Abstract
Mammalian sperm acquire fertilizing capacity after residing in the female tract, where physiological changes named capacitation take place. In animals with external fertilization as amphibians, gamete interactions are first established between sperm and molecules of the egg jelly coat released into the medium. Since dejellied oocytes are not normally fertilized, the aim of this study was to determine if the jelly coat of the toad Bufo arenarum promotes a “capacitating” activity on homologous sperm. We found that sperm incubation in diffusible substances of the jelly coat (Egg Water) for 90–180 sec is sufficient to render sperm transiently capable of fertilizing dejellied oocytes. The fertilizing state was correlated with an increase of protein tyrosine phosphorylation and a decrease of sperm cholesterol content. Inhibition of either the increase in tyrosine phosphorylation or cholesterol efflux affected the acquisition of fertilizing capacity. Phosphorylation and fertilization could be promoted with NaHCO3, and also by addition of beta cyclodextrin. Moreover, sperm could gain the ability to fertilize dejellied oocytes in the presence of these compounds. These data indicate that sperm should undergo a series of molecular changes to gain fertilizing capacity; these changes are reminiscent of mammalian sperm capacitation and take place before the acrosome reaction.
Keywords: fertilization, spermatozoa, capacitation, jelly coat, amphibia, phosphorylation
INTRODUCTION
Mammalian sperm do not fertilize an egg immediately upon ejaculation. Over 50 years ago, independent reports suggested that ejaculated mammalian spermatozoa require a period of residence in the female reproductive tract before being capable of fertilization (Autin, 1952;Chang, 1951). The changes that take place during this period confer to the spermatozoa the ability to adhere to the zona pellucida, to undergo the acrosome reaction and initiate oocyte fusion. This process is known as “capacitation”, and includes changes in intracellular pH, alterations in membrane lipid architecture and protein distribution, and initiation of complex signal transduction pathways (Baldi et al., 2002;Visconti et al., 2002;Visconti and Kopf, 1998). In animals with external fertilization as amphibians, gamete interactions are first established between the spermatozoa and molecules released into the medium by the egg jelly coat (JC) (Miceli and Cabada, 1998). This JC surrounds the vitelline envelope (analogous to the zona pellucida of mammals), and is formed by the oviduct secretion during ovulation. Some molecules of the JC are released from the jelly matrix during spawning. The solution containing the substances that diffuse from the JC is called Egg Water (EW).
The requirement of the jelly coats for amphibian fertilization was established many years ago (Kambara, 1953;Newport, 1851). The passage of sperm through the jelly layers has been regarded as an important step in fertilization, and was sometimes proposed to be a sperm “capacitating” requisite, by analogy with the concept developed in mammals (Shivers and James, 1970). EW of the toad Bufo arenarum was reported to “activate” homologous free spermatozoa before they penetrate into the jelly coats (Barbieri and Cabada, 1969;Barbieri and del Pino, 1975;Barbieri and Raisman, 1969). Dejellied oocytes of different amphibian species can still be fertilized after reintroduction of the diffusible jelly components (EW) in the insemination media (Barbieri, 1976;Barbieri and Oterino, 1972;Barbieri and Villecco, 1966;Elinson, 1971;Katagiri, 1973). Unlike sea urchin, a necessary condition for Bufo spermatozoa to fertilize the oocytes is to reach the vitelline envelope with its acrosome intact, or at least not completely reacted (Omata and Katagiri, 1996;Raisman et al., 1980;Yoshizaki and Katagiri, 1982). Acrosomal integrity is rapidly lost when spermatozoa are incubated in hypotonic solutions (resembling the osmolarity of the jelly at the time of fertilization). It was shown that L-HGP, a component of the EW, was able to avoid spontaneous acrosome breakdown caused by hypoosmotic stress (Arranz and Cabada, 2000;Krapf et al., 2006). As a consequence, acrosome integrity might be maintained until spermatozoa contact and bind to the vitelline envelope (Barisone et al., 2007).
The first demonstration of a physiological role for egg jelly macromolecules in amphibian fertilization was reported by al-Anzi and Chandler (1998). Allurin, a 21 kDa protein from Xenopus EW, exhibits sperm chemoattractant activity (Olson et al., 2001;Xiang et al., 2004;Xiang et al., 2005).
In this paper we report that short exposure to EW rendered sperm transiently capable of fertilizing dejellied oocytes. The fertilizing state was correlated with an increase of protein phosphorylation in tyrosine residues, and with sperm cholesterol loss. Moreover, these physiological modifications could be mimicked in-vitro with incubations in media containing combinations of NaHCO3 and the synthetic cholesterol acceptor methyl-β-cyclodextrin. As far as we are aware, this is the first report of sperm physiological changes induced by the jelly coat that take place at early stages of fertilization in amphibians.
MATERIALS AND METHODS
Reagents
Tyrphostin A1, A25 and genistein were purchased from Calbiochem (San Diego, CA) (kindly provided by Dr Patricia Miranda). H89 (N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide), chelerythrine, dibutyryl cAMP and IBMX were obtained from Sigma. Methyl-β-cyclodextrin (MβCD) was from Aquaplex, Cyclodextrin Technologies Development (Gainesville, Fl) (kindly provided by Dr Luis S. Mayorga). Anti-phosphotyrosine antibody (clone 4G10) was obtained from Upstate (Lake Placid, NY). All other reagents were of the highest analytical grade.
Animals
Bufo arenarum sexually mature specimens (150 g) were collected in the neighborhood of Rosario city, and maintained in the dark in a moist chamber between 15 and 17°C until used. Experiments were performed in accordance with the guide for the care and use of laboratory animals of Facultad de Ciencias Bioquímicas y Farmacéuticas, UNR.
Preparation of gametes
Sperm suspensions were obtained as described by elsewhere (Valz-Gianinet et al., 1991). After washing, spermatozoa were suspended in ice cold Ringer ST medium to a final concentration of 1 – 1.4 × 108 cells/ml and used within 3 hrs. Uterine oocytes (referred to as oocytes) were obtained according to Barisone et al (2007). Oocytes were dejellied as described (Barisone et al., 2007).
Egg water
EW was obtained as described (Diaz Fontdevila et al., 1991). Final protein concentration was 0.3 mg/ml (Arranz and Cabada, 2000). EW solutions were referred to as percentages of EW (30–80% EW), instead of stating the EW protein concentration since its chemical composition includes glycoproteins, inorganic ions, and lipids (Diaz Fontdevila et al., 1991;Ishihara et al., 1984;Katagiri, 1973).
Assessment of tyrosine phosphorylation of sperm proteins
Sperm suspensions were diluted to 1.4 ×107 cells/ml in the appropriate media depending on the experiment performed. Osmotic pressure of media was in all cases similar to Ringer ST. When the effect of NaHCO3 was analyzed, a modified Ringer media was used (80 mM NaCl instead of 110 mM, 25 mM Hepes, pH 7.6) adding NaCl to maintain constant osmolarity. It is important to notice that addition of NaHCO3 did not significantly changed the pH. After the incubation period, sperm were concentrated by centrifugation at 650 × g for 5 min (4°C), the sperm pellet resuspended in 10 μl of ice cold lysis buffer (1% Triton X-100, 1mM NaVO3, 1 mM PMSF, 5 mM EDTA, 150 mM NaCl, 10 mM Tris pH 7.6) and stored 10 min on ice with 3 × 5 sec vortexing. Samples were then centrifuged at 18000 × g for 5 min (4°C), the supernatant mixed with sample buffer containing 50 mM DTT, incubated 10 min at 70°C and then subjected to SDS-PAGE (Laemmli, 1970) in 8% gels. Each lane was loaded with 7 × 106 cells. Proteins were transferred to nitrocellulose membranes (Hybond-ECL, Amersham Biosciences, UK) at 250 mA (constant) for 2 h at 4°C. Immunodetection was performed using a dilution 1/2000 (0.5 μg/ml final concentration) of a monoclonal antibody against anti-phosphotyrosine (clone 4G10) following manufacture’s directions, and a dilution 1/5000 of a secondary HRP labelled antibody provided with the enhanced chemiluminescence detection kit (ECL, Amersham Biosciences, UK). For specificity controls, 10 mM phosphotyrosine was added to the antibodies solution and incubated with constant rotation for 30 min at 20°C prior to use in the immunoblots.
Cholesterol measurements
Aliquots (8×106 cells) of B. arenarum spermatozoa were incubated for 15 min at 22°C with the specified MβCD or EW concentration in Ringer ST (500 μl final volume) and concentrated by centrifugation at 650 × g for 5 min at 4°C. Pellets and supernatants were individually lyophilized for 6 h. Free cholesterol was determined basically as described by Gamble et al (1978). In brief, to each tube was added 20 μl of a solution of 20 mM sodium desoxycholate and 1% Triton X-100, followed by 25 μl of 95% ethanol and stored at 4°C for 30 min. Cholesterol was measured by the method of cholesterol oxidase/peroxidase (Wiener, Rosario, Argentina) in a final volume of 1 ml, and analyzed in a Gilford Response spectrophotometer at 505 nm (Allain et al., 1974).
In vitro fertilization
In vitro fertilization was carried out at 20°C, inseminating jelly intact oocyte strings (110–170 oocytes) with 1 × 104 sperm/ml, or jelly free oocytes with 1 × 106 sperm/ml in 10% Ringer medium (0.1X Ringer/10 mM Tris pH 7.6). When the effect of NaHCO3 was analyzed, NaCl was added to maintain constant osmolarity, using modified Ringer medium as stated above for the assessment of tyrosine phosphorylation. After 15 min, oocytes and embryos were transferred to fresh 10% Ringer. Fertilization was evaluated with a stereomicroscope by recording two or four cells embryos.
Statistical Analysis
Statistical analyses were performed with the ANOVA test. Models were further tested according to Nagarsenker (1984), and Shapiro and Wilk (1965). Significance (p) and sample size (n) were indicated in each figure legend.
RESULTS
Effect of EW on the fertilizing capacity of sperm
Studies in a number of amphibian species suggest that diffusible factors that are released from the jelly coat play a role in fertilization (Cabada, 1975;Katagiri, 1973;Katagiri, 1986;Olson and Chandler, 1999;Shivers and James, 1971). EW has a direct effect on sperm and may increase their ability to penetrate the oocyte’s surface by an increase in sperm motility or other mechanisms. If these mechanisms correspond to a “capacitating” activity of EW, its presence would no longer be necessary once sperm have undergone capacitation. Thus, it should be possible to expose spermatozoa to EW and fertilize dejellied oocytes in EW free media. To test this hypothesis, sperm suspensions were incubated in 80% EW (since it is the maximum concentration achievable after addition of Ringer St medium) before inseminating jelly free oocytes. When sperm were not previously exposed to EW, only 4.3% of oocytes were fertilized (Fig. 1). However, the effect on fertilization was especially pronounced when sperm were incubated in EW for 1.5 or 3 min (48 and 43% of fertilization respectively). Longer incubation periods promoted a lowering in the fertilization rates, down to zero. Due to the technical procedure, EW concentration in the insemination media was 10% in all the conditions assayed. The fact that fertilization could proceed without the further participation of EW suggests that the jelly components promote a change in sperm physiology.
FIG. 1.

Effect of sperm-incubation in EW on fertilization rates of dejellied eggs. Sperm were incubated in Ringer ST media containing 80% EW for the indicated periods before inseminating dejellied oocytes. Fertilized oocytes were observed at 2 or 4 cells stage embryos. Data represent the mean ± SEM (n=3). The means of groups that have different letters differ significantly (p<0.05).
Effect of EW on sperm cholesterol content
Capacitation of mouse sperm requires the presence of BSA in the incubation medium (Visconti et al., 1995a). The role of BSA in capacitation of mammalian sperm has been postulated to involve the removal of cholesterol from the plasma membrane (Suzuki and Yanagimachi, 1989;Visconti et al., 1999a;Visconti et al., 1999b). As EW has a number of proteins potentially capable of binding cholesterol, cholesterol loss could play a role in the acquisition of fertilizing capacity of B. arenarum sperm upon contact with the jelly coat. To test this hypothesis, we incubated Bufo sperm in Ringer ST media (110 mM NaCl, 2 mM KCl, 1.4 mM CaCl2, 10 mM Tris pH 7.6) with or without EW, and measured sperm cholesterol content. The use of Ringer ST medium would prevent an acrosome breakdown, and a consequently membrane loss induced by hypotonic stress (Arranz and Cabada, 2000). Cholesterol content of untreated sperm (freshly prepared and stored on ice for no longer than 1 hr) was 0.80 ± 0.025 pg/sperm (mean ± SEM, n = 6). As observed in Fig. 2A, an EW concentration-dependent efflux of cholesterol was observed. Maximal cholesterol removal was achieved with the maximum EW concentration tested.
FIG. 2.

Sperm cholesterol content and its role in fertilization. A: Sperm suspensions (8 × 106 cells) were incubated 15 minutes in the specified EW concentration. Cholesterol sperm content was normalized within each experiment with respect to the cholesterol content of the untreated control sample (100% equivalent to 0.80 ± 0.025 pg/sperm). Data represent the mean ± SEM (n=4). The means of groups that have different letters differ significantly (p<0.01). B: Intact oocyte strings were incubated for 90 min in 10% Ringer at 22 ºC containing the specified cholesterol-SO4− concentration, before insemination with 1 × 104 sperm/ml. After 15 min, the strings were transferred to fresh 10% Ringer. Between 110 and 170 oocytes were used in each experiment. The means of groups that have different letters differ significantly (mean ± SEM; n=3; p<0.002).
Cholesterol efflux may represent a sperm regulatory step for the acquisition of fertilizing capacity. If sperm cholesterol loss was a key element for acquisition of sperm fertilizing capacity, this process could be impaired by preincubation with the cholesterol analogue, cholesterol-SO4−. Preincubation with cholesterol-SO4− would block cholesterol-binding sites in the egg jelly, lowering their ability to bind cholesterol from sperm. As shown in Fig. 2B, preincubation of gametes with increasing concentrations of cholesterol-SO4− lowered fertilization rates of intact oocytes, suggesting that the cholesterol efflux from B. arenarum sperm is necessary for acquisition of fertilizing capacity.
Time and concentration-dependence of protein tyrosine phosphorylation on EW incubation
While the molecular bases underlying the capacitating events in mammalian sperm are not fully understood, they are consistent with the activation of signal transduction cascades. Among them, capacitation is associated with an increase in tyrosine phosphorylation of a subset of proteins in sperm from several mammalian species, including the mouse (Visconti et al., 1995a), cow (Galantino-Homer et al., 1997), human (Leclerc et al., 1996;Osheroff et al., 1999), pig (Kalab et al., 1998) and hamster (Devi et al., 1999;Kulanand and Shivaji, 2001;Visconti et al., 1999c).
To examine if there is correlation between the acquisition of fertilizing capacity and protein tyrosine phosphorylation in B. arenarum, sperm were incubated under conditions that support fertilization. After incubations, phosphorylation on tyrosine residues was analyzed. When sperm suspensions were incubated in EW, a concentration-dependent increase in the phosphorylation of tyrosine residues was observed in proteins from Mr 50,000 to 200,000 (Fig. 3A). Phosphorylation was observed 30 sec after the addition of 80% EW (Fig. 3B). Ponceau S staining was carried out to show equal protein loads in each lane (Fig. 3B, right panel). These proteins were specifically phosphorylated on tyrosine residues since the immunoreactivity was completely abolished when the antibody solution was preincubated with 10 mM O-phosphotyrosine for 30 min (not shown). Fig. 3C shows the immunoblotting EW proteins, indicating that the phosphoproteins detected do not belong to EW.
FIG. 3.
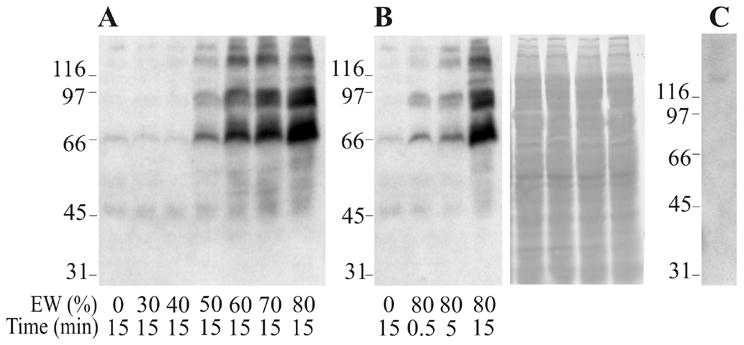
Time course and concentration-dependence of EW-induced protein phosphorylation in B. arenarum sperm. A and B: Sperm suspensions were incubated in EW (0–80%) for 0.5 to 15 min at 22°C. Each lane contains 1% triton X-100 soluble proteins of 7 × 106 spermatozoa subjected to Western immunoblotting analysis by using the anti-PY antibody. The right blot of panel B was stained with Ponceau S as a loading control. C: EW (12 μg) assayed with anti-PY antibody. Molecular weight standards are indicated at the left of each blot (Mr × 103).
Effects of tyrosine kinase inhibitors on sperm protein phosphorylation and fertilization
To find out if phosphorylation inhibition had any effect on fertilization, sperm were incubated in media containing the tyrosine-kinase inhibitor tyrphostin A25. After 30 min, sperm were exposed to 80% EW. Phosphorylation was inhibited in a concentration dependent manner, with an IC50 (50% phosphorylation inhibition) of about 2 μM (Fig. 4A), based on densitometric analysis (Gel-Pro Analyzer, Version 3.0, Media Cybernetics). No inhibition was observed when the inactive analogue Tyrphostin A1 was assayed at 200 μM. Genistein also displayed an inhibitory effect on phosphorylation with an IC50 between 2 and 20 μM (data not shown).
FIG. 4.

Effect of protein kinase inhibitors on protein tyrosine phosphorylation and fertilization. A: Sperm were incubated for 30 min at 19 ºC in Ringer ST containing tyrphostin A25 or A1 before exposure to 80% EW for 15 min at 22 ºC. Phosphoproteins were detected as in Fig. 3. While tyrphostin A25 inhibited protein tyrosine phosphorylation, the inactive analogue tyrphostin A1 had no effect at 200 μM. B: Sperm were incubated for 30 min at 19 ºC in Ringer ST containing tyrphostin A25 or A1 before inseminating jelly-intact oocytes, keeping the inhibitor concentration in the insemination media. Two or 4 cells embryos were counted. Data represent the mean ± SEM, n=3. The means of groups that have different letter differ significantly (p<0.0002).
Since we observed that sperm incubated in EW were capable of fertilizing dejellied oocytes in a condition that tyrosine phosphorylation is detected, we examined whether this phosphorylation has functional consequences. Sperm suspensions were incubated in different concentrations of tyrphostin A25 for 30 min. These suspensions were then used to inseminate intact oocytes, a condition where fertilizing capacity is naturally acquired. As shown in Fig. 4B, inhibition of tyrosine phosphorylation was accompanied by a significant reduction of fertilization rates. However, no effect was observed when sperm were preincubated in the presence of the inactive analogue tyrphostin A1, correlated with no inhibition of tyrosine phosphorylation. The inhibitor concentration was held during all the experiment by keeping the inhibitor in both the incubation and the insemination media. The effect of tyrphostin A25 on fertilization did not appear to be due to any effect on the oocyte itself, since insemination of jellied oocytes in tyrphostin A25 is achieved with untreated sperm (data not shown).
Relationship between cholesterol efflux and sperm tyrosine phosphorylation
EW components were able to sequester cholesterol from B. arenarum spermatozoa. However, in addition to cholesterol removal, EW molecules could induce on sperm other physiological modifications. We examined whether cholesterol removal by cyclodextrin in medium devoid of EW was sufficient to activate phosphorylation of B. arenarum sperm proteins. β-Cyclodextrins are water-soluble cyclic heptasaccharides able to effectively solubilize non-polar substances (Pitha et al., 1988). They have been widely used to promote cholesterol efflux from a wide variety of cells (Kilsdonk et al., 1995;Yancey et al., 1996). Sperm cholesterol content was measured after incubations in Ringer ST media with different concentrations of methyl-β-cyclodextrin (MβCD). As shown in Fig. 5A, a concentration-dependent cholesterol efflux was observed. At 1 and 2 mM MβCD, the loss of cholesterol was around one half of the total loss. Maximum cholesterol removal was observed with 10 mM MβCD, the maximum concentration tested. To analyze a possible vitality loss caused by cholesterol removal or toxic drug effects, sperm vitality was assessed by trypan blue staining after the MβCD treatment. Samples showed high vitality percentages ranging between 88% and 90% for MβCD concentrations between 0.5 and 2 mM (Fig. 5B). Lower vitality (74 and 68%) was observed at 5 and 10 mM MβCD. Therefore, Bufo sperm lose approximately 20% of cholesterol maintaining membrane integrity. Interestingly, the amount of cholesterol removed after incubation for 15 min in 80% EW (Fig. 2A) was analogous to the amount removed with 2 mM MβCD during the same period (Fig. 5A). To study a possible relationship between cholesterol efflux and tyrosine phosphorylation, sperm suspensions were incubated either for 30 sec or 15 min in media containing 80% EW, 2 mM MβCD or 80% EW pre-incubated with 20 μM cholesterol-sulfate. As shown in Fig. 5C, 2 mM MβCD failed in promoting tyrosine phosphorylation. However, when 80% EW was pre-incubated with 20 μM cholesterol-sulfate for 30 min prior to sperm exposure, a concentration sufficient to block fertilization (Fig. 2B), the increase in protein tyrosine phosphorylation was inhibited. Altogether, these experiments suggest that similar to what it is observed for mammalian fertilization, cholesterol efflux is necessary but not sufficient for the promotion of sperm protein tyrosine phosphorylation.
FIG. 5.

Sperm cholesterol efflux in MβCD and its relation with tyrosine phosphorylation. A: Sperm were incubated for 15 min at 22 °C in Ringer ST media containing the indicated concentrations of MβCD. Remaining cholesterol (open circles) and released cholesterol (filled circles) were normalized within each experiment with respect to the cholesterol content of the untreated control sample (100%). Cholesterol remaining at 0.5 mM MβCD or higher concentrations were significantly less than in control sample (p < 0.001). There were no significant differences between the cholesterol remaining at 0.5 mM and 1 mM, and between the cholesterol remaining at 2 mM and 5 mM MβCD. Data represent mean ± SEM (n = 4). B: Assessment of B. arenarum sperm vitality was performed with trypan blue staining after incubations in MβCD. The results express percentages of unstained spermatozoa normalized within each experiment with respect to the unstained spermatozoa of the 0 mM MβCD treatment (100%). Sperm vitality was in all cases significantly less than in control (p<0.001). At least 200 spermatozoa were counted in each case (p < 0.001) (mean ± SEM, n=3). C: Sperm were incubated for the indicated periods of time in Ringer ST, 80% EW, 2 mM MβCD or 80% EW preincubated 30 min with 20 μM cholesterol sulfate. Protein tyrosine phosphorylation was detected as in Fig. 3.
Role of MβCD and HCO3− in mediating the fertilizing capacity acquisition of B. arenarum sperm
The presence of NaHCO3 is required for in-vitro capacitation of mouse sperm. The associated changes of tyrosine phosphorylation occur upon activation of sperm adenyl cyclase induced by HCO3− (Visconti et al., 1995a). Membrane permeable cAMP analogues can substitute for NaHCO3 in promoting phosphorylation, through direct activation of PKA (Visconti et al., 1995b). We examined whether tyrosine phosphorylation of Bufo sperm proteins could be, as in the case of mouse sperm, stimulated by NaHCO3. Sperm suspensions were incubated in modified Ringer ST media (see Materials and Methods) containing different NaHCO3 concentrations. The ionic strength was kept constant in all samples. As shown in Fig. 6A, 30 mM NaHCO3 did induce protein tyrosine phosphorylation. Interestingly, when sperm were pre-incubated with 2 mM MβCD for 15 min prior to exposure to different concentrations of NaHCO3, tyrosine phosphorylation was induced at lower concentrations. A striking biphasic effect was displayed by NaHCO3 when sperm were pre-treated with MβCD: tyrosine phosphorylation was induced at 1 and 5 mM NaHCO3 but no phosphorylation was observed at higher concentrations. To test wether PKA could also play a role in B. arenarum sperm tyrosine phosphorylation, we incubated sperm with the membrane permeable cAMP analogue dibutyryl-cAMP. However, db-cAMP did not induce protein phosphorylation in incubations up to 2 hrs and concentrations up to 1 mM in the absence or presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) (Fig. 6B).
FIG. 6.
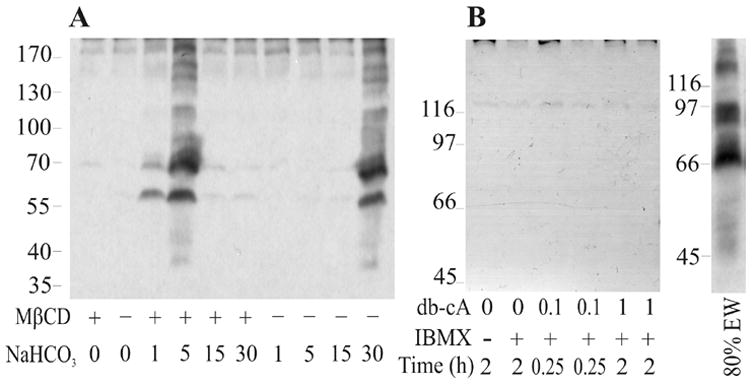
Dependence of tyrosine phosphorylation induced by NaHCO3 on MβCD pre-treatment and stimulation of PKA by a cAMP analogue. A: Sperm suspensions were incubated in the absence or presence of 2 mM MβCD for 15 min prior to exposure to the specified NaHCO3 concentrations (in mM) for 15 min. B: Sperm were incubated in the presence of db-cAMP (db-cA, in mM) and 0.1 mM IBMX as specified. Right panel shows a sample incubated with 80% EW for 15 min at 22 ºC. Protein tyrosine phosphorylation was detected as in Fig. 3A.
Since NaHCO3 supported tyrosine phosphorylation, we examined if NaHCO3 could substitute for EW in supporting acquisition of sperm fertilizing capacity. As previously demonstrated, dejellied oocytes inseminated in the absence of EW or with sperm that were not previously exposed to EW, were not fertilized (Fig. 1). We performed two sets of experiments where different NaHCO3 concentrations were tested on sperm with respect to their ability to fertilize dejellied oocytes. Only in one set of experiments sperm were previously incubated in the presence of 2 mM MβCD. Under no preincubation with MβCD, sperm were able to fertilize when insemination was carried out in media containing 30 mM NaHCO3 (Fig. 7). Fertilization rates were four times higher in 30 mM NaHCO3 than in 1 to 15 mM. However, when sperm were previously incubated in media containing 2 mM MβCD, fertilization was observed at lower NaHCO3 concentrations. Both results demonstrate that acquisition of fertilizing sperm capacity could be supported in the absence of EW. In vitro fertilization performed at higher concentrations of NaHCO3 with sperm previously incubated in media containing MβCD 2 mM displayed reduced fertilization rates (Fig. 7); interestingly, these conditions are correlated with the levels of protein tyrosine phosphorylation shown in Fig. 6A.
FIG. 7.

Effects of MβCD incubation on the ability of sperm to fertilize dejellied oocytes in media containing NaHCO3. Sperm suspensions were incubated for 15 min in the absence or presence of 2 mM MβCD and diluted ten times before inseminating dejellied oocytes in media containing the specified NaHCO3 concentrations. Embryos and oocytes (at least 110) were recorded after 2–4 hours. Data represent the mean ± SEM (n=4). The means of groups that have different letters differ significantly (p<0.05).
DISCUSSION
Successful fertilization by free-spawning organisms such as amphibians can occur only if a series of constraints are overcome before the sperm makes contact with the egg. Bufo sperm reach the egg envelope with an intact acrosome (Raisman et al., 1980;Yoshizaki and Katagiri, 1982). In B. arenarum, spermatozoa can bind to dejellied oocytes but no fertilization takes place (Krapf et al., unpublished results). Accordingly, previous reports showed that the acrosome of B. japonicus sperm bound to the vitelline envelope of dejellied oocytes is not reacted (Omata and Katagiri, 1996). These data indicate that spermatozoa depend on egg jelly components to fertilize, suggesting a key role of the jelly coat in the sperm acquisition of fertilizing capacity.
One of the characteristics of mammalian sperm capacitation is the ability of the acrosome-intact spermatozoa to undergo the acrosome reaction in response to ZP stimulation (Ward and Storey, 1984). Thus, the similarity between the unresponsiveness of acrosome reaction to egg envelope components in non-capacitated mammalian sperm with the lack of fertilizing capacity of Bufo sperm when no EW is present was of interest. We were able to set up conditions where fertilization of dejellied Bufo oocytes was restored by short preincubations of sperm in EW media before insemination. Olson and Chandler (1999) have shown different results in X. laevis, where incubation of sperm in EW did not restore fertilization of dejellied eggs. In those experiments, the preincubation media contained approximately 10-times less diffusible proteins and the osmolarity was about 10-times higher than our preincubation medium. Moreover, the authors restricted the preincubation periods to 5 min. Considering the short period of fertilizing capacity that Bufo sperm displayed in our conditions, it could be possible that they failed to find a “fertility window” under their conditions. We showed that this presumably “capacitated state” of B. arenarum sperm, as first hypothesized in amphibians by Shivers and James over 35 years ago (1970), correlates to physiological changes caused by the jelly coat components to the sperm. These changes are not related to the occurrence of an acrosome reaction, since EW of B. arenarum does not promote acrosome exocytosis of homologous spermatozoa (Arranz and Cabada, 2000;Krapf et al., 2006).
In mammals, these changes are associated with sperm cholesterol efflux and protein tyrosine phosphorylation. We measured the amount of cholesterol in B. arenarum sperm and found it to be similar to that of X. laevis (Bernardin et al., 1992). After incubations of 15 minutes in Ringer ST supplemented with 80% EW, approximately 20% of cholesterol was released from sperm. This efflux was dependent on EW concentration. Since a fast efflux was noticed, it is most likely that the pool of cholesterol released was located in the sperm plasma membrane (Yancey et al., 1996). This cholesterol loss could not be ascribed to an acrosome breakdown, since the acrosome reaction is not induced under the assay conditions (Arranz and Cabada, 2000). We found that the cholesterol efflux is a prerequisite for fertilization since the incubation of oocytes with cholesterol-SO4− impairs fertilization.
In addition, the fertilizing capacity acquired during incubation in EW was found to be correlated with the tyrosine phosphorylation of a subset of proteins of Mr 50,000 to 200,000. The induced phosphorylation could be detected within 30 sec and found it to be maximal at 15 min, with no further increase thereafter (not shown). Inhibition of tyrosine phosphorylation could be accomplished with the tyrosine kinase inhibitor tyrphostin A25. The role of tyrosine kinases during fertilizing capacity acquisition was further supported by the inhibitory effects of tyrphostin A25 on fertilization of jelly intact oocytes.
In mouse sperm, increasing of cytoplasmatic cAMP levels follows direct activation of a sperm adenyl cyclase by HCO3− (Okamura et al., 1985;Visconti et al., 1990). Unlike the modulation of mouse sperm phosphorylation by cAMP (Visconti et al., 1995b), a cAMP analogue could not substitute for EW in promoting protein tyrosine phosphorylation. However, tyrosine phosphorylation of B. arenarum sperm proteins was induced by NaHCO3. The regulation of the transmembrane signal transduction pathway leading to the promotion of tyrosine phosphorylation by EW or HCO3−, could be modulated by cholesterol removal. The removal of cholesterol was necessary for activation of phosphorylation, although it could be circumvented with high NaHCO3 concentration (Fig. 7). This fact became evident when both tyrosine phosphorylation promoted by EW and fertilization of intact oocytes were impaired when cholesterol release from sperm was abrogated. How cholesterol removal regulates this pathway is still not known. Previous studies suggested that cholesterol modifies biophysical properties of biological membranes, including the ability of membrane proteins to undergo conformational changes that may control their functions (Cross and Overstreet, 1987;Rochwerger and Cuasnicu, 1992). Thus, high concentrations of cholesterol in the membrane might inhibit membrane protein function. This indirect effect of cholesterol on membrane protein function might stabilize those membrane and transmembrane events that are part of the intrinsic regulatory nature of fertilizing capacity acquisition. The dependence of the EW-induced increase in tyrosine phosphorylation on cholesterol membrane content could possibly be ascribed to an increase of the permeability of the sperm to certain ions, such as HCO3−, upon removal of cholesterol from the sperm plasma membrane. In fact, this could be similar to the regulation of some membrane-associated ion transporters by sterols (Shouffani and Kanner, 1990;Vemuri and Philipson, 1989). Cholesterol might concentrate in specialized plasma membrane microdomains known as lipid rafts. In this regard, the removal of cholesterol upon contact with components of the egg jelly or EW could mediate the disruption of lipid raft domains causing a shift in the overall membrane fluidity of the sperm plasma membrane, playing a role in the transmembrane movement of HCO3− (Sleight et al., 2005).
The effect of NaHCO3 on phosphorylation could be related to intracellular pH changes rather than direct adenyl cyclase activation, since a rise of intracellular cAMP concentration did not promote phosphorylation. In this regard, a Na+/HCO3− cotransporter was identified and localized in the flagellar plasma membrane of sea urchin (Gunaratne et al., 2006) and mouse sperm (Demarco et al., 2003). Alkalinization of intracellular pH was found to alter the phosphorylation pattern of epididymal bovine sperm proteins, but this effect could also occur via kinases other than PKA or potentially on protein phosphatases (Carr and Acott, 1989). A possible role of PKA in Bufo sperm capacitation can not be discarded yet.
In summary, we identified physiological modifications that take place in B. arenarum sperm before the acrosome reaction. These changes are guided by female factors secreted by the oviduct that are found in the jelly coat. We could correlate these modifications (rise in tyrosine phosphorylation and cholesterol efflux) with the acquisition of fertilizing capacity. Moreover, HCO3− was shown to be a key element of both tyrosine phosphorylation induction and acquisition of fertilizing capacity. Using this ion in combination with MβCD, we defined an EW-free medium for fertilization. We can conclude that capacitation-like changes of spermatozoa take place in an animal with external fertilization as B. arenarum, suggesting that sperm capacitation is not exclusive of mammalian species.
Acknowledgments
We thank M Leiva, L Racca and H Bottai for statistical analyses. This study was supported by NIH HD38082 and HD44044 (to PEV), and grants from ANPCyT (PICT0108545) and CONICET (PIP6428) (to MOC and SEA). DK is a fellow of CONICET; MOC is a member of the Research Career of CONICET.
Abbreviations footnote
- EW
egg water
- MβCD
methyl-β-cyclodextrin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- al Anzi B, Chandler DE. A sperm chemoattractant is released from Xenopus egg jelly during spawning. Dev Biol. 1998;198:366–375. [PubMed] [Google Scholar]
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- Arranz SE, Cabada MO. Diffusible highly glycosylated protein from Bufo arenarum egg-jelly coat: biological activity. Mol Reprod Dev. 2000;56:392–400. doi: 10.1002/1098-2795(200007)56:3<392::AID-MRD10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature. 1952;170:326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Bonaccorsi L, Forti G. Signal transduction pathways in human spermatozoa. J Reprod Immunol. 2002;53:121–131. doi: 10.1016/s0165-0378(01)00089-4. [DOI] [PubMed] [Google Scholar]
- Barbieri FD. Diffusible factors in anuran fertilization. Acta Physiol Lat Am. 1976;26:1–9. [PubMed] [Google Scholar]
- Barbieri FD, Cabada M. The role of the diffusible factor released by the egg jelly in fertilization of the toad egg. Experientia. 1969;25:1312–1313. doi: 10.1007/BF01897520. [DOI] [PubMed] [Google Scholar]
- Barbieri FD, del Pino EJ. Jelly coats and diffusible factor in Anuran fertilization. Arch Biol (Liege) 1975;86:311–321. [Google Scholar]
- Barbieri FD, Oterino JM. A study of the diffusible factor released by the jelly of the eggs of the toad, Bufo arenarum. Dev Growth Differ. 1972;14:107–117. doi: 10.1111/j.1440-169X.1972.00107.x. [DOI] [PubMed] [Google Scholar]
- Barbieri FD, Raisman JS. Non-gametic factors involved in the fertilization of Bufo arenarum oocytes. Embryologia (Nagoya) 1969;10:363–372. [PubMed] [Google Scholar]
- Barbieri FD, Villecco EI. A fertilizin like substance in the jelly coat of the toad Bufo arenarum. Arch Zool Ital. 1966;51:227. [Google Scholar]
- Barisone GA, Krapf D, Correa-Fiz F, Arranz SE, Cabada MO. Glycoproteins of the vitelline envelope of Amphibian oocyte: Biological and molecular characterization of ZPC component (gp41) in Bufo arenarum. Mol Reprod Dev. 2007;74:629–640. doi: 10.1002/mrd.20635. [DOI] [PubMed] [Google Scholar]
- Bernardin G, Gornati R, Rapelli S, Rossi F, Berra B. Lipids of Xenopus laevis Spermatozoa. Dev Growth Differ. 1992;34:329–335. doi: 10.1111/j.1440-169X.1992.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Cabada MO. Some female coordinating factors in amphibian fertilization. Dev Growth Differ. 1975;17:187–195. doi: 10.1111/j.1440-169X.1975.00187.x. [DOI] [PubMed] [Google Scholar]
- Carr DW, Acott TS. Intracellular pH regulates bovine sperm motility and protein phosphorylation. Biol Reprod. 1989;41:907–920. doi: 10.1095/biolreprod41.5.907. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–698. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- Cross NL, Overstreet JW. Glycoconjugates of the human sperm surface: distribution and alterations that accompany capacitation in vitro. Gamete Res. 1987;16:23–35. doi: 10.1002/mrd.1120160104. [DOI] [PubMed] [Google Scholar]
- Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. Involvement of a Na+/HCO-3 cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278:7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- Devi KU, Jha K, Shivaji S. Plasma membrane-associated protein tyrosine phosphatase activity in hamster spermatozoa. Mol Reprod Dev. 1999;53:42–50. doi: 10.1002/(SICI)1098-2795(199905)53:1<42::AID-MRD5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Diaz Fontdevila MF, Bloj B, Cabada MO. Effect of Egg Water from Bufo arenarum on the fertilizing capacity of homologous spermatozoa. J Exp Zool. 1991;257:408–414. [Google Scholar]
- Elinson RP. Fertilization of partially jellied and jellyless oocytes of the frog Rana pipiens. J Exp Zool. 1971;176:415–428. doi: 10.1002/jez.1401760405. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol Reprod. 1997;56:707–719. doi: 10.1095/biolreprod56.3.707. [DOI] [PubMed] [Google Scholar]
- Gamble W, Vaughan M, Kruth HS, Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- Gunaratne HJ, Nomura M, Moy GW, Vacquier VD. A sodium bicarbonate transporter from sea urchin spermatozoa. Gene. 2006;375:37–43. doi: 10.1016/j.gene.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Hosono J, Kanatani H, Katagiri C. Toad egg–jelly as a source of divalent cations essential for fertilization. Dev Biol. 1984;105:435–442. doi: 10.1016/0012-1606(84)90300-2. [DOI] [PubMed] [Google Scholar]
- Kalab P, Peknicova J, Geussova G, Moos J. Regulation of protein tyrosine phosphorylation in boar sperm through a cAMP-dependent pathway. Mol Reprod Dev. 1998;51:304–314. doi: 10.1002/(SICI)1098-2795(199811)51:3<304::AID-MRD10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kambara S. Role of jelly envelopes of toad eggs in fertilization. Annot Zool Jpn. 1953;26:78–84. [Google Scholar]
- Katagiri C. Chemical analysis of toad egg-jelly in relation to its ‘spermcapacitating’ activity. Dev Growth Differ. 1973;15:81–92. doi: 10.1111/j.1440-169X.1973.00081.x. [DOI] [PubMed] [Google Scholar]
- Katagiri C. The role of oviducal secretions in mediating gamete fusion in the toad, Bufo bufo japonicus. Adv Exp Med Biol. 1986;207:151–166. doi: 10.1007/978-1-4613-2255-9_10. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Krapf D, Vidal M, Arranz SE, Cabada MO. Characterization and biological properties of L-HGP, a glycoprotein from the amphibian oviduct with acrosome-stabilizing effects. Biol Cell. 2006;98:403–413. doi: 10.1042/BC20050051. [DOI] [PubMed] [Google Scholar]
- Kulanand J, Shivaji S. Capacitation-associated changes in protein tyrosine phosphorylation, hyperactivation and acrosome reaction in hamster spermatozoa. Andrologia. 2001;33:95–104. doi: 10.1046/j.1439-0272.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de LE, Gagnon C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–692. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- Miceli DC, Cabada MO. Amphibian fertilization. Trends in Comp Biochem Physiol. 1998;5:249–265. [Google Scholar]
- Nagarsenker PB. On Bartlett’s test for homogeneity of variances. Biometrika. 1984;71:405–407. [Google Scholar]
- Newport G. On the impregnation of the ovum in the amphibian. Philosophical Transactions of the Royal Society London (First Series Part 1) 1851;141:169–242. [Google Scholar]
- Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–9705. [PubMed] [Google Scholar]
- Olson JH, Chandler DE. Xenopus laevis egg jelly contains small proteins that are essential to fertilization. Dev Biol. 1999;210:401–410. doi: 10.1006/dbio.1999.9281. [DOI] [PubMed] [Google Scholar]
- Olson JH, Xiang X, Ziegert T, Kittelson A, Rawls A, Bieber AL, Chandler DE. Allurin, a 21-kDa sperm chemoattractant from Xenopus egg jelly, is related to mammalian sperm-binding proteins. Proc Natl Acad Sci U S A. 2001;98:11205–11210. doi: 10.1073/pnas.211316798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata S, Katagiri C. Involvement of carbohydrate moieties of the toad egg vitelline coat in binding with fertilizing sperm. Dev Growth Differ. 1996;38:663–672. doi: 10.1046/j.1440-169X.1996.t01-5-00010.x. [DOI] [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;5:1017–1026. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- Pitha J, Irie T, Sklar PB, Nye JS. Drug solubilizers to aid pharmacologists: amorphous cyclodextrin derivatives. Life Sci. 1988;43:493–502. doi: 10.1016/0024-3205(88)90150-6. [DOI] [PubMed] [Google Scholar]
- Raisman JS, Cunio RW, Cabada MO, del Pino EJ, Mariano MI. Acrosome breackdown in Leptodactilys chaquensis (Amfibia Anura) spermatozoa. Dev Growth Differ. 1980;22:289–297. doi: 10.1111/j.1440-169X.1980.00289.x. [DOI] [PubMed] [Google Scholar]
- Rochwerger L, Cuasnicu PS. Redistribution of a rat sperm epididymal glycoprotein after in vitro and in vivo capacitation. Mol Reprod Dev. 1992;31:34–41. doi: 10.1002/mrd.1080310107. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. Distributional Fitting, Assumption Testing. Biometrika. 1965;52:591–611. [Google Scholar]
- Shivers CA, James JM. Capacitation of frog sperm. Nature. 1970;227:183–184. doi: 10.1038/227183a0. [DOI] [PubMed] [Google Scholar]
- Shivers CA, James JM. Fertilization of antiserum-inhibited frog eggs with “capacitated” sperm. Biol Reprod. 1971;5:229–235. doi: 10.1093/biolreprod/5.3.229. [DOI] [PubMed] [Google Scholar]
- Shouffani A, Kanner BI. Cholesterol is required for the reconstruction of the sodium- and chloride-coupled, gamma-aminobutyric acid transporter from rat brain. J Biol Chem. 1990;265:6002–6008. [PubMed] [Google Scholar]
- Sleight SB, Miranda PV, Plaskett NW, Maier B, Lysiak J, Scrable H, Herr JC, Visconti PE. Isolation and proteomic analysis of mouse sperm detergent-resistant membrane fractions: evidence for dissociation of lipid rafts during capacitation. Biol Reprod. 2005;73:721–729. doi: 10.1095/biolreprod.105.041533. [DOI] [PubMed] [Google Scholar]
- Suzuki F, Yanagimachi R. Changes in the distribution of intramembranous particles and filipin-reactive membrane sterols during in vitro capacitation of golden hamster spermatozoa. Gamete Res. 1989;23:335–347. doi: 10.1002/mrd.1120230310. [DOI] [PubMed] [Google Scholar]
- Valz-Gianinet JN, del Pino EJ, Cabada MO. Glycoproteins from Bufo arenarum vitelline envelope with fertility-impairing effect on homologous spermatozoa. Dev Biol. 1991;146:416–422. doi: 10.1016/0012-1606(91)90243-v. [DOI] [PubMed] [Google Scholar]
- Vemuri R, Philipson KD. Influence of sterols and phospholipids on sarcolemmal and sarcoplasmic reticular cation transporters. J Biol Chem. 1989;264:8680–8685. [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995a;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. beta-cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999a;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59:1–6. doi: 10.1095/biolreprod59.1.1. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995b;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Muschietti JP, Flawia MM, Tezon JG. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim Biophys Acta. 1990;1054:231–236. doi: 10.1016/0167-4889(90)90246-a. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999b;214:429–443. doi: 10.1006/dbio.1999.9428. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Stewart-Savage J, Blasco A, Battaglia L, Miranda P, Kopf GS, Tezon JG. Roles of bicarbonate, cAMP, and protein tyrosine phosphorylation on capacitation and the spontaneous acrosome reaction of hamster sperm. Biol Reprod. 1999c;61:76–84. doi: 10.1095/biolreprod61.1.76. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;53:133–150. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- Ward CR, Storey BT. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev Biol. 1984;104:287–296. doi: 10.1016/0012-1606(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Xiang X, Burnett L, Rawls A, Bieber A, Chandler D. The sperm chemoattractant “alluring” is expressed and secreted from the Xenopus oviduct in a hormone-regulated manner. Dev Biol. 2004;275:343–355. doi: 10.1016/j.ydbio.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Xiang X, Kittelson A, Olson J, Bieber A, Chandler D. Allurin, a 21 kD sperm chemoattractant, is rapidly released from the outermost jelly layer of the Xenopus egg by diffusion and medium convection. Mol Reprod Dev. 2005;70:344–360. doi: 10.1002/mrd.20201. [DOI] [PubMed] [Google Scholar]
- Yancey PG, Rodrigueza WV, Kilsdonk EP, Stoudt GW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration Of kinetic pools and mechanism of efflux. J Biol Chem. 1996;271:16026–16034. doi: 10.1074/jbc.271.27.16026. [DOI] [PubMed] [Google Scholar]
- Yoshizaki N, Katagiri C. Acrosome reaction in sperm of Bufo bufo japonicus. Gamete Res. 1982;6:342–352. [Google Scholar]


