Abstract
Cardiomyocytes selected from murine embryonic stem cells (ESCs) using the cardiac-specific promoter alpha-myosin heavy chain were embedded into collagen and fibronectin scaffolds. A custom-built device was used to expose these constructs to mechanical loading (10% stretch at 1, 2, or 3 Hz) or no loading. Constructs were evaluated using reverse transcriptase polymerase chain reaction, histology, and immunohistochemistry. Mechanical loading significantly affected gene expression, and these changes were dependent on the frequency of stretch. A 1 Hz cyclical stretch resulted in significantly lower gene expression, whereas a 3 Hz cyclical stretch resulted in significantly greater gene expression than in unstretched controls. These constructs also developed cardiac-specific cell structures similar to those found in vivo. This study describes a 3-dimensional model to examine the direct effect of mechanical loading on the differentiation of ESC-derived cardiomyocytes embedded in a defined extracellular matrix scaffold. A technique was also developed to isolate the areas within the constructs undergoing the most homogeneous strain so that the effect of mechanical loading on gene expression could be directly evaluated. These experiments emphasize that ESC-derived cardiomyocytes are actively responding to cues from their environment and that those cues can drive phenotypic control and cardiomyocyte differentiation.
INTRODUCTION
Cardiovascular disease is the leading cause of morbidity and mortality in developed countries and is the number one cause of death in the United States. Mortality is high because adult cardiomyocytes lose their ability to proliferate during early development and cannot regenerate when injured by ischemia or infarction. This inability to regenerate also makes repair of congenital malformations challenging. New attempts at repairing cardiac tissue have focused on tissue engineering. This technology uses 3-dimensional (3D) scaffolds embedded with cells to produce biological substitutes to mimic the structure and function of living tissue for the replacement of diseased tissue in vivo. Attempts to produce a viable substitute for myocardial tissue include cardiac tissue–engineered constructs made using natural1,2 and synthetic3–5 substrates populated with undifferentiated embryonic stem cells (ESCs);6 mesenchymal stem cells;7 and embryonic,4,8,9 fetal,10 and neonatal cardiomyocytes.1,3 These tissue-engineered constructs have been used as in vitro models of cardiac tissue1,3,4,11 and have also been placed in vivo to ascertain their survival and efficacy as replacement tissue.5,6,10 Several investigators have shown that, when grafted into the heart, these constructs can become vascularized and exhibit contractile behavior similar to native cardiac tissue.5,12 Vascularized constructs have also been created in vitro on polymer scaffolds seeded with mixed populations of human embryonic cardiomyocytes, endothelial cells, and embryonic fibroblasts.4 In addition, recent studies have shown that a construct made from a bilayered sheet of neonatal cardiomyocytes became electrically coupled with its host myocardium in vivo13 and that these sheets could be fabricated into beating myocardial tubes.14
Other studies have also shown the effect that physiologic stimuli can have on these tissue-engineered constructs. Cardiac constructs have been grown using a variety of culture conditions, including bioreactors, electrical stimulation,2 perfusion chambers,3 and static stretch,15 however they are most often subjected to cyclical stretch.9,12,16 Over the past 10 years, the Eschenhagen group has been applying mechanical loads to cardiomyocyte-enriched tissue constructs. The application of mechanical loads to these constructs increased their force of contraction and facilitated the organization of cellular structures in vitro, which further enhanced their ability to differentiate and improved their cardiac function in vivo.12 Most recently, their constructs showed better contractile function with a greater number of new blood vessels when the cardiomyocyte-enriched population of cells was replaced with cells obtained from whole heart digestion.17 Finally, they have shown that these constructs could be layered in vitro to form a variety of shapes that could be useful for implantation.17
Despite these positive developments, the vast majority of cardiac tissue–engineered constructs are still created using non-renewable cell sources, making them impracticable from a clinical perspective. In an attempt to address this, a 3D construct created with a renewable cell source was pursued. The focus of the present study was to examine the biochemical and histological effects of long-term mechanical loading on ESC-derived cardiomyocytes embedded in a 3D scaffold. The expression of 6 cardiac genes were evaluated: 2 transcription factors and 4 sarcomeric. To evaluate gene expression, the regions within the constructs were isolated where the applied load was most homogeneous. Polymerase chain reaction (PCR) analysis showed that mechanical loading significantly altered gene expression. These changes in gene expression were dependent on the frequency of stretch. In addition, sarcomeric structures and gap junctions were more organized in the mechanically loaded constructs than in the unstretched constructs.
MATERIALS AND METHODS
Selection and culture of the ESC-derived cardiomyocytes
Undifferentiated murine J1 ESCs (generously provided by the laboratory of Jaenisch18) were cultured in flasks coated with 0.1% gelatin (DIFCO Laboratories, Liverpool, Australia) and maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 15% fetal bovine serum (FBS) (Sigma, St. Louis, MO), 50 U/mL penicillin, 50 μg/mL streptomycin, 4 mM L-glutamine, 0.1 mM nonessential amino acids, 0.03% 2-mercaptoethanol, and 103 U/mL leukemia inhibitory factor (LIF) (Chemicon, Temecula, CA). Undifferentiated ESCs were transfected and selected using the method of Klug et al.19 Briefly, the ESCs were transfected using lipofectamine 2000 with a 4.5-kb α-myosin heavy chain (MHC) promoter (kindly provided by Dr. Jeff Robbins, University of Cincinnati) according to the manufacturer’s protocol. Transfected ESCs were then selected by culturing for 7 days in the presence of 300 μg/mL of hygromycin (Sigma). All other reagents were obtained from Invitrogen (Carlsbad, CA) unless specified.
To initiate differentiation, the ESCs were dissociated using 0.25% trypsin– ethylenediaminetetraacetic acid (EDTA) and resuspended in differentiation medium (the previously described culture medium without LIF and using only 10% FBS). The cells were plated onto flasks coated with 0.1% gelatin at 800 cells/cm2 and then cultured for 5 days without medium change. Subsequently, the cells were cultured in differentiation medium containing 10−4 M L-ascorbic acid (Sigma).20 On Day 10, the cells were dissociated using 0.25% trypsin-EDTA, resuspended in differentiation medium containing L-ascorbic acid, and cultured in T75 flasks at 3×106 cells per flask in the presence of 300 μg/mL G418 sulfate (Invitrogen) for 7 days. Only the cells resistant to G418 survived selection in culture. Upon completion of G418 selection, the cells were expanded and frozen as early-passage cardiomyocytes.
Construct creation and culture
Acid-soluble type I collagen, 0.4 mg/mL (Upstate Cell Signaling Solutions, Charlottesville, VA), was mixed with 2×DMEM (Invitrogen) containing 20% FBS (Fig. 1). The solution was then neutralized with 0.1N sodium hydroxide. After this, 10 μg/mL of fibronectin (Sigma) was added. Finally, this mixture was combined with a cell solution containing 6×106 cells in differentiation medium containing L-ascorbic acid. To prevent premature polymerization, the construct components were maintained and mixed together at 4°C. A 1-mL volume of the cell, collagen, and fibronectin solution was carefully pipetted into each of the 6 ring-shaped wells of specially designed Teflon molds and placed in a humidified tissue culture incubator in 5% carbon dioxide at 37°C, where they quickly polymerized into gels. After 30 min at 37°C, 30 mL of differentiation medium containing L-ascorbic acid was added to each dish. Medium was exchanged every 48 h for the 7 days that the constructs were cultured in the mold.
FIG. 1.
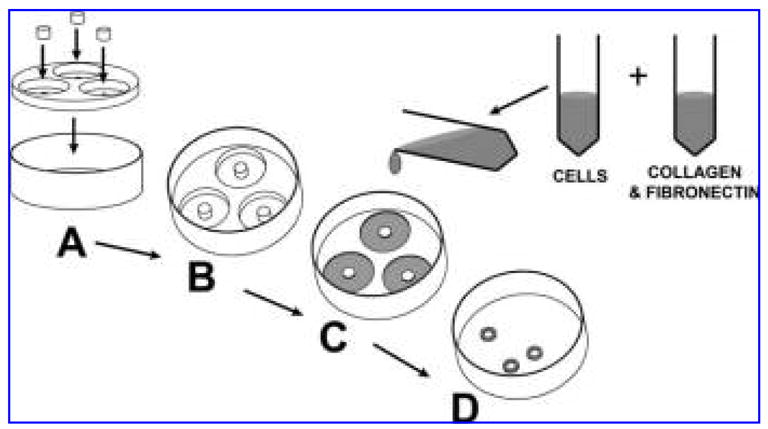
Gelation method for creating the ring-shaped tissue construct. Assemble the Teflon mandrel and mold together (A) and place in a 10-cm culture dish (B). Combine the collagen and fibronectin with the cells and carefully transfer 1 mL of the solution into each well of the mold (C). Allow gel to polymerize at 37°C in a cell-culture incubator. After 7 days, the constructs have substantially shrunk in size (D) and are removed from the mold for subsequent use in the device.
Mechanical loading device
The design of the device used in this research is conceptually similar to a design used by Zimmermann, et al., in which ring-shaped constructs are placed around 2 rods and stretched using a computer-controlled mechanism.21 Although the concept is similar, the device used in this experiment was uniquely designed to fit around a 6-well plate that housed all the constructs, including the controls. The device was separated into 3 units for housing, driving, and loading the constructs (Fig. 2). The housing unit was designed to accommodate a standard, commercially available 6-well tissue culture plate (Fig. 2G) that was sandwiched between the aluminum holding piece (Fig. 2E) and the aluminum base plate (Fig. 2L). The lid of the 6-well plate (Fig. 2D) loosely rested above the holding piece to allow for gas exchange. These pieces were fastened securely together with 4 bolts (Fig. 2F), enabling the housing and loading units to be independently detached from the driving unit and platform, allowing for easy transportation to and from the incubator and biosafety hood for periodic medium exchange. The driving unit included a computer-controlled stepper motor (Fig. 2A) and linear actuator (Fig. 2B) that was securely fastened to the aluminum device platform (Fig. 2O), which served as a clamping and stabilization mechanism. The driving unit was controlled using LabView software (National Instruments, Austin, TX).
FIG. 2.
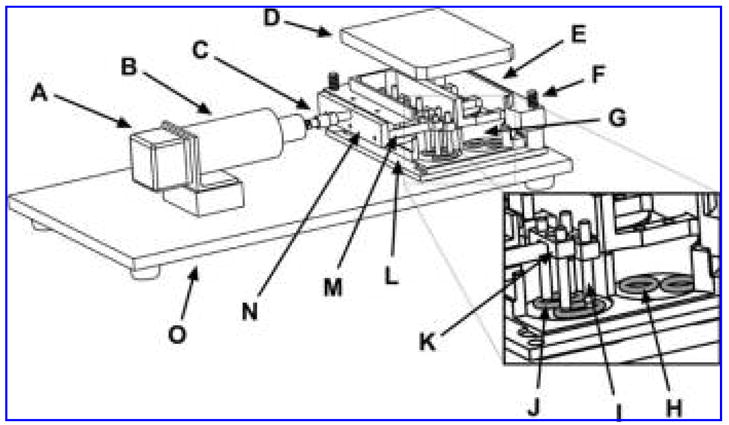
Exploded view and cut-out of mechanical loading device with stepper motor (A), linear actuator (B), connection rod (C), 6-well plate lid (D), aluminum holding piece (E), bolt (F), 6-well plate (G), control construct (H), Teflon construct holding rod (I), experimental construct (J), Teflon double loaders (K), base plate (L), loading bar (M), loading bar holding piece (N), device platform (O).
The loading unit rigidly attached to the driving unit via a connection rod and a quick connect (Fig. 2C). Movement of the linear actuator was transferred to the constructs through a combination of a loading bar holding piece (Fig. 2N), Teflon loading bars (Fig. 2M), Teflon double loaders (Fig. 2K), and Teflon construct holding rods (Fig. 2I). Thus, the same mechanical deformation could be simultaneously applied to 6 experimental constructs (Fig. 2J). An additional 6 unloaded constructs (Fig. 2H) were maintained in the remaining 3 wells. While the experiment was in progress, the housing unit fit snugly in the groove of the device platform (Fig. 2O), preventing movement of the 3 components (driving, loading, and housing) relative to each other.
Mechanical loading device experiments
Twelve constructs were placed into the device after 7 days of culture in the mold. Six were randomly selected and subjected to a continuous, cyclical mechanical load that stretched the constructs to 10% of their initial length at frequencies of 1, 2, and 3 Hz for a period of 3 days. Percentage stretch was defined as
where Lfinal is the final desired construct length, and Linitial is the initial construct length. The remaining 6 constructs were left unstretched in the remaining 3 wells and functioned as the controls. All constructs were cultured in differentiation medium containing L-ascorbic acid. Medium exchange occurred daily. The constructs lengthened along the direction of stretch due to the cyclical mechanical load. To account for this, the distance between the construct holding rods was increased 1% daily when the medium was exchanged.
Construct evaluation
The goal of this study was to evaluate the effects of mechanical loading on ESC-derived cardiomyocytes embedded in 3D constructs. Because previous research has shown that central regions of stretched collagen gels have a more homogeneous strain profile than the ends,22,23 only the homogenously stretched, mid-sections of the constructs were used.
Determination of gene expression
Before extracting the ribonucleic acid (RNA) from the cells, the mechanically loaded constructs were sectioned to separate the mid-sections of the construct from their respective end pieces (Fig. 3). Once sectioned, the mid-sections of the constructs were prepared for reverse transcriptase (RT) PCR. RNA was isolated using the Qiagen RNeasy Kit (Qiagen, Valencia, CA) and was reverse transcribed into complementary deoxyribonucleic acid (cDNA) using Superscript III (Invitrogen). Quantitative RT-PCR was performed using the Cepheid Smart Cycler (Cepheid, Sunnyvale, CA). The expression of the α-cardiac actin, α-skeletal actin, α-MHC, β-MHC, myocyte enhancer factor (MEF)2C, (GATA)-4, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) genes were examined using Platinum Quantitative PCR SuperMix-UDG (Invitrogen). The primer sequences for each gene have been described previously.24 Gene expression data are presented as the fold change in gene expression of the mechanically loaded constructs with respect to the unstretched constructs. All data were normalized to the reference gene GAPDH. Values greater than 1.0 represented greater gene expression, and values less than 1.0 represented lower gene expression. Results were calculated using the comparative cycle threshold method.25 Additional analyses were conducted on the end pieces of the mechanically loaded constructs for comparison with their respective mid-sections.
FIG. 3.
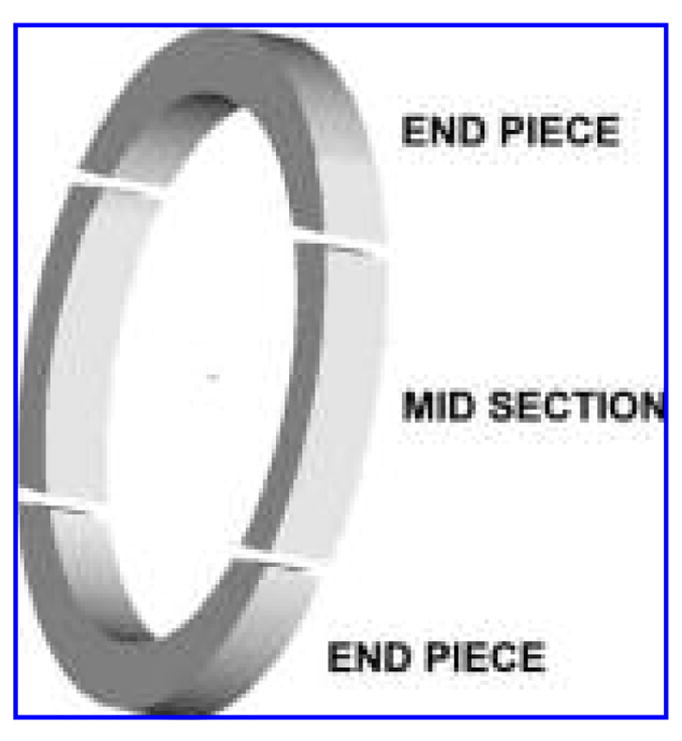
Schematic of a sectioned construct in preparation for the isolation of ribonucleic acid.
Histology and immunohistochemistry
Constructs were removed from the device and fixed overnight at 4°C in 4% neutral buffered formalin (pH 7.4). Using standard methods, the constructs were embedded in paraffin blocks, care being taken to maintain their final orientation to the device. Hematoxylin and eosin (H&E) and trichrome stains were applied using standard methods.
For immunohistochemical stains, sections were deparaffinized and rehydrated before antigen retrieval, which was performed using standard methods. The primary antibodies used were titin (Developmental Studies Hybridoma Bank, Iowa City, IA), pancadherin (Sigma), and connexin-43 (Sigma). The slides were then incubated with a Hoechst 33258 nuclear stain (1:1000) combined with the secondary antibody. The secondary antibodies used for visualization were Cy3-conjugated goat anti-mouse immunoglobulin (Ig)M, μ chain specific, Cy3-conjugated goat anti-mouse IgG (H + L), and Cy3-conjugated goat anti-rabbit IgG (H + L) (secondary antibodies from Jackson Immuno-Research Laboratories, West Grove, PA). Finally, the slides were incubated with 1.0% Sudan Black B (Sigma) to block any autofluorescence.26 Adult and neonatal rat heart tissues were used as positive controls, and the absence of the primary antibodies on construct sections were used as the negative controls. The stained sections were imaged using the MetaMorph imaging system (Molecular Devices, Sunnyvale, CA).
Statistics
Statistical significance was determined using analysis of variance in conjunction with post hoc Tukey tests with a significance level of 0.05. All statistical tests were performed using StatView (SAS Institute, Inc., Cary, NC).
RESULTS
Gene expression
Compared with the unstretched constructs, GATA-4 was significantly down-regulated at 1 Hz whereas mechanical loading at 2 and 3 Hz had no effect on the 2 cardiomyo-cyte transcription factors, MEF2C and GATA-4 (Fig. 4). Expression of the 4 sarcomeric genes, however, was dependent on the frequency of stretch (Fig. 4). Mechanically loading the constructs at 1 Hz significantly decreased the expression of α-skeletal actin, α-MHC, and β-MHC, whereas α-cardiac actin was unaffected. Mechanically loading the constructs at 2 Hz significantly increased the expression of β-MHC and had no effect on the expression of the other 3 sarcomeric genes. In contrast, mechanical loading at 3 Hz significantly up-regulated α-skeletal actin, α-cardiac actin, and α-MHC, whereas β-MHC was unchanged.
FIG. 4.
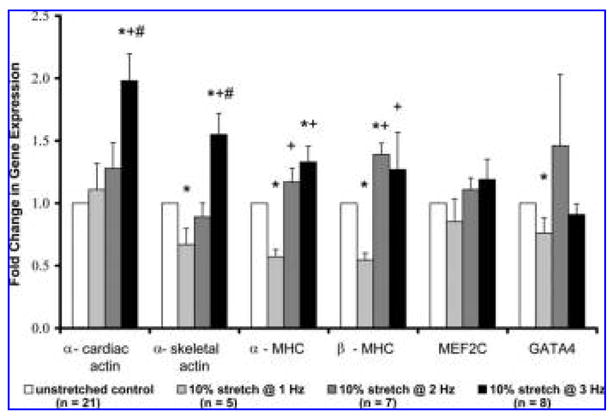
The effect of mechanical loading at 1, 2, and 3 Hz for 3 days on gene expression. The fold change in gene expression was determined by comparing the gene expression of the mechanically loaded constructs with the control constructs and normalized to glyceraldehyde 3-phosphate dehydrogenase. Data are expressed as means ± standard errors. P-values were determined using Tukey’s test for pairwise multiple comparisons in conjunction with analysis of variance. * Indicates significant difference from unstretched control constructs; + and # indicate significant differences from 10% stretch at 1 and 2 Hz, respectively p <0.05. MHC, myosin heavy chain; MEF, myocyte enhancer factor.
PCR analysis was also performed to compare the gene expression of the mid-sections and the end pieces of mechanically loaded constructs. The expression of α-cardiac actin, α-skeletal actin, and α-MHC were significantly greater in the mid-sections of the constructs (1.69±30.32, 1.545±3 0.166, 1.64±30.24, respectively) than at their ends, indicating that the homogeneous strain distribution in the midsections of the constructs influenced the cells more than the distribution in the end pieces.
Histology and immunohistochemistry
After analysis of gene expression, histological and immunohistochemical evaluation were performed. Because gene expression was shown not to increase in the constructs exposed to mechanical loads at 1 Hz and 2 Hz, only un-stretched control constructs and the 3 Hz mechanically loaded constructs were studied and compared with adult and neonatal cardiac tissue.
Standard H&E staining revealed a dense boundary layer of cells and a homogeneous distribution of cells throughout the interior of the 3 Hz mechanically loaded constructs. Longitudinal alignment of cells within these constructs was pervasive, with long strands of cells containing elongated nuclei (Fig. 5D) resembling the myofibers found in the neonatal heart (Fig. 5B). Along with distinct boundary layers that were 4 to 6 cells wide, it was also common to find side-by-side layering of cells within the central regions of the mechanically loaded constructs. In contrast, cellular alignment was noted sporadically throughout the unstretched constructs (Fig. 5C). In addition, unstretched constructs had a less-pronounced boundary layer and a less-homogeneous cell distribution throughout the interior of the scaffold. When the mid-sections and the end pieces of the mechanically loaded constructs were compared, histological staining showed better alignment and organization of cells in the mid-sections of the constructs (Fig. 6A) compared with the rounded and random orientation of the cells observed in the end pieces (Fig. 6B).
FIG. 5.
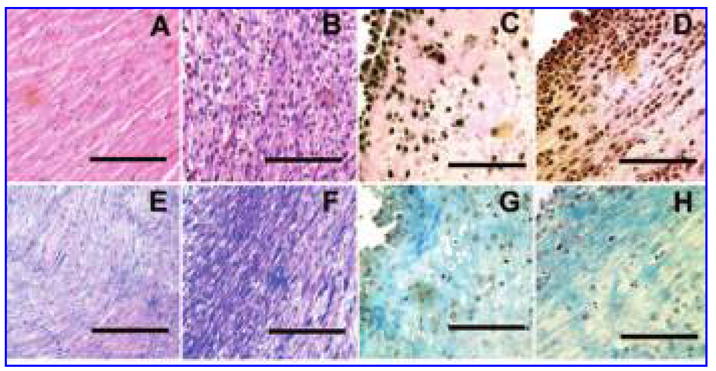
Hematoxylin and eosin staining of adult rat heart (A), neonatal rat heart (B), unstretched construct at day 3 (C), construct exposed to a mechanical load of 10% stretch at 3 Hz for 3 days (D). Trichrome staining of adult rat heart (E), neonatal rat heart (F), unstretched construct at day 3 (G), construct exposed to a mechanical load of 10% stretch at 3 Hz for 3 days (H). Bar = 100 μm. Color images available online at www.liebertpub.com/ten.
FIG. 6.
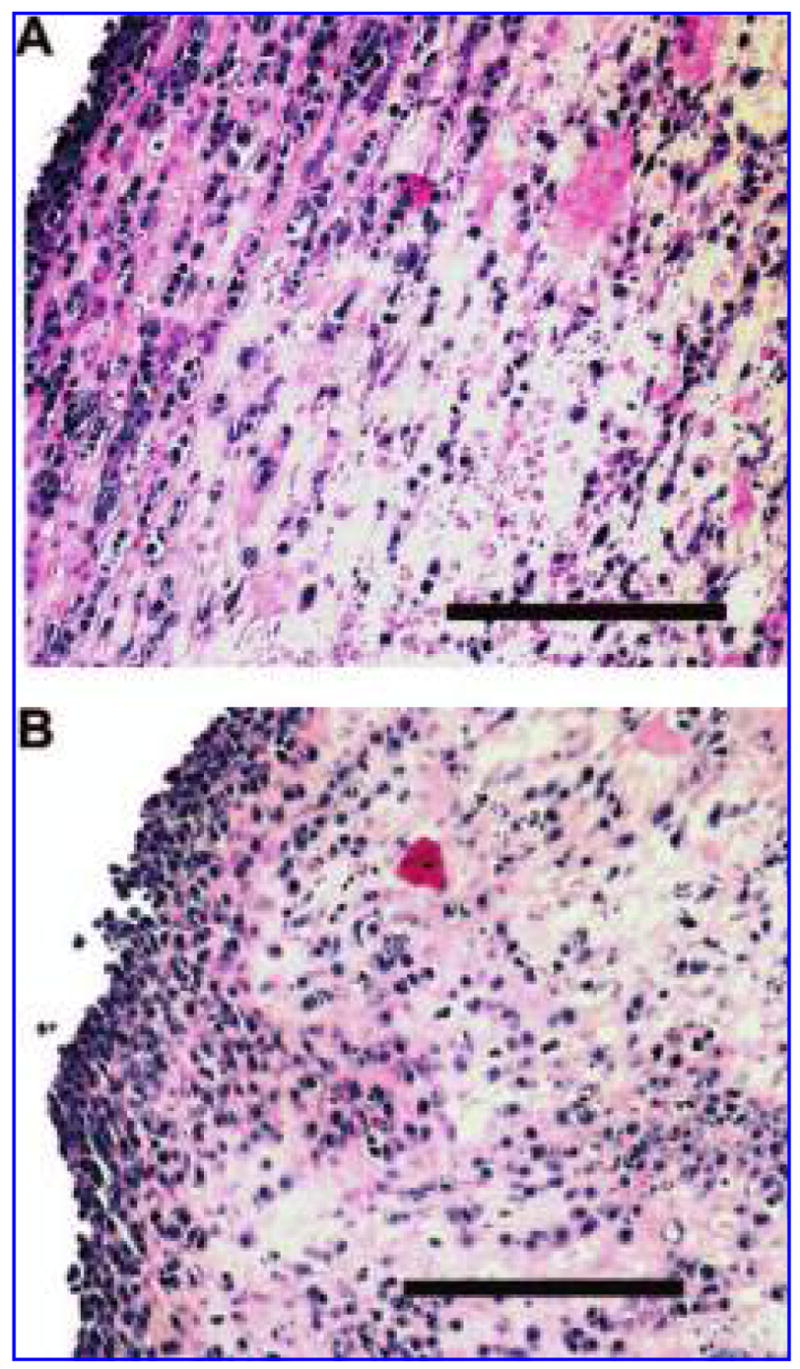
Hematoxylin and eosin staining of embryonic stem cell–derived cardiomyocytes embedded in collagen and fibronectin scaffolds. Cellular alignment is more pronounced and cell distribution is more homogenous in mid-sections of mechanically loaded constructs (A) than in the respective end pieces (B). Bar = 100 μm. Color images available online at www.liebertpub.com/ten.
Trichrome staining was used to examine collagen alignment and distribution in the constructs. Collagen alignment was more evident in the 3 Hz mechanically loaded constructs and presented as thin interwoven strands between the cells (Fig. 5H). This type of collagen organization was similar to that observed in the neonatal heart (Fig. 5E, F).27,28 In contrast, the collagen was more disorganized in the un-stretched constructs (Fig. 5G).
Immunohistochemical staining was performed to identify sarcomeric structures using the titin antibody and to identify cell-to-cell junctions using the pancadherin and connexin-43 antibodies (Fig. 7). These proteins were present in all of the constructs, although there were noticeable differences in the amounts and organization of the proteins between the un-stretched and the mechanically loaded constructs. Although there were some cells within the unstretched constructs that had organized sarcomeric structures, the majority of the sarcomeric proteins appeared to be randomly distributed (Fig. 7A). In contrast, mechanical loading resulted in constructs in which the cells were more elongated with distinct myofilaments and organized sarcomeric structures (white arrows in Fig. 7B).
FIG. 7.
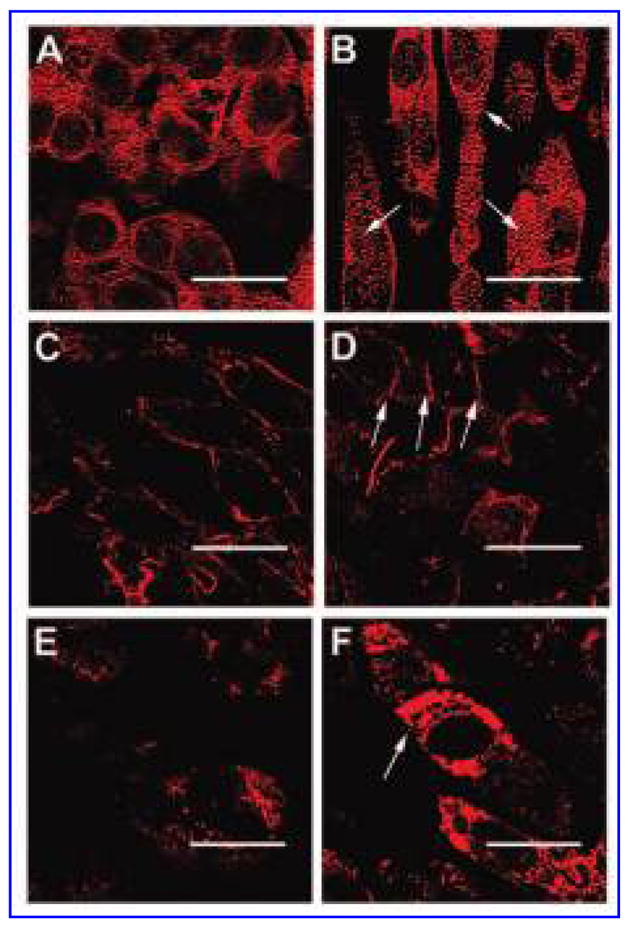
Immunohistochemical staining of titin in an unstretched construct at day 3 (A) and a construct exposed to a mechanical load of 10% stretch at 3 Hz for 3 days (B). Immunohistochemical staining of pancadherin in an unstretched construct at day 3 (C) and a construct exposed to a mechanical load of 10% stretch at 3 Hz for 3 days (D). Immunohistochemical staining of connexin-43 in an unstretched construct at day 3 (E) and a construct exposed to a mechanical load of 10% stretch at 3 Hz for 3 days (F). Bar = 25 μm. White arrows indicate sarcomeric structural organization within the cells (B) or point to cell–cell junctions between the cells (D and F). Color images available online at www.liebertpub.com/ten.
Pancadherin was present in equal amounts in the control and experimental constructs. In control constructs, the antibody was observed around the entire periphery of the cells (Fig. 7C), which resembled the staining observed in the neonatal tissue. In addition to showing staining around the periphery of the cells, mechanically loaded constructs also displayed localization of pancadherin between cells, indicating end-to-end intercellular junctions (white arrows in Fig. 7D), similar to the staining observed in adult heart tissue. In contrast, there were clear differences between the labeling of connexin-43 in the mechanically loaded and unstretched constructs. The labeling of connexin-43 was punctate and diffuse throughout the control constructs (Fig. 7E), whereas in the mechanically loaded constructs, it was more prevalent, similar to the staining observed in cardiac tissue constructs made with neonatal cardiomyocytes.2,12 In addition, connexin-43 labeling sometimes appeared concentrated between cells, demarcating gap junctions (white arrows in Fig. 7F).
DISCUSSION
The primary focus of this work was to study the effects of mechanical loading on the expression of cardiac-specific sarcomeric genes and 2 commonly studied cardiac transcriptions factors in 3D cultures of ESC-derived cardio-myocytes. After isolating the regions within the constructs that were exposed to the most homogeneous strain, we demonstrated that mechanical loading significantly affected gene expression and that these changes in gene expression were dependent on the frequency of stretch.
In this study, comparative analysis of gene expression and immunohistochemical staining was only assessed in the midsections of cardiac tissue–engineered constructs. This selective analysis was employed because of work by Krishnan et al. and Karamichos et al. in which they detailed the strain distributions within cell-seeded collagen gels caused by an applied mechanical load.22,23 Their work showed relatively homogeneous strain distributions in the central regions of their constructs and regions of highly inhomogeneous strain near the ends of the constructs. Because of these studies, we hypothesized that inhomogeneous strain distributions between the mid-section and end pieces of the constructs would result in spatial effects on gene expression and possibly even construct structural development. Detailed analysis confirmed this hypothesis. Although sectioning constructs, as we have done here, may not be useful when examining total construct behavior (i.e., mechanical properties or contractile behavior), it proved to be an effective technique for studying gene expression and structural organization in constructs that have previously been difficult to evaluate due to their inherent non-linear mechanical properties.29
Mechanically loading the ESC-derived cardiomyocyte-embedded constructs at a frequency of 1 Hz resulted in a statistically significant down-regulation of GATA-4. The in vivo regulatory effects of GATA-4 on α-MHC and its importance in late embryonic heart development are well established.30–33 Although the mechanism resulting in the down-regulation seen here remains unknown, it is possible that the 1 Hz stretch resulted in depressed gene expression because it may have been less than the intrinsic beat rate of these ESC-derived cardiomyocytes.34–36 Regardless of the mechanisms responsible for the down-regulation of GATA-4, it is likely that the reduced expression of GATA-4 at 1 Hz was responsible for the down-regulation seen in α-skeletal actin, α-MHC, and β-MHC, because GATA-4, in part, regulates the expression of many genes, including α-skeletal actin,37 α-MHC,38 β-MHC,39 atrial natriuretic factor (ANF),40 brain natriuretic factor,40 and cardiac troponin T.41
In contrast, a 3 Hz cyclical stretch had no effect on the expression of either of the cardiomyocyte transcription factors: GATA-4 and MEF2C. This response was more consistent with the literature, which has shown that GATA-4 and MEF2C are first expressed during embryogenesis42,43 and then maintain their expression once the cardiomyocytes are terminally differentiated.44 Other studies have shown that short-term up-regulation of GATA-4 can be achieved in vitro with electrical stimulation, although these events were transient, with GATA-4 quickly returning to its baseline expression while still being electrically paced.45 Mechanically loading our constructs at 3 Hz did, however, affect sarcomeric gene expression. The significant up-regulation in the expression of α-cardiac actin, α-skeletal actin, and α-MHC is similar to findings from previous studies that applied static mechanical loads46–51 or electrical stimulation52–54 to monolayer cultures of neonatal or adult cardiomyocytes. These studies found that immediate-early genes, stress-activated kinases, ANF, and the structural genes desmin, α-skeletal actin, MHC, and myosin light chain-2 were all up-regulated in 2D models of hypertrophy. Although data collected from the stimulation of monolayer cultures are compelling, only 1 study has examined the effect that cyclical mechanical loading has on the gene expression of neonatal cardiomyocytes embedded in 3D scaffolds. In that study, mechanical loading resulted in a 2-fold increase in ANF and a 1.4-fold increase in α-sarcomeric actin, with no change in β-MHC1 levels similar to those seen in this study.
Greater gene expression also translated into better structural organization. Sarcomeric banding was more organized in constructs exposed to a 3 Hz cyclical stretch than in un-stretched control constructs, and staining of connexin-43 and pancadherin was more localized at intercellular junctions, indicating gap junction formation. Unlike the sparse, disorganized distribution of cells in unstretched constructs, histological staining revealed dense boundary layers, as well as side-by-side layering of cells within the central regions of mechanically loaded constructs. Trichrome staining also showed collagen in abundance and presented as interwoven strands between the cells in the mechanically loaded group. These data suggest that the changes in gene expression and the better structural organization observed in the ESC-derived cardiomyocytes, cultured in a 3D collagen and fibronectin construct and subjected to mechanical loading of 3 Hz, exhibit changes similar to those observed in 3D cultures of neonatal cardiomyocytes.
Despite the up-regulation of the cardiac-specific genes α-cardiac actin, α-skeletal actin, α-MHC, and β-MHC and the more organized sarcomeric organization and gap junction formation, a beating construct was not attained. Several studies have shown that ESC-derived cardiomyocytes obtained through genetic manipulation synchronously contract while in monolayer culture and when seeded onto polyurethane films.19,34,55,56 In these cases, the cells had been cultured for an extended period of time, forming confluent monolayers or dense aggregates. In the present study, it is possible that the cells were capable of beating in small aggregates or independently within the constructs, although full construct contraction was not visualized. It is also possible that the embedded cells were not mature enough (i.e., a complete switch from the fetal genes, α-skeletal actin and β-MHC to the mature genes, α-cardiac actin and α-MHC) or not in culture long enough to elicit contractions with enough force to cause a structure as large as the construct to contract.
CONCLUSION
This study provides techniques to investigate the effects of long-term mechanical loading on a pure population of ESC-derived cardiomyocytes in a well-defined 3D environment. A custom-built device was used to mechanically load the constructs and was uniquely designed to fit around a 6-well plate that housed all the constructs, including the controls. The ESC-derived cardiomyocytes were successfully cultured while embedded in a defined ECM scaffold, and we observed that mechanical loading significantly affected gene expression and construct morphology. A technique was also developed to isolate the areas within the constructs undergoing the most homogeneous strain so that the effects of mechanical loading on gene expression could be directly evaluated.
This study emphasizes that ESC-derived cardiomyocytes are actively responding to cues from their environment in a frequency-dependent manner and that those cues can drive phenotypic control and potentially cardiac tissue development. Other studies have shown that mechanical loading enhances contractile function, causes cellular hypertrophy, and up-regulates gene expression in neonatal and adult cardiomyocytes. However, the effect that the frequency of stimulation (mechanical or electrical) has on gene expression is rarely investigated. Furthermore, data such as these do not exist for ESC-derived cardiomyocytes. We believe that expression of GATA-4, or some upstream modulator of GATA-4, is dependent on the frequency of stretch in these early-stage cardiomyocytes and merits further investigation. Understanding this mechanotransduction pathway may play an important role if these early-stage cardiomyocytes are to be used in cardiac tissue–engineering applications.
Acknowledgments
We thank Dr. May Lam for developing the ESC culture technique and selecting the ESCs, Gary Seibel (University of Maryland) for component machining, and the LSUHSC histopathology laboratory for histology. This work was supported by NIH grant HL-076498 and the Joe W. and Dorothy Brown Foundation.
References
- 1.Fink C, Ergun S, Kralisch D, Remmers U, Weil J, Eschenhagen T. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. Faseb J. 2000;14:669. doi: 10.1096/fasebj.14.5.669. [DOI] [PubMed] [Google Scholar]
- 2.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 4.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. Faseb J. 2006;20:708. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 6.Ke Q, Yang Y, Rana JS, Chen Y, Morgan JP, Xiao YF. Embryonic stem cells cultured in biodegradable scaffold repair infarcted myocardium in mice. Sheng Li Xue Bao. 2005;57:673. [PubMed] [Google Scholar]
- 7.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 8.Evans HJ, Sweet JK, Price RL, Yost M, Goodwin RL. Novel 3D culture system for study of cardiac myocyte development. Am J Physiol Heart Circ Physiol. 2003;285:H570. doi: 10.1152/ajpheart.01027.2002. [DOI] [PubMed] [Google Scholar]
- 9.Guo XM, Zhao YS, Chang HX, Wang CY, E LL, Zhang XA, Duan CM, Dong LZ, Jiang H, Li J, Song Y, Yang XJ. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. 2006;113:2229. doi: 10.1161/CIRCULATIONAHA.105.583039. [DOI] [PubMed] [Google Scholar]
- 10.Li RK, Yau TM, Weisel RD, Mickle DA, Sakai T, Choi A, Jia ZQ. Construction of a bioengineered cardiac graft. J Thorac Cardiovasc Surg. 2000;119:368. doi: 10.1016/S0022-5223(00)70193-0. [DOI] [PubMed] [Google Scholar]
- 11.Kofidis T, Akhyari P, Wachsmann B, Boublik J, Mueller-Stahl K, Leyh R, Fischer S, Haverich A. A novel bioartificial myocardial tissue and its prospective use in cardiac surgery. Eur J Cardiothorac Surg. 2002;22:238. doi: 10.1016/s1010-7940(02)00256-7. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann WH, Didie M, Wasmeier GH, Nixdorff U, Hess A, Melnychenko I, Boy O, Neuhuber WL, Weyand M, Eschenhagen T. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002;106:I151. [PubMed] [Google Scholar]
- 13.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, Nishiyama N, Tanimoto K, Hagiwara Y, Satoh T, Fukuda K, Okano T, Ogawa S. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 14.Sekine H, Shimizu T, Yang J, Kobayashi E, Okano T. Pulsatile myocardial tubes fabricated with cell sheet engineering. Circulation. 2006;114:I87. doi: 10.1161/CIRCULATIONAHA.105.000273. [DOI] [PubMed] [Google Scholar]
- 15.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. Faseb J. 1997;11:683. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 16.Gonen-Wadmany M, Gepstein L, Seliktar D. Controlling the cellular organization of tissue-engineered cardiac constructs. Ann N Y Acad Sci. 2004;1015:299. doi: 10.1196/annals.1302.025. [DOI] [PubMed] [Google Scholar]
- 17.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 18.Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 19.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan L, Weiss JA, Wessman MD, Hoying JB. Design and application of a test system for viscoelastic characterization of collagen gels. Tissue Eng. 2004;10:241. doi: 10.1089/107632704322791880. [DOI] [PubMed] [Google Scholar]
- 23.Karamichos D, Brown RA, Mudera V. Complex dependence of substrate stiffness and serum concentration on cell-force generation. J Biomed Mater Res A. 2006;78:407. doi: 10.1002/jbm.a.30814. [DOI] [PubMed] [Google Scholar]
- 24.White SM, Claycomb WC. Embryonic stem cells form an organized, functional cardiac conduction system in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H670. doi: 10.1152/ajpheart.00841.2004. [DOI] [PubMed] [Google Scholar]
- 25.Fransson M, Adner M, Erjefalt J, Jansson L, Uddman R, Cardell LO. Up-regulation of Toll-like receptors 2, 3 and 4 in allergic rhinitis. Respir Res. 2005;6:100. doi: 10.1186/1465-9921-6-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49:1565. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- 27.Yost MJ, Simpson D, Wrona K, Ridley S, Ploehn HJ, Borg TK, Terracio L. Design and construction of a uniaxial cell stretcher. Am J Physiol Heart Circ Physiol. 2000;279:H3124. doi: 10.1152/ajpheart.2000.279.6.H3124. [DOI] [PubMed] [Google Scholar]
- 28.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984;104:86. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 29.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. 2. New York: Springer-Verlag; 1993. [Google Scholar]
- 30.Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 2001;15:839. doi: 10.1101/gad.875201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 32.Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275:235. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zweigerdt R, Burg M, Willbold E, Abts H, Ruediger M. Generation of confluent cardiomyocyte monolayers derived from embryonic stem cells in suspension: a cell source for new therapies and screening strategies. Cytotherapy. 2003;5:399. doi: 10.1080/14653240310003062. [DOI] [PubMed] [Google Scholar]
- 35.Meyer N, Jaconi M, Landopoulou A, Fort P, Puceat M. A fluorescent reporter gene as a marker for ventricular specification in ES-derived cardiac cells. FEBS Lett. 2000;478:151. doi: 10.1016/s0014-5793(00)01839-1. [DOI] [PubMed] [Google Scholar]
- 36.Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE, Fleischmann BK. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Mol Cell Biol. 2000;20:7550. doi: 10.1128/mcb.20.20.7550-7558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275:38949. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 39.Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol Cell Biol. 1999;19:4355. doi: 10.1128/mcb.19.6.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grepin C, Dagnino L, Robitaille L, Haberstroh L, Antakly T, Nemer M. A hormone-encoding gene identifies a pathway for cardiac but not skeletal muscle gene transcription. Mol Cell Biol. 1994;14:3115. doi: 10.1128/mcb.14.5.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ip HS, Wilson DB, Heikinheimo M, Tang Z, Ting CN, Simon MC, Leiden JM, Parmacek MS. The GATA-4 transcription factor transactivates the cardiac muscle-specific troponin C promoter-enhancer in nonmuscle cells. Mol Cell Biol. 1994;14:7517. doi: 10.1128/mcb.14.11.7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem. 2001;276:30245. doi: 10.1074/jbc.M102174200. [DOI] [PubMed] [Google Scholar]
- 43.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fijnvandraat AC, van Ginneken AC, Schumacher CA, Boheler KR, Lekanne Deprez RH, Christoffels VM, Moorman AF. Cardiomyocytes purified from differentiated embryonic stem cells exhibit characteristics of early chamber myocardium. J Mol Cell Cardiol. 2003;35:1461. doi: 10.1016/j.yjmcc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Xia Y, McMillin JB, Lewis A, Moore M, Zhu WG, Williams RS, Kellems RE. Electrical stimulation of neonatal cardiac myocytes activates the NFAT3 and GATA4 pathways and up-regulates the adenylosuccinate synthetase 1 gene. J Biol Chem. 2000;275:1855. doi: 10.1074/jbc.275.3.1855. [DOI] [PubMed] [Google Scholar]
- 46.Kent RL, McDermott PJ. Passive load and angiotensin II evoke differential responses of gene expression and protein synthesis in cardiac myocytes. Circ Res. 1996;78:829. doi: 10.1161/01.res.78.5.829. [DOI] [PubMed] [Google Scholar]
- 47.Komuro I, Kudo S, Yamazaki T, Zou Y, Shiojima I, Yazaki Y. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. Faseb J. 1996;10:631. doi: 10.1096/fasebj.10.5.8621062. [DOI] [PubMed] [Google Scholar]
- 48.Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992;267:10551. [PubMed] [Google Scholar]
- 49.Simpson DG, Sharp WW, Borg TK, Price RL, Terracio L, Samarel AM. Mechanical regulation of cardiac myocyte protein turnover and myofibrillar structure. Am J Physiol. 1996;270:C1075. doi: 10.1152/ajpcell.1996.270.4.C1075. [DOI] [PubMed] [Google Scholar]
- 50.Vandenburgh HH, Solerssi R, Shansky J, Adams JW, Henderson SA. Mechanical stimulation of organogenic cardiomyocyte growth in vitro. Am J Physiol. 1996;270:C1284. doi: 10.1152/ajpcell.1996.270.5.C1284. [DOI] [PubMed] [Google Scholar]
- 51.Watson PA, Hannan R, Carl LL, Giger KE. Desmin gene expression in cardiac myocytes is responsive to contractile activity and stretch. Am J Physiol. 1996;270:C1228. doi: 10.1152/ajpcell.1996.270.4.C1228. [DOI] [PubMed] [Google Scholar]
- 52.Ivester CT, Tuxworth WJ, Cooper GT, McDermott PJ. Contraction accelerates myosin heavy chain synthesis rates in adult cardiocytes by an increase in the rate of translational initiation. J Biol Chem. 1995;270:21950. doi: 10.1074/jbc.270.37.21950. [DOI] [PubMed] [Google Scholar]
- 53.McDonough PM, Glembotski CC. Induction of atrial natriuretic factor and myosin light chain-2 gene expression in cultured ventricular myocytes by electrical stimulation of contraction. J Biol Chem. 1992;267:11665. [PubMed] [Google Scholar]
- 54.McDonough PM, Hanford DS, Sprenkle AB, Mellon NR, Glembotski CC. Collaborative roles for c-Jun N-terminal kinase, c-Jun, serum response factor, and Sp1 in calcium-regulated myocardial gene expression. J Biol Chem. 1997;272:24046. doi: 10.1074/jbc.272.38.24046. [DOI] [PubMed] [Google Scholar]
- 55.Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, Pasumarthi KB, Field LJ. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 56.Alperin C, Zandstra PW, Woodhouse KA. Polyurethane films seeded with embryonic stem cell-derived cardiomyocytes for use in cardiac tissue engineering applications. Biomaterials. 2005;26:7377. doi: 10.1016/j.biomaterials.2005.05.064. [DOI] [PubMed] [Google Scholar]


