Abstract
Recent studies revealed that the Wnt receptor Frizzled-5 (Fzd5) is required for eye and retina development in zebrafish and Xenopus, however, its role during mammalian eye development is unknown. In the mouse embryo, Fzd5 is prominently expressed in the pituitary, distal optic vesicle and optic stalk, then later in the progenitor zone of the developing retina. To elucidate the role of Fzd5 during eye development, we analyzed embryos with a germline disruption of the Fzd5 gene at E10.25, just before embryos die due to defects in yolk sac angiogenesis. We observed severe defects in optic cup morphogenesis and lens development. However, in embryos with conditional inactivation of Fzd5 using Six3-Cre we observed no obvious early eye defects. Analysis of Axin2 mRNA expression and TCF/LEF-responsive reporter activation demonstrate that Fzd5 does not regulate the Wnt/β-catenin pathway in the eye. Thus, the function of Fzd5 during eye development appears to be species-dependent.
Keywords: mouse, eye, optic cup, morphogenesis, retina, lens, wnt, Frizzled, pituitary
Introduction
Cellular and tissue-tissue interactions regulate development of the central nervous system (CNS) and a paramount question is what is the nature of the signals involved in these interactions. The vertebrate eye represents an excellent and challenging CNS model since it contains multiple cell and tissue types that must coordinate their development to form a functional unit. Eye development becomes apparent when the ventral diencephalic neuroepithelium evaginates to form the optic vesicles. The neural retina and retinal pigment epithelium become patterned in the distal and proximal domains of the optic vesicle, respectively. Adjacent extraocular tissues such as the surrounding mesenchyme, the overlying surface ectoderm and the ventral diencephalon regulate these patterning events. Interaction between the distal optic vesicle and surface (lens) ectoderm leads to invagination, formation of the optic cup and subsequent development of the lens. Several genes have been shown to control optic cup morphogenesis and differentiation of ocular tissues (for reviews, see: Fuhrmann et al., 2000; Chow and Lang, 2001; Lang, 2004; Yang, 2004; Donner et al., 2006; Adler and Canto-Soler, 2007; Medina-Martinez and Jamrich, 2007).
The Wnt family of secreted glycoproteins (about 19 genes in mammals) regulates key developmental processes in the CNS such as proliferation, apoptosis, stem cell maintenance, lineage decision, differentiation and axon guidance. Several Wnt pathway components are expressed in developing ocular tissues and modulation of Wnt signaling has revealed the importance of these signals at multiple stages of eye development (Wang et al., 1996; Borello et al., 1999; Rasmussen et al., 2001; Jin et al., 2002; Fuhrmann et al., 2003; see below and for reviews, see Van Raay and Vetter, 2004; Fuhrmann, 2008). Wnt proteins bind to surface receptors and act via three major pathways, all of which signal via Frizzled (Fzd) receptors, and about ten Fzds are known in vertebrates to date. However, the specificity of Wnt and Fzd receptors for these pathways is often dependent on the cellular context and species. The best-characterized pathway is the Wnt/β-catenin (canonical) pathway, in which low-density lipoprotein receptor-related proteins (LRP) act as co-receptors and β-catenin represents the key player to activate transcription of target genes. Upon activation, β-catenin is stabilized and translocates into the nucleus where it interacts with high mobility group (HMG) box transcription factors such as TCF or LEF forming a transcriptional activator complex. (For details, see: http://www.stanford.edu/~rnusse/wntwindow.html) Alternatively, Wnt/Fzd signaling can stimulate intracellular Ca2+ release or activate the planar cell polarity pathway that involves activation of Jun- and MAP-kinases. Wnt/Fzd signaling is modulated by extracellular signals such as secreted frizzled-related proteins (SFRPs), Dickkopf proteins (Dkk) or Wnt-inhibitory factor (WIF).
Recent studies in different vertebrates demonstrate that both canonical and non-canonical Wnt pathways regulate events during early eye development. Non-canonical Wnt/Fzd signaling is required during eye field formation in frog and zebrafish (Cavodeassi et al., 2005; Maurus et al., 2005). Overexpression of Fzd3 in Xenopus embryos leads to formation of ectopic eyes and the Wnt pathway modulator SFRP1 is required for normal development of the eye field in medaka fish (Rasmussen et al., 2001; Esteve et al., 2004). However, it is not clear from these studies whether Fzd3 and SFRP1 regulate canonical or non-canonical Wnt/Fzd signaling. Support for a role of Wnt/β-catenin signaling is evident from mice with a homozygous deletion of the co-receptor LRP6 that exhibit severe eye defects such as microphthalmia and coloboma (Pinson et al., 2000; Stump et al., 2003). In addition, analysis of transgenic LEF/TCF-dependent reporter lines in zebrafish, frog and mice suggest that Wnt/β-catenin signaling is active in developing ocular tissues (Dorsky et al., 2002; Liu et al., 2003; 2006; Maretto et al., 2003), and that in Xenopus it regulates Sox2 expression and retinal neurogenesis (Van Raay et al., 2005). Early conditional disruption of the canonical Wnt pathway, however, revealed that β-catenin is necessary for correct lamination but dispensible for retinal specification and cell cycle exit in mouse (Fu et al., 2006). Thus, these studies suggest that both canonical and non-canonical Wnt signaling control different aspect of eye development and that the actual role of these pathways can differ among vertebrate species. Interestingly, Wnt/β-catenin signaling needs to be suppressed in the developing lens ectoderm to ensure normal morphogenesis of lens and eye (Miller et al., 2006; Smith et al., 2005)
Fzd5 is unique since it is almost exclusively expressed in the eye during early embryonic development in frog, zebrafish, chick and mouse suggesting a specific, non-redundant role in the regulation of early eye development (Borello et al., 1999; Sumanas and Ekker, 2001; Fuhrmann et al., 2003; Cavodeassi et al., 2005; Van Raay et al., 2005). Surprisingly, recent studies suggest that Fzd5 can activate either non-canonical Wnt signaling in zebrafish or the Wnt/β-catenin pathway in frog and, in addition, exerts different functions in both species during eye development. In zebrafish, Fzd5 mediates non-canonical Wnt-11 signaling and promotes eye field formation (Cavodeassi et al., 2005). In frog, Fzd5 is strongly expressed in the optic vesicle and controls the neural potential of retinal progenitors by regulating the expression of the competence factor Sox2 (Sumanas and Ekker, 2001; Van Raay et al., 2005). Thus, these studies indicate that Fzd5 function during eye development appears to be dependent on the cellular context and on the species. The question arises, therefore, how Fzd5 functions in mammals, specifically in mouse.
Here, we analyze the expression of Fzd5 and its role during mouse retinal development using mice with a targeted deletion of Fzd5 (Ishikawa et al., 2001). In Fzd5−/− embryos, early eye patterning appears to be largely normal, however, germline deletion of Fzd5 results in a failure of optic cup morphogenesis and loss of gene expression in retina and lens at embryonic day 10.5 (E10.5) just before the embryos die due to defects in yolk sac angiogenesis. These eye defects, however, are likely secondary and result from aberrations caused by an earlier requirement for Fzd5 in non-ocular tissues, since conditional inactivation of a LoxP-flanked allele of Fzd5 using Six3-Cre results in the formation of normal optic cups with normal gene expression. Surprisingly, analysis of mice transgenic for a TCF/LEF reporter and Axin2 expression reveal that Fzd5 does not activate Wnt/β-catenin signaling in the developing mouse eye.
Results
Frizzled-5 is expressed in the optic vesicle and in the developing pituitary
Previous studies revealed that mouse Fzd5 is expressed in the eye at E9.5 (Borello et al., 1999; Ishikawa et al., 2001). To obtain a more detailed analysis of the spatial and temporal expression pattern of Fzd5, we performed in situ hybridization at different developmental stages. At E8.0 and E9.0 (6–8 and 12–14 somites, respectively), whole mount in situ hybridization showed that Fzd5 is expressed broadly within the anterior neural plate encompassing the eye anlage and becomes restricted to the anterior edge of the neural plate as well as the ventral diencephalon (Fig. 1A, B). At E9.0 and E10.5, Fzd5 is expressed in the ventral forebrain, in the presumptive retina and optic stalk of the optic primordia (Fig. 1B–D, 2A). Analysis of sections revealed that neither the overlying lens ectoderm, lens vesicle nor the presumptive retinal pigment epithelium (RPE) express Fzd5 (Fig. 2A–D). At E13.5, Fzd5 expression is reduced within the regions of neuronal differentiation such as the ganglion cell layer but is maintained within the undifferentiated region of the neural retina (Fig. 2C). In addition, expression along the dorsal optic nerve was observed at this stage (Fig. 2C; arrowhead). At E15.5 and postnatal day 1 (P1), Fzd5 expression remained in the neuroblastic layer (Fig. 2D; not shown). In addition, the presumptive outer nuclear layer (ONL), which contains fewer dividing cells at this age, exhibits a mottled pattern of Fzd5 (Fig. 2D).
Figure 1. Fzd5 expression pattern in ventral forebrain structures.
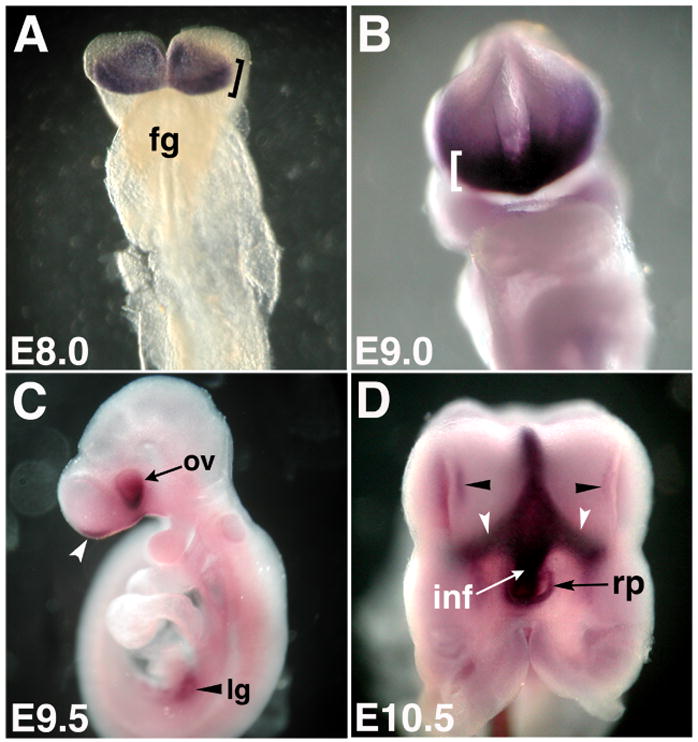
Whole mount in situ hybridization of Fzd5 mRNA expression showing frontal (A, B), lateral (C) and ventral views (D) of the embryonic mouse head. (A) Fzd5 is expressed in the anterior neural plate at E8 (bracket, 6–8 somites; fg: foregut). (B) At E9, Fzd5 expression is detectable in the anterior forebrain (bracket). (C) At E9.5, Fzd5 is expressed in the optic vesicle (ov) and optic stalk as well as in the olfactory placode (white arrowhead) and lung buds (black arrowhead). (D) By E10.5, Fzd5 is expressed in the ventral forebrain, Rathke’s pouch (rp) and infundibulum (inf). Fzd5 expression was also detected in the olfactory placode (black arrowheads) and along the optic stalks (white arrowheads). E: embryonic day, Lg: lung
Figure 2. Fzd5 expression in the embryonic and perinatal mouse eye.
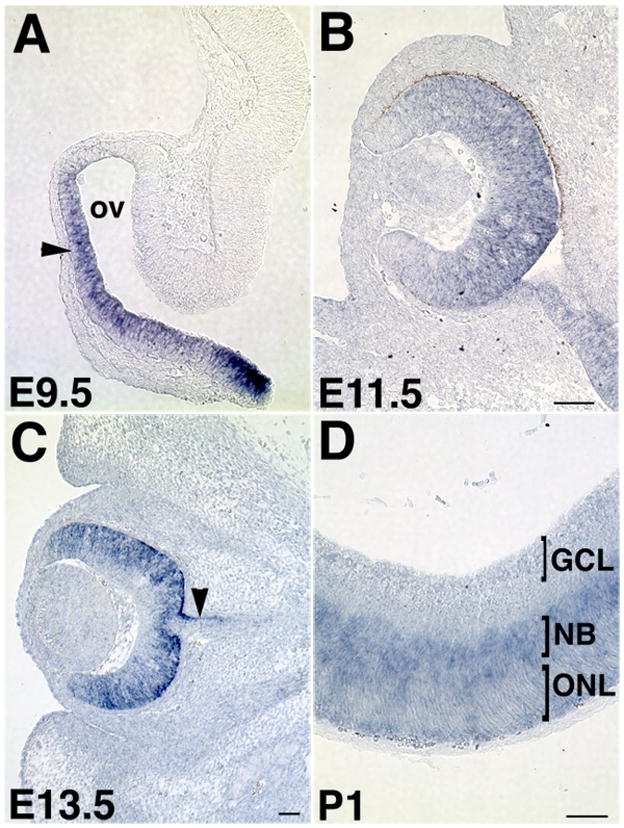
(A) Fzd5 mRNA is expressed in the presumptive neural retina (arrowhead) and optic stalk of the optic vesicle (ov) at E9.5 but not in the future RPE or overlying lens ectoderm. (B) Fzd5 expression is maintained in the neural retina and optic stalk at E11.5. (C) By E13.5, Fzd5 expression is decreased in the presumptive ganglion cell layer (GCL), but maintained in the progenitor zone. In addition, some Fzd5 expression is present in the dorsal optic stalk (arrowhead). (D) Subsequently, Fzd5 becomes restricted to the neuroblastic layer (NBL) in the P1 retina and is downregulated in the future outer nuclear layer (ONL). P: postnatal day. All scale bars are 100μm.
During pituitary organogenesis, Fzd5 is broadly expressed in the anterior neural plate at E8 encompassing the region that will give rise to the telencephalon, olfactory epithelium, anterior hypothalamus and pituitary (6–8 somites; Fig. 3A). At E9, Fzd5 expression is detectable in the ventral forebrain as well as the oral ectoderm (Fig. 3B, C), which will produce the placode that forms Rathke’s pouch (Baker and Bronner-Fraser, 2001; Scully and Rosenfeld, 2002). By E9.5 and E10.5, Fzd5 is expressed in the ventral forebrain, Rathke’s pouch and infundibulum (Fig. 1D, 3D, E; not shown). Fzd5 expression is also detectable in the medial olfactory placode (Fig. 3F; arrowhead) and along the midline of the ventral forebrain (Fig. 3C, E). At E11.5, low levels of expression were detected in the developing infundibulum, however, expression in Rathke’s pouch appeared to be downregulated as well as in the ventral forebrain and medial olfactory epithelium (not shown). Our data demonstrate that Fzd5 is expressed in both the neural and non-neural anlage of the developing pituitary from the early open neural plate stages of mouse development through E10.5. This expression pattern corresponds to early specification, commitment and patterning of oral ectoderm and ventral diencephalon to the pituitary fate.
Figure 3. Expression of Fzd5 mRNA in the developing pituitary.
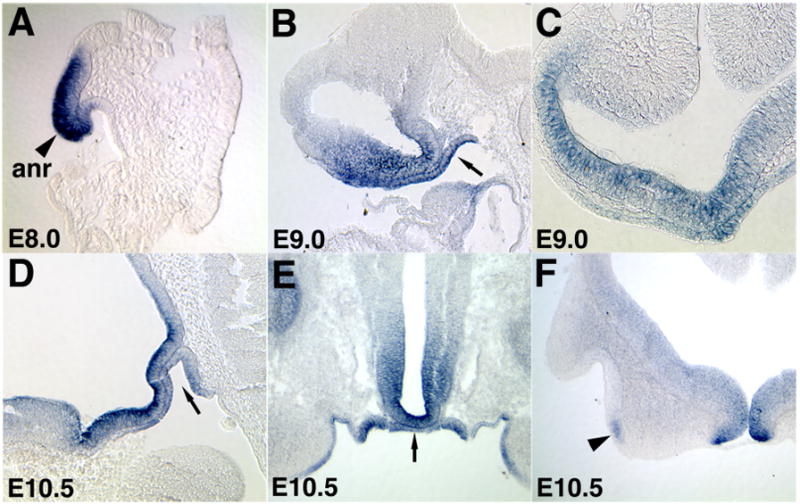
(A) During pituitary organogenesis, Fzd5 is expressed in the anterior neural plate at E8 (anr; sagittal section; 6–8 somites). At E9, Fzd5 expression is detectable in the ventral forebrain (sagittal section in B, coronal section in C) as well as the oral ectoderm (B, arrow). By E10.5, Fzd5 is expressed in the ventral forebrain (sagittal section in D, coronal section in E), oral ectoderm (arrow in D) and Rathke’s pouch (arrow in E). Fzd5 expression was also detected in the olfactory placode (F; black arrowhead, coronal section).
Fzd5 expression does not coincide with regions of Wnt/β-catenin pathway activity in the optic vesicle and ventral forebrain
In mouse, Fzd5 activates the Wnt/β-catenin (canonical) pathway during yolk sac angiogenesis and maturation of intestinal Paneth cells (Ishikawa et al., 2001; Van Es et al., 2005), and in Xenopus Fzd5 regulates Wnt/β-catenin signaling in the optic vesicle. To determine whether canonical signaling is active in the mouse optic vesicle and in the ventral forebrain, we analyzed mice transgenic for a LEF/TCF-responsive promoter that drives expression of LacZ (TOPGAL; DasGupta and Fuchs, 1999). Activation of this reporter is first detected in the developing eye at E9.5 but this activity is restricted to the dorsal most portion of the optic vesicle (Fig. 4A) as previously shown by Maretto et al. (2003) in a different reporter line. Interestingly, Fzd5 expression and the pattern of canonical Wnt activity detected by the TOPGAL reporter mice are mutually exclusive at the optic vesicle stage (compare Fig. 2A with Fig. 4A). In addition, expression of the canonical target gene Axin2 starts in the dorsal optic vesicle at E9.75 (Fig. 4C). This expression pattern overlaps with the TOPGAL reporter, but not with the Fzd5 domain of the optic vesicle. Furthermore, the ventral forebrain, Rathke’s pouch and infundibulum did not show TOPGAL reporter expression between E9.5 and E11.5 in regions that overlapped with Fzd5 (not shown). Therefore, our observations suggest that Fzd5 does not activate the β-catenin-dependent Wnt pathway during early eye and pituitary development in mouse.
Figure 4. Fzd5 does not appear to inhibit or activate the canonical pathway.
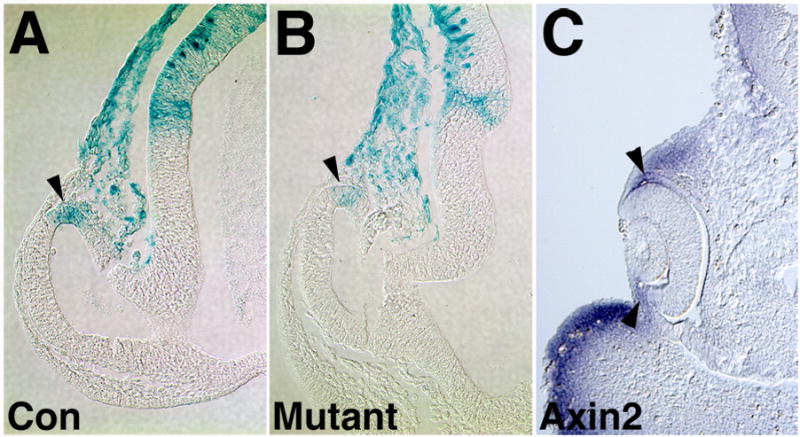
Control (A) or Fzd5−/− embryos (B) carrying the TOPGAL transgene were analyzed by Xgal staining. Activation of the TOPGAL reporter is restricted to the dorsal optic vesicle in Fzd5−/− embryos at E9.5, similar to control embryos (A, B; arrowheads). Expression of Axin2 at 30 somites is restricted to the dorsal optic cup (arrowhead in C).
To determine if Fzd5 function is required for eye development, we analyzed embryos with a germline deletion of Fzd5 (Ishikawa et al., 2001). Homozygous embryos die at E10.75 due angiogenesis defects in yolk sac and placenta (Ishikawa et al., 2001), thus, we were limited in our analysis to the optic vesicle and optic cup stages. If Fzd5 does not signal through activation of TCF/LEF, it may utilize one of the alternative Wnt/Fz signaling pathways and, in addition, may repress canonical Wnt signaling as shown for Fzd5 in zebrafish and for other Fzd receptors (Westfall et al., 2003; Roman-Roman et al., 2004). However, Fzd5−/− embryos carrying the TOPGAL transgene did not show ectopic activation of the reporter in the distal and ventral optic vesicle suggesting that Fzd5 does not inhibit the canonical pathway (Fig. 4B).
Frizzled-5 mutant mice exhibit abnormal eye development, increased cell death and decreased retinal proliferation by the optic cup stage
Histological analysis of Fzd5−/− embryos between E9.75 and E10.75 revealed a severe defect in optic cup morphogenesis compared with control littermates (Fig. 5C–D). Mutant optic vesicles fail to invaginate and, instead, a vesicle-like structure remains, in which the outer portion is partially thickened, reminiscent of the developing neural retina. In addition, lens development does not proceed beyond a rudimentary lens pit (Fig. 5D, inset). This suggests that Fzd5 may be required for morphogenesis of the optic cup and lens. We performed additional experiments to further analyze the Fzd5−/− phenotype in more detail. The loss of Fzd5 function could cause cells to undergo apoptosis and/or prematurely exit the cell cycle and differentiate, since Wnt/Fzd signaling is known to regulate both proliferation and cell survival within the developing nervous system (Chen et al., 2001; Chenn and Walsh, 2002; Megason and McMahon, 2002; Zechner et al., 2003). While at E9.5, few apoptotic cells in the distal optic vesicle of mutant embryos are detectable (not shown), significantly more Tunel-labeled cells are present by E10.25, compared with controls (Fig. 5E, F; arrows). The increase of the number of TUNEL-labeled cells in the presumptive retina of the optic vesicle is significant (Fzd5−/−: 41.14 ± 5.84; control: 5.05 ± 1.09; P<0.00002; n = 4 optic vesicles, 2 embryos per genotype analyzed) and extends far beyond normal cell death observed in the developing optic vesicle (Laemle et al., 1999). At E10.5, an increase of apoptosis was also observed in regions that do not express Fzd5 such as the dorsal diencephalon and spinal cord reflecting compromised development of the whole embryo due to defects in angiogenesis of yolk sac and placenta (not shown; Ishikawa et al., 2001). Furthermore, premature differentiation in mutant optic vesicles did not occur since Tuj-1 positive cells were not observed in either the mutant or control embryos at E9.5 or E10.0, which is consistent with previous studies (not shown; Philips et al., 2005). Another explanation for the observed defects in Fzd5−/−optic vesicles could be a decrease in proliferation of retinal progenitor cells. Using BrDU incorporation to determine effects on proliferation, we observed that at E10, the neural retina domain in the mutant optic vesicle exhibited changes in proliferation compared with controls; mutant optic vesicles showed a significant decrease in the number of proliferating cells in the distal and ventral portion but not in the dorsal diencephalon, which does not express Fzd5 (Fig. 5G; Supplemental Fig. 1). Thus, at this age, disruption of Fzd5 affects proliferation in the Fzd5 expression domains of the optic vesicle specifically. Therefore, it is likely that an increase in cell death and a concomitant decrease of proliferation contribute to formation of defective optic cups in Fzd5−/− embryos.
Figure 5. Fzd5−/− embryos fail to develop an optic cup.
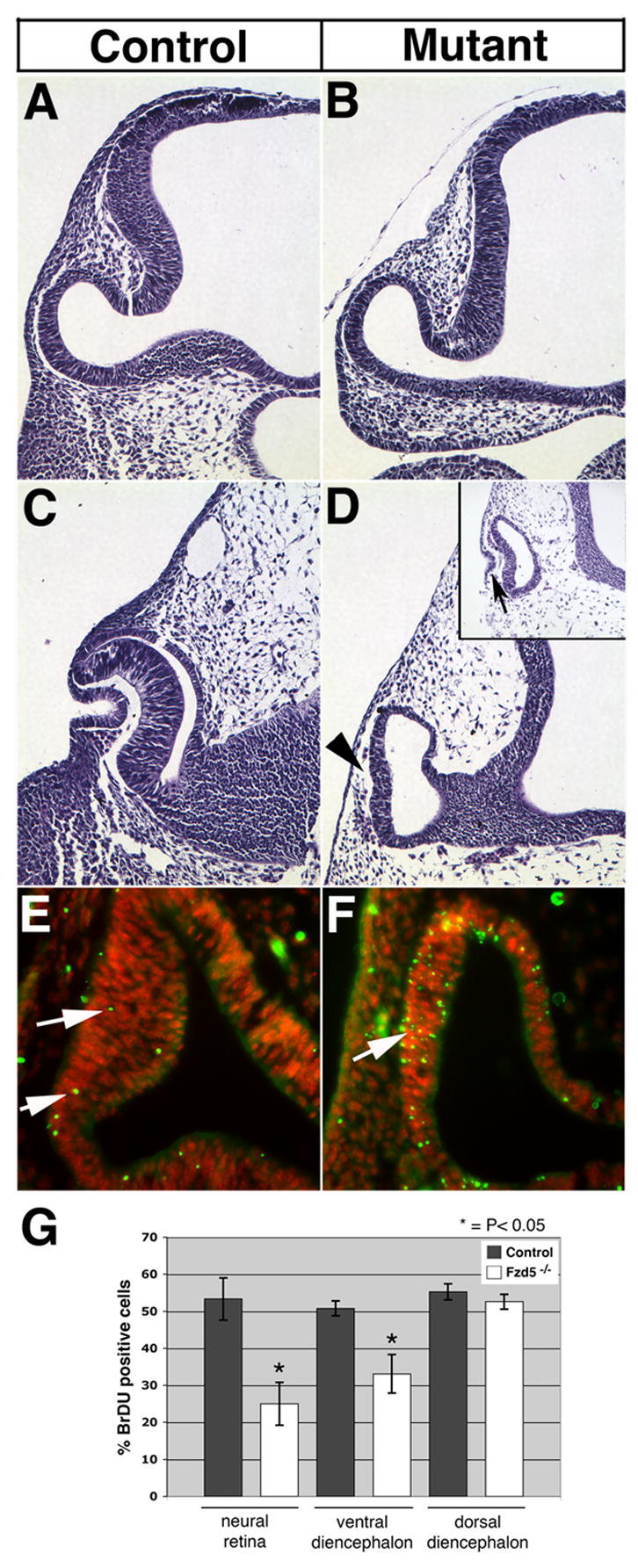
Morphology of control (wildtypes, A, C) and Fzd5−/− embryos (B, D) was analyzed by hematoxylin and eosin staining using frontal sections of paraffin embedded tissue, dorsal is toward the top in all panels. Fzd5−/− and control embryos develop a morphologically normal optic vesicle at E9.5 (compare A and B). By E10.75, the Fzd5−/− eye has failed to form the bilayered optic cup, unlike the control littermates (compare C and D; arrowhead). The lens placode does invaginate to some extent in Fzd5−/− embryos (inset in D; arrow). Control (E) or mutant embryos (F) were analyzed for Tunel labeling (green; DAPI: red). At E10 (30 somites), Fzd5−/− embryos showed increased Tunel labeling in the presumptive neural retina compared with controls (F; arrow). To determine effects on proliferation, BrdU incorporation was determined at E10 (G). The proportion of BrdU positive cells was significantly reduced in the distal (25.03 ± 14.14) and ventral optic vesicle (33.1 ± 12.67) in Fzd5−/− embryos in comparison to controls (distal: 53.35 ± 5.67; ventral: 50.80 ± 5.10; n = 3 embryos). In contrast, proliferation did not significantly change in the dorsal diencephalon where Fzd5 is not expressed (55.27 ± 5.27 in controls versus 52.62 ± 5.05 in Fzd−/− embryos; n = 2 embryos). Solid bars: control; open bars: Fzd−/− embryos.
General patterning of the optic cup is normal in Frizzled-5 mutant mice
To determine whether defects in optic cup morphogenesis in Fzd5−/− embryos are caused by abnormal specification of ocular tissues, we analyzed expression of more than 20 different genes expressed in the eye between E9.5 and E10.5 (see list in Experimental Procedures). The paired homeodomain transcription factor Pax6 is required for optic cup formation, proliferation and multipotency of retinal progenitor cells (Hogan et al., 1986; Hill et al., 1991; Walther and Gruss, 1991; Ashery-Padan et al., 2000; Ashery-Padan and Gruss, 2001; Marquardt et al., 2001). In Fzd5−/− optic cups, Pax6 expression is normal in the neural retina, future RPE and lens ectoderm suggesting that initial specification of the eye occurs normally (Fig. 6B). Furthermore, the homeodomain transcription factor Chx10 is exclusively expressed in retinal progenitors and regulates proliferation (Liu et al., 1994; Burmeister et al., 1996). Although proliferation can be decreased in the mutant optic vesicle (Fig. 5G, H), Chx10 expression was normal compared to control embryos (Fig. 6C, D). Recently, functional analyzes in frog revealed that Fzd5 regulates expression of Sox2 and Xath5 in the developing retina (Van Raay et al., 2005). To test whether Sox2 and Math5 are similarly dependent on Fzd5 function in mouse, we analyzed expression of both genes in Fzd5−/− embryos. In Fzd5−/− mice, Sox2 expression is present in the presumptive retina between E9.5 and E10.5 (n = 7, not shown). Since Fzd5−/− embryos die approximately one day before onset of Math5 expression, we examined optic vesicle explants grown for 3 days in culture. However, Math5 expression was detectable in both control optic vesicle explants (4/10 explants) and mutant explants (3/10 explants; Supplemental Fig. 2). Since a similar proportion of explants expressed Math5 for both controls and mutants (no significant difference, P = 1.0), Fzd5 does not appear to be required for Math5 expression in mouse. The function of Fzd5 to promote Math5 expression during eye development, therefore, is not evolutionary conserved and appears to be species-dependent.
Figure 6. Patterning of the ventral and distal optic vesicle occurs normally in Fzd5−/−embryos.
At E10.5, Pax6 is expressed in the developing neural retina, lens (arrowhead in B) and presumptive RPE of control (A) and Fzd5−/− embryos (B). Similarly, Chx10 is present in the neural retina of control (C) and in the distal region of the optic vesicle in mutant embryos (D). Pax2 protein is present in the optic vesicle and stalk at 27 somites in mutant embryos (E) similar to the expression observed in control embryos (F). Ventral expression of Vax2 is also normal in Fzd5−/− optic vesicles at 30 somites (compare G, H).
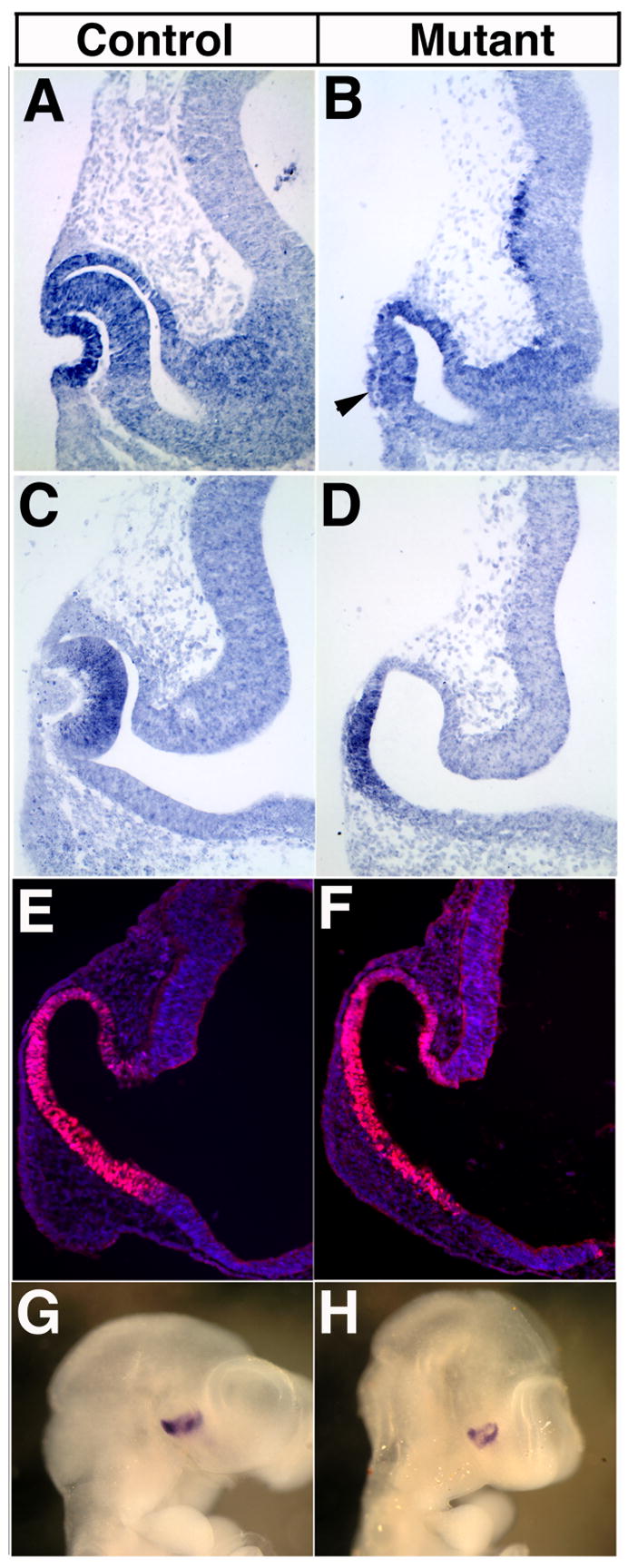
Since Fzd5 is expressed in the optic stalk, we next addressed whether ventral patterning is altered in Fzd5−/− eyes. We analyzed the expression of Pax2, a transcription factor that is initially expressed in the developing neural retina and optic stalk during early eye development (Otteson et al., 1998; Schwarz et al., 2000). We observed normal Pax2 protein expression in the optic vesicle, with stronger expression detected in the ventral region of the neural retina and optic stalk (Fig. 6F). The homeodomain protein Vax2 is expressed in the ventral optic vesicle and is required for ventral patterning (Barbieri et al., 1999; Mui et al., 2005). Similarly to Pax2, we found that Vax2 expression in Fzd5−/− optic cups is normal at E10–E10.5 (Fig. 6H). In addition, analysis of FGF8 expression confirmed that patterning of the optic stalk is not disturbed (n = 2, not shown). Analysis of expression of the bHLH transcription factor Mitf revealed that the presumptive RPE is specified normally (n = 2, not shown). Therefore, patterning of the proximodistal and dorsoventral axes in the optic vesicle appear to be normal without Fzd5 function. No changes of expression of BMP7, Hes1, Hes5 and TBX5 were detectable in Fzd5−/− eyes (n = 2–4, not shown). However, we observed changes in expression of FGF15, which is one of the most abundant FGFs expressed in the distal optic vesicle (McWhirter et al., 1997, Wright et al., 2004). FGF15 is initially expressed in the Fzd5−/− optic vesicle but is decreased at 30 somites (not shown) and is absent at 33 somites (Fig. 7B).
Figure 7. Effects of germline and conditional inactivation of Fzd5 on FGF15, FoxE3 and Mab21L1 expression in the mouse optic vesicle.
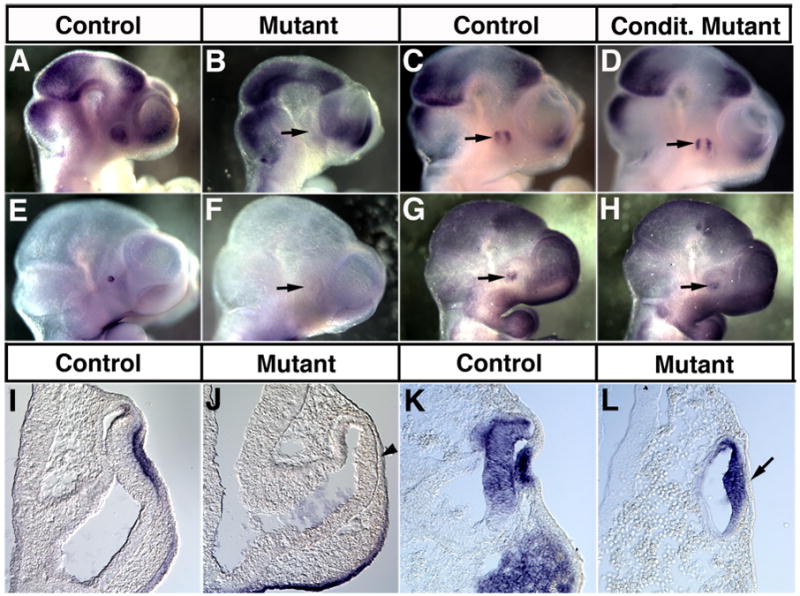
Whole mount in situ hybridization for FGF15 (A, B: 33 somites, C, D: 35 somites) and FoxE3 (E, F: 33 somites, G, H: 30 somites) showing lateral views. Coronal sections of FoxE3 (I, J: 30 somites) and Mab21L1 expression (K, L: 30 somites) are shown. Controls (A, C, E, G, I, K), embryos with a germline mutation (B, F, J, L) or a conditional disruption of Fzd5 (D, H) are presented. Germline disruption of Fzd5 leads to an absence of FGF15 (arrow in B), FoxE3 (arrow in F, arrowhead in J) and Mab21L1 expression (L, arrow) in the optic vesicle or lens placode. Embryos with conditional disruption of Fzd5 were generated using Six3-Cre heterozygous for the Fzd5 null allele and one floxed Fzd5 allele. Conditional Fzd5−/− embryos exhibit a normal expression pattern of FGF15 and FoxE3 in the optic vesicle (arrows in D, H, respectively).
Lens development is altered in Fzd5−/− embryos
Invagination of the optic vesicle and formation of the lens vesicle require tight interaction between lens ectoderm and optic vesicle as well proper gene expression in the lens ectoderm such as Pax6, Six3, Sox2 and Mab21L1 (for reviews, see Lang, 2004; Medina-Martinez and Jamrich, 2007). In Fzd5−/− eyes, the lens placode does form only rudimentarily as shown in Fig. 5D (inset), however, several genes required for lens placode formation are expressed normally. For example, Pax6 mRNA and protein expression is detectable in the lens placode up to E10.5 (Fig. 6B, not shown). Other lens placodal markers such as Six3 and Sox2 are expressed in Fzd5−/− lens ectoderm at E10 (not shown). Furthermore, genes expressed in the distal optic vesicle and shown to be necessary for lens formation such as Pax6, BMP4 and Mab21L2 are present in the presumptive retina of mutant embryos (n = 2–4, Fig. 6B, not shown). Thus, early stages of induction and specification of the lens placode appear to be normal in Fzd5−/− embryos (Lang, 2004; Donner et al., 2006; Medina-Martinez and Jamrich, 2007). However, later stages of lens development are affected in Fzd5−/− eyes. Mab21L1, a member of the Mab gene family, is essential for lens placode formation in mouse (Yamada et al., 2003). We observed that at E9.5 (25 somites, not shown), Mab21L1 is initially present in the lens placode but is undetectable at E10 (30 somites; Fig. 7L). Interestingly, Mab21L1 expression in the distal optic vesicle appears to be normal, thus, its expression is differentially affected in the presumptive retina and lens placode in Fzd5−/− eyes. The forkhead transcription factor FoxE3 regulates lens vesicle closure and separation and is dependent on Mab21L1 function (Blixt et al., 2000; Brownell et al., 2000; Yamada et al., 2003). In Fzd5−/− eyes, expression of FoxE3 was not induced in the lens ectoderm between E9.5 and E10.25 (n = 3, Fig. 7F, J) consistent with the downregulation of Mab21L1 expression. FoxE3 negatively regulates expression of the homeodomain protein Prox-1 in the lens (Blixt et al., 2000; Medina-Martinez et al., 2005). However, we did not observe premature expression of Prox-1 in the lens ectoderm of Fzd5−/− eyes up to 35 somites (n=2; not shown).
Eye morphogenesis proceeds normally in conditional Fzd5−/− embryos
Since embryos with a homozygous germline disruption of Fzd5 die at E10.75 due to a defect in yolk sac angiogenesis (Ishikawa et al., 2001), we extended our analysis to embryos with Cre-mediated inactivation of a LoxP-flanked Fzd5 allele (Van Es et al., 2005). To inactivate the Fzd5 gene specifically in the optic vesicle, we used a Six3-Cre transgenic mouse line heterozygous for the Fzd5 null allele. Six3-Cre expresses Cre recombinase in the distal and ventral optic vesicle similar to Fzd5 (Furuta et al., 2000). Cre recombinase activity was confirmed by crosses with ROSA-26 reporter mice showing LacZ expression in the distal and ventral optic vesicle at E10.5 (Supplemental Fig. 3A; Soriano, 1999). In addition, we observed reduced Fzd5 mRNA expression in the eye of conditional Fzd5−/− embryos at E9.5 in the distal and ventral optic vesicle where Six3-Cre is expressed (Supplemental Fig. 3C). The residual Fzd5 expression in the Six3-Cre expression domain is consistent with the fact that the germline Fzd5 null allele is an insertional mutant that does not remove the coding region (Ishikawa et al., 2001), and that there may also be transcript for the remaining part of the 3′UTR of the conditional Fzd5 allele (Van Es et al., 2005). Furthermore, we cannot exclude that the Six3-Cre line recombines the floxed Fzd5 gene incompletely in some cells of the distal optic vesicle. However, we found a non-cell autonomous effect on the hyaloid vasculature in conditional Fzd5−/− embryos as early as E14.5 and in the postnatal eye (to be described elsewhere). Therefore, we are confident that Six3-Cre-mediated inactivation of Fzd5 in the developing retina is successful. However, following conditional inactivation of Fzd5, surprisingly, we observed that no defects in optic cup and lens morphogenesis occur and FoxE3 and FGF15 expression are not altered in comparison to control embryos (Fig. 7C, D, G, H; Supplemental Fig. 4A, B). In addition, proliferation and total cell number is not affected at E10.25 (Supplemental Fig. 4C, D). This suggests that Fzd5, although highly expressed in the optic vesicle, is not directly required for optic cup morphogenesis and lens formation in mouse. However, we cannot completely exclude the possibility that unanticipated Fzd5 expression domains before E9 (e.g. mesenchyme or surface ectoderm) are not eliminated by Six3-Cre allowing eye morphogenesis to proceed normally in conditional Fzd5−/−embryos. Finally, it is also possible that eye morphogenesis is highly dependent on proper systemic vascularization and blood circulation of the embryo.
Discussion
We have shown in mouse that Fzd5 is expressed at early stages of eye and pituitary development. TCF/LEF reporter activity and Axin2 expression are not detectable in these domains of Fzd5 expression suggesting that this receptor does not activate Wnt/β-catenin signaling at the ages examined. We examined the function of Fzd5 more precisely during eye development and observed that a failure of optic cup morphogenesis occurs in Fzd5−/−embryonic eyes. However, expression of genes required for retinal neurogenesis such as Math5 and Sox2 are not dependent on Fzd5 function. These observations reveal that Fzd5 is dispensable for early mouse eye development in contrast to other vertebrates such as zebrafish and frog.
Potential role of Fzd5 during pituitary development
Pituitary organogenesis occurs through a series of signaling events, and the Wnt family of secreted glycoproteins is one of the extrinsic signals thought to normally regulate this process (for review, see Zhu et al., 2007). However, except for confirmation of Fzd2 expression during pituitary development, and in vitro assays that implicate Wnt/β-catenin signaling in transcriptional control of pituitary gene expression, the Frizzled receptor(s) that regulate pituitary organogenesis remain elusive (Treier et al., 1998; Douglas et al., 2001; Kioussi et al., 2002). Our studies identify Fzd5 as a candidate receptor for mediating Wnt signals during early events of pituitary organogenesis in mouse, since Fzd5 is transiently expressed in Rathke’s pouch and oral ectoderm between E9 and E10.5. Mouse Fzd5 is most closely related to Xenopus Fzd5, human Fzd5, and zebrafish Fzd8c (Kim et al., 1998; Ishikawa et al., 2001; Sumanas and Ekker, 2001). However, recent observations suggest that frog Fzd5 is also expressed in the developing pituitary, ventral diencephalon, and hypothalamus at late neurula/early tailbud stages (personal communication; M.L. Vetter and K.B. Moore). The expression pattern of Fzd5 in zebrafish appears to be very similar in the eye field and diencephalon (Cavodeassi et al., 2005). Thus, the expression of Fzd5 in different vertebrates is evolutionarily conserved across species and is consistent with a potential role of Fzd5 during pituitary development.
In the developing pituitary, Fzd5 function appears to be independent of TCF/LEF transcriptional activity since TCF/LEF reporter activity and expression of the target gene Axin2 are not detectable in the Fzd5 domain between E9 and E10.5 (Fig. 4, not shown; Olson et al., 2006). These observations are in agreement with a previous study revealing that Wnt/β-catenin signaling in the developing pituitary is tightly regulated (Olson et al., 2006). While after E10.5, β-catenin is necessary to control cell determination events, premature activation of Wnt/β-catenin signaling disrupts formation of Rathke’s pouch (Olson et al., 2006). The transient expression of Fzd5 before E10.5 could indicate a role in repressing Wnt/β-catenin signaling. However, at E10, we did not observe ectopic Axin2 and TOPGAL reporter expression in the pituitary in Fzd5−/− embryos (not shown). Furthermore, in Fzd5−/− embryos, we observed no obvious defects in Rathke’s pouch formation as well as BMP4, Hes1 or Sox2 expression in transversal sections at E10 (not shown). This suggests that initial formation and patterning of the pituitary in mouse occurs independently of Fzd5 and more detailed studies are necessary to determine the precise role of Fzd5.
The function of Fzd5 during early eye development is not evolutionary conserved
Fzd5 is strongly expressed in the distal and ventral optic vesicle as well as in retinal progenitors (our study; Wang et al., 1996; Borello et al., 1999; Ishikawa et al., 2001; Kim et al., 2001). This spatial and temporal expression pattern at the optic vesicle stage is very similar to Fzd5 expression in frog and zebrafish (Sumanas and Ekker, 2001; Cavodeassi et al., 2005; Van Raay et al., 2005). In chick, however, expression starts later at the optic cup stage in the dorsal portion and extends subsequently throughout the whole retina (Fuhrmann et al., 2003; Kubo et al., 2003). In the differentiating and adult retina, Fzd5 expression is quite different in these species; in mouse, Fzd5 is present in the inner nuclear layer, whereas in frog, Fzd5 expression becomes restricted to the ciliary margin (Blackshaw et al., 2004; Van Raay et al., 2005). In chick, Fzd5 expression disappears entirely around the time when later born retinal cell types such as rod photoreceptors and Muller Glia start to exit the cell cycle (embryonic day 7; Fuhrmann et al., 2003). However, in all species examined Fzd5 appears to be expressed in the majority of progenitors that reside in the proliferative zone during retinal histogenesis.
In our study, conditional inactivation of Fzd5 in the optic vesicle shows that it is not directly required for optic cup morphogenesis and lens development. One explanation could be functional redundancy. Recent expression analysis in mouse indicates that several Fzd receptors are expressed in retinal progenitors in the mouse embryo, including Fzd3, Fzd4, Fzd6 and Fzd7 (Liu et al., 2003). Furthermore, previous studies show that Fzd receptors can compensate for each other. In fly, absence of expression of both Fz and Drosophila (D)Fz2 produces more severe patterning defects in the embryo than with loss of function of either Fz or DFz2 alone (Bhat, 1998; Kennerdell and Carthew, 1998; Bhanot et al., 1999; Chen and Struhl, 1999). In mouse, only combined deletion of Fzd3 and Fzd6 results in orientation defects of hair bundles in the inner ear (Wang et al., 2006). Therefore, it is possible that loss of Fzd5 function in retinal progenitors in mouse is compensated by other Fzd receptors.
The actual function of Fzd5 during eye development appears to be species-dependent. In chick, Fzd5 appears to be involved in retinotectal pathfinding but an earlier role of Fzd5 has not been investigated (Schmitt et al., 2006). In zebrafish, Fzd5 is expressed in the eye field and is sufficient to produce ectopic eyes by promoting eye field formation (Cavodeasssi et al., 2005). Conversely, knock-down of Fzd5 results in smaller eye fields and Wnt11 was identified as a good candidate ligand for Fzd5. Interestingly, here Wnt11/Fzd5 appears to antagonize the Wnt/β-catenin pathway, which inhibits eye field formation, possibly by promoting posterization of the forebrain (Cavodeassi et al., 2005). In addition, it is postulated that Wnt11/Fzd5 signaling directly regulates morphogenetic movements of cells in the eye field through activation of a noncanonical Wnt pathway. In Xenopus, blocking Fzd5 function results in reduced eye size, inhibition of neurogenic genes expression and increased formation of Muller Glia. This effect is due to loss of Sox2 and can be correlated with loss of Wnt/β-catenin signaling. These observations indicate that Fzd5 controls the neurogenic potential of retinal progenitors in the developing Xenopus eye. However, our observations in mouse are not consistent with the results in zebrafish and frog. We did not observe a change of expression of eye patterning markers such as Pax6 or of genes required for neurogenesis such as Sox2 or Math5 in Fzd5−/− eyes. Loss of function of LRP6 results in variable eye defects but does not lead to changes in expression of Sox2, further suggesting that Sox2 expression in the developing mouse eye is not dependent on Fzd5 (Smith et al., 2005; Stump et al., 2003). Overall, our observations reveal that, although germline deletion of Fzd5 can cause a defect in optic cup morphogenesis in mouse, the Fzd5 gene is not directly involved in this process as shown by conditional disruption. It may be that the eye phenotype results indirectly from defective angiogenesis in yolk sac and placenta, however, it is not clear whether these two phenotypes are linked. We conclude that Fzd5 function is highly context dependent –regulation of eye field formation or retinal neurogenesis - and species dependent in frog, zebrafish, chick and mouse.
Fzd5 does not appear to activate the canonical pathway in ventral diencephalon derivatives in mouse
In the presence of its co-receptor LRP6, Fzd5 binds Wnts such as Wnt5A or Wnt7A and activates the Wnt/β-catenin (canonical) pathway in different in vitro and in vivo systems. Human Fzd5 induces a secondary axis in frog when overexpressed with Wnt5A and the soluble extracellular cysteine-rich domain can inhibit Wnt3A-induced β-catenin accumulation (He et al., 1997; Kemp et al., 2007). In PC12 cells, Fzd5 interacts with LRP6 and Wnt7A to activate the canonical pathway (Caricasole et al., 2003). In mouse, Fzd5 is expressed in neonatal and adult intestinal crypts and mediates differentiation of Paneth cells through nuclear localization of β-catenin and activation of TFC/LEF transcription factors (Van Es et al., 2005). Furthermore, Ishikawa et al. showed that mouse Fzd5 induces a secondary axis with head structures in frog embryos when co-injected into blastomeres with Wnt2 or Wnt5A (Ishikawa et al., 2001). These results demonstrate that mouse Fzd5 can activate Wnt/β-catenin signaling. Surprisingly, in the mouse optic vesicle, we have no evidence that canonical signaling is active where Fzd5 is expressed. Our analysis of TCF/LEF reporter activation in TOPGAL mice rather revealed that reporter expression and Fzd5 expression are mutually exclusive; Fzd5 is expressed in the distal and ventral optic vesicle whereas the TOPGAL reporter is expressed dorsally. Another TCF/LEF reporter shows a similar dorsal expression domain in the optic vesicle (BATgal; Maretto et al., 2003). Furthermore, Axin2 expression, a very reliable read out for canonical Wnt signaling, is consistent with these results. Our observations suggest that Fzd5 does not activate or suppress the canonical pathway in the embryonic mouse eye. In fact, Wnt/β-catenin signaling might not play a prominent role in retina proliferation and differentiation in mouse (for review, see Fuhrmann, 2008). Conditional ablation of β-catenin in the optic vesicle leads to cell adhesion and lamination defects but does not interfere with optic cup morphogenesis and retinal cell differentiation (Fu et al., 2006). In agreement with these observations in mouse, TCF/LEF reporters are not active in the developing central retina in chick and zebrafish (Dorsky et al., 2002; Cho and Cepko, 2006; Lee et al., 2006). This supports the notion that canonical Wnt signaling does not regulate neurogenesis or proliferation in the central retina of other vertebrates, with the exception in frog. Interestingly, more recent studies demonstrate that Wnt/β-catenin signaling is sufficient and required in controlling differentiation of the peripheral retina into ciliary body and iris (Cho and Cepko, 2006; Liu et al., 2007). Furthermore, Wnt/β-catenin may also function to regulate proliferation of progenitors in the embryonic and adult ciliary margin zone (Ahmad et a., 2000; Tropepe et al., 2000; Kubo et al., 2003; 2005; Inoue et al., 2006; Sun et al., 2006; Asami et al., 2007). Since the frog retina grows by generating new cells from the ciliary margin zone, the function of Wnt/β-catenin signaling in peripheral proliferation may indeed be conserved across vertebrates.
Interestingly, Weeraratna et al. (2002) showed that the Fzd5 receptor in combination with Wnt5A is required for PKC activation in primary metastatic melanoma cells. In zebrafish, Fzd5 appears to antagonize canonical Wnt signaling possibly by activation a non-canonical pathway and, interestingly, overexpression can also induce formation of a secondary axis (Cavodeassi et al., 2005). Thus, depending on the cellular context, Fzd5 might be able to activate a non-canonical Wnt pathway. Similarly, other Fzd receptors such as Fzd3 and Fzd4 have been shown to activate both canonical and non-canonical Wnt pathways (Sheldahl et al., 1999; Umbhauer et al., 2000; Mikels and Nusse, 2006). Thus, the same Fzd receptor can activate different Wnt pathways in the same species, which is tightly regulated depending on the tissue-specific context.
Experimental Procedures
Mice
Mice carrying null alleles of the Frizzled-5 gene (referred to as Fzd5−/−) were generated as previously described (Ishikawa et al., 2001). Mice transgenic for the TOPGAL reporter were generously provided by E. Fuchs (The Rockefeller University, New York; DasGupta and Fuchs, 1999). To obtain Fzd5−/− embryos harboring the TOPGAL transgene, mice heterozygous for TOPGAL reporter and the Fzd5 null allele were mated. Noon of the day observing the vaginal plug was considered 0.5 DPC. To precisely match mutant embryos with similar aged control littermates, somites were counted. Embryos were fixed in 4% paraformaldehyde in PBS for two hours at room temperature or overnight at 4°C. Fzd5−/−mice were genotyped as previously published (Ishikawa et al., 2001) and using the following primer pair to amplify the neomycin resistance cassette: 5′ cgatgaatccagaaaagcgg 3′(forward), 5′ gcttgggtggagaggctatt 3′ (reverse). TOPGAL mice were genotyped using the following primer pair: 5′ cgatgaatccagaaaagcgg 3′ (foward); 5′ gcttgggtggagaggctatt 3′ (reverse).
Mice with floxed Fzd5 alleles (Fzd5LoxP/LoxP) were previously described (Van Es et al., 2005). For Cre-mediated recombination, Yasuhide Furuta (University of Texas) generously provided Six3-Cre transgenic mice (Furuta et al., 2000). Cre recombinase activity was examined using Rosa26R reporter mice and labeling with X-gal substrate (Soriano, 1999). To obtain conditional Fzd5 mutant embryos, Six3-Cre females heterozygous for the Fzd5 null allele were generated and crossed with Fzd5LoxP/LoxP males. Littermates from the same litter were used as controls. Genotyping of the floxed Fzd5 allele was performed using primers complementary to Neo (pn5b: 5′ cta aag cgc atg ctc cag act 3′) and to Fzd5 downstream of the stopcodon (sj2: 5′ cct tta gca aag agt cct aac 3′) generating a 700 bp PCR product. The wildtype allele was identified using a third primer (f5x: 5′ aga gga ggc ctt ata gag cg 3′), which generates a 250 bp product in combination with sj2 using 31 cycles with an annealing temperature of 55°C. Genotyping of the Six3-Cre transgene was performed as previously described (Furuta et al. 2000).
Whole-mount and section in situ hybridization
Whole mount in situ hybridization using digoxygenin-labeled riboprobes was performed as previously described (Henrique et al., 1995). Templates for Axin2 (Zeng et al., 1997), BMP4 (Furuta and Hogan, 1998), BMP7 (Furuta et al., 1997), Chx10 (Green et al., 2003), FGF8 (Moon and Capecchi, 2000), FGF15 (McWirter et al., 1997), FoxE3 (Blixt et al., 2000), Fzd5 (IMAGE clone ID#445088), Hes1 (Brown et al., 1998), Hes5 (Takebayashi et al., 1995), Mab21L1 (Yamada et al., 2003), Mab21L2 (Yamada et al., 2004), Math5 (Brown et al., 1998), Mitf (Hodgkinson et al., 1993), Pax2 (Nornes et al., 1990), Pax6 (Brown et al., 1998), Prox1 (Oliver et al., 1993), Six3 (Oliver et al., 1995), Sox2 (Avilion et al., 2003), Tbx5 (Chapman et al., 1996) and Vax2 (Barbieri et al., 1999) were used. For each marker, between two and seven mutant embryos were analyzed. In situ hybridization on paraffin sections was performed as previously described (Perron et al., 1998) with riboprobes for Fzd5, Pax6 (Brown et al., 1998) and Chx10 with the following modifications to the hybridization buffer: 1mg/mL Torula RNA, 1X Denhardts, 0.1% CHAPS; no blocking reagent was added. In some experiments, 5% polyvinyl alcohol (MW-30-70K, Sigma) was added to the reaction buffer. Expression was visualized using NBT/BCIP tablets (Roche) dissolved in 10% polyvinyl alcohol. For Fzd5 in situ hybridization, sense controls confirmed the specificity of the antisense probe (data not shown).
Math5 expression was analyzed using optic vesicle explant cultures. At E9.5, embryos were dissected and optic vesicles including extraocular tissues carefully removed. Single explants were incubated at 37°C and 5% CO2 for three days in 4-well dishes (Nunc, Denmark) in serum-free 250 μl DMEM/F12 media (Gibco) with Sigma I-1884 supplement (Rachel et al., 2002). After fixation, explants were dehydrated and stored at −80°C until processed for whole mount in situ hybridization as described above. Ten optic vesicle explants of five embryos heterozygous (control) or homozygous for the Fzd5 null allele were analyzed for Math5 mRNA expression. Statistical analysis was performed using Fisher’s Exact Test.
Cell death and proliferation analysis
Cryostat sections (12–16 μm) were analyzed for apoptotic cell death using the Fluorescein In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s instructions. At least 8 embryos for each genotype between E9.75 and E10.25 were examined. For quantification, three 16 μm-sections of each optic vesicle of two embryos for each genotype were counterstained with Dapi, imaged, and the percentage of TUNEL-labeled cells was determined in the distal portion of the optic vesicle. Statistical analysis was performed using the T-test.
Pregnant females were injected subcutaneously with 75mg BrDU/kg body weight 30 min before embryo dissection. Paraffin sections of BrDU-labeled embryos were processed for immunohistochemical analysis using a rat anti-BrDU antibody (1:10, Immunologicals Direct; 0BT0030S). For visualization, donkey anti-rat Alexa488-conjugated antibody secondary antibody was used. To visualize cell nuclei, sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI; Roche). For each Fzd5−/− and control optic vesicle, at least three embryos were analyzed. For proliferation of the dorsal diencephalon, two embryos were analyzed. In order to quantify cell numbers, DAPI and BrdU fluorescent images were obtained using a Spot digital camera (Diagnostic Instruments) on a Nikon Eclipse E800 epifluorescent microscope. The central sections at the level of the optic vesicle were determined for the left and right sides of the embryo and images of the six central most, alternating sections of the optic vesicle, ventral diencephalon and dorsal diencephalon were obtained. Using NIH Image software, the total number of DAPI positive cells or BrdU positive cells was counted for each region and the proportion of BrdU positive cells was determined. No significant difference was detected between the right and the left side of the mutant or wild type embryos; therefore, further statistical analysis (T-test) combined the left and right side of each respective region.
For the proliferation analysis of conditional Fzd5−/− optic cups, three conditional heterozygous and conditional Fzd5−/− embryos were analyzed similar as described above. Four or five alternating sections were obtained (similar to described above for the BrDU analysis) and processed for rabbit anti-phospho-Histone H3 (pHH3; 1:1000; Upstate, #06-570), Dapi labeling and imaging. The proportion of pHH3-labeled cells was manually determined in the optic cup and analyzed for statistical significance using the T-test.
Immunohistochemistry
Antibodies used for immunohistochemical detection on paraffin or cryostat sections were mouse monoclonal anti-Tuj-1 (Covance; MMS-435P), rabbit anti-Pax2 (Zymed, #71-6000) and Pax-6 (Philips et al., 2005). Alexa-conjugates of secondary antibodies (Molecular Probes) in appropriate combination with the primary antibodies were used.
Supplementary Material
Representative examples of control (A) and Fzd5−/− optic vesicles (B) at 30 somites showing an abnormal decrease of BrdU positive cells (green; Dapi = blue) upon loss of Fzd5 function.
Optic vesicles were cultures for 3 days and examined for Math5 expression using whole mount in situ hybridization. Math5 starts to become expressed in one or both optic vesicle explants of control embryos (arrows in A; 4 explants from 3 different embryos, total of 10 explants, n = 5 embryos) and usually in one explant of Fzd5−/− embryos (arrow in B; 3 explants from 3 different embryos, total of 10 explants, n = 5 embryos) suggesting that Fzd5 is not required for Math5 expression in the embryonic mouse eye.
Cre recombinase activity was confirmed by crosses with ROSA-26 reporter mice showing X-gal labeling in the distal and ventral optic vesicle at E10.5 (A, asteriks marks expression in the optic stalk). To determine the extent of loss of Fzd5 expression in conditional Fzd5 mutants, three sets of control and conditional mutant embryos were labeled for Fzd5 at E9.5 using whole mount in situ hybridization. Fzd5 mRNA expression is strongly expressed in the distal optic vesicle of controls (B; arrowhead) and reduced in conditional Fzd5−/− embryos at 25 somites (C; arrowhead).
Histological examination using hematoxylin and eosin staining of cryostat sections revealed no morphological difference between conditional heterozygous (A) and conditional mutant optic cups (B) at E10.25 (n = 3 embryos). Analysis of phospho-Histone H3 labeling showed no significant difference in the neural retina of conditional heterozygous and conditional mutant optic cups at E10.25 (2.81 ± 0.76 and 3.46 ± 0.20, respectively; n = 3 embryos). In addition, the total cell number, determined by counting the number of Dapi-labeled nuclei, does not change significantly upon conditional disruption of Fzd5 (235.2 ± 21.85 in conditional heterozygous versus 213.23 ±62.91 in conditional mutant optic cups; n = 3 embryos). Scale bars in B and D = 100 μm.
Acknowledgments
We are grateful to Elaine Fuchs and Yasuhide Furuta for providing TOPGAL and Six3-Cre mice, respectively. We thank Grant Mastik for Pax-6 antibody, Suzi Mansour for providing cDNA constructs and Ben Atkins, Erin Callahan, Annie Chen, Amber Mathiesen, Scott Spritzer for technical help. We thank Ed Levine, Rich Dorsky, Daneen Wellik and Richard Lang for helpful comments and Nadean Brown and Kathy Moore for critical reading of the manuscript. This work was supported by an unrestricted grant from Research to Prevent Blindness, Inc., to the Dept. of Ophthalmology and Visual Sciences, University of Utah.
Funded by: NIH, R01 EY14954, NIH Core Grant, EY014800, unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, University of Utah
References
- Adler R, Canto-Soler MV. Molecular mechanisms of optic vesicle development: complexities, ambiguities and controversies. Dev Biol. 2007;305:1–13. doi: 10.1016/j.ydbio.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–521. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- Asami M, Sun G, Yamaguchi M, Kosaka M. Multipotent cells from mammalian iris pigment epithelium. Dev Biol. 2007;304:433–446. doi: 10.1016/j.ydbio.2006.12.047. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr Opin Cell Biol. 2001;13:706–714. doi: 10.1016/s0955-0674(00)00274-x. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Barbieri AM, Lupo G, Bulfone A, Andreazzoli M, Mariani M, Fougerousse F, Consalez GG, Borsani G, Beckmann JS, Barsacchi G, Ballabio A, Banfi S. A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci U S A. 1999;96:10729–10734. doi: 10.1073/pnas.96.19.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development. 1999;126:4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Bhat KM. frizzled and frizzled 2 play a partially redundant role in wingless signaling and have similar requirements to wingless in neurogenesis. Cell. 1998;95:1027–1036. doi: 10.1016/s0092-8674(00)81726-2. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, Yung R, Asch E, Ohno-Machado L, Wong WH, Cepko CL. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt A, Mahlapuu M, Aitola M, Pelto-Huikko M, Enerback S, Carlsson P. A forkhead gene, FoxE3, is essential for lens epithelial proliferation and closure of the lens vesicle. Genes Dev. 2000;14:245–254. [PMC free article] [PubMed] [Google Scholar]
- Borello U, Buffa V, Sonnino C, Melchionna R, Vivarelli E, Cossu G. Differential expression of the Wnt putative receptors Frizzled during mouse somitogenesis. Mech Dev. 1999;89:173–177. doi: 10.1016/s0925-4773(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- Brownell I, Dirksen M, Jamrich M. Forkhead Foxe3 maps to the dysgenetic lens locus and is critical in lens development and differentiation. Genesis. 2000;27:81–93. doi: 10.1002/1526-968x(200006)27:2<81::aid-gene50>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Burmeister M, Novak J, Liang MY, Basu S, Ploder L, Hawes NL, Vidgen D, Hoover F, Goldman D, Kalnins VI, Roderick TH, Taylor BA, Hankin MH, McInnes RR. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat Genet. 1996;12:376–384. doi: 10.1038/ng0496-376. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Ferraro T, Iacovelli L, Barletta E, Caruso A, Melchiorri D, Terstappen GC, Nicoletti F. Functional characterization of WNT7A signaling in PC12 cells: interaction with A FZD5 x LRP6 receptor complex and modulation by Dickkopf proteins. J Biol Chem. 2003;278:37024–37031. doi: 10.1074/jbc.M300191200. [DOI] [PubMed] [Google Scholar]
- Cavodeassi F, Carreira-Barbosa F, Young RM, Concha ML, Allende ML, Houart C, Tada M, Wilson SW. Early stages of zebrafish eye formation require the coordinated activity of Wnt11, Fz5, and the Wnt/beta-catenin pathway. Neuron. 2005;47:43–56. doi: 10.1016/j.neuron.2005.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Chen CM, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Donner AL, Lachke SA, Maas RL. Lens induction in vertebrates: variations on a conserved theme of signaling events. Semin Cell Dev Biol. 2006;17:676–685. doi: 10.1016/j.semcdb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Sheldahl LC, Moon RT. A transgenic Lef1/beta-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol. 2002;241:229–237. doi: 10.1006/dbio.2001.0515. [DOI] [PubMed] [Google Scholar]
- Douglas KR, Brinkmeier ML, Kennell JA, Eswara P, Harrison TA, Patrianakos AI, Sprecher BS, Potok MA, Lyons RH, Jr, MacDougald OA, Camper SA. Identification of members of the Wnt signaling pathway in the embryonic pituitary gland. Mamm Genome. 2001;12:843–851. doi: 10.1007/s00335-001-2076-0. [DOI] [PubMed] [Google Scholar]
- Esteve P, Lopez-Rios J, Bovolenta P. SFRP1 is required for the proper establishment of the eye field in the medaka fish. Mech Dev. 2004;121:687–701. doi: 10.1016/j.mod.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Fu X, Sun H, Klein WH, Mu X. Beta-catenin is essential for lamination but not neurogenesis in mouse retinal development. Dev Biol. 2006;299:424–437. doi: 10.1016/j.ydbio.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. Wnt signaling in eye organogenesis. Organogenesis. 2008 doi: 10.4161/org.4.2.5850. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609. doi: 10.1242/dev.127.21.4599. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Stark MR, Heller S. Expression of Frizzled genes in the developing chick eye. Gene Expr Patterns. 2003;3:659–662. doi: 10.1016/s1567-133x(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26:130–132. [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Green ES, Stubbs JL, Levine EM. Genetic rescue of cell number in a mouse model of microphthalmia: interactions between Chx10 and G1-phase cell cycle regulators. Development. 2003;130:539–552. doi: 10.1242/dev.00275. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–90. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Hogan BL, Horsburgh G, Cohen J, Hetherington CM, Fisher G, Lyon MF. Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J Embryol Exp Morphol. 1986;97:95–110. [PubMed] [Google Scholar]
- Inoue T, Kagawa T, Fukushima M, Shimizu T, Yoshinaga Y, Takada S, Tanihara H, Taga T. Activation of canonical Wnt pathway promotes proliferation of retinal stem cells derived from adult mouse ciliary margin. Stem Cells. 2006;24:95–104. doi: 10.1634/stemcells.2005-0124. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Burrus LW, Erickson CA. The expression patterns of Wnts and their antagonists during avian eye development. Mech Dev. 2002;116:173–176. doi: 10.1016/s0925-4773(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Kemp CR, Willems E, Wawrzak D, Hendrickx M, Agbor Agbor T, Leyns L. Expression of Frizzled5, Frizzled7, and Frizzled10 during early mouse development and interactions with canonical Wnt signaling. Dev Dyn. 2007;236:2011–2019. doi: 10.1002/dvdy.21198. [DOI] [PubMed] [Google Scholar]
- Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- Kim AS, Lowenstein DH, Pleasure SJ. Wnt receptors and Wnt inhibitors are expressed in gradients in the developing telencephalon. Mech Dev. 2001;103:167–172. doi: 10.1016/s0925-4773(01)00342-2. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park HC, Yeo SY, Hong SK, Choi JW, Kim CH, Weinstein BM, Huh TL. Characterization of two frizzled8 homologues expressed in the embryonic shield and prechordal plate of zebrafish embryos. Mech Dev. 1998;78:193–201. doi: 10.1016/s0925-4773(98)00137-3. [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b inhibits differentiation of retinal progenitor cells in the absence of Notch activity by downregulating the expression of proneural genes. Development. 2005;132:2759–2770. doi: 10.1242/dev.01856. [DOI] [PubMed] [Google Scholar]
- Laemle LK, Puszkarczuk M, Feinberg RN. Apoptosis in early ocular morphogenesis in the mouse. Brain Res Dev Brain Res. 1999;112:129–133. doi: 10.1016/s0165-3806(98)00153-9. [DOI] [PubMed] [Google Scholar]
- Lang RA. Pathways regulating lens induction in the mouse. Int J Dev Biol. 2004;48:783–791. doi: 10.1387/ijdb.041903rl. [DOI] [PubMed] [Google Scholar]
- Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133:4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- Liu H, Mohamed O, Dufort D, Wallace VA. Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn. 2003;227:323–334. doi: 10.1002/dvdy.10315. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BL, Ren JC, Taketo MM, van der Kooy D, Wallace VA. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev Biol. 2007;308:54–67. doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Liu IS, Chen JD, Ploder L, Vidgen D, van der Kooy D, Kalnins VI, McInnes RR. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron. 1994;13:377–393. doi: 10.1016/0896-6273(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Maurus D, Heligon C, Burger-Schwarzler A, Brandli AW, Kuhl M. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. Embo J. 2005;24:1181–1191. doi: 10.1038/sj.emboj.7600603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter JR, Goulding M, Weiner JA, Chun J, Murre C. A novel fibroblast growth factor gene expressed in the developing nervous system is a downstream target of the chimeric homeodomain oncoprotein E2A-Pbx1. Development. 1997;124:3221–3232. doi: 10.1242/dev.124.17.3221. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Brownell I, Amaya-Manzanares F, Hu Q, Behringer RR, Jamrich M. Severe defects in proliferation and differentiation of lens cells in Foxe3 null mice. Mol Cell Biol. 2005;25:8854–8863. doi: 10.1128/MCB.25.20.8854-8863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Martinez O, Jamrich M. Foxe view of lens development and disease. Development. 2007;134:1455–1463. doi: 10.1242/dev.000117. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Smith AN, Taketo MM, Lang RA. Optic cup and facial patterning defects in ocular ectoderm beta-catenin gain-of-function mice. BMC Dev Biol. 2006;6:14. doi: 10.1186/1471-213X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat Genet. 2000;26:455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui SH, Kim JW, Lemke G, Bertuzzi S. Vax genes ventralize the embryonic eye. Genes Dev. 2005;19:1249–1259. doi: 10.1101/gad.1276605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nornes HO, Dressler GR, Knapik EW, Deutsch U, Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990;109:797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- Oliver G, Mailhos A, Wehr R, Copeland NG, Jenkins NA, Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Otteson DC, Shelden E, Jones JM, Kameoka J, Hitchcock PF. Pax2 expression and retinal morphogenesis in the normal and Krd mouse. Dev Biol. 1998;193:209–224. doi: 10.1006/dbio.1997.8794. [DOI] [PubMed] [Google Scholar]
- Perron M, Kanekar S, Vetter ML, Harris WA. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev Biol. 1998;199:185–200. doi: 10.1006/dbio.1998.8939. [DOI] [PubMed] [Google Scholar]
- Philips GT, Stair CN, Young Lee H, Wroblewski E, Berberoglu MA, Brown NL, Mastick GS. Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev Biol. 2005;279:308–321. doi: 10.1016/j.ydbio.2004.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Rachel RA, Dolen G, Hayes NL, Lu A, Erskine L, Nowakowski RS, Mason CA. Spatiotemporal features of early neuronogenesis differ in wild-type and albino mouse retina. J Neurosci. 2002;22:4249–4263. doi: 10.1523/JNEUROSCI.22-11-04249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JT, Deardorff MA, Tan C, Rao MS, Klein PS, Vetter ML. Regulation of eye development by frizzled signaling in Xenopus. Proc Natl Acad Sci U S A. 2001;98:3861–3866. doi: 10.1073/pnas.071586298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Roman S, Shi DL, Stiot V, Hay E, Vayssiere B, Garcia T, Baron R, Rawadi G. Murine Frizzled-1 behaves as an antagonist of the canonical Wnt/beta-catenin signaling. J Biol Chem. 2004;279:5725–5733. doi: 10.1074/jbc.M309233200. [DOI] [PubMed] [Google Scholar]
- Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y. Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping. Nature. 2006;439:31–37. doi: 10.1038/nature04334. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Scully KM, Rosenfeld MG. Pituitary development: regulatory codes in mammalian organogenesis. Science. 2002;295:2231–2235. doi: 10.1126/science.1062736. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Sumanas S, Ekker SC. Xenopus frizzled-5: a frizzled family member expressed exclusively in the neural retina of the developing eye. Mech Dev. 2001;103:133–136. doi: 10.1016/s0925-4773(01)00327-6. [DOI] [PubMed] [Google Scholar]
- Sun G, Asami M, Ohta H, Kosaka J, Kosaka M. Retinal stem/progenitor properties of iris pigment epithelial cells. Dev Biol. 2006;289:243–252. doi: 10.1016/j.ydbio.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Takebayashi K, Akazawa C, Nakanishi S, Kageyama R. Structure and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-5. Identification of the neural precursor cell-specific promoter element. J Biol Chem. 1995;270:1342–1349. doi: 10.1074/jbc.270.3.1342. [DOI] [PubMed] [Google Scholar]
- Treier M, Gleiberman AS, O’Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. doi: 10.1101/gad.12.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- Umbhauer M, Djiane A, Goisset C, Penzo-Mendez A, Riou JF, Boucaut JC, Shi DL. The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling. Embo J. 2000;19:4944–4954. doi: 10.1093/emboj/19.18.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Moore KB, Iordanova I, Steele M, Jamrich M, Harris WA, Vetter ML. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron. 2005;46:23–36. doi: 10.1016/j.neuron.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci. 2004;26:352–358. doi: 10.1159/000082277. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem. 1996;271:4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TJ, Ladher R, McWhirter J, Murre C, Schoenwolf GC, Mansour SL. Mouse FGF15 is the ortholog of human and chick FGF19, but is not uniquely required for otic induction. Dev Biol. 2004;269:264–275. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Hasegawa T, Osumi N, Koseki H, Takahashi N. Cell-autonomous involvement of Mab21l1 is essential for lens placode development. Development. 2003;130:1759–1770. doi: 10.1242/dev.00399. [DOI] [PubMed] [Google Scholar]
- Yamada R, Mizutani-Koseki Y, Koseki H, Takahashi N. Requirement for Mab21l2 during development of murine retina and ventral body wall. Dev Biol. 2004;274:295–307. doi: 10.1016/j.ydbio.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15 doi: 10.1016/j.semcdb.2003.09.004. 9-1-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- Zhu X, Gleiberman AS, Rosenfeld MG. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev. 2007;87:933–963. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative examples of control (A) and Fzd5−/− optic vesicles (B) at 30 somites showing an abnormal decrease of BrdU positive cells (green; Dapi = blue) upon loss of Fzd5 function.
Optic vesicles were cultures for 3 days and examined for Math5 expression using whole mount in situ hybridization. Math5 starts to become expressed in one or both optic vesicle explants of control embryos (arrows in A; 4 explants from 3 different embryos, total of 10 explants, n = 5 embryos) and usually in one explant of Fzd5−/− embryos (arrow in B; 3 explants from 3 different embryos, total of 10 explants, n = 5 embryos) suggesting that Fzd5 is not required for Math5 expression in the embryonic mouse eye.
Cre recombinase activity was confirmed by crosses with ROSA-26 reporter mice showing X-gal labeling in the distal and ventral optic vesicle at E10.5 (A, asteriks marks expression in the optic stalk). To determine the extent of loss of Fzd5 expression in conditional Fzd5 mutants, three sets of control and conditional mutant embryos were labeled for Fzd5 at E9.5 using whole mount in situ hybridization. Fzd5 mRNA expression is strongly expressed in the distal optic vesicle of controls (B; arrowhead) and reduced in conditional Fzd5−/− embryos at 25 somites (C; arrowhead).
Histological examination using hematoxylin and eosin staining of cryostat sections revealed no morphological difference between conditional heterozygous (A) and conditional mutant optic cups (B) at E10.25 (n = 3 embryos). Analysis of phospho-Histone H3 labeling showed no significant difference in the neural retina of conditional heterozygous and conditional mutant optic cups at E10.25 (2.81 ± 0.76 and 3.46 ± 0.20, respectively; n = 3 embryos). In addition, the total cell number, determined by counting the number of Dapi-labeled nuclei, does not change significantly upon conditional disruption of Fzd5 (235.2 ± 21.85 in conditional heterozygous versus 213.23 ±62.91 in conditional mutant optic cups; n = 3 embryos). Scale bars in B and D = 100 μm.


