Abstract
OBJECTIVE
The objective of this study was to use volumetric MRI to study brain volumes in 10- to 14-year-old children with and without intrauterine exposure to cocaine, alcohol, cigarettes, or marijuana.
METHODS
Volumetric MRI was performed on 35 children (mean age: 12.3 years; 14 with intrauterine exposure to cocaine, 21 with no intrauterine exposure to cocaine) to determine the effect of prenatal drug exposure on volumes of cortical gray matter; white matter; subcortical gray matter; cerebrospinal fluid; and total parenchymal volume. Head circumference was also obtained. Analyses of each individual substance were adjusted for demographic characteristics and the remaining 3 prenatal substance exposures.
RESULTS
Regression analyses adjusted for demographic characteristics showed that children with intrauterine exposure to cocaine had lower mean cortical gray matter and total parenchymal volumes and smaller mean head circumference than comparison children. After adjustment for other prenatal exposures, these volumes remained smaller but lost statistical significance. Similar analyses conducted for prenatal ethanol exposure adjusted for demographics showed significant reduction in mean cortical gray matter; total parenchymal volumes; and head circumference, which remained smaller but lost statistical significance after adjustment for the remaining 3 exposures. Notably, prenatal cigarette exposure was associated with significant reductions in cortical gray matter and total parenchymal volumes and head circumference after adjustment for demographics that retained marginal significance after adjustment for the other 3 exposures. Finally, as the number of exposures to prenatal substances grew, cortical gray matter and total parenchymal volumes and head circumference declined significantly with smallest measures found among children exposed to all 4.
CONCLUSIONS
These data suggest that intrauterine exposures to cocaine, alcohol, and cigarettes are individually related to reduced head circumference; cortical gray matter; and total parenchymal volumes as measured by MRI at school age. Adjustment for other substance exposures precludes determination of statistically significant individual substance effect on brain volume in this small sample; however, these substances may act cumulatively during gestation to exert lasting effects on brain size and volume.
Keywords: pregnancy, illicit substance, MRI, children, brain
What's Known on This Subject.
MRI neuroimaging has been used to study the effect of prenatal exposure to alcohol on the human brain but comparatively little data have been gathered using these methods to study the effects of prenatal exposure to cigarettes, cocaine or marijuana.
What This Study Adds.
This study adds MRI neuroimaging data on the lasting effect of prenatal exposures to alcohol, cigarettes, cocaine and marijuana on human brain structure in early adolescence. Moreover, it provides new information about the persisting potential effects of prenatal exposure to combinations of these substances.
SCIENTIFIC UNCERTAINTY PERSISTS as to whether intrauterine cocaine exposure (IUCE) exerts a lasting effect on brain structure and development in humans. Early observation that head circumference (HC) at birth was reduced in children who were exposed to cocaine raised concern that structural development of brain in children was adversely affected as a result of IUCE.1–3 Animal and nonhuman primate studies suggested that IUCE may exert deleterious effects on the developing brain.4,5 Postnatal reductions of cortical neuron number and lamination have been observed in rodent models of IUCE.5 It is interesting that upregulation of proapoptotic proteins has been reported to occur in cerebral cortex of IUCE fetal mice relative to controls.6–8 IUCE has been associated with diminished neuronal density and altered cortical organization of postnatal brain in nonhuman primates9; however, the validity of extrapolating these results from mammalian models to humans remains unclear. Animal studies involve known dosages of single, pure drug preparations administered at precisely defined points in gestation. In contrast to animal studies, human studies of cocaine use by pregnant women present additional analytic challenges that consist not only of widely varying drug concentration, purity, and patterns of use during pregnancy but also of concurrent cigarette, alcohol, or marijuana use during the course of the pregnancy10–12 The effect of variability in the pattern of human intrauterine exposures is exacerbated by differences in social and clinical risk factors between cocaine-using and non-using pregnant women, which can be statistically but not experimentally controlled.13
Concern about structural consequences of these intrauterine exposures is heightened by an ample literature that documents cognitive and behavioral consequences associated with prenatal exposures to cocaine, alcohol, cigarettes, or marijuana. Some, although not all, neurobehavioral studies of infants, toddlers, and children with IUCE reported deficits in language development, early executive function, learning, and self-regulation.14–18 Prenatal exposure to alcohol has been associated with cognitive and behavioral deficits in executive function, processing speed, and inattentiveness that persist into childhood even in children without stigmata of fetal alcohol syndrome (FAS).19–22 Human newborns with intrauterine nicotine exposure demonstrated increased tone and excitability as compared with control infants,23 delayed babbling at 8 months of age,24 increased impulsivity as young children, and working memory and visuospatial skills deficits throughout childhood.25–27 An inverse relationship between prenatal cigarette exposure and general intelligence persisted into adolescence.28 Children with intrauterine marijuana exposure have demonstrated poorer scores on intelligence tests,29,30 increased impulsivity, hyperactivity, decreased attentiveness, increased rates of delinquency, and externalizing problems26,31–35 Thus, investigation of the effect of 1 prenatal exposure on subsequent brain structure and function must take into consideration the contributions of other prenatal exposures. In this study, we acquired volumetric MRI data and applied quantitative segmentation techniques to evaluate potential differences in brain tissue volumes of children with and without IUCE while adjusting for intrauterine exposures to alcohol, cigarettes, and marijuana.
METHODS
Participants
The parent sample was recruited by trained interviewer/recruiters who screened maternity and nursery charts 7 days a week on the postpartum floor of Boston City Hospital, from October 1990 to March 1993, using informed consent and protocols approved by the Boston Medical Center institutional review board. Potential participants for the IUCE group were initially identified by review of medical charts for mother's report of cocaine use in pregnancy or positive prenatal or postnatal urine drug screen for cocaine metabolites from mother or infant. Unexposed dyads roughly comparable to cocaine-exposed mother–infant dyads in ethnicity (African American/African Caribbean versus other) were preferentially approached for recruitment soon after delivery. All mother–infant dyads met the following criteria: (1) infant gestational age ≥36 weeks; (2) no NICU care; (3) no major congenital malformation; (4) no diagnosis of FAS in the neonatal chart or physical examination; (5) no history of HIV seropositivity in mother's or infant's medical chart; (6) mother's ability to communicate fluently in English; (7) no neonatal or maternal urine drug screen or medical chart evidence of mother's use during pregnancy of illegal opiates, methadone, amphetamines, phencyclidine, barbiturates, or hallucinogens; and (8) mother's age ≥18 years. Additional details about sample characteristics and recruitment are reported elsewhere.36
Mothers who participated in the study were identified as either lighter users, heavier users, or nonusers of cocaine by interview and biological markers obtained by clinicians and study personnel. Meconium specimens from enrolled infants were analyzed by radioimmuno-assay for the cocaine metabolite benzoylecgonine, opiates, amphetamines, benzodiazepines, and cannabinoids using a modified method of Ostrea, published in detail elsewhere.36,37
During the period of study recruitment at Boston Medical Center, urine testing for metabolites of illicit drugs was performed for clinical indications at the discretion of health care personnel but was not universal. Clinical indications at the time included no prenatal care, a jittery infant, unusual maternal behavior, or a known history of substance use. When available in the medical chart, we documented the results of urine drug enzyme-multiplied immunoassay technique assays obtained for clinical purposes during prenatal or perinatal care of mother or infant. After recruitment and written informed consent, we collected additional urine samples from study mothers to determine benzoylecgonine, opiates, amphetamines, benzodiazepines, and cannabinoids by radioimmunoassay using commercial kits (Abuscreen RIA; Roche Diagnostics Systems, Inc, Montclair, NJ). Participants were protected by a writ of confidentiality.
Exposure Classification
All exposed or unexposed mother–infant dyads had at least 1 biological marker, either urine from mother or infant or meconium, to identify their exposure or lack of exposure to cocaine during pregnancy. In the parent sample, the mean days of self-reported cocaine use during pregnancy was 20.6 days (range: 0−264 days). The mean meconium concentration was 1143 ng of benzoylecgonine/gram of meconium (range: 0−17 950 ng/g). The sample median was 1100 ng/g in meconium at birth or 9 days of self-reported use during pregnancy. Before source study data were analyzed, a composite measure of heavier use was a priori defined as the top quartile of meconium concentration for cocaine metabolites (benzoylecgonine >3314 ng/g meconium) and/or top quartile days of self-reported use (>61 days) during the entire pregnancy. All other use was classified as “lighter.” By this method, 45 users (33% of the users) were classified as heavier users. All other users (n = 93) were classified as “lighter users.”
Pragmatic as well as scientific considerations influenced this either/or definition of exposure level. Women are more likely to underreport rather than overreport illicit substance use during pregnancy38; therefore, women who reported days of use in the top quartile were a priori considered heavier users, even if the meconium benzoylecgonine level was not in the top quartile because not all infants who are exposed to cocaine prenatally have positive meconium assays.39 Moreover, meconium samples were not obtained from 14% of study infants; therefore, whichever indicator (self-report or meconium assay) demonstrated higher exposure was used to determine exposure category. For this study, we recruited participants from the parent sample of children had who IUCE and fit additional criteria listed next and whose intrauterine cocaine exposure was >1100 ng/g meconium, the parent sample median.
A total of 123 infants were initially identified by perinatal clinicians as cocaine exposed on the basis of maternal antepartum or peripartum urine drug screen cocaine metabolite results. Five of 134 infants who were clinically considered to be cocaine unexposed were excluded from the final sample because no confirmatory biological marker was available, and 15 others were reclassified because urine or meconium assays were positive for cocaine metabolites, leaving 114 unexposed infants in the sample. In all, 138 infants were found to demonstrate evidence of IUCE. Together, the unexposed and exposed infants constituted a neonatal study sample of 252 initially consented. In all, 216 (86%) of the neonatal sample had meconium assays performed. No infant demonstrated amphetamine, opiate, or phencyclidine exposure by urine or meconium assay or by maternal report.
The Addiction Severity Index, a validated tool for substance abuse report, as well as additional study-specific interview questions was used to determine maternal alcohol, cigarette, and marijuana use during pregnancy.40 Alcohol, cigarette, and marijuana use was reported as number of drinks per week; number of cigarettes smoked per day; and number of days of use of alcohol, cigarettes, and marijuana, respectively, during the last 30 days of pregnancy. Data were acquired similarly for use of these substances during pregnancy before the last 30 days. This permitted determination and use of average daily volume and average daily cigarettes for alcohol and cigarette use, respectively. Because marijuana is stored for long periods in body fat, marijuana metabolite concentration in meconium constitutes a less valid indication of total dosage than that of cocaine metabolites.37 In addition, one third of the marijuana users identified on the basis of meconium/urine assay denied its use on interview. Consequently, construction of a reliable index of marijuana dosage proved difficult. In the following analyses, marijuana exposure was coded as exposed or unexposed on the basis of any positive self-report or any positive urine/meconium assay.
Cohort Selected for MRI
We recruited children for this study from the parent sample who were African American/African Caribbean until a sample size of 40 was attained, 16 with IUCE >1100 ng/g meconium, the parent sample median, and 24 otherwise demographically comparable children who had no IUCE. All children were right-handed. In addition to the initial exclusion criteria, the sample for this neuroimaging study excluded children with the following complications that might confound MRI interpretation: (1) history of central nervous system radiation, (2) loss of consciousness after head trauma, (3) history of seizures, (4) history of lead poisoning requiring chelation therapy, (5) history of intracranial surgery, and (6) history of systemic illness or medical treatment that might adversely affect brain structure. Children were recruited after parent/guardian informed consent and child assent were obtained using protocols approved by the Boston Medical Center institutional review board and the Committee on Clinical Investigation of Children's Hospital Boston.
MRI Scans
Each of the 40 children underwent imaging using a 1.5 T GE LX MR scanner (GE, Milwaukee, WI) operating at the GE 12.0 platform at Children's Hospital Boston. No sedation was used for image acquisition, but patients were acclimatized to the scanner environment. After a T1-weighted localizing scan was obtained, a high-resolution 3-dimensional T1-weighted Fourier Transform Spoiled Gradient neuroanatomic whole-brain acquisition was obtained (24 cm field of view, 1.5 mm contiguous coronal slice thickness, 120 slices, repetition time/echo time = 40/4, matrix 256 × 192, flip angle = 20°, scan time 11 minutes 20 seconds). Subsequently, whole-brain T2-weighted and proton density data were acquired using a dual echo (DE) fast spin echo pulse sequence (echo train length [ETL] = 8, 3-mm skip, 3-mm interleaved coronal slices, 2 acquisitions, repetition time/echo time/echo time 2 = 4000/14/84, 192 × 256, 20 cm field of view, 1 number of excitations [NEX], scan time 10 minutes and 25 seconds).
MRI Quality
Complete data sets were obtained from all 40 children scanned. Each raw data set was reviewed by a member of the research team (Dr Rivkin) for data quality. A neuroradiologist blinded to the identity of study children (Dr Robson) provided a clinical reading of each MRI data set. Scans of 5 children (2 with IUCE, 3 control subjects) possessed unacceptable movement artifact precluding their further analysis. Thus, 35 children (14 with IUCE, 21 without IUCE; 88% of children scanned) produced data sets that were fully analyzed.
Postimaging Data Analysis
Postimaging data processing and quantitative volumetric analyses were conducted on computer workstations using a LINUX platform. A sequence of established and validated image processing algorithms was used to segment each of the MRI slices into separate tissue classes.41–46 Briefly, each MRI slice in each data set was segmented into cerebral cortical gray matter (CGM), subcortical gray matter (SCGM; to include caudate, putamen, globus pallidus, and substantia nigra, bilaterally), white matter (WM), and cerebrospinal fluid (CSF; including ventricular and extra-axial CSF). These algorithms were designed to reduce imaging system noise, identify a linear transformation, and resample the DE spin-echo images according to the aforementioned linear transform to align the coronal DE spin-echo images with the coronal spoiled gradient images to form a 3-channel data set to classify tissue types on the basis of the MR intensity in the 3 channels and identify tissue-class surfaces for 3-dimensional visualization. From tissue segmentation of each slice, whole-brain absolute tissue volumes were determined in milliliters for CGM, WM, SCGM, and CSF (Fig 1), permitting calculation of total parenchymal volume (TOT; CGM + WM + SCGM) and total intracranial volume (TICV; CGM + WM + SCGM + CSF). Data were examined both as volumes referenced to TICV and as absolute volumes.
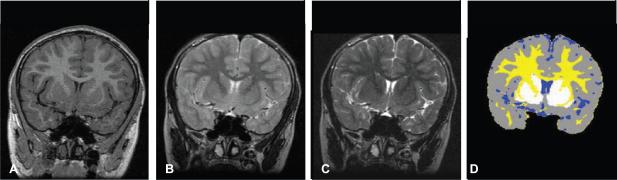
FIGURE 1.
Example of brain tissue segmentation. A, Representative coronal T1-weighted image of a single individual. B and C, T2-weighted images of same slice in A, taken at each of two different echo times (TE); TE = 14 milliseconds (B) and TE = 84 milliseconds (C). D, Resulting segmentation of same slice as in images A through C, to label CGM in gray, WM in yellow, SCGM in white, and CSF in blue.
Segmentations and volumetric analyses were undertaken by 2 members of the research team (Mr Davis and Ms Lemaster) and reviewed by a third team member (Dr Rivkin), all of whom were masked to subject group membership. Reviewers' consistency in tissue classification was assessed throughout the study with no more than 4% variance in tissue volumes found on quality certification data sets performed by both reviewers.
Data Analysis
All volumetric data and head circumference measurements were included in analysis. To ensure comparability with the cocaine variable, alcohol, tobacco, and marijuana were also collapsed to exposed/unexposed. First, bivariate analyses were performed to examine the effect of each individual exposure on HC and brain tissue volumes without adjustment for demographic variables or other exposures. Second, an initial multiple linear regression analysis discerned the effect of each individual exposure adjusted for child's gender and age at scan (demographic features). Such adjustment was performed because age and gender can affect brain size. Given that the children studied were both male and female and represented ages ranging from 10 to 13.8 years, adjustment for these features was essential to avoid misattribution of possible effects of age and gender to prenatal drug exposures. Because the impact of the birth mother's level of highest educational attainment was tested and found not to correlate with any outcome, it was not retained in final models. Third, a second-level, multiple linear regression analysis for each exposure incorporated the first model and adjusted for the remaining exposures. Thus, for comparison of children with IUCE and those with none, mean values were adjusted for gender and age at scan as well as for simultaneous cigarette, alcohol, and marijuana exposure. Similarly, for comparison of children with intrauterine alcohol exposure and those with none, mean values were adjusted for demographic features as well as for simultaneous cocaine, cigarette, and marijuana exposure. Similar analyses were conducted for intrauterine cigarette exposure and for intrauterine marijuana exposure. Associations with 2-tailed P < .05 were judged to be statistically significant.
Small HC in bivariate analyses was associated with each of the 4 substance exposures studied (see “Results”). Because HC has been demonstrated to correlate with brain volumes, analyses were performed using absolute volumes.47–49 Analyses were also performed using a ratio of absolute tissue volumes divided by TICV, which yielded no statistically significant differences among groups for any of the cerebral tissue outcome variables; however, children who were exposed to cocaine, to alcohol, and to cigarettes each demonstrated reductions in HC at the time of measurement that were statistically significant as compared with control subjects. Furthermore, significant reduction of HC was found in children who were exposed prenatally to ≥2 of these substances. Because the principal determinant of head size is brain size and because reduction of HC accompanied prenatal drug exposure for the majority of the exposed children, we reasoned that adjustment for HC or its proxy, intracranial cavity volume, factored out the effect of the exposure as well. As a result, all analyses were performed using absolute tissue volumes.
RESULTS
Subjects
Thirty-five children were included for final analysis of MRI data. The group comprised 14 children with IUCE and 21 with no IUCE. Characteristics of children in the study sample are provided in Table 1. Among the children with IUCE, prenatal exposures to maternally used alcohol, cigarettes, or marijuana were common; however, 8 (38%) children with no IUCE had prenatal exposures to alcohol, cigarettes, or marijuana as well. Multiple prenatal exposures occurred in 15 of the 35 children studied. Among children with IUCE, 11 (78.5%) of the 14 had prenatal exposure to ≥2 of the other substances studied, whereas only 2 (9.5%) of the 21 children with no IUCE had such exposures. Finally, 43% of the children in the exposed group of this study had exposures to cocaine and 2 other substances, whereas 35% had exposures to cocaine and the 3 other substances (Table 2).
TABLE 1.
Study Sample Characteristics
| Characteristic | Children With IUCE | Control Children |
|---|---|---|
| No. of children | 14 | 21 |
| Age, mean (range), y | 12.3 (10.0−13.8) | 12.4 (10.0−13.2) |
| Handedness | All right-handed | All right-handed |
| Gender (M/F) | 8/6 | 12/9 |
| Gestational age at birth, mean (SD), wk | 39.7 (1.5) | 40.3 (1.6) |
| IQ score at 10.5 y, mean (SD)a | 90.3 (11.1) | 85.9 (12.5) |
Wechsler Intelligence Scale for Children full-scale IQ score.
TABLE 2.
Prenatal Exposure to Maternal Cocaine, Ethanol, Cigarette, and Marijuana Use
|
IUCE Group (n = 14) |
Control Group (n = 21)a |
||
|---|---|---|---|
| Parameter | n (%) | Parameter | n (%) |
| Combined prenatal substance exposureb | Combined prenatal substance exposureb | ||
| Cocaine alone | 1 (7.1) | None | 13 (61.9) |
| Cocaine + 1 other substance | 2 (14.3) | 1 Substance | 6 (28.6) |
| Cocaine + 2 other substances | 6 (42.9) | 2 Substances | 1 (4.8) |
| Cocaine + 3 other substances | 5 (35.7) | 3 Substances | 1 (4.8) |
| Exposure by substance type | Exposure by substance type | ||
| Cocaine alone | 1 (7.1) | None | 13 (61.9) |
| Cocaine + ethanol | 9 (64.3) | Ethanol | 2 (9.5) |
| Cocaine + cigarettes | 12 (85.7) | Cigarettes | 6 (28.6) |
| Cocaine + marijuana | 8 (57.1) | Marijuana | 3 (14.3) |
Control group had no IUCE.
Cocaine, ethanol, cigarettes, or marijuana used by children's mothers during pregnancy.
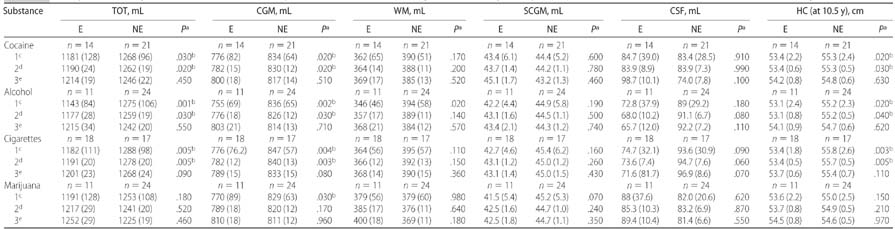
Children with IUCE demonstrated significantly smaller HC than those with no IUCE in both the unadjusted analysis (53.4 cm [SE 2.2] vs 55.3 cm [SE: 2.4]; P = .02) and the analysis that was adjusted for demographic features (53.4 cm [SE: 0.6] vs 55.3 cm [SE: 0.5]; P = .03). CGM and TOT were also significantly reduced in both the unadjusted analysis (CGM: 776 mL [SE: 82] vs 834 mL [SE: 64; P = .02]; TOT: 1181 mL [SE: 128] vs 1268 mL [SE: 96; P = .03]) and the analysis that was adjusted for demographics (CGM: 782 mL [SE: 15] vs 830 mL [SE: 12; P = .02]; TOT: 1190 mL [SE: 24] vs 1262 mL [SE: 19; P = .02]) for children with IUCE as compared with children with no IUCE. No significant differences in WM, SCGM, or CSF were found between children with and without IUCE in either of these analyses (Table 3).
TABLE 3.
Unadjusted and Adjusted Effects of Intrauterine Exposures to Cocaine, Alcohol, Cigarettes, or Marijuana by Substance on Brain MRI Tissue Volumes and HC in Linear Regression Models
E indicates exposed; NE, nonexposed. All HC values reflect group means of individual HC measurements obtained from each child at 10.5 years of age.
P value for significance level of corresponding tissue volume difference between E and NE groups (P values are 2-tailed).
P values statistically significant at the .05 level.
Mean volumes of unadjusted analyses with SD.
Mean volumes adjusted for gender and age at examination with SE.
Mean volumes adjusted for gender, age at examination and other drug exposures with SE.
Children with intrauterine alcohol exposure showed significantly smaller HC compared with those with no prenatal alcohol exposure (53.1 cm [SE: 2.4] vs 55.2 cm [SE: 2.3; P = .02]) for the unadjusted analysis and after adjustment for demographic features (53.1 cm [SE: 0.8] vs 55.2 cm [SE: 0.5; P = .04]). Volumetric analyses revealed that CGM, WM, and TOT were significantly reduced in the unadjusted analysis (CGM: 755 mL [SE: 69] vs 836 [SE: 65; P = .002]; WM: 346 mL [SE: 46] vs 394 cm [SE: 58; P = .02]; TOT: 1143 mL [SE: 84] vs 1275 mL [SE: 106; P = .001]), whereas only CGM and TOT were significantly reduced after adjustment for demographic features (CGM: 776 mL [SE: 18] vs 826 mL [SE: 12; P = .03]; TOT: 1177 mL [SE: 28] vs 1259 mL [SE: 19; P = .03]) among children with prenatal exposure to alcohol as compared with those with no such exposure. No significant intergroup differences were observed between groups in SCGM or CSF volumes for the unadjusted analysis or for SCGM, WM, or CSF volumes for the analysis adjusted for demographic features (Table 3).
Children with intrauterine cigarette exposure demonstrated significantly smaller HC (53.4 cm vs 55.8 cm) as compared with those with no prenatal cigarette exposure for both the unadjusted analysis (53.4 cm [SE: 1.8] vs 55.8 cm [SE: 2.6]; P = .003) and analysis adjusted for demographics (53.4 cm [SE 0.5] vs 55.7 cm [SE 0.5]; P = .005). Volumetric analyses revealed that CGM and TOT were significantly reduced in both the unadjusted analysis (CGM: 776 mL [SE: 76.2] vs 847 [SE 57; P = .004]; TOT: 1182 mL [SE: 111] vs 1288 mL [SE: 98; P = .005]) and the analysis adjusted for demographic features (CGM: 782 mL [SE: 12] vs 840 [SE: 13; P = .003]; TOT: 1191 mL [SE: 20] vs 1278 mL [SE: 20; P = .005]). No difference between groups was observed for WM, SCGM, or CSF volumes in both the unadjusted analysis and the analysis that was adjusted for demographics (Table 3).
Children with prenatal marijuana exposure demonstrated a trend toward smaller mean HC as compared with those with no prenatal marijuana exposure in both the unadjusted analysis and the analysis that was adjusted for demographics, but the difference did not reach significance. Although CGM and TOT were reduced in the unadjusted analysis and the analysis that was adjusted for demographic features, only in the unadjusted volumetric analysis did the CGM reduction reach significance (770 mL [SE: 89] vs 829 mL [SE 63]; P = .03). No significant difference in any other cerebral tissue volume was found in the analysis that was adjusted for demographic features (Table 3).
After the foregoing unadjusted and first-level multiple regression analyses, a second multiple regression analysis for each exposure was performed and incorporated the first model and added adjustments for the remaining exposures. It is interesting that although the trend for group differences in cerebral tissue volumes persisted, the individual effects of cocaine and alcohol, respectively, were no longer statistically significant after controlling for the other exposures. Notably, CGM and TOT remained reduced and retained marginal significance (CGM: P = .08; TOT: P = .09) in the analysis of intrauterine cigarette exposure after adjustment for simultaneous exposures to cocaine, alcohol, and marijuana (see Table 3 for summary of intergroup differences by substance for the unadjusted and both adjusted analyses).
Additional analysis was performed to compare children who were exposed prenatally to ≥2 substances with control subjects, adjusted for gender and age at examination. This analysis revealed significant reductions in CGM, TOT, and HC (776 mL vs 841 mL [P = .004]; 1175 mL vs 1269 mL [P = .007]; 53.4 cm vs 55.7 cm [P = .02], respectively; Table 4). Next, analysis of variance was performed to examine the cumulative effect on CGM, TOT, and HC of prenatal exposure to ≥1 of these substances (Table 5) as compared with nonexposed children with the independent variable the count of the number of substances to which the child was exposed in utero. A global mean P value for the analysis of variance models of .01 for both CGM and TOT indicated that reductions in these volumes relative to the unexposed were significantly associated with number of substances of exposure. A similar pattern was apparent for HC, which demonstrated a global mean P = .07. Table 5 demonstrates that CGM, TOT, and HC all declined as the number of substances to which study children were prenatally exposed exceeded 1. Indeed, this analysis of variance demonstrated that the smallest volumes of CGM, TOT, and HC were found in association with prenatal exposures to all 4 substances: cocaine, cigarettes, alcohol, and marijuana (CGM: 731 mL [exposed] vs 853 mL [unexposed; P = .002]; TOT: 1129 mL [exposed] vs 1287 mL [unexposed; P = .006]; HC: 52.3 cm [exposed] vs 55.7 cm [unexposed; P = .008]).
TABLE 4.
Adjusted Effect of Prenatal Exposure to >2 Substances on MRI Volumes, HC
| Parameter | Adjusted Mean Adjusted for Gender and Age at Examination |
P | |
|---|---|---|---|
| Exposed to ≥2 Substances (n = 15) | Unexposed (n = 13) | ||
| TOT, mL | 1175 (22) | 1269 (24) | .007a |
| CGM, mL | 776 (14) | 841 (15) | .004a |
| WM, mL | 356 (13) | 383 (14) | .180 |
| Basal ganglia, mL | 43.1 (1.3) | 45.4 (1.4) | .260 |
| CSF, mL | 80.3 (8.8) | 86.1 (9.5) | .660 |
| HC (at 10.5 y), cm | 53.4 (0.6) | 55.7 (0.7) | .020a |
All P values are 2-tailed.
P values statistically significant at the .05 level.
TABLE 5.
One-Way ANOVA for Effect of Number of Substances in Prenatal Exposure on CGM, TOT, and HC
| Parameter |
No. of Substances in Prenatal Exposure |
Global P | ||||
|---|---|---|---|---|---|---|
| 0 (n = 13) | 1 (n = 7) | 2 (n = 3) | 3 (n = 7) | 4 (n = 5) | ||
| TOT, mL | 1287 | 1292 (.91) | 1131 (.02) | 1194 (.06) | 1129 (.006) | .01 |
| CGM volume, mL | 853 | 827 (.42) | 772 (.06) | 788 (.04) | 731 (.002) | .01 |
| HC, cm | 55.7 | 54.9 (.46) | 54.5 (.41) | 53.6 (.05) | 52.3 (.008) | .07 |
All P values are 2-tailed. Numbers in parentheses are P values for difference between exposure group and group with no prenatal substance exposure; prenatal exposures are cocaine, cigarettes, alcohol, and marijuana. ANOVA indicates analysis of variance.
DISCUSSION
In this study, multiple regression analyses of absolute brain tissue volumes and head size revealed that prenatal exposures to cocaine, alcohol, and cigarettes individually were associated with reductions in HC and CGM and TOT volumes in 10- to 14-year-old children relative to comparison subjects after controlling for age at scan and gender. Importantly, these reductions reached bivariate statistical significance for cocaine, alcohol, and cigarette exposures, individually. Additional analyses of each exposure in which additional adjustment was made for the remaining 3 exposures revealed that the observed trend to reductions in CGM and TOT volumes associated with prenatal cocaine or alcohol exposure persisted but was no longer statistically significant. In distinction, the effects of prenatal cigarette exposure on CGM and TOT retained marginal significance despite such adjustment (P = .08 and .09, respectively). Last, analysis of variance provided evidence of an inverse relationship between the number of substances to which these children were prenatally exposed and the reduction found in CGM, TOT, and HC.
To our knowledge, this is the first volumetric magnetic resonance neuroimaging study to report whole-brain parenchymal volume and CGM volume reductions in older children that may be related to the individual effects of multiple intrauterine exposures, including cocaine. Our data indicate that, in addition to cocaine, prenatal exposures to tobacco and alcohol could play roles individually or in combination with IUCE in the observed brain tissue volume and HC reductions.
These findings are consistent with those of other investigators. Diminished HC has been linked repeatedly to intrauterine exposure. Prenatal cocaine exposure has been associated with reduced HC at birth14,50,51 with both an inverse dosage effect and trimester-specific effects of prenatal cocaine exposure on HC reported.3,52,53 Similarly, maternal cigarette use has been associated with significant reduction of newborn HC.54,55 Finally, prenatal alcohol exposure during pregnancy has been associated with reduced HC both at birth and as late in childhood as 14 years of age56,57 in children who do not manifest physical features of FAS.
The reported reductions of both CGM and TOT volumes are consistent with other observations about brain structure reported with respect to intrauterine exposures to cocaine, alcohol, cigarettes, or marijuana. Use of recently developed quantitative neuroimaging techniques to study the brain structure and function of children with IUCE has been limited. In the parent sample of this study, cranial ultrasonography revealed that neonates who were exposed to higher (top quartile) levels of prenatal cocaine demonstrated more frequent occurrence of caudothalamic groove subependymal hemorrhage than was found in lighter exposed or unexposed newborns after adjustment for a variety of confounding variables, including prenatal exposure to cigarettes, alcohol, and marijuana.36 Other studies using similar samples have shown no such effects.58 Use of MRI morphometry and magnetic resonance spectroscopy to examine the subsequent effect of IUCE on the brain of school-age children showed no morphometric difference between children with and without IUCE; however, frontal WM creatine was elevated by magnetic resonance spectroscopy in children with IUCE.59 Although concomitant exposures to cigarettes or alcohol were recognized in both groups, statistical adjustment for these exposures was not attempted. Another study that sought correlation between cognitive performance and mean diffusivity measured with diffusion tensor imaging found higher mean diffusivity in anterior callosal and right frontal projection fibers and lower scores on executive function measures among children who had IUCE compared with those without such exposure.60 Notably, these investigators found that prenatal exposures to alcohol and marijuana as well as an interaction between prenatal exposures to marijuana and cocaine affected prefrontal WM mean diffusivity. Although we found an association between alcohol exposure and WM volume in the bivariate analysis of this study, we did not find any group differences in WM volume associated with cocaine exposure; however, our data are volumetric and do not include diffusion tensor imaging and spectroscopy data.
Similarly, compelling evidence supports the deleterious effect of prenatal alcohol exposure on brain development. Neuropathologic study of brain from children with FAS indicated alcohol-associated derailment of the migrational phase of normal brain development. These findings included microcephaly, widespread leptomeningeal heterotopias, and schizencephaly.61,62 In addition, midline WM abnormalities such as agenesis and dysgenesis of the corpus callosum as well as septo-optic dysplasia have been found on autopsy and in vivo through MRI study.63,64 Volumetric MRI studies of children with prenatal exposure to alcohol including children without stigmata of FAS revealed reduced intracranial vault size; volume reduction of corpus callosum, basal ganglia, and cerebellum; and abnormal ratios of gray matter to WM in temporoparietal regions of brain, persistent even in adolescence.65–72
A growing body of evidence indicates a lasting adverse effect of prenatal exposure to maternal cigarette use. Mammalian studies demonstrated that prenatal exposure to nicotine can upregulate nicotinic cholinergic receptors in developing brain, shortening the proliferative phase of brain development and thereby allowing earlier onset of neuronal differentiation as compared with comparison subjects.73,74 Volumetric MRI study of brain tissue volumes in adult smokers as compared with nonsmokers revealed reduced gray matter volume in prefrontal cortex in both hemispheres and left anterior cingulate.75 Although these findings related to postnatal cigarette exposure could represent effects of chronic smoking, predisposing traits that lead to smoking, or some combination of these factors, they raise the possibility that prenatal cigarette exposure may exert structural consequences on developing brain.
Data are less available regarding the structural consequences of prenatal marijuana exposure on brain development. Wilson et al76 raised the question of developmental sensitivity of brain to marijuana exposure in their volumetric MRI study that demonstrated proportionately less CGM and more WM in children who initiated marijuana use before the age of 17 as compared with those who did not use marijuana or who initiated use after the 17 years of age.
The limitations of this study must be recognized. First, because not all cases had drug screens from both members of each dyad (urine from mothers and urine or meconium from control infants), we cannot exclude the possibility that in addition to those who were reclassified after recruitment because of positive screen results, there were other exposed children misclassified as “controls.” Second, our definition of heavy cocaine exposure (top quartile for this sample, ≥61 days of reported use during pregnancy) differed from the definition used by others in other cohorts to define heavy cocaine use (≥2 times per week throughout pregnancy), and it is, therefore, possible that our inability to detect an effect of heavy exposure on HC or brain tissue volumes in the analysis adjusted for both demographic and other substance exposures may have been attributable to a cohort that was overall less heavily exposed than some other cohorts.77,78 Comparisons across cohorts are difficult because the potency and contamination of illicit substances, such as cocaine, vary across time and by geographic location so that number of days of use can only approximate the actual “dosage” experienced by the fetus. Third, the total number of children studied was small. This small number prohibits determination of interactions among the prenatal exposures that may have affected the outcomes. Furthermore, stratification by gender was not possible. Finally, the sample studied was exclusively African American or African Caribbean and poor; therefore, generalization of these findings to the general population of children in the United States is not possible at this time. It is possible that study of a larger sample would allow effects of individual and combined prenatal exposures on the MRI outcome measures to emerge that are not apparent now.
Our data indicate that prenatal exposures to cocaine, cigarettes, and alcohol may each individually exert an adverse effect on CGM and TOT volumes that can be detected subsequently in children who are 10 to 14 years of age using volumetric MRI. Furthermore, prenatal exposures to these substances in combination may exert deleterious and lasting consequences on brain structure. Prenatal exposure to increasing numbers of substances was associated with significant reduction in TOT, CGM, and HC. Although firm conclusions about the discrete individual effects of prenatal cocaine, alcohol, or cigarettes on brain volume in the children of our small sample cannot be made, these data are consistent with a possible, lasting effect of each and raise concern that exposure to combinations of these 4 substances during the prenatal period may have an enduring effect on brain structure in children.
The clinical implication of these results suggests that prenatal care and counsel of pregnant women not only should include emphasis on the potential lasting consequences on children of use of individual substances (whether legal or illegal) by their pregnant mothers but also should stress that substances such as cocaine, cigarettes, alcohol, and marijuana may produce cumulative effects on brain structure that are detectable at school age. Furthermore, assistance should be offered to reduce use of all of these substances. From a scientific perspective, additional systematic neuroimaging studies of larger samples are needed to clarify the effect of dosage of each substance, threshold of effect, interaction of substances with each other, and potential moderating effects of demographic variables such as age and gender.
ACKNOWLEDGMENTS
This work was supported by National Institute on Drug Abuse grants R01 DA06532 (Dr Frank); R01 RR021885 (Dr Warfield), and R01 GM074068 (Dr Warfield), and Children's Hospital MRDDRC HD018655 (Dr Rivkin).
Abbreviations
- IUCE
intrauterine cocaine exposure
- HC
head circumference
- FAS
fetal alcohol syndrome
- DE
dual echo
- CGM
cortical gray matter
- SCGM
subcortical gray matter
- WM
white matter
- CSF
cerebrospinal fluid
- TOT
total parenchymal volume
- TICV
total intracranial volume
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: preschool development at 3 years of age. J Pediatr Psychol. 2006;31(1):41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer LT, Salvator A, Arendt R, Minnes S, Farkas K, Kliegman R. Effects of cocaine/polydrug exposure and maternal psychological distress on infant birth outcomes. Neurotoxicol Teratol. 2002;24(2):127–135. doi: 10.1016/s0892-0362(01)00208-2. [DOI] [PubMed] [Google Scholar]
- 3.Richardson GA. Prenatal cocaine exposure. A longitudinal study of development. Ann N Y Acad Sci. 1998;846:144–152. [PubMed] [Google Scholar]
- 4.Nassogne MC, Gressens P, Evrard P, Courtoy PJ. In contrast to cocaine, prenatal exposure to methadone does not produce detectable alterations in the developing mouse brain. Brain Res Dev Brain Res. 1998;110(1):61–67. doi: 10.1016/s0165-3806(98)00094-7. [DOI] [PubMed] [Google Scholar]
- 5.Gressens P, Kosofsky BE, Evrard P. Cocaine-induced disturbances of corticogenesis in the developing murine brain. Neurosci Lett. 1992;140(1):113–116. doi: 10.1016/0304-3940(92)90694-3. [DOI] [PubMed] [Google Scholar]
- 6.Nassogne MC, Louahed J, Evrard P, Courtoy PJ. Cocaine induces apoptosis in cortical neurons of fetal mice. J Neurochem. 1997;68(6):2442–2450. doi: 10.1046/j.1471-4159.1997.68062442.x. [DOI] [PubMed] [Google Scholar]
- 7.Nassogne MC, Evrard P, Courtoy PJ. Selective neuronal toxicity of cocaine in embryonic mouse brain cocultures. Proc Natl Acad Sci U S A. 1995;92(24):11029–11033. doi: 10.1073/pnas.92.24.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novikova SI, He F, Bai J, Badan I, Lidow IA, Lidow MS. Cocaine-induced changes in the expression of apoptosis-related genes in the fetal mouse cerebral wall. Neurotoxicol Teratol. 2005;27(1):3–14. doi: 10.1016/j.ntt.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Lidow MS. Consequences of prenatal cocaine exposure in nonhuman primates. Brain Res Dev Brain Res. 2003;147(1−2):23–36. doi: 10.1016/j.devbrainres.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Woods NS, Behnke M, Eyler FD. Cocaine use among pregnant women: socioeconomic, obstetrical, and psychological issues. In: Lewis M, Bendersky M, editors. Mothers, Babies, and Cocaine: The Role of Toxins in Development. Erlbaum; Hillsdale, NJ: 1995. pp. 305–332. [Google Scholar]
- 11.Bendersky M, Alessandri S, Gilbert P, Lewis M. Characteristics of pregnant substance abusers in two cities in the northeast. Am J Drug Alcohol Abuse. 1996;22(3):349–362. doi: 10.3109/00952999609001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIDA . National Pregnancy and Health Survey: Drug Use Among Women Delivering Live Births, 1992. US Dept of Health and Human Services; Rockville, MD: 1993. [Google Scholar]
- 13.Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. JAMA. 2001;285(12):1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandstra ES, Morrow CE, Vogel AL, et al. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicol Teratol. 2002;24(3):297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- 15.Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K. Birth outcome from a prospective, matched study of prenatal crack/cocaine use: II—interactive and dose effects on neurobehavioral assessment. Pediatrics. 1998;101(2):237–241. doi: 10.1542/peds.101.2.237. [DOI] [PubMed] [Google Scholar]
- 16.Potter SM, Zelazo PR, Stack DM, Papageorgiou AN. Adverse effects of fetal cocaine exposure on neonatal auditory information processing. Pediatrics. 2000;105(3) doi: 10.1542/peds.105.3.e40. Available at: www.pediatrics.org/cgi/content/full/105/3/e40. [DOI] [PubMed]
- 17.Delaney-Black V, Covington C, Templin T, et al. Expressive language development of children exposed to cocaine prenatally: literature review and report of a prospective cohort study. J Commun Disord. 2000;33(6):463–480. doi: 10.1016/s0021-9924(00)00033-2. quiz 480−481. [DOI] [PubMed] [Google Scholar]
- 18.Singer LT, Arendt R, Minnes S, et al. Cognitive and motor outcomes of cocaine-exposed infants. JAMA. 2002;287(15):1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Burden MJ, Jacobson SW, Jacobson JL. Relation of prenatal alcohol exposure to cognitive processing speed and efficiency in childhood. Alcohol Clin Exp Res. 2005;29(8):1473–1483. doi: 10.1097/01.alc.0000175036.34076.a0. [DOI] [PubMed] [Google Scholar]
- 21.Burden MJ, Jacobson SW, Sokol RJ, Jacobson JL. Effects of prenatal alcohol exposure on attention and working memory at 7.5 years of age. Alcohol Clin Exp Res. 2005;29(3):443–452. doi: 10.1097/01.alc.0000156125.50577.ec. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29(8):1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- 23.Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111(6 pt 1):1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- 24.Obel C, Henriksen TB, Hedegaard M, Secher NJ, Ostergaard J. Smoking during pregnancy and babbling abilities of the 8-month-old infant. Paediatr Perinat Epidemiol. 1998;12(1):37–48. [PubMed] [Google Scholar]
- 25.Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23(5):421–430. doi: 10.1016/s0892-0362(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 26.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 1998;20(3):293–306. doi: 10.1016/s0892-0362(97)00091-3. [DOI] [PubMed] [Google Scholar]
- 27.Fried PA, Watkinson B. Visuoperceptual functioning differs in 9- to 12-year olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2000;22(1):11–20. doi: 10.1016/s0892-0362(99)00046-x. [DOI] [PubMed] [Google Scholar]
- 28.Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2003;25(4):427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 29.Day NL, Richardson GA, Goldschmidt N, et al. Effect of prenatal marijuana exposure on the cognitive development of off-spring at age three. Neurotoxicol Teratol. 1994;16(2):169–175. doi: 10.1016/0892-0362(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 30.Fried PA. Behavioral outcomes in preschool and school-age children exposed prenatally to marijuana: a review and speculative interpretation. NIDA Res Monogr. 1996;164:242–260. [PubMed] [Google Scholar]
- 31.Fried PA, O'Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J Dev Behav Pediatr. 1992;13(6):383–391. [PubMed] [Google Scholar]
- 32.Gray KA, Day NL, Leech S, Richardson GA. Prenatal marijuana exposure: effect on child depressive symptoms at ten years of age. Neurotoxicol Teratol. 2005;27(3):439–448. doi: 10.1016/j.ntt.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: effects on attention and impulsivity of 6-year-olds. Neurotoxicol Teratol. 1999;21(2):109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- 34.Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24(3):309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- 35.Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22(3):325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 36.Frank DA, McCarten KM, Robson CD, et al. Level of in utero cocaine exposure and neonatal ultrasound findings. Pediatrics. 1999;104(5 pt 1):1101–1105. doi: 10.1542/peds.104.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ostrea EM, Knapp DK, Ostrea AR, Tannenbaum L, Saleri V. A prospective study comparing systematic interview and analysis of maternal hair and meconium to determine illicit drug use during pregnancy. Pediatr Res. 1994;35:A245. [Google Scholar]
- 38.Lester BM, ElSohly M, Wright LL, et al. The Maternal Lifestyle Study: drug use by meconium toxicology and maternal self-report. Pediatrics. 2001;107(2):309–317. doi: 10.1542/peds.107.2.309. [DOI] [PubMed] [Google Scholar]
- 39.Ostrea EM, Brady M, Gause S, Raymundo AL, Stevens M. Drug screening of newborns by meconium analysis: a large-scale, prospective, epidemiologic study. Pediatrics. 1992;89(1):107–113. [PubMed] [Google Scholar]
- 40.Kosten TR, Rounsaville BJ, Kleber HD. Concurrent validity of the addiction severity index. J Nerv Ment Dis. 1983;171(10):606–610. doi: 10.1097/00005053-198310000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Warfield SK, Kaus M, Jolesz FA, Kikinis R. Adaptive, template moderated, spatially varying statistical classification. Med Image Anal. 2000;4(1):43–55. doi: 10.1016/s1361-8415(00)00003-7. [DOI] [PubMed] [Google Scholar]
- 42.Hüppi PS, Maier SE, Peled S, et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44(4):584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- 43.Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115(2):286–294. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 44.Shah DK, Guinane C, August P, et al. Reduced occipital regional volumes at term predict impaired visual function in early childhood in very low birth weight infants. Invest Ophthalmol Vis Sci. 2006;47(8):3366–3373. doi: 10.1167/iovs.05-0811. [DOI] [PubMed] [Google Scholar]
- 45.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130(Pt 3):667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 46.Mewes AU, Huppi PS, Als H, et al. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118(1):23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- 47.Cooke RW, Lucas A, Yudkin PL, Pryse-Davies J. Head circumference as an index of brain weight in the fetus and newborn. Early Hum Dev. 1977;1(2):145–149. doi: 10.1016/0378-3782(77)90015-9. [DOI] [PubMed] [Google Scholar]
- 48.Bray PF, Shields WD, Wolcott GJ, Madsen JA. Occipitofrontal head circumference: an accurate measure of intracranial volume. J Pediatr. 1969;75(2):303–305. doi: 10.1016/s0022-3476(69)80404-x. [DOI] [PubMed] [Google Scholar]
- 49.Wickett JC, Vernon PA, Lee DH. Relationships between factors of intelligence and brain volume. Person Individ Diff. 2000;29(6):1095–1122. [Google Scholar]
- 50.Sallee FR, Katikaneni LP, McArthur PD, Ibrahim HM, Nesbitt L, Sethuraman G. Head growth in cocaine-exposed infants: relationship to neonate hair level. J Dev Behav Pediatr. 1995;16(2):77–81. [PubMed] [Google Scholar]
- 51.Mirochnick M, Frank DA, Cabral H, Turner A, Zuckerman B. Relation between meconium concentration of the cocaine metabolite benzoylecgonine and fetal growth. J Pediatr. 1995;126(4):636–638. doi: 10.1016/s0022-3476(95)70367-5. [DOI] [PubMed] [Google Scholar]
- 52.Bateman DA, Chiriboga CA. Dose-response effect of cocaine on newborn head circumference. Pediatrics. 2000;106(3) doi: 10.1542/peds.106.3.e33. Available at: www.pediatrics.org/cgi/content/full/106/3/e33. [DOI] [PubMed]
- 53.Bada HS, Das A, Bauer CR, et al. Gestational cocaine exposure and intrauterine growth: maternal lifestyle study. Obstet Gynecol. 2002;100(5 pt 1):916–924. doi: 10.1016/s0029-7844(02)02199-3. [DOI] [PubMed] [Google Scholar]
- 54.Källén K. Maternal smoking during pregnancy and infant head circumference at birth. Early Hum Dev. 2000;58(3):197–204. doi: 10.1016/s0378-3782(00)00077-3. [DOI] [PubMed] [Google Scholar]
- 55.Shankaran S, Das A, Bauer CR, et al. Association between patterns of maternal substance use and infant birth weight, length, and head circumference. Pediatrics. 2004;114(2) doi: 10.1542/peds.114.2.e226. Available at: www.pediatrics.org/cgi/content/full/114/2/e226. [DOI] [PubMed]
- 56.Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26(10):1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- 57.Smith IE, Coles CD, Lancaster J, Fernhoff PM, Falek A. The effect of volume and duration of prenatal ethanol exposure on neonatal physical and behavioral development. Neurobehav Toxicol Teratol. 1986;8(4):375–381. [PubMed] [Google Scholar]
- 58.Behnke M, Davis Eyler F, Conlon M, Wobie K, Stewart Woods N, Cumming W. Incidence and description of structural brain abnormalities in newborns exposed to cocaine. J Pediatr. 1998;132(2):291–294. doi: 10.1016/s0022-3476(98)70447-0. [DOI] [PubMed] [Google Scholar]
- 59.Smith LM, Chang L, Yonekura ML, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107(2):227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warner TD, Behnke M, Eyler FD, et al. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118(5):2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clarren SK, Alvord EC, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92(1):64–67. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- 62.Peiffer J, Majewski F, Fischbach H. Alcohol embryo and fetopathy. J Neurol Sci. 1979;41(2):125–137. doi: 10.1016/0022-510x(79)90033-9. [DOI] [PubMed] [Google Scholar]
- 63.Clarren SK. Central nervous system malformations in two offspring of alcoholic women. Birth Defects Orig Artic Ser. 1977;13(3D):151–153. [PubMed] [Google Scholar]
- 64.Johnson VP, Swayze VW, II, Sato Y, Andreasen NC. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genet. 1996;61(4):329–339. doi: 10.1002/(SICI)1096-8628(19960202)61:4<329::AID-AJMG6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 65.Autti-Rämö I, Autti T, Korkman M, Kettunen S, Salonen O, Valanne L. MRI findings in children with school problems who had been exposed prenatally to alcohol. Dev Med Child Neurol. 2002;44(2):98–106. doi: 10.1017/s0012162201001748. [DOI] [PubMed] [Google Scholar]
- 66.Sowell ER, Thompson PM, Mattson SN, et al. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12(3):515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- 67.Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001;57(2):235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- 68.Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: size reduction in lobules I-V. Alcohol Clin Exp Res. 1996;20(1):31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 69.Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: a decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127(1):35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- 70.Mattson SN, Riley EP, Sowell ER, Jernigan TL, Sobel DF, Jones KL. A decrease in the size of the basal ganglia in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20(6):1088–1093. doi: 10.1111/j.1530-0277.1996.tb01951.x. [DOI] [PubMed] [Google Scholar]
- 71.Sowell ER, Thompson PM, Peterson BS, et al. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002;17(4):1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- 72.Sowell ER, Thompson PM, Mattson SN, et al. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12(8):856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- 73.Levin ED, Wilkerson A, Jones JP, Christopher NC, Briggs SJ. Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Brain Res Dev Brain Res. 1996;97(2):207–215. doi: 10.1016/s0165-3806(96)00144-7. [DOI] [PubMed] [Google Scholar]
- 74.Slikker W, Jr, Xu ZA, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35(8−9):703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- 75.Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 76.Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19(1):1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- 77.Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999;11(2):195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- 78.Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Chiodo LM. New evidence for neurobehavioral effects of in utero cocaine exposure. J Pediatr. 1996;129(4):581–590. doi: 10.1016/s0022-3476(96)70124-5. [DOI] [PubMed] [Google Scholar]