blocked with 5% nonfat dry milk and probed with the appropriate primary (Abs to phosphorylated proteins 1:600; actin or total Akt Ab 1:1000) and secondary HRP-conjugated secondary Abs (1:1000). The blots were developed using the ECL kit (Amersham). The Image J program was used to quantify band intensity. Background intensity was subtracted from each sample and then fold change was determined as follows: (treated sample band intensity / untreated sample band intensity)
ELISA
One × 105 day 5 MDMs were adhered to each well of a 96-well plate for 2 h at 37°C, washed to remove lymphocytes, and then incubated with RHH medium with APP-SP-A (10 μg/ml) or RHH for 2 h at 37°C. Cells were washed with and re-suspended in RHH, and incubated for an additional 10 min at 37°C to allow for internalization of bound SP-A. Cells were then incubated with RHH alone, LPS (1 ng/ml), or Pam3Cys (10 ng/ml) for 1 h. Cell supernatants were collected and centrifuged at 200 × g for 5 min to remove dead cells, and TNFα was measured by ELISA. Quantitative ELISAs were performed using the Quantikine ELISA kits from R & D Systems, according to the manufacturer’s instructions.
NFκB assay
Four × 105 day 5 MDMs were adhered to each well of a 12-well tissue culture plate for 2 h at 37°C, washed to remove lymphocytes, and then incubated with APP-SP-A (10 μg/ml) or RHH medium for 10 min. The cells were then washed with RHH, and incubated for another 10 min at 37°C to allow for internalization of all bound SP-A. Pam3Cys (500 ng/ml) or LPS (500 ng/ml) was added to appropriate wells for 1 h. Media was removed and nuclear extracts from MDMs (2 wells combined to make 8 × 105 MDMs/sample) were prepared using the Transfactor extraction kit (Clontech; Mountain View, CA). The nuclear lysates were assayed for the presence of p65 in wells pre-coated with the DNA-binding consensus sequence by using the Colorimetric TransFactor kit (Clontech) and samples were read at 655 nm. Untreated, SP-A + LPS or Pam3Cys, and SP-A groups were compared to LPS or Pam3Cys. LPS or Pam3Cys were set to 100% p65. Percent inhibition was determined as follows: ((experimental cell group – LPS or Pam3Cys cell group) / (LPS or Pam3Cys cell group) × 100). 100 minus percent inhibition = percent p65 compared to LPS or Pam3Cys.
Statistical analysis
An unpaired one-tailed Student’s t test was used to analyze differences between two groups (e.g. +/- SP-A) and a one-way ANOVA with post-Tukey test was used to analyze differences among multiple test groups.
RESULTS
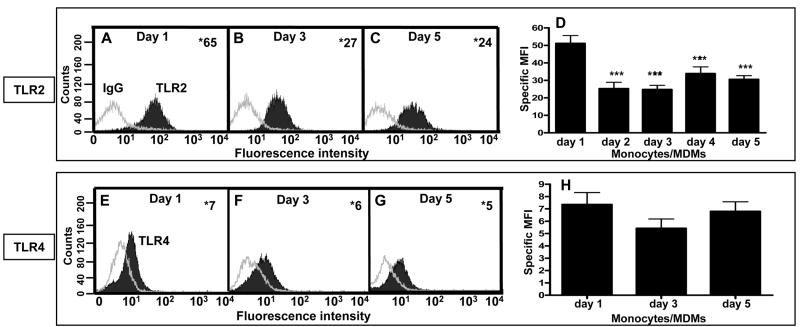
TLR2 surface protein expression decreases as human monocytes differentiate into macrophages, while TLR4 surface expression remains unchanged
Studies of TLR2 and TLR4 expression and function have been conducted largely in transfected murine cell lines or murine macrophages, with less data available in human monocytes and very limited data in human macrophages. There are significant differences in the structures of murine and human TLRs as well as in their responsiveness to various stimuli, such as LPS (35-41). Therefore, we began our studies by determining the basal surface protein expression of TLR2 and TLR4 by flow cytometry using an established model of human monocytes and monocyte-derived macrophages (MDMs) (4). Such a model allows us to assess changes in TLR expression during differentiation of cells from monocytes to macrophages in individual donors. The results of a typical experiment are displayed as histograms (Fig. 1A,B,C,E,F,G) and cumulative data are presented as bar graphs (Fig. 1D,H). The data show that primary human monocytes express the highest level of TLR2 and this expression decreases when monocytes differentiate into macrophages. Monocytes and macrophages express equivalent amounts of TLR4, indicating that TLR4 expression remains unchanged during differentiation. By flow cytometry, TLR2 and TLR4 were both found to be expressed on the surface of human alveolar macrophages (data not shown).
Figure 1.
Human monocytes and macrophages express TLR2 and TLR4. Peripheral blood mononuclear cells (PBMCs) were isolated from human blood and incubated in Teflon wells with autologous serum. The monocytes differentiate into macrophages (MDM) by day 5. 1-(A,E), 3-(B,F), or 5- (C,G) day old PBMCs were harvested and incubated with either an APC-conjugated human TLR2 mAb (or an APC-conjugated subtype control mAb) or a PE-conjugated human TLR4 mAb (or a PE-conjugated subtype control mAb). The stained cells were analyzed using flow cytometry by gating on the monocytes or macrophages (4), and a representative experiment is shown. The number in the top right corner represents the specific mean fluorescence intensity (MFI; TLR2 or TLR4 MFI minus subtype control MFI). (D, H) Bar graph with cumulative data (triplicate samples in each experiment; n = 5 for TLR2; n = 4 for TLR4). One-way ANOVA with post Tukey test *** p < 0.005 compared to day 1 cells (± SEM).
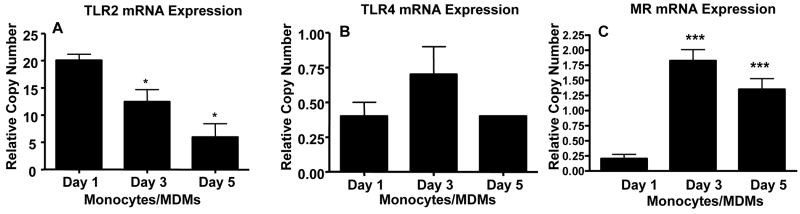
TLR2, but not TLR4, mRNA expression decreases as human monocytes differentiate into macrophages
Previous data on TLR2 and TLR4 mRNA expression in monocytes/macrophages are inconsistent, which likely reflects differences in cell type and activation state of the cells (38,41-44). We used real-time PCR to determine steady state mRNA levels of TLR2 and TLR4 in human monocytes and macrophages. The results are displayed as bar graphs (Fig. 2) and cumulative data are in table 1. TLR2 mRNA expression is greatest in day 1 monocytes and steadily decreases thereafter, while TLR4 mRNA expression changes to a much lesser degree during differentiation into macrophages. Expression of MR mRNA was used as a positive control since its expression is known to increase during monocyte differentiation into macrophages (4,45).
Figure 2.
TLR2 steady state mRNA levels decrease, while TLR4 levels vary to only a small extent, as monocytes differentiate into macrophages. 1-, 3-, and 5- day old PBMCs in Teflon wells were harvested and adhered to a 12-well tissue culture plate in RPMI containing 10% autologous serum. After washing away lymphocytes, the monocytes or MDMs were lysed in Trizol and total RNA was isolated. mRNA was converted to cDNA and real time PCR was performed. TLR2 (A), TLR4 (B), and MR (C) amplification was normalized to the β actin housekeeping gene and the relative copy number was determined. A and B are representative experiments (mean ± SD, triplicate samples) and C are cumulative data (mean ± SEM) [n = 6 (TLR2, TLR4); n = 4 (MR)]. The MR was used as a positive control as a macrophage marker. One-way ANOVA with post-Tukey test. * p < 0.05 compared to day 1 monocytes. ***p < 0.005 compared to day 1 monocytes.
Table I.
TLR2 and TLR4 steady state mRNA levels in human monocytes and macrophagesa
| % Change RCNc compared to day 1 monocytes | ||
|---|---|---|
| Receptorb | Day 3 monocytes | Day 5 MDMs |
| TLR2 | -51 ± 13* | -65 ± 10* |
| TLR4 | 68 ± 40 | -18 ± 11 |
| MR | 1193 ± 415*** | 923 ± 474*** |
TLR2, TLR4, and MR mRNA levels were measured by real time PCR
RNA was isolated and converted to cDNA and real time PCR was performed using TLR2, TLR4, and MR taqman gene expression kits.
Relative copy number (RCN) = E-ΔCt × 100. E is the efficiency (2 = 100% efficiency) ΔCt = Ct(target) – Ct(reference).
p < 0.05 relative to day 1 monocytes,
p < 0.005 relative to day 1 monocytes.
Mean ± SEM (n = 6 for TLR2, TLR4) and n = 4 for MR)
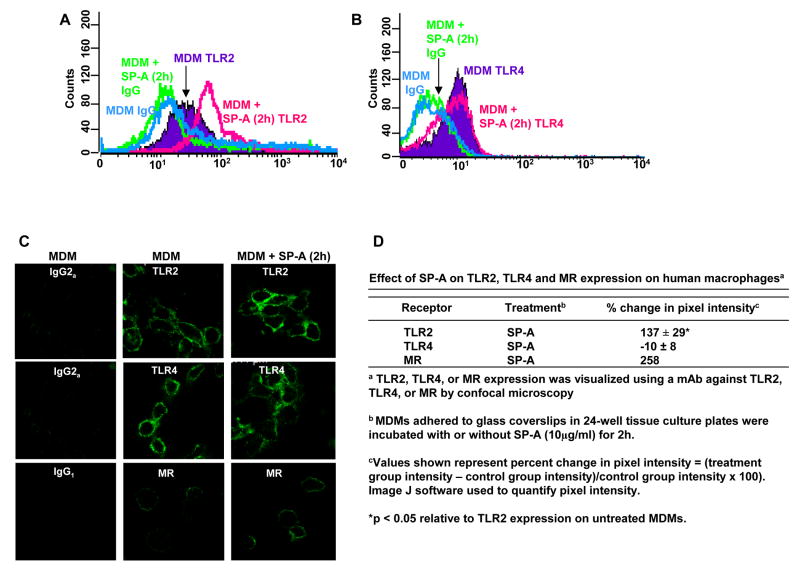
SP-A up-regulates expression of TLR2, but not TLR4, on human macrophages
SP-A binds to one or more receptors on macrophages (22) which triggers signaling events that alter the biology of these cells (28,46,47). Previous work from our laboratory has shown that SP-A increases MR expression on human MDMs, while another laboratory found that SP-A increases SR-A expression on rat macrophages (4,5); both receptors are known PRRs. The effect(s) of SP-A on TLR expression and function in human macrophages has not been explored. We used flow cytometry to determine whether SP-A regulated the basal levels of TLR2 and TLR4 surface expression on MDMs. A typical experiment is displayed as histograms in Fig. 3A (TLR2) and 3B (TLR4) with SP-A incubation for 2h. Incubation with SP-A for 1, 6, and 20 h showed equivalent results (data not shown). Our results show that SP-A increases TLR2, but not TLR4, expression on human macrophages. We next sought to determine whether the increase in TLR2 expression is due to a unique function of SP-A and/or SP-A-specific receptor engagement or whether the function could be shared with other structurally related proteins such as the complement protein C1q which also shares some functional similarities with SP-A (28). We determined that C1q does not regulate TLR2 expression (-8 ± 9 %, mean ± SD, n = 2).
Figure 3.
SP-A increases TLR2, but not TLR4, surface expression on MDMs. 5-day old PBMCs in Teflon wells were incubated with or without SP-A (10 μg/ml) for 2 h (A,B). Following the same protocol as in figure 1, SP-A-treated and untreated MDMs were incubated with either a human APC-conjugated TLR2 mAb or APC-conjugated subtype control mAb (A) or human PE-conjugated TLR4 mAb or PE-conjugated subtype control mAb (B). The stained cells were analyzed using flow cytometry by gating on the MDMs and the average of triplicate samples is shown in this experiment which is representative of n = 4 (A) and n = 5 (B). 5-day old MDMs in monolayer culture on glass coverslips were incubated with or without SP-A (10 μg/ml) for 2h. After washing, cells were fixed in paraformaldehyde (no permeabilization) and stained with mouse anti-human TLR2 mAb, mouse anti-human TLR4 mAb, mouse anti-human MR mAb, or subtype control mAb followed by a secondary Alexa-488-conjugated anti-mouse Ab. Glass coverslips were mounted and visualized by confocal microscopy. A representative experiment is shown in (C) [n = 3 (TLR2); n = 2 (TLR4, MR)]. Cumulative data are shown in (D). A Student t-test was performed. * p < 0.05 relative to TLR2 expression on untreated MDMs. The MR was used as a positive control.
To complement our data with SP-A, we used confocal microscopy to visualize TLR surface expression. Consistent with the flow cytometry data, SP-A increased TLR2, but not TLR4, surface expression on MDMs (Fig. 3C, 3D). MR staining was used as a positive control since SP-A increases MR surface expression on MDMs (4).
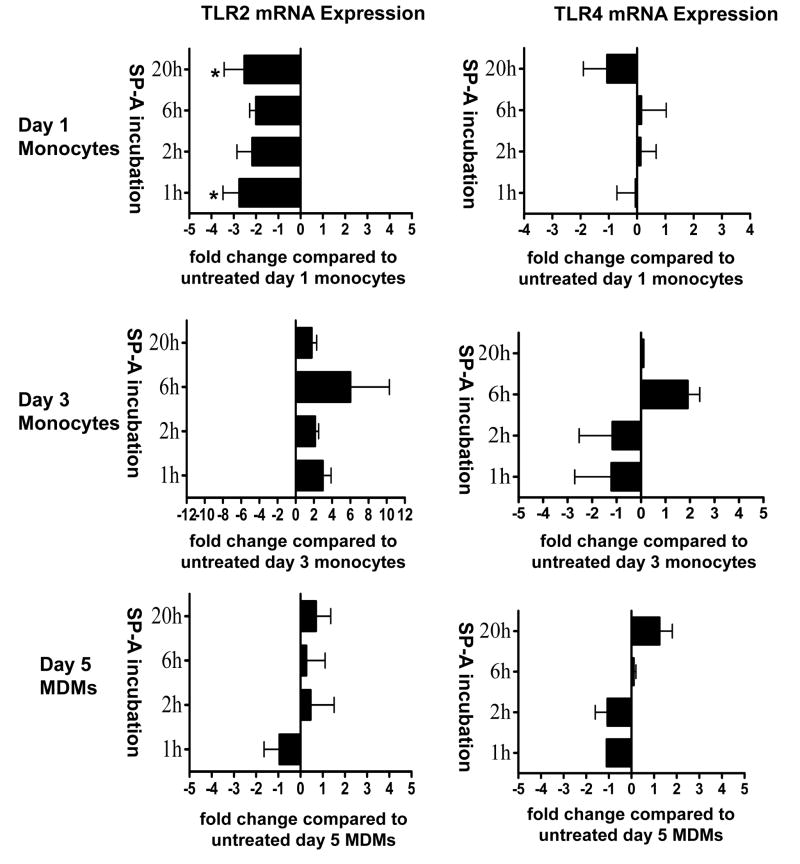
SP-A regulates TLR2, but not TLR4, mRNA expression in a differentiation-dependent manner
After determining that SP-A regulates TLR2, but not TLR4 surface protein expression, we next examined whether SP-A regulates TLR2 through a transcriptional mechanism. We used real time PCR to examine whether SP-A regulates TLR2 mRNA expression in monocytes and macrophages. We examined SP-A regulation of TLR4 mRNA expression for comparison. The results are displayed as bar graphs (Fig. 4). While SP-A decreased TLR2 mRNA expression in day 1 monocytes and increased its expression to a small extent in day 3 monocytes; importantly, it did not regulate TLR2 mRNA expression in day 5 MDMs. In contrast to its effects on TLR2 mRNA levels, SP-A had very little effect on TLR4 mRNA expression in monocytes and macrophages. Thus, the effect of SP-A on regulating TLR2 protein expression on macrophages (as opposed to monocytes), appears to be via a post-translational mechanism.
Figure 4.
SP-A regulates steady state mRNA expression of TLR2, but not TLR4, during monocyte differentiation into macrophages. SP-A (10 μg/ml) was added to 1-, 3-, and 5-day old PBMCs in selected Teflon wells for 1, 2, 6, or 20h. PBMCs were harvested and monocytes/macrophages adhered to a 12-well tissue culture plate in autologous serum. After washing away lymphocytes, the monocytes or MDMs were lysed in Trizol and total RNA was isolated. mRNA was converted to cDNA and real time PCR was performed. TLR2 and TLR4 mRNA amplification was normalized to the β actin housekeeping gene. The fold change was determined by comparing SP-A-treated samples to samples with no SP-A. Cumulative data are shown (± SEM) [TLR2: n = 6 (d5 20h); n = 5 (d5 2h); n = 4 (d1 1h, 2h, 6h, 20h; d3 1, 2h; d5 1h, 6h); n = 2 (d3 6h, 20h)] [TLR4: n = 6 (d1 2h); n = 5 (d1 1h, 20h); n = 4 (d1 6h); n = 3 (d3 1h, 2h; d5 2h, 20h); n = 2 (d3 6h, d5 6h); n = 1 (d3 20h; d5 20h)]. Student t-test was performed. * p < 0.05 compared to day 1 monocytes
SP-A diminishes TNFα secretion from macrophages stimulated with TLR2 or TLR4 ligands
Once we established that SP-A differentially regulates TLR2 and TLR4 expression on macrophages, we studied whether SP-A regulates TLR function, since changes in expression do not always correlate with changes in function. When TLR2 or TLR4 is activated by an appropriate ligand, a signaling cascade is initiated that can result in the production of pro-inflammatory cytokines. We determined whether SP-A regulates the secretion of one of the major pro-inflammatory cytokines, TNFα, in response to LPS (TLR4 ligand) or Pam3Cys (TLR2 ligand) by macrophages. We found that SP-A markedly decreased TNFα secretion by MDMs in the presence of either ligand (Table II). Thus, although SP-A regulates the expression of only TLR2, these data suggest that SP-A can alter the biological activity of both TLR2 and TLR4.
Table II.
Effect of SP-A on TNFα secretion from human macrophagesa
| Treatment | Ligand addedb | % decrease in TNFαc | n |
|---|---|---|---|
| SP-A | none | 0 | 3 |
| SP-A | LPS | 82 ± 12* | 3 |
| SP-A | Pam3Cys | 88 ± 13* | 3 |
TNFα secretion by MDMs was measured by ELISA
Day 5 MDMs adhered to a 96-well tissue culture plate were incubated with or without SP-A (10μg/ml) for 2h, and then with or without the indicated TLR ligand
Values shown represent percent change in TNFα secretion = (treatment group TNFα secretion – control group TNFα secretion)/control group secretion × 100). Control group is MDMs with no treatment or ligand added; Mean TNFα values (pg/ml) for LPS, Pam3Cys, SP-A + LPS and SP-A + Pam3Cys were 108, 108, 19 and 13, respectively.
p < 0.05 relative to TNFα secretion by untreated MDMs.
SP-A regulates the phosphorylation of IkBα and the nuclear translocation of p65 in macrophages stimulated with TLR2 or TLR4 ligands
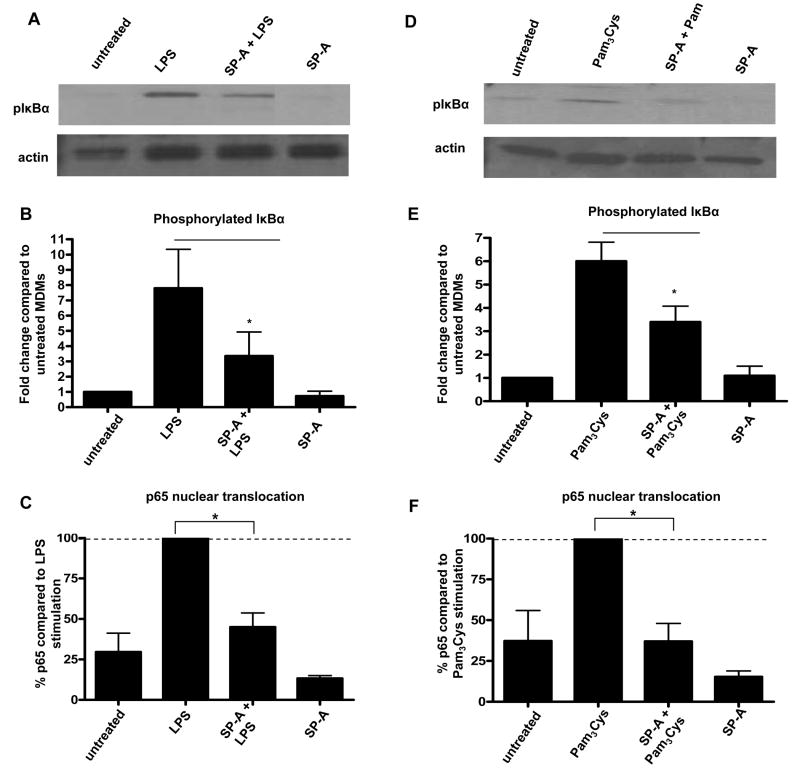
Since SP-A regulates the biological outcome of TLR signaling pathways, we chose to study the effect of SP-A on the phosphorylation of specific protein kinases of these pathways, a prerequisite for kinase activity. We first examined whether SP-A regulates the phosphorylation of the inhibitory protein, IκBα. IκBα is a key regulator of NFκB activation, since it binds to the NFκB complex in the cytosol to prevent translocation of NFκB to the nucleus and subsequent transcription of NFκB-regulated genes. After IκBα is phosphorylated, it is eventually degraded, which allows the NFκB complex to translocate to the nucleus (48,49). As shown in Figure 5, we found that SP-A decreases the phosphorylation of IκBα in MDMs in response to TLR ligands. SP-A does not regulate the level of total IκBα protein in these cells (data not shown).
Figure 5.
SP-A regulates the phosphorylation of IκBα in macrophages following the addition of TLR ligands. 5 day old MDMs were adhered to a 12-well tissue culture plate, washed, and incubated overnight in autologous serum at 37°C. Cells were incubated with or without SP-A (10 μg/ml) for 10 min. After washing, cells were incubated for an additional 10 min at 37°C to internalize any bound SP-A. LPS (10 min) or Pam3Cys (5 min) was added to appropriate wells. MDMs were lysed and SDS-PAGE and Western blots were performed using pIκBα or actin (control) Abs. Representative Western blots are shown (A, D) and bar graphs (B, E), generated by densitometric analysis, represent cumulative data (duplicate samples in each experiment; mean ± SEM; n = 4, LPS 10 min incubation or n = 4 Pam3Cys 5 min incubation). (C, F) 5 day old MDMs were adhered to a 12-well tissue culture plate, washed, and incubated overnight in autologous serum at 37°C. Cells were incubated with or without SP-A (10 μg/ml) for 10 min. After washing, cells were incubated for an additional 10 min at 37°C to internalize any bound SP-A. LPS (1 h) or Pam3Cys (1 h) was added to appropriate wells, media was removed, and nuclear extracts were prepared. p65 nuclear translocation was measured according to the manufacturers of the colorimetric TransFactor kit. (mean ± SEM; n = 3) A Student t-test was performed. * p < 0.05 compared to LPS or Pam3Cys
After determining that SP-A decreases the phosphorylation of IκBα, we next examined whether SP-A directly regulates the nuclear translocation of the NFκB complex. We observed that SP-A significantly decreases the nuclear translocation of p65, a major component of the NFκB complex, in the presence of TLR ligands (Figure 5).
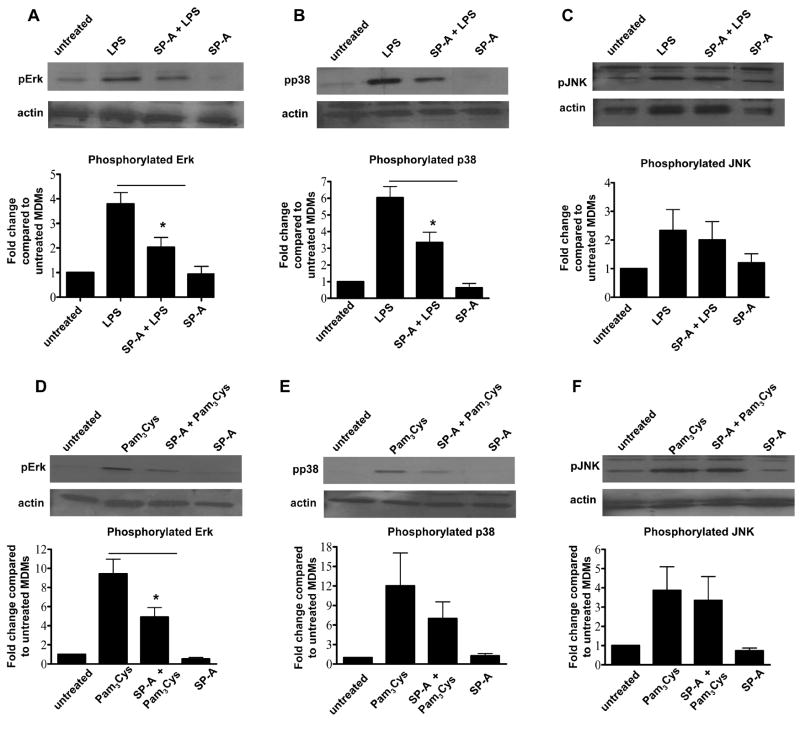
SP-A selectively regulates the phosphorylation of MAP kinases in macrophages stimulated with TLR2 or TLR4 ligands
A recent report has shown the ability of SP-A to activate SHP-1, a tyrosine phosphatase, and thereby decrease the phosphorylation of p38, one member of the MAP kinase family, in response to LPS (28). Thus, we sought to determine whether SP-A regulates the phosphorylation of each of the MAP kinases in the presence of TLR ligands in human macrophages. These results would provide further insight as to whether SP-A directly regulates NFκB activity or if the effect is upstream of this transcriptional complex. We found that while SP-A decreases the phosphorylation of p38 and Erk, it does not affect the phosphorylation of JNK in MDMs, as assayed by Western blot analysis (Fig. 6).
Figure 6.
SP-A selectively regulates the phosphorylation of MAP kinases in macrophages following the addition of TLR ligands. Following the same protocol as in figure 5, treated MDMs were lysed and SDS-PAGE and Western blots were performed using pErk (A,D), pp38 (B,E), pJNK (C,F) or actin (control) Abs. Representative Western blots are shown and bar graphs generated by densitometric analysis, represent cumulative data (duplicate samples in each experiment; mean ± SEM; n = 4 for pErk, LPS and Pam3Cys 15min incubation; n = 4 for pp38, LPS and Pam3Cys 15min incubation; and n = 3 for pJNK, LPS and Pam3Cys 10min incubation). A Student t-test was performed. * p < 0.05 compared to LPS and Pam3Cys.
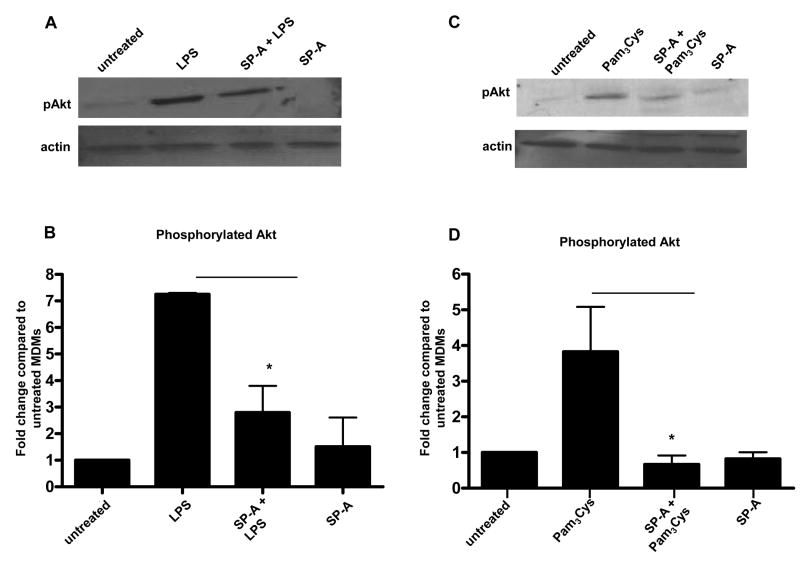
SP-A regulates the phosphorylation of Akt in human macrophages stimulated with TLR2 or TLR4 ligands
Having determined that SP-A regulates the phosphorylation of IκBα and members of the MAP kinase family, we sought to identify a novel upstream target of SP-A’s activity that is known to regulate both NFκB and MAP kinases. The protein kinase Akt has been shown to both positively and negatively regulate NFκB and cytokine production, depending on the stimulus, cell type, and activation state of the cell (34,50-55). Akt can also regulate the MAP kinase pathway and thereby affect the activation of AP-1 and NFκB (51). In support of this possibility, we had previously determined that phosphoinositide 3-kinase (PI3K) activity is involved in the up-regulation of MR expression by SP-A (33). PI3Ks are activated either directly through TLR2 or through MyD88 for the TLR4 pathway (55-57). PI3K activation can lead to the phosphorylation and activation of Akt (58-60). Therefore, we chose to study whether SP-A regulates the phosphorylation of Akt. Using Western blots, we found that SP-A decreased the phosphorylation of Akt in the presence of LPS or Pam3Cys in MDMs (Figure 7). Total Akt expression was not affected by the presence of SP-A (data not shown).
Figure 7.
SP-A regulates the phosphorylation of Akt in human macrophages following the addition of TLR ligands. 5-day old MDMs were adhered to a 12-well tissue culture plate, washed, and incubated overnight in autologous serum at 37°C. Cells were incubated with or without SP-A (10 μg/ml) for 10 min. After washing, cells were incubated for an additional 10 min at 37°C to internalize any bound SP-A. LPS (30 min) or Pam3Cys (15 min) was added to appropriate wells. MDMs were lysed and SDS-PAGE and Western blots were performed with lysates using pAkt Ab or actin Ab (control). Representative Western blots are shown (A, C) and bar graphs (B, D), generated by densitometric analysis, represent cumulative data (duplicate samples in each experiment; mean ± SEM; n = 3 LPS; n = 4 Pam3Cys). A Student t-test was performed. * p < 0.05 compared to LPS or Pam3Cys.
DISCUSSION
AM recognize and remove microbes through increased activity of a subset of PRRs. Two major PRRs are TLR2 and TLR4 which, depending on the stimulus, initiate pro-inflammatory responses or these inflammatory responses can be inhibited by the activation of TLR negative regulators. TLR2 and TLR4 initiate an inflammatory immune response by binding to a wide variety of PAMPS (61,62) through the extracellular leucine-rich repeat domains of these receptors which lead to the activation of the TLR cytoplasmic signaling domains. TLR2 and TLR4 signaling can indirectly activate the adaptive immune response through the production of inflammatory cytokines which, in turn, can lead to dendritic cell maturation and migration to lymph nodes where Ag-derived peptide presentation to T cells occurs (63).
We used our primary human monocyte/ macrophage differentiation cell model to address several unanswered questions with regard to TLR2 and TLR4 expression and activity on these cells. We were able to discern important differences in the transcriptional and post-translational mechanisms for TLR2 and TLR4 expression between monocytes and macrophages, both with respect to basal levels and following SP-A stimulation. Further, our studies have identified a new signaling pathway targeted by SP-A, i.e. involvement of the major cell regulator Akt, members of the MAP kinase family, and a key downstream regulator of NFκB, IKBα, ultimately leading to a marked reduction in the production of TNFα.
There are important differences between human and murine TLRs with regard to steady-state mRNA levels, surface protein expression, protein structure, and regulation. Some of the TLR2 differences include: little homology between human and murine promoter regions, expression on murine but not human T cells, and different regulatory elements controlling the expression of the murine and human TLR2 genes (41). Similarly, the TLR4 human and murine genes are regulated differently, and murine TLR4 mRNA is more broadly expressed than human TLR4 mRNA (35,64). Also, while there is strong sequence homology between the human and murine TLR4 promoters, there are differences in the structures of these promoters, which could account for the differences in expression (35). Since the majority of TLR studies involve murine cell lines and primary murine cells, we chose to focus our attention on whether human monocytes and MDMs expressed TLR2 and TLR4.
We observed the highest TLR2 surface protein and mRNA expression in day 1 monocytes, and this expression decreased as monocytes differentiated into macrophages. Unlike TLR2, TLR4 surface protein expression stayed constant, and TLR4 mRNA expression varied only slightly as monocytes differentiated into macrophages. The differences in basal surface protein expression and mRNA steady-state levels between TLR2 and TLR4 suggested that they may also be differentially regulated by SP-A. Our TLR2 mRNA data are consistent with a previous finding by Haehnel et al. where TLR2 mRNA expression (Northern blot analysis) in monocytes decreased after 24 hours of adherence to a tissue culture plate (41). Although others have shown TLR2 and TLR4 mRNA expression in human monocytes or MDMs (38,44,65), these studies did not measure mRNA levels during monocyte differentiation into macrophages. Our cell surface protein expression data are consistent with a previous report that showed more TLR2 and TLR4 surface protein expression on human monocytes compared to human AM (66).
We found that while SP-A up-regulated TLR2 surface protein expression, it did not affect TLR4 surface expression on macrophages. Interestingly, SP-A decreased TLR2 mRNA expression in day 1 monocytes, but increased to a small extent (not significant) mRNA expression in day 3 cells. Importantly, SP-A did not regulate TLR2 mRNA expression in day 5 macrophages. These data show that SP-A differentially regulates TLR2 mRNA expression during monocyte differentiation into macrophages. Due to the lack of TLR2 mRNA regulation by SP-A in day 5 MDMs, our data support that SP-A up-regulation of TLR2 surface expression on MDMs is most likely the result of a post-translational mechanism, possibly by translocating intracellular pools of pre-formed TLR2 to the cell surface. Confocal microscopy experiments using fixed and permeabilized MDMs suggest that intracellular pools of TLR2 exist (unpublished observation). Our previous work indicated a similar mechanism for SP-A’s up-regulation of the MR on macrophages (4) and there are reports that TLR2 and TLR4 can be found within intracellular vesicles (67,68).
We examined the relationship between SP-A’s effects on TLR expression and its effects on TLR function since effects on expression and function do not always correlate. There are contradictory data regarding the role of SP-A and TLRs in the inflammatory response. By using CHO cells and TLR4-deficient/wild type mice, one group observed that SP-A can activate the NFκB signaling pathway and up-regulate the synthesis of cytokines, such as TNFα, by relying predominantly on a functional TLR4 complex (69), while another group did not observe SP-A activation of NFκB (70). Differences in these results may depend on the SP-A purification process used by the researchers. Purified SP-A used by the first group contained higher concentrations of LPS compared to that of the second group. This difference in LPS contamination could result in the pro-inflammatory effects by SP-A which have been observed (71).
We observed a significant reduction, but not a complete elimination, of TNFα secretion when macrophages were pretreated with SP-A and then treated with either the TLR4 ligand, LPS, or the TLR2 ligand, Pam3Cys. Our TLR4 data are consistent with previous publications that showed that SP-A decreases TNFα secretion in the presence of LPS (28,72-74). Overall, these data provide further evidence for the role of SP-A in dampening the pro-inflammatory response in the lung environment.
Recently, SP-A was shown to directly associate with TLR2 and TLR4 proteins and thereby attenuate a pro-inflammatory response in rat macrophages and murine cell lines (46,75). In our studies, we used a protocol which allows for complete internalization of SP-A in MDMs prior to adding the TLR ligands (33). Following incubation with SP-A, we used specific mAbs to readily detect the TLRs (increased in the case of TLR2), indicating that surface TLR binding sites were not occupied by SP-A and were free to bind other ligands. Thus, our studies point to a different mechanism independent of TLR blockade by SP-A, whereby SP-A binds to one or more of its receptors, initiating a signaling cascade that results in altered expression and activity of the TLRs. Several potential SP-A receptors have been identified, including SIRPα, calreticulin/CD91 (28) and SP-R210 (76,77).
We found that SP-A significantly decreased the phosphorylation of IκBα in macrophages following the addition of the TLR2 and TLR4 ligands. Our data contradicts previous findings by Wu et al., which did not find an effect of SP-A on the phosphorylation of IκBα in the presence of LPS (70). The differences could be due to the low concentration of LPS that was used to stimulate the cells and the difference in cell type. Additionally, our data show that SP-A directly regulates the NFκB complex by decreasing the nuclear translocation of p65.
We found that SP-A decreases the phosphorylation of p38 and Erk, but not JNK in the presence of TLR ligands. SP-A regulation of the phosphorylation of p38 in the presence of LPS are consistent with previous findings by Gardai et al. using murine RAW cells and human macrophages (28). However, they reported that SP-A also regulated the phosphorylation of JNK, although the data were not shown. If RAW cells were used for their pJNK experiments, the differences in our findings could be explained by their use of a murine macrophage-like cell line versus our human MDMs. Our data suggest that JNK is regulated by distinct mechanisms compared to p38 and Erk. While the p38, Erk, and JNK pathways can be activated by unique upstream signaling molecules, there are overlapping upstream and downstream kinases, which could explain why we observed that SP-A regulated only two of the three MAP kinases (78).
We previously found that PI3K was involved in SP-A’s increase in MR expression on macrophages (33). Also, previous studies have shown that when TLR2 or TLR4 is activated by appropriate ligands, PI3Ks are activated (79). The activation of PI3K initiates a signaling cascade that results in the phosphorylation and activation of Akt. Akt is a kinase that is involved in numerous cell functions, one of which is regulation of NFκB (80-82). The literature provides evidence for a potential link between Akt, MAP kinases and the activation of AP-1 and NFκB (51). We found that SP-A decreased the phosphorylation of Akt in the presence of Pam3Cys and LPS, which is associated with the decrease in phosphorylation of MAP kinases and IκBα; finally leading to a diminished pro-inflammatory response.
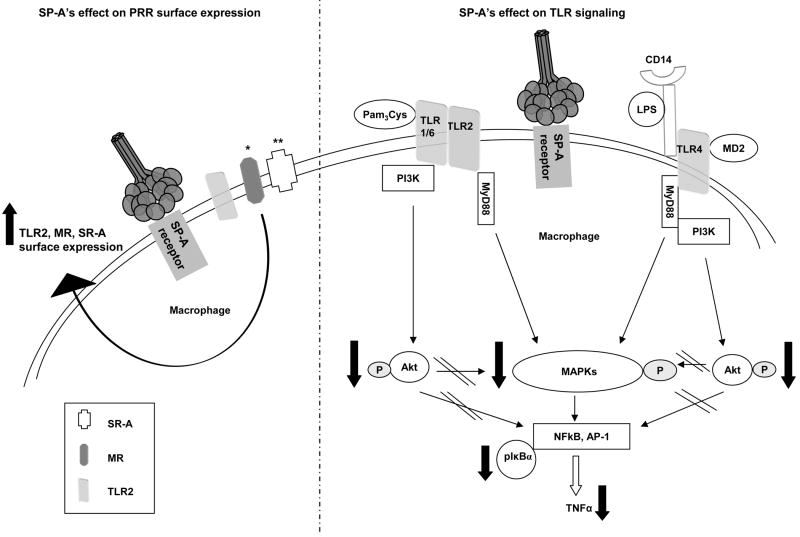
Based on our previous work and current work, and the literature, we propose a model of TLR modulation by SP-A (Figure 8), where SP-A binds to its macrophage receptor(s) and signals TLR2 intracellular pools to travel to the cell surface, which increases TLR2 surface expression. Simultaneously, SP-A signals also regulate TLR activity by diminishing pro-inflammatory cytokine production as the result of a decrease in the phosphorylation of a key regulator of NFκB, IκBα, and a decrease in the nuclear translocation of p65. SP-A down-regulates kinases upstream of IκBα by decreasing the phosphorylation of Akt and MAP kinases in response to TLR ligands. Therefore, while SP-A increases TLR2 expression, which can enhance pathogen recognition, it limits TLR signaling so that the lung is not damaged by an over-reactive inflammatory response.
Figure 8.
SP-A is a key regulator of TLR expression and signaling in macrophages. SPA binds to its receptor(s) leading to increased expression of TLR2 but not TLR4 (this report), the MR *(4) and SR-A ** (5). Simultaneously, SP-A regulates TLR activity in response to agonists by decreasing the phosphorylation of key TLR signaling proteins, including Akt and MAP kinases. Finally, SP-A decreases the phosphorylation of IκBα and nuclear translocation of p65 which results in diminished pro-inflammatory cytokine production.
There is accumulating evidence that SP-A regulates the macrophage surface expression and/or function of a subset of PRRs including the MR, SR-A and, based on this report, TLR2 and TLR4, and controls the host inflammatory response and microbicidal activity against various pathogens and particulates routinely encountered in the lung (Figure 8). This is a critical function of SP-A that preserves the normal architecture of the alveolar environment and gas exchange, and provides for effective microbial clearance and sterility of the alveolar environment.
Acknowledgments
We thank Dr. Mark Wewers for performing bronchoscopies in order to obtain BAL for the human alveolar macrophage experiments. Also, we thank Dr. Francis McCormack and Dr. Bruce Trapnell for providing BAL from alveolar proteinosis patients for SP-A isolation and purification. We acknowledge the support of two core facilities at the Ohio State University; the Dorothy M. Davis Heart and Lung Research Institute Flow Cytometry Core Laboratory and the Campus Microscopy and Imaging Facility.
This work was supported by the NIH AI059639 (LSS).
Abbreviations used in this study
- MDMs
monocyte-derived macrophages
- MR
mannose receptor
- TLR2
Toll-like receptor 2
- TLR4
Toll-like receptor 4
- SP-A
surfactant protein-A
- LPS
lipopolysaccharide
- Pam3Cys
Pam3Cys-Ser-(Lys)4 hydrochloride
Footnotes
DISCLOSURES The authors have no financial conflict of interest.
References
- 1.Zhang P, Summer WR, Bagby GJ, Nelson S. Innate immunity and pulmonary host defense. Immunol Rev. 2000;173:39–51. doi: 10.1034/j.1600-065x.2000.917306.x. [DOI] [PubMed] [Google Scholar]
- 2.Fels A, Cohn ZA. The alveolar macrophage. J Appl Physiol. 1986;60:353–369. doi: 10.1152/jappl.1986.60.2.353. [DOI] [PubMed] [Google Scholar]
- 3.Lohmann-Matthes ML, Steinmuller C, Franke-Ullmann G. Pulmonary macrophages. Eur Respir J. 1994;7:1678–1689. [PubMed] [Google Scholar]
- 4.Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- 5.Kuronuma K, Sano H, Kato K, Kudo K, Hyakushima N, Yokota S, Takahashi H, Fujii N, Suzuki H, Kodama T, Abe S, Kuroki Y. Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J Biol Chem. 2004;279:21421–21430. doi: 10.1074/jbc.M312490200. [DOI] [PubMed] [Google Scholar]
- 6.Wewers MD, Rennard SI, Hance AJ, Bitterman PB, Crystal RG. Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest. 1984;74:2208–2218. doi: 10.1172/JCI111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balter MS, Toews GB, Peters-Golden M. Different patterns of arachidonate metabolism in autologous human blood monocytes and alveolar macrophages. J Immunol. 1989;142:602–608. [PubMed] [Google Scholar]
- 8.Oren R, Farnham AE, Saito K, Milofsky E, Karnovsky ML. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons CR, Ball EJ, Toews GB, Weissler JC, Stastny P, Lipscomb MF. Inability of human alveolar macrophages to stimulate resting T cells correlates with decreased antigen-specific T cell-macrophage binding. J Immunol. 1986;137:1173–1180. [PubMed] [Google Scholar]
- 10.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–180. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 12.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann P, Wiesmuller KH, Metzger J, Jung G, Bessler WG. Induction of tumor cytotoxicity in murine bone marrow-derived macrophages by two synthetic lipopeptide analogues. Biol Chem Hoppe Seyler. 1989;370:575–582. doi: 10.1515/bchm3.1989.370.1.575. [DOI] [PubMed] [Google Scholar]
- 14.Muller MR, Pfannes SD, Ayoub M, Hoffmann P, Bessler WG, Mittenbuhler K. Immunostimulation by the synthetic lipopeptide P3CSK4: TLR4-independent activation of the ERK1/2 signal transduction pathway in macrophages. Immunology. 2001;103:49–60. doi: 10.1046/j.1365-2567.2001.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poltorak A, He X, Smirnova I, Liu MY, Van HC, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 16.Vabulas RM, hmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 18.Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J Immunol. 2002;168:1435–1440. doi: 10.4049/jimmunol.168.3.1435. [DOI] [PubMed] [Google Scholar]
- 19.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 20.Pattle RE. Properties, function and origin of the alveolar lining layer. Nature. 1955;175:1125–1126. doi: 10.1038/1751125b0. [DOI] [PubMed] [Google Scholar]
- 21.Clements JA. Surface tension of lung extracts. Proc Soc Exp Biol Med. 1957;95:170–172. doi: 10.3181/00379727-95-23156. [DOI] [PubMed] [Google Scholar]
- 22.Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 23.Voss T, Eistetter H, Schafer KP, Engel J. Macromolecular organization of natural and recombinant lung surfactant protein SP 28-36. J Mol Biol. 1988;201:219–227. doi: 10.1016/0022-2836(88)90448-2. [DOI] [PubMed] [Google Scholar]
- 24.King R, Simon D, Horowitz PM. Aspects of secondary and quaternary structure of surfactant protein A from canine lung. Biochim Biophys Acta. 1989;1001:294–301. doi: 10.1016/0005-2760(89)90114-8. [DOI] [PubMed] [Google Scholar]
- 25.Kuroki Y, Voelker DR. Pulmonary surfactant proteins. J Biol Chem. 1994;269:25943–25946. [PubMed] [Google Scholar]
- 26.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 27.Kudo K, Sano H, Takahashi H, Kuronuma K, Yokota S, Fujii N, Shimada K, Yano I, Kumazawa Y, Voelker DR, Abe S, Kuroki Y. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. J Immunol. 2004;172:7592–7602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- 28.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 29.Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA, Zweier JL, McCormack FX, Schlesinger LS. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol. 2004;172:6866–6674. doi: 10.4049/jimmunol.172.11.6866. [DOI] [PubMed] [Google Scholar]
- 30.Crowther JE, Schlesinger LS. Endocytic pathway for surfactant protein A in human macrophages: binding, clathrin-mediated uptake, and trafficking through the endolysosomal pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L334–L342. doi: 10.1152/ajplung.00267.2005. [DOI] [PubMed] [Google Scholar]
- 31.Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol. 1995;155:5343–5351. [PubMed] [Google Scholar]
- 32.Gavrilin MA, Bouakl IJ, Knatz NL, Duncan MD, Hall MW, Gunn JS, Wewers MD. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103:141–146. doi: 10.1073/pnas.0504271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beharka AA, Crowther JE, McCormack FX, Denning GM, Lees J, Tibesar E, Schlesinger LS. Pulmonary surfactant protein a activates a phosphatidylinositol 3-kinase/calcium signal transduction pathway in human macrophages: participation in the up-regulation of mannose receptor activity. J Immunol. 2005;175:2227–2236. doi: 10.4049/jimmunol.175.4.2227. [DOI] [PubMed] [Google Scholar]
- 34.Rajaram MV, Ganesan LP, Parsa KV, Butchar JP, Gunn JS, Tridandapani S. Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J Immunol. 2006;177:6317–6324. doi: 10.4049/jimmunol.177.9.6317. [DOI] [PubMed] [Google Scholar]
- 35.Lichtinger M, Ingram R, Hornef MW, Bonifer C, Rehli M. Transcription factor PU.1 controls transcription start site positioning and alternative TLR4 promoter usage. J Biol Chem. 2007;282:26874–26883. doi: 10.1074/jbc.M703856200. [DOI] [PubMed] [Google Scholar]
- 36.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting Edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 38.Siren J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J Immunol. 2005;174:1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- 39.Nahori MA, Fournie-Amazouz E, Que-Gewirth NS, Balloy V, Chignard M, Raetz CR, Saint G, I, Werts C. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J Immunol. 2005;175:6022–6031. doi: 10.4049/jimmunol.175.9.6022. [DOI] [PubMed] [Google Scholar]
- 40.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–359. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]
- 41.Haehnel V, Schwarzfischer L, Fenton MJ, Rehli M. Transcriptional regulation of the human toll-like receptor 2 gene in monocytes and macrophages. J Immunol. 2002;168:5629–5637. doi: 10.4049/jimmunol.168.11.5629. [DOI] [PubMed] [Google Scholar]
- 42.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 43.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 44.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 45.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- 47.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 48.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 49.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 50.Fang H, Pengal RA, Cao X, Ganesan LP, Wewers MD, Marsh CB, Tridandapani S. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J Immunol. 2004;173:360–366. doi: 10.4049/jimmunol.173.1.360. [DOI] [PubMed] [Google Scholar]
- 51.Kao SJ, Lei HC, Kuo CT, Chang MS, Chen BC, Chang YC, Chiu WT, Lin CH. Lipoteichoic acid induces nuclear factor-kappaB activation and nitric oxide synthase expression via phosphatidylinositol 3-kinase, Akt, and p38 MAPK in RAW 264.7 macrophages. Immunology. 2005;115:366–374. doi: 10.1111/j.1365-2567.2005.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Islam S, Hassan F, Mu MM, Ito H, Koide N, Mori I, Yoshida T, Yokochi T. Piceatannol prevents lipopolysaccharide (LPS)-induced nitric oxide (NO) production and nuclear factor (NF)-kappaB activation by inhibiting IkappaB kinase (IKK) Microbiol Immunol. 2004;48:729–736. doi: 10.1111/j.1348-0421.2004.tb03598.x. [DOI] [PubMed] [Google Scholar]
- 53.Kim HG, Shrestha B, Lim SY, Yoon DH, Chang WC, Shin DJ, Han SK, Park SM, Park JH, Park HI, Sung JM, Jang Y, Chung N, Hwang KC, Kim TW. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells. Eur J Pharmacol. 2006;545:192–199. doi: 10.1016/j.ejphar.2006.06.047. [DOI] [PubMed] [Google Scholar]
- 54.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 55.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 56.Koyasu S. The role of PI3K in immune cells. Nat Immunol. 2003;4:313–319. doi: 10.1038/ni0403-313. [DOI] [PubMed] [Google Scholar]
- 57.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 58.Stokoe D, Stephens LR, Copeland T, Gaffney PR, Reese CB, Painter GF, Holmes AB, McCormick F, Hawkins PT. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 59.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17:313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 60.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 61.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 62.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 63.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 64.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 65.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 66.Droemann D, Goldmann T, Tiedje T, Zabel P, Dalhoff K, Schaaf B. Toll-like receptor 2 expression is decreased on alveolar macrophages in cigarette smokers and COPD patients. Respir Res. 2005;6:68. doi: 10.1186/1465-9921-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flo TH, Halaas O, Torp S, Ryan L, Lien E, Dybdahl B, Sundan A, Espevik T. Differential expression of Toll-like receptor 2 in human cells. J Leukoc Biol. 2001;69:474–481. [PubMed] [Google Scholar]
- 68.Latz E, Visintin A, Lien E, Fitzgerald KA, Monks BG, Kurt-Jones EA, Golenbock DT, Espevik T. Lipopolysaccharide rapidly traffics to and from the Golgi apparatus with the toll-like receptor 4-MD-2-CD14 complex in a process that is distinct from the initiation of signal transduction. J Biol Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 69.Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M. Cutting Edge: The immunostimulatory activity of the lung surfactant protein-A involves toll-like receptor 4. J Immunol. 2002;168:5989–5992. doi: 10.4049/jimmunol.168.12.5989. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Adam S, Hamann L, Heine H, Ulmer AJ, Buwitt-Beckmann U, Stamme C. Accumulation of inhibitory kappaB-alpha as a mechanism contributing to the anti-inflammatory effects of surfactant protein-A. Am J Respir Cell Mol Biol. 2004;31:587–594. doi: 10.1165/rcmb.2004-0003OC. [DOI] [PubMed] [Google Scholar]
- 71.Wright JR, Zlogar DF, Taylor JC, Zlogar TM, Restrepo CI. Effects of endotoxin on surfactant protein A and D stimulation of NO production by alveolar macrophages. Am J Physiol. 1999;276:L650–L658. doi: 10.1152/ajplung.1999.276.4.L650. [DOI] [PubMed] [Google Scholar]
- 72.Borron P, McIntosh JC, Korfhagen TR, Whitsett JA, Taylor J, Wright JR. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Physiol Lung Cell Mol Physiol. 2000;278:L840–L847. doi: 10.1152/ajplung.2000.278.4.L840. [DOI] [PubMed] [Google Scholar]
- 73.McIntosh JC, Mervin-Blake S, Conner E, Wright JR. Surfactant protein A protects growing cells and reduces TNF-α activity from LPS-stimulated macrophages. Am J Physiol Lung Cell Mol Physiol. 1996;271:L310–L319. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- 74.Arias-Diaz J, Garcia-Verdugo I, Casals C, Sanchez-Rico N, Vara E, Balibrea JL. Effect of surfactant protein A (SP-A) on the production of cytokines by human pulmonary macrophages. Shock. 2000;14:300–306. doi: 10.1097/00024382-200014030-00010. [DOI] [PubMed] [Google Scholar]
- 75.Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, Akino T, Kuroki Y. Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-α secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J Biol Chem. 2002;277:6830–6837. doi: 10.1074/jbc.M106671200. [DOI] [PubMed] [Google Scholar]
- 76.Chroneos ZC, Abdolrasulnia R, Whitsett JA, Rice WR, Shepherd VL. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem. 1996;271:16375–16383. doi: 10.1074/jbc.271.27.16375. [DOI] [PubMed] [Google Scholar]
- 77.Yang CH, Szeliga J, Jordan J, Faske S, Sever-Chroneos Z, Dorsett B, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, Whitsett JA, Chroneos ZC. Identification of the surfactant protein A receptor 210 as the unconventional myosin 18A. J Biol Chem. 2005;280:34447–34457. doi: 10.1074/jbc.M505229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 79.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 80.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 81.Brazil DP, Park J, Hemmings BA. PKB binding proteins. Getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 82.Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]