Abstract
Retinoids, a group of structural and functional analogs of vitamin A, are known to regulate a large number of essential biological processes and to suppress carcinogenesis. The effects of retinoids are mainly mediated by nuclear retinoid receptors, which include retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Each receptor has three subtypes (α, β, and γ) and each subtype has different isoforms. Retinoic acid receptor-β (RAR-β) has four isoforms that have different affinities to retinoids and different biological functions. Loss of expression of RAR-β2 during cancer development is associated with tumorigenesis and retinoid resistance; induction of its expression, on the other hand, can suppress carcinogenesis. Expression of another isoform, RAR-β4, is increased in various types of cancer. RAR-β4 transgenic mice develop hyperplasia and neoplasia in various tissues, and induction of RAR-β4 expression increases the growth of tumor cells that do not express RAR-β2. Future studies will focus on molecular pathways involving RAR-β2 and the role of RAR-β4 in cancer development.
Keywords: Retinoids, RAR-β2, RAR-β4, biomarker, methylation, tumorigenesis
1. Introduction
Retinoids, a group of structural and functional analogs of vitamin A, regulate several essential biological processes [1,2]. Pharmacologically, they have been recognized as important in modulating cell growth, differentiation, and apoptosis and in suppressing carcinogenesis in a broad range of tissue types in vitro, in animal models, and in clinical trials [see reviews in 3,4]. The ability of all-trans retinoic acid (RA) to induce the differentiation of acute promyelocytic leukemia (PML) cells into granulocytes is the basis for its therapeutic activity, and it was its effectiveness in this regard that led the U.S. Food and Drug Administration to approve RA as a therapeutic agent for PML. Promising results of retinoid studies in vitro and in vivo led to retinoid compounds being tested in a battery of clinical prevention and treatment trials being carried out all over the world. In clinical chemoprevention trials, retinoids showed activity in patients with oral leukoplakia [5, 6], cervical dysplasia [7], bronchial metaplasia [8], actinic keratosis [9], and second primary tumors in the aerodigestive tract [10,11]. However, findings from several other studies indicated that retinoids were not effective and could even be harmful [12–15]. Large randomized trials conducted in Europe and the United States showed that moderate doses of natural or synthetic vitamin A compounds were ineffective in reversing premalignancy or in suppressing recurrence of primary tumors [12,13]. Thus, the use of retinoids as chemopreventive agents in human cancers has gone from a zenith in the 1990s to something of a nadir nowadays. Current research efforts focus mainly on the molecular mechanisms underlying the actions of retinoids [16–21] and on use of retinoid X receptor-selective or retinoid receptor-independent retinoids in the prevention of human cancers [22–25].
The effects of retinoids are mediated mainly by two classes of nuclear retinoid receptors: retinoic acid receptors (RARs) and retinoid X receptors (RXRs), both of which are members of the steroid hormone receptor superfamily. Each receptor has three subtypes (α, β, and γ) and each subtype has different isoforms [26,27]. In humans, mRNA for RAR-α is expressed in most tissues; RAR-β expression is prevalent in neural tissues but hardly detectable in skin; and RAR-γ is expressed predominantly in the skin [26,27]. As for the RXRs, RXR-β is found in nearly all tissues; RXR-α is abundantly expressed in the liver, kidney, spleen, and skin; and RXR-γ expression seems to be restricted to mostly muscle and brain [26,27]. Changes in the expression of these receptors have been thought to cause neoplasia and malignant transformation in human cells [see reviews in ref. 3,4]. For example, chromosomal translocations that fuse the PML gene to the RAR-α gene form the chimeric genes PML-RARα and RARα-PML during the malignant transformation of PML [28], and recent evidence suggests that these fused genes are causally linked to the pathogenesis of the disease [29–31]. The RAR- β gene is rearranged in human hepatocellular carcinoma as a result of insertion of a hepatitis B virus sequence [32], although the importance of the rearrangement in causing hepatocellular carcinoma warrants further investigation. However, most alterations in nuclear retinoid receptors result from the reduction or loss of their expression in various premalignant and malignant tissues and cells. For example, loss of RAR-γ expression has been noted in skin cancer or premalignant lesions [33], but loss of RAR-β2 expression is common in a wide variety of cancers [3,4]. Because much of the work published to date has focused on role of RAR-β2 in various cancers, the focus of this review is on the molecular role of RAR-β in regulating cell growth and differentiation and in suppressing carcinogenesis.
2. Loss of RAR-β2 expression as a biomarker in solid tumors
Since the 1990s, loss of the expression of nuclear retinoid receptors, including RAR-β2, in various cancer cell lines has been detected by northern blotting or reverse transcriptase- polymerase chain reaction techniques [34–36]. Our group was the first to analyze the expression of transcripts of these receptors in formalin-fixed and paraffin-embedded tissues by using in situ hybridization [37]. Afterwards, many studies have demonstrated that among these receptors, loss of RAR-β2 is the most common and the loss is progressive in premalignant and malignant tissues and cells (Table 1), including those of the head and neck [38,39], breast [40,41], lung [42,43], esophagus [44,45], pancreas [46], cervix [47], and prostate [48,49]. The observations that RAR-β2 was upregulated in patients after treatment with 13-cis RA and that increased expression of RAR-β2 correlated with clinical response [39] suggest that RAR-β2 has an important role in suppressing carcinogenesis. After these findings were published, the methylation status of the RAR-β2 gene promoter was used as a biomarker for the early detection of malignancy or as an intermediate end-point marker to monitor the efficacy of chemoprevention agents in clinical trials [50–60].
Table 1.
Suppression of Nuclear Retinoid Receptors in Premalignant Lesions and Tumors
| Lesion | Lost receptor expression |
|---|---|
| Oral premalignant lesions | RAR-β2 |
| Bronchial squamous metaplasia | RAR-β2 |
| Head and neck cancer | RAR-β2 |
| Lung cancer | RAR-β2, RAR-γ, RXR-β |
| Pancreatic cancer | RAR-β2 |
| Esophageal cancer | RAR-β2 |
| Breast cancer | RAR-β2, RAR-α |
| Prostate cancer | RAR-β2, RXR-β |
Methylation of the RAR-β2 gene promoter, along with methylation of other gene promoters, has been evaluated as a biomarker of breast cancer risk [50,51]. In one study, methylation of the RAR-β2 gene promoter occurred in 32% of benign breast samples from patients with cancer but in only 9% of similar samples from patients without breast cancer [50]. In another study, random periareolar fine needle aspiration samples from the mammary glands showed methylation of the RAR-β2 gene promoter in 69% of primary breast cancers tested, and methylation was positively associated with increasing cytologic abnormality in those samples [51]. Hypermethylation of the RAR-β2 gene promoter is also an important factor in predicting early recurrence of lung cancer and may be useful as a prognostic marker in non-small cell lung cancer (NSCLC) as well, although the clinical implications of these findings need further investigation [52]. Methylation analysis of the RAR-β2 gene promoter can also aid in the diagnosis of primary lung cancer in bronchial aspirate samples [53] or in serum DNA [54].
In chemoprevention studies, RAR-β2 has often been used as an intermediate end-point biomarker in clinical trials of various retinoids [39,43,57,58,61]. However, evidence from preclinical studies suggests that RAR-β2 may also be useful for monitoring the efficacy of other agents, such as inhibitors of the epidermal growth factor receptor (EGFR) and β-cryptoxanthin [59,60].
3. Molecular mechanisms responsible for loss of RAR-β2 expression
The mechanisms underlying the loss of RAR-β2 gene expression are not fully understood [reviewed in 3,4]. Early studies showed that loss of RAR-β2 expression in some forms of cancer (e.g., NSCLC) resulted from chromosome 3p deletion [62]), but in others (e.g., head and neck cancer) neither homozygous deletions nor gene rearrangements have been found [34]. Since those findings were published, other studies have shown that transcriptional deregulation can silence RAR-β2 expression through decreased levels of co-activators, the presence of co-repressors, or epigenetic mechanisms such as histone deacetylation [63–68]. Expression of RAR-β2 also depends on the cellular level of retinoids because this receptor is itself an RA–inducible gene. Indeed, RAR-β2 expression is selectively reduced in several organs during vitamin A–deficient states and is enhanced by RA, as demonstrated in studies of rats [69,70]. In humans, some premalignant tissues are deficient in vitamin A because of reduced uptake of vitamin A from serum or abnormally elevated catabolism of intracellular retinoids [71,72]. We found that the binding of an anti-RA antibody to tissue sections of premalignant lesions was much lower than the binding to normal oral mucosa, suggesting that the lesions had lower levels of retinoids [72]. Lack of RAR-α expression may also contribute to lost RAR-β2 expression. RAR-α regulates RAR-β2 transcription by mediating dynamic changes of RAR-β2 chromatin in the presence and absence of RA. Interfering with RA signaling by silencing RAR-α exacerbates the repression of chromatin for RAR-β2 and leads to RAR-β2 transcriptional silencing. RAR-β2 silencing is also associated with resistance to the growth-inhibitory effect of RA, indicating that RAR-β2 silencing and RA resistance result from impaired integration of RA signaling at RAR-β2 chromatin [73].
Nevertheless, many recent studies have shown that the diminished expression of RAR- β2 in the development of different human cancers results from epigenetic silencing by methylation of cytosine-phospho-guanosine (CpG) islands in the promoter region of the gene [50–56]. Moreover, treatment with 5-aza-2-deoxycytidine, a DNA demethylation agent, was able to induce RAR-β expression in various cancer cell lines [74–77], further supporting the notion that RAR-β2 is a tumor suppressor gene. Cigarette smoke causes morphologic changes and the loss of RAR-β2 expression in lung tissues of animals [78], and cigarette smoke and the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone have been shown to induce the methylation of the RAR-β2 gene promoter in murine lung cancer [79]. Our own studies similarly showed that benzo[a]pyrene diol epoxide (BPDE), a carcinogen present in tobacco smoke and environmental pollution, and bile acid, a tumor promoter in the gastrointestinal tract, suppressed RAR-β2 expression in premalignant and malignant esophageal cells and that BPDE induced the methylation of the RAR-β2 gene promoter [19,80]. Notably, epigallocatechin gallate, a chemopreventive agent extracted from the tea plant, has been shown to bind to DNA methyltransferases and reactivate RAR-β2 expression in esophageal cancer cells [81]. Thus, as discussed in section 2, methylation of the RAR-β2 gene promoter is being evaluated as a biomarker for the early detection or prediction of prognosis for various forms of cancer in humans.
In patients with NSCLC, hypermethylation of the RAR-β gene promoter has different effects on the development of second primary lung cancers (SPLCs) depending on smoking status [82]. In one study, Kim et al [82] showed that current smokers developed more SPLCs when the RAR-β gene promoter was unmethylated than when it was hypermethylated. In contrast, the development of SPLCs in former smokers was higher in patients with hypermethylated than unmethylated RAR-β. This group suggested that in current smokers, the continuous high oxygen tension and free radicals induce apoptosis, which could inhibit the development of SPLC. However, another explanation is that overexpression of RAR-β4 may be important in the development of SPLCs in active smokers. Three observations prompted this idea: First, expression of both RAR-β2 and RAR-β4 is controlled by the same RAR-β promoter (P2; details are given below); second, methylation of the RAR-β gene promoter shuts down the expression of both isoforms; and third, RAR-β2 expression is lost but RAR-β4 expression is increased in various forms of cancer, including lung [83]. Collectively, these observations support the idea that hypermethylation of the RAR-β gene promoter may have a protective effect in tumors in which RAR-β4 is overexpressed. Previous studies have also revealed that exposure to tobacco smoke or BPDE suppresses RAR-β2 expression through methylation of the RAR-β gene (19,79,80). This may be true for active smokers, but unmethylated RAR-β could trigger RAR-β4 expression in various cancers [84–86], although the hypothesis that tobacco smoke can increase RAR-β4 expression requires further testing. In contrast, hypermethylation of the RAR-β gene reduces RAR-β2 expression, thereby losing any RAR-β2-protective effects in the suppression of tumorigenesis; therefore nonsmokers or former smokers with hypermethylated RAR-β tend to develop more SPLCs.
In contrast to previous evidence of the importance of RAR-β2 in suppressing cancer development, our own studies showed that expression of RAR-β in patients with early-stage NSCLC was unexpectedly associated with very poor prognosis [87]. Overexpression of RAR-β correlated with the increased expression of cyclooxygenase-2 (COX-2), an enzyme known to be present at elevated levels in progressive carcinogenesis and a marker of poor prognosis in various malignancies [88], and with increased levels of telomerase [89]. These findings were unexpected, as they contradict the concept of RAR-β2 functioning as a tumor suppressor gene. In seeking possible mechanisms for this effect, we recently found that reduced expression of RAR-β2 correlated with increased RAR-β4 expression in esophageal cancer tissues [84]. This finding may help to clarify this apparent discrepancy between studies, because the in situ hybridization technique we used in the NSCLC studies cannot distinguish among RAR-β isoforms (β1, β2, and β4). Given the finding that the ratio of RAR-β4 to RAR-β2 expression in human lung and breast cancer cell lines is higher than that in normal cells [83–86] and that reduced RAR-β2 expression correlated with increased RAR-β4 expression [84], we speculate that the in situ hybridization–detected RAR-β mRNA in NSCLC tissues is the RAR-β4 isoform, which has oncogenic effects on cells. This speculation, however, requires further investigation.
4. RAR-β isoforms
Differences in the actions of P1 and P2, the two known RAR-β promoters (P1 initiates transcription of isoforms 1 and 3, and P2 initiates transcription of isoforms 2 and 4), and alternative splicing [26,27,90,91] give rise to four major isoforms of RAR-β in mice (β1, β2, β3, and β4) and three in humans (β1, β2, and β4). Additional isoforms (e.g., RAR-β5 and RAR-β1') have been identified in human cancer cells [92,93]. Briefly, RAR-β1 is a fetal isoform that may be a master developmental gene in humans; it is also expressed in small-cell lung cancer [94]. In one study involving transgenic mice, RAR-β1 was found to have unique tumor suppressor activity that could not be entirely compensated by the overexpression of RAR-β2 and the suppression of RAR-β4 [95]. RAR-β2 is the most abundant and the major RA-inducible isoform, and thus the term RAR-β in the literature usually refers to the RAR-β2 isoform. RAR-β4 is generated by alternative splicing from the same primary transcripts as those generating RAR-β2 and is initiated by the CUG codon [90]. The cDNA sequence of RAR-β4 in regions B to F is identical to that of the same regions of RAR-β2. The A region of RAR-β4—only 4 amino acids long—is much shorter than that of RAR-β2 [90] (Fig. 1). Moreover, this non-AUG codon seems to be relatively inefficient, resulting in alternative use of an internal methionine codon at +448 bp that yields an RAR-β isoform in which all amino acid sequences at the N-terminal of the second finger of the DNA binding domain are truncated. Because the full-length and truncated RAR-β4 proteins retain the ability to heterodimerize with RXR-α and to interact with transcription cofactors but lack the DNA-binding capacity to regulate gene expression [85,90,96], RAR-β4 and the truncated RAR-β4 may act structurally as a dominant-negative form of RAR-β2 [96]. In addition, RAR-β4 has an elevated Kd value for RA binding and cannot inhibit AP-1 activity, as RAR-β2 can [97]. Little is known as yet about the most recently discovered isoforms, RAR-β5 and RAR-β1'. RAR-β5 is expressed in human breast cancer cells, and its detection correlates with the RA resistance of estrogen-negative breast cancer cells [92]. RAR-β1' has antitumor activity in lung cancer [93]. In summary, the various RAR-β isoforms in humans have different affinities to RA (at least with regard to RAR-β2 and RAR-β4) and different biological functions (e.g., the RAR-β2 protein is a tumor suppressor whereas RAR-β4 has oncogenic properties).
Fig. 1.

Comparison of RAR-β2 and RAR-β4 cDNA (modified from ref. 90). A. Organization of RAR cDNA is shown at the top and RAR-β isoforms at the bottom. The location of sequences that encode regions B to F and the 3’-untranslated region (UTR) common to all RAR-β isoforms is indicated, as well as those of the 5’-UTR and A region, which is isoform-specific. Numbers correspond to nucleotide positions in the human RAR-β2 cDNA sequence. The “A” regions of RAR-β2 and RAR-β4 are represented as a solid box. The region of RAR-β2 that is spliced to give RAR-β4 (between 266–619 bp) is indicated with a dashed line. B. Sequence and schematic representation of donor splice sites for RAR-β4 and RAR-β2 (positions 265 and 619, respectively).
The etiology of RAR-β4 upregulation in cancer cells remains unknown. We speculate that altered expression of microRNAs (miRNA) in cancer tissues may be responsible. miRNA is a class of naturally occurring small noncoding RNA, 18 to 22 nucleotides long, that posttranslationally silences gene expression through binding to complementary target mRNAs, thereby degrading these mRNAs or inhibiting their being translated into protein [see reviews in 98]. This discovery has greatly broadened our understanding of gene regulation mechanisms. Although comprehensive knowledge of the specific mRNA targets of miRNAs is lacking [98,99], bioinformatics analyses may predict their mRNA targets; for example, miR-16, miR-128, and miR-30e can target the RAR-β gene [98]. Further investigation of this exciting discovery is warranted.
5. Role of RAR-β2 and RAR-β4 in human carcinogenesis
To date, several convincing lines of evidence have shown correlations between loss of RAR-β2 expression and increased carcinogenesis in humans, but only a few studies have linked RAR-β4 expression with cancer development. With respect to RAR-β2, lung carcinoma cells expressing transfected RAR-β2 are less tumorigenic in nude mice than the vector-control-transfected cells [100], and transgenic mice expressing antisense RAR-β2 develop lung cancer [101]. Transfection with an RAR-β2 expression vector can suppress the growth of various cancer cell lines and restore the sensitivity of those cells to RA treatment [see reviews in 3,4]. Knocking out RAR-β2 by homologous recombination in F9 mouse teratocarcinoma cells resulted in the loss of RA-associated growth arrest and changes in cell morphology and differentiation [102]. Our own findings have shown that induction of RAR-β2 expression in esophageal cancer cells suppressed tumor cell growth and colony formation and induced apoptosis [18]. Others have shown that RAR-β2 also suppressed breast cancer cell metastasis in a mouse xenograft model [103]. In another study, loss of RAR-β2 expression in patients with neuroblastoma correlated with poor prognosis [104]. Also, esophageal, lung, and breast cancer cell lines that do not express RAR-β2 are resistant to retinoid treatment [35,44,73]. Finally, in one study of oral dysplastic tissues, half of the cells cultured from those tissues had become immortalized, as indicated by loss of RAR-β2 and p16 expression, mutations in p53, and induction of hTERT mRNA [105]. Treating these cells with 5-aza-2-deoxycytidine reversed one of the immortal phenotypes and led to re-expression of both RAR-β2 and p16 [106]. A subsequent study by the same group showed that only the loss of RAR-β and p16 was responsible for the immortalization of oral dysplastic cells [107].
Expression of RAR-β4, on the other hand, was found to be increased in esophageal cancer tissues and the increase was associated with reduced expression of RAR-β2 [84]. Pharmacologic concentrations of RA have been shown to increase RAR-β4 expression in esophageal cancer cell lines (our unpublished data) and in breast cancer cells (86). Several other studies have shown that hyperplasia and neoplasia developed spontaneously in various tissues in RAR-β4 transgenic mice [83] and that induction of RAR-β4 expression enhanced the growth of cancer cells that do not express RAR-β2 (our unpublished data). As noted earlier in this review, RAR-β4 has an elevated Kd value for RA binding and cannot inhibit AP-1 activity, as does RAR-β2 [97]. Collectively, these findings suggest that RAR-β4 either may be an oncogene or may have oncogenic effects. Further investigation of the effects of RAR-β4 on tumor cell growth, tumorigenesis, and gene expression will clarify whether RAR-β4 is a dominant-negative form of RAR-β2 or an oncogene. Such knowledge can be put to use in the development of better strategies to control cancer development.
6. Molecular pathways involved in the mediation of tumor suppression by RAR-β2
Although the precise molecular mechanisms responsible for RAR-β2-mediated antitumor activity are not fully understood, we previously found that RAR-β2 suppressed the expression of EGFR, activating protein-1 (AP-1), and COX-2 and the phosphorylation of extracellular signal- regulated protein kinases 1 and 2 (Erk1/2) [19]. We also found that COX-2 expression was downregulated in RAR-β2 -transfected esophageal cancer cells and that the restoration of sensitivity to RA was mediated by suppression of COX-2 expression [18]. Further, we unexpectedly found that stable transfection with RAR-β2 cDNA did not restore the sensitivity of COX-2–negative esophageal cancer cells to RA or inhibit tumor formation in nude mouse xenograft models. These findings indicate that the antitumor effect of RAR-β2 may require the suppression of COX-2 expression (our unpublished data). To further substantiate this notion, we studied the expression of RAR-β2 and COX-2 mRNA in tissue specimens and found RAR-β2 expression to be associated with low levels of COX-2 expression in esophageal cancer tissues. We also found that after a 3-month treatment with 13-cis RA, the induction of RAR-β2 expression in human oral leukoplakia tissues correlated with a reduction in COX-2 expression and a clinical response (our unpublished data). Collectively, our findings suggest that the antitumor activity of RAR-β2 occurs through the suppression of COX-2 expression, although further study is needed to determine whether manipulation of COX-2 expression in these cells could antagonize this antitumor activity. We also determined that downregulation of COX-2 expression by RAR-β2 took place through the inhibition of EGFR, with subsequent decreases in ERK1/2 phosphorylation and AP-1 expression [19]. Most importantly, we showed that induction of COX-2 expression by the carcinogen BPDE depended on both the expression and the inhibition of RAR-β in esophageal cells [19] (Fig. 2). A previous study showed that stable transfection with RAR-β2 exhibited strong inhibition of AP-1 activity in tumor cells, even in the absence of RA. Moreover, expression of the endogenous AP-1–responsive gene collagenase I was strongly repressed in cancer cells stably transfected with RAR-β2 (108). Another line of evidence comes from a study showing that a high-fat diet reduced the expression of PPAR-γ and RAR-β2 mRNA, increased COX-2 and β-catenin levels, and increased the number of aberrant crypt foci in rat colon tissue. However, vitamin A was able to prevent these high-fat-diet–induced alterations of PPAR-γ and RAR-β2 and the increases in COX-2 and β-catenin [109]—further evidence that that RAR-β2 can suppress COX-2 expression.
Fig. 2.
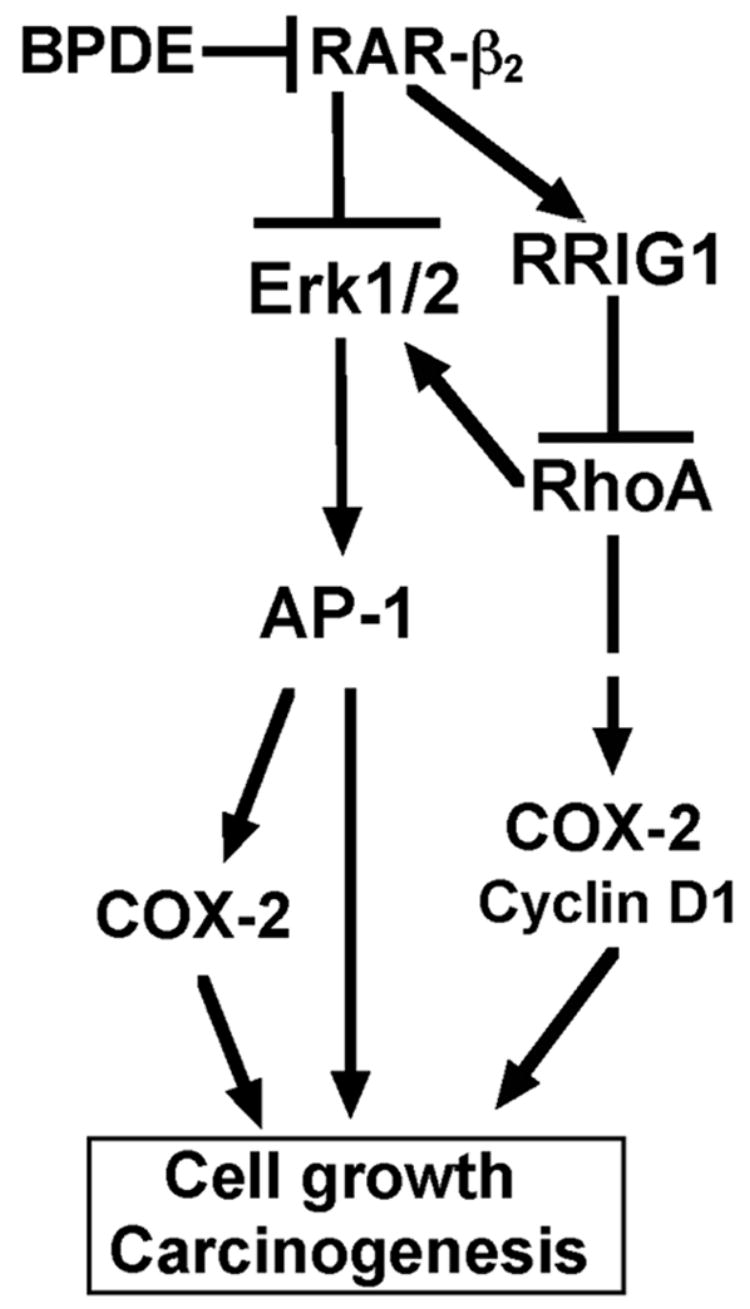
A molecular pathway triggered by RAR-β2 for the suppression of human carcinogenesis. Benzo(a)pyrene diol epoxide (BPDE) exposure is a risk factor in the development of esophageal cancer. BPDE is thought to act by suppressing RAR-β2 expression and, in turn, upregulating EGFR expression and Erk1/2 phosphorylation, resulting in the induction of AP-1 and COX-2 expression. Further, BPDE can educe the expression of retinoid receptor-induced gene-1 (RRIG1), perhaps through reduction of RAR-β2. RRIG1 mediates RAR-β2 effects on the regulation of cancer cell growth and gene expression. RRIG1 protein binds to and inhibits RhoA activity and consequently suppresses Erk1/2 phosphorylation and expression of COX-2 and cyclin D1, resulting in reductions in tumor cell colony formation, invasion, and proliferation.
A cDNA microarray analysis comparison of a parental and an RAR-β2–transfected lung cancer cell line revealed 27 genes expressed at different levels between the two cell lines [16]. Several of the affected genes code for proteins whose functions augment apoptosis or host immune response. Other investigators identified and characterized genes that are differentially expressed in wild-type cells and RAR-β2−/ − F9 teratocarcinoma cells by using subtractive hybridization and DNA array analysis [17]. The identified genes, which encode transcription factors, cell surface signal-transduction molecules, and metabolic enzymes, included c-myc, FOG1, GATA6, glutamate dehydrogenase, Foxq1, Hic5, Meis1a, Dab2, midkine, and the PDGF-α receptor [17]. Again, induction of RAR-β2 expression suppressed the potential for metastasis of breast cancer cells. Another cDNA microarray study showed that RAR-β2 induced the expression of tumor-cell antigens (CTAG1 and CTAG2), the innate immune response (e.g., RIG-I/DDX58), and a tumor suppressor gene (TYRP1) but reduced the expression of several genes involved in cell adhesion (LSAMP, PCDH11Y, and CD64), nutrient availability (FABP6, SLC38A2, PCSK4, SRPB1), and transcription/AP-1 activity (HOXB7 and JUN) [20].
We identified and cloned a retinoid receptor-induced gene, RRIG1, which is expressed differentially by RAR-β2-positive and -negative esophageal cancer cells, by using restriction fragment differential display–polymerase chain reaction techniques [21]. RRIG1 is expressed in a broad range of normal tissues but is lost in various types of cancer [21,110]. RRIG1 mediates the effect of RAR-β2 in the regulation of cancer cell growth and gene expression [21]. The RRIG1 protein is expressed in the cell membrane and binds to and inhibits small GTPase RhoA activity. Whereas induction of RRIG1 expression inhibits RhoA activation and f-actin formation and consequently reduces colony formation, invasion, and proliferation of esophageal cancer cells, antisense RRIG1 increases RhoA activity and f-actin formation and thus induces the colony formation, invasion, and proliferation of these cells. These findings indicate the existence of a novel molecular pathway involving RAR-β2 regulation of RRIG1 expression and RRIG1–RhoA interactions [21]. Further studies of RRIG1 and the pathways involved may translate into better control of esophageal cancer.
In summary, a large body of evidence has established that RAR-β2 participates in the regulation of cell growth and the suppression of carcinogenesis, although the molecular mechanisms of action deserve further study. However, the role of RAR-β4 in cancer development and its molecular signaling transduction pathways has remained virtually unexplored.
7. Future directions
The associations between loss of RAR-β2 expression and tumorigenesis, and induction of RAR-β2 expression and the suppression of cancer development, have been well established [3,4]. However, expression of the closely related isoform RAR-β4 is increased in various types of cancer [83–86], RAR-β4 transgenic mice developed hyperplasia and neoplasia in various tissues [83], and induction of RAR-β4 expression increased growth of tumor cells that do not express RAR-β2 (our unpublished data). Therefore, future studies are needed of the molecular pathways triggered by RAR-β2 to suppress cancer development, as are other studies of the role of RAR-β4 in cancer development. Issues remaining to be clarified include whether RAR-β4-mediated oncogenic effects on normal and cancerous cells are attributable to its modulation of RAR-β2-mediated signal pathways, or whether RAR-β4 acts on separate signaling pathways leading to malignant transformation and cancer development. Answers to these questions will provide crucial clues for resolving the occasionally contradictory and controversial findings from studies of retinoids for cancer prevention and treatment.
Acknowledgments
This work was supported by the National Cancer Institute Grant R29 CA74835, R21 CA10226, and R01 CA117895.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochem Biophys Acta. 1980;605:33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- 2.Sporn MB, Roberts AB, Goodman DS. The Retinoids: Biology, Chemistry, and Medicine. 2. Raven Press; New York: 1994. [Google Scholar]
- 3.Xu X-C, Lotan R. Aberrant expression and function of retinoid receptors in cancer. In: Nau H, Blaner WS, editors. Handbook of experimental pharmacology. Springer; Berlin and New York: 1999. pp. 323–343. [Google Scholar]
- 4.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 5.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 6.Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- 7.Meyskens FL, Jr, Surwit E, Moon TE, Childers JM, Davis JR, Dorr RT, et al. Enhancement of regression of cervical intraepithelial neoplasia II (moderate dysplasia) with topically applied all-trans-retinoic acid: a randomized trial. J Natl Cancer Inst. 1994;86:539–543. doi: 10.1093/jnci/86.7.539. [DOI] [PubMed] [Google Scholar]
- 8.Misset JL, Mathe G, Santelli G, Gouveia J, Homasson JP, Sudre MC, et al. Regression of bronchial epidermoid metaplasia in heavy smokers with etretinate treatment. Cancer Detect Prev. 1986;9:167–170. [PubMed] [Google Scholar]
- 9.Moon TE, Levine N, Cartmel B, Bangert JL, Rodney S, Dong Q, et al. Effect of retinol in preventing squamous cell skin cancer in moderate-risk subjects: a randomized, double-blind, controlled trial. Southwest Skin Cancer Prevention Study Group. Cancer Epidemiol Biomarkers Prev. 1997;6:949–956. [PubMed] [Google Scholar]
- 10.Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 11.Pastorino U, Infante M, Maioli M, Chiesa G, Buyse M, Firket P, et al. Adjuvant treatment of stage I lung cancer with high-dose vitamin A. J Clin Oncol. 1993;11:1216–1222. doi: 10.1200/JCO.1993.11.7.1216. [DOI] [PubMed] [Google Scholar]
- 12.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 13.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 14.Lippman SM, Lee JJ, Karp DD, Vokes EE, Benner SE, Goodman GE, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–618. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 15.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, Benner SE, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 16.Toulouse A, Loubeau M, Morin J, Pappas JJ, Wu J, Bradley WE. RARbeta involvement in enhancement of lung tumor cell immunogenicity revealed by array analysis. FASEB J. 2000;14:1224–1232. doi: 10.1096/fasebj.14.9.1224. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang Y, Faria TN, Chambon P, Gudas LJ. Identification and characterization of retinoic acid receptor beta(2) target genes in F9 teratocarcinoma cells. Mol Cancer Res. 2003;1:619–630. [PubMed] [Google Scholar]
- 18.Li M, Song S, Lippman SM, Zhang XK, Liu X, Lotan R, Xu XC. Induction of retinoic acid receptor-beta suppresses cyclooxygenase-2 expression in esophageal cancer cells. Oncogene. 2002;21:411–418. doi: 10.1038/sj.onc.1205106. [DOI] [PubMed] [Google Scholar]
- 19.Song S, Lippman SM, Zou Y, Ye X, Xu X-C. Induction of cyclooxygenase-2 by benzo[a]pyrene diol epoxide through inhibition of retinoic acid receptor-β2 expression. Oncogene. 2005;24:8268–8276. doi: 10.1038/sj.onc.1208992. [DOI] [PubMed] [Google Scholar]
- 20.Wallden B, Emond M, Swift ME, Disis ML, Swisshelm K. Antimetastatic gene expression profiles mediated by retinoic acid receptor beta 2 in MDA-MB-435 breast cancer cells. BMC Cancer. 2005;5:140–153. doi: 10.1186/1471-2407-5-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang ZD, Lippman SM, Wu TT, Lotan R, Xu XC. RRIG1 mediates effects of retinoic acid receptor-β_ on tumor cell growth and gene expression through binding to and inhibiting RhoA. Cancer Res. 2006;66:7111–7118. doi: 10.1158/0008-5472.CAN-06-0812. [DOI] [PubMed] [Google Scholar]
- 22.Grubbs CJ, Lubet RA, Atigadda VR, Christov K, Deshpande AM, Tirmal V, et al. Efficacy of new retinoids in the prevention of mammary cancers and correlations with short-term biomarkers. Carcinogenesis. 2006;27:1232–1239. doi: 10.1093/carcin/bgi308. [DOI] [PubMed] [Google Scholar]
- 23.Lippman SM, Lee JJ, Martin JW, El-Naggar AK, Xu X, Shin DM, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res. 2006;12:3109–3114. doi: 10.1158/1078-0432.CCR-05-2636. [DOI] [PubMed] [Google Scholar]
- 24.Wu K, Dupre E, Kim H, Tin UC, Bissonnette RP, Lamph WW, et al. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res Treat. 2006;96:147–157. doi: 10.1007/s10549-005-9071-1. [DOI] [PubMed] [Google Scholar]
- 25.Xu H, Cheepala S, McCauley E, Coombes K, Xiao L, Fischer SM, S M, et al. Chemoprevention of skin carcinogenesis by phenylretinamides: retinoid receptor-independent tumor suppression. Clin Cancer Res. 2006;12:969–979. doi: 10.1158/1078-0432.CCR-05-1648. [DOI] [PubMed] [Google Scholar]
- 26.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 27.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de The H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean AA. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Zhou J, Peres L, Riaucoux F, Honore N, Kogan S, et al. sumoylation site in PML/RARA is essential for leukemic transformation. Cancer Cell. 2005;7:143–153. doi: 10.1016/j.ccr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Peres L, Honore N, Nasr R, Zhu J, de The H. Dimerization-induced corepressor binding and relaxed DNA-binding specificity are critical for PML/RARA-induced immortalization. Proc Natl Acad Sci U S A. 2006;103:9238–9243. doi: 10.1073/pnas.0603324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rego EM, Ruggero D, Tribioli C, Cattoretti G, Kogan S, Redner RL, Pandolfi PP. Leukemia with distinct phenotypes in transgenic mice expressing PML/RAR alpha, PLZF/RAR alpha or NPM/RAR alpha. Oncogene. 2006;25:1974–1979. doi: 10.1038/sj.onc.1209216. [DOI] [PubMed] [Google Scholar]
- 32.Garcia M, de The H, Tiollais P, Samarut J, Dejean A. A hepatitis B virus pre-S-retinoic acid receptor beta chimera transforms erythrocytic progenitor cells in vitro. Proc Natl Acad Sci U S A. 1993;90:89–93. doi: 10.1073/pnas.90.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finzi E, Blake MJ, Celano P, Skouge J, Diwan R. Cellular localization of retinoic acid receptor-gamma expression in normal and neoplastic skin. Am J Pathol. 1992;140:1463–1471. [PMC free article] [PubMed] [Google Scholar]
- 34.Hu L, Crowe DL, Rheinwald JG, Chambon P, Gudas LJ. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51:3972–3981. [PubMed] [Google Scholar]
- 35.Houle B, Leduc F, Bradley WE. Implication of RAR-β in epidermoid (squamous) lung cancer. Genes Chromosomes Cancer. 1991;3:358–366. doi: 10.1002/gcc.2870030506. [DOI] [PubMed] [Google Scholar]
- 36.Swisshelm K, Ryan K, Lee X, Tsou HC, Peacocke M, Sager R. Down-regulation of retinoic acid receptor β in mammary carcinoma cell lines and its up-regulation in senescing normal mammary epithelial cells. Cell Growth Differ. 1994;5:133–141. [PubMed] [Google Scholar]
- 37.Xu XC, Clifford JL, Hong WK, Lotan R. Detection of nuclear retinoic acid receptor mRNA in histological tissue sections using nonradioactive in situ hybridization histochemistry. Diagn Mol Pathol. 1994;3:122–131. doi: 10.1097/00019606-199406000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoic acid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54:3580–3587. [PubMed] [Google Scholar]
- 39.Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, et al. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 40.Widschwendter M, Berger J, Daxenbichler G, Muller-Holzner E, Widschwendter A, Mayr A, et al. Loss of retinoic acid receptor beta expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Res. 1997;57:4158–4161. [PubMed] [Google Scholar]
- 41.Xu XC, Sneige N, Liu X, Nandagiri R, Lee JJ, Lukmanji F, et al. Progressive decrease in nuclear retinoic acid receptor beta messenger RNA level during breast carcinogenesis. Cancer Res. 1997;57:4992–4996. [PubMed] [Google Scholar]
- 42.Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, et al. Suppression of nuclear retinoic acid receptor β in non-small cell lung cancer in vivo: Implications in lung cancer development. J Natl Cancer Inst. 1997;89:624–629. doi: 10.1093/jnci/89.9.624. [DOI] [PubMed] [Google Scholar]
- 43.Xu XC, Lee JS, Lee JJ, Morice RC, Liu X, Lippman SM, et al. Nuclear retinoid acid receptor beta in bronchial epithelium of smokers before and during chemoprevention. J Natl Cancer Inst. 1999;91:1317–1321. doi: 10.1093/jnci/91.15.1317. [DOI] [PubMed] [Google Scholar]
- 44.Xu XC, Liu X, Tahara E, Lippman SM, Lotan R. Expression and up-regulation of retinoic acid receptor-beta is associated with retinoid sensitivity and colony formation in esophageal cancer cell lines. Cancer Res. 1999;59:2477–2483. [PubMed] [Google Scholar]
- 45.Qiu H, Zhang W, El-Naggar AK, Lippman SM, Lin P, Lotan R, et al. Loss of retinoic acid receptor-beta expression is an early event during esophageal carcinogenesis. Am J Pathol. 1999;155:1519–1523. doi: 10.1016/s0002-9440(10)65467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu XC, Stier U, Rosewicz S, El-Naggar AK, Lotan R. Selective suppression of nuclear retinoic acid receptor beta gene expression in human pancreatic cancers. Int J Oncol. 1996;8:445–451. doi: 10.3892/ijo.8.3.445. [DOI] [PubMed] [Google Scholar]
- 47.Xu XC, Mitchell MF, Silva E, Jetten A, Lotan R. Decreased expression of retinoic acid receptors, transforming growth factor beta, involucrin, and cornifin in cervical intraepithelial neoplasia. Clin Cancer Res. 1999;5:1503–1508. [PubMed] [Google Scholar]
- 48.Lotan Y, Xu XC, Shalev M, Lotan R, Williams R, Wheeler TM, et al. Differential expression of nuclear retinoid receptors in normal and malignant prostates. J Clin Oncol. 2000;18:116–121. doi: 10.1200/JCO.2000.18.1.116. [DOI] [PubMed] [Google Scholar]
- 49.Bastian PJ, Ellinger J, Heukamp LC, Kahl P, Muller SC, von Rucker A. Prognostic value of CpG island hypermethylation at PTGS2, RAR-beta, EDNRB, and other gene loci in patients undergoing radical prostatectomy. Eur Urol. 2006 doi: 10.1016/j.eururo.2006.08.008. in press. [DOI] [PubMed] [Google Scholar]
- 50.Lewis CM, Cler LR, Bu DW, Zochbauer-Muller S, Milchgrub S, Naftalis EZ, et al. Promoter hypermethylation in benign breast epithelium in relation to predicted breast cancer risk. Clin Cancer Res. 2005;11:166–172. [PubMed] [Google Scholar]
- 51.Bean GR, Scott V, Yee L, Ratliff-Daniel B, Troch MM, Seo P, et al. Retinoic acid receptor-beta2 promoter methylation in random periareolar fine needle aspiration. Cancer Epidemiol Biomarkers Prev. 2005;14:790–798. doi: 10.1158/1055-9965.EPI-04-0580. [DOI] [PubMed] [Google Scholar]
- 52.Kim YT, Lee SH, Sung SW, Kim JH. Can aberrant promoter hypermethylation of CpG islands predict the clinical outcome of non-small cell lung cancer after curative resection? Ann Thorac Surg. 2005;79:1180–1188. doi: 10.1016/j.athoracsur.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 53.Grote HJ, Schmiemann V, Geddert H, Rohr UP, Kappes R, Gabbert H, et al. Aberrant promoter methylation of p16(INK4a), RARB2 and SEMA3B in bronchial aspirates from patients with suspected lung cancer. Int J Cancer. 2005;116:720–725. doi: 10.1002/ijc.21090. [DOI] [PubMed] [Google Scholar]
- 54.Fujiwara K, Fujimoto N, Tabata M, Nishii K, Matsuo K, Hotta K, et al. Identification of epigenetic aberrant promoter methylation in serum DNA is useful for early detection of lung cancer. Clin Cancer Res. 2005;11:1219–1225. [PubMed] [Google Scholar]
- 55.Wang Y, Fang MZ, Liao J, Yang GY, Nie Y, Song Y, et al. Hypermethylation-associated inactivation of retinoic acid receptor beta in human esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9:5257–5263. [PubMed] [Google Scholar]
- 56.Jeronimo C, Henrique R, Hoque MO, Ribeiro FR, Oliveira J, Fonseca D, et al. Quantitative RARbeta2 hypermethylation: a promising prostate cancer marker. Clin Cancer Res. 2004;10:4010–4014. doi: 10.1158/1078-0432.CCR-03-0643. [DOI] [PubMed] [Google Scholar]
- 57.Ayoub J, Jean-Francois R, Cormier Y, Meyer D, Ying Y, Major P. Placebo-controlled trial of 13-cis-retinoic acid activity on retinoic acid receptor-beta expression in a population at high risk: implications for chemoprevention of lung cancer. J Clin Oncol. 1999;17:3546–3552. doi: 10.1200/JCO.1999.17.11.3546. [DOI] [PubMed] [Google Scholar]
- 58.Kurie JM, Lotan R, Lee JJ, Lee JS, Morice RC, Liu DD, et al. Treatment of former smokers with 9-cis-retinoic acid reverses loss of retinoic acid receptor-beta expression in the bronchial epithelium: results from a randomized placebo-controlled trial. J Natl Cancer Inst. 2003;95:206–214. doi: 10.1093/jnci/95.3.206. [DOI] [PubMed] [Google Scholar]
- 59.Grunt TW, Puckmair K, Tomek K, Kainz B, Gaiger A. An EGF receptor inhibitor induces RAR-beta expression in breast and ovarian cancer cells. Biochem Biophys Res Commun. 2005;329:1253–1259. doi: 10.1016/j.bbrc.2005.02.104. [DOI] [PubMed] [Google Scholar]
- 60.Lian F, Hu KQ, Russell RM, Wang XD. beta-Cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int J Cancer. 2006;119:2084–2089. doi: 10.1002/ijc.22111. [DOI] [PubMed] [Google Scholar]
- 61.Berg WJ, Nanus DM, Leung A, Brown KT, Hutchinson B, Mazumdar M, et al. Up-regulation of retinoic acid receptor beta expression in renal cancers in vivo correlates with response to 13-cis-retinoic acid and interferon-alpha-2a. Clin Cancer Res. 1999;5:1671–1675. [PubMed] [Google Scholar]
- 62.Geradts J, Chen JY, Russell EK, Yankaskas JR, Nieves L, Minna JD. Human lung cancer cell lines exhibit resistance to retinoic acid treatment. Cell Growth Differ. 1993;4:799–809. [PubMed] [Google Scholar]
- 63.Lin B, Chen GQ, Xiao D, Kolluri SK, Cao X, Su H, et al. Orphan receptor COUP-TF is required for induction of retinoic acid receptor beta, growth inhibition, and apoptosis by retinoic acid in cancer cells. Mol Cell Biol. 2000;20:957–970. doi: 10.1128/mcb.20.3.957-970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lefebvre B, Brand C, Flajollet S, Lefebvre P. Down-regulation of the tumor suppressor gene RAR-β2 through the PI3K/Akt signaling pathway. Mol Endocrinol. 2006;20:2109–2121. doi: 10.1210/me.2005-0321. [DOI] [PubMed] [Google Scholar]
- 65.Wang XF, Qian DZ, Ren M, Kato Y, Wei Y, Zhang L, et al. Epigenetic modulation of retinoic acid receptor beta2 by the histone deacetylase inhibitor MS-275 in human renal cell carcinoma. Clin Cancer Res. 2005;11:3535–3542. doi: 10.1158/1078-0432.CCR-04-1092. [DOI] [PubMed] [Google Scholar]
- 66.Sirchia SM, Ren M, Pili R, Sironi E, Somenzi G, Ghidoni R, et al. Endogenous reactivation of the RARß2 tumor suppressor gene epigenetically silenced in breast cancer. Cancer Res. 2002;62:2455–2461. [PubMed] [Google Scholar]
- 67.Ferrari N, Pfahl M, Levi G. Retinoic acid receptor gamma1 (RARgamma1) levels control RARbeta2 expression in SK-N-BE2(c) neuroblastoma cells and regulate a differentiation-apoptosis switch. Mol Cell Biol. 1998;18:6482–6492. doi: 10.1128/mcb.18.11.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suh YA, Lee HY, Virmani A, Wong J, Mann KK, Miller WH, Jr, et al. Loss of retinoic acid receptor ß gene expression is linked to aberrant histone H3 acetylation in lung cancer cell lines. Cancer Res. 2002;62:3945–3949. [PubMed] [Google Scholar]
- 69.Verma AK, Shoemaker A, Simsiman R, Denning M, Zachman RD. Expression of retinoic acid nuclear receptors and tissue transglutaminase is altered in various tissues of rats fed a vitamin A-deficient diet. J Nutr. 1992;122:2144–2152. doi: 10.1093/jn/122.11.2144. [DOI] [PubMed] [Google Scholar]
- 70.Kato S, Mano H, Kumazawa T, Yoshizawa Y, Kojima R, Masushige S. Effect of retinoid status on alpha, beta and gamma retinoic acid receptor mRNA levels in various rat tissues. Biochem J. 1992;286:755–760. doi: 10.1042/bj2860755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo X, Ruiz A, Rando RR, Bok D, Gudas LJ. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis. 2000;21:1925–1933. doi: 10.1093/carcin/21.11.1925. [DOI] [PubMed] [Google Scholar]
- 72.Xu XC, Zile MH, Lippman SM, Lee JS, Lee JJ, Hong WK, et al. Anti-retinoic acid (RA) antibody binding to human premalignant oral lesions which occurs less frequently than binding to normal tissue increases after 13-cis-RA treatment in vivo and is related to RA receptor beta expression. Cancer Res. 1995;55:5507–5511. [PubMed] [Google Scholar]
- 73.Ren M, Pozzi S, Bistulfi G, Somenzi G, Rossetti S, Sacchi N. Impaired retinoic acid (RA) signal leads to RARbeta2 epigenetic silencing and RA resistance. Mol Cell Biol. 2005;25:10591–10603. doi: 10.1128/MCB.25.23.10591-10603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Di Croce L, Raker VA, Corsaro M, Fazi F, Fanelli M, Faretta M. Methyltransferase recruitment and DNA hypermethylation of target promoters by an oncogenic transcription factor. Science. 2002;295:1079–1082. doi: 10.1126/science.1065173. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Fang MZ, Liao J, Yang GY, Nie Y, Song Y. Hypermethylation-associated inactivation of retinoic acid receptor beta in human esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9:5257–5263. [PubMed] [Google Scholar]
- 76.Virmani AK, Rathi A, Zochbauer-Muller S, Sacchi N, Fukuyama Y, Bryant D. Promoter methylation and silencing of the retinoic acid receptor-beta gene in lung carcinomas. J Natl Cancer Inst. 2000;92:1303–1307. doi: 10.1093/jnci/92.16.1303. [DOI] [PubMed] [Google Scholar]
- 77.Cote S, Momparler RL. Activation of the retinoic acid receptor beta gene by 5-aza-2'-deoxycytidine in human DLD-1 colon carcinoma cells. Anticancer Drugs. 1997;8:56–61. doi: 10.1097/00001813-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 79.Vuillemenot BR, Pulling LC, Palmisano WA, Hutt JA, Belinsky SA. Carcinogen exposure differentially modulates RAR-beta promoter hypermethylation, an early and frequent event in mouse lung carcinogenesis. Carcinogenesis. 2004;25:623–629. doi: 10.1093/carcin/bgh038. 25. [DOI] [PubMed] [Google Scholar]
- 80.Song S, Xu XC. Effect of benzo[a]pyrene diol epoxide on expression of retinoic acid receptor-beta in immortalized esophageal epithelial cells and esophageal cancer cells. Biochem Biophys Res Commun. 2001;281:872–877. doi: 10.1006/bbrc.2001.4433. [DOI] [PubMed] [Google Scholar]
- 81.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 82.Kim JS, Lee H, Kim H, Shim YM, Han J, Park J, et al. Promoter methylation of retinoic acid receptor beta 2 and the development of second primary lung cancers in non-small-cell lung cancer. J Clin Oncol. 2004;22:3443–3450. doi: 10.1200/JCO.2004.11.135. [DOI] [PubMed] [Google Scholar]
- 83.Berard J, Gaboury L, Landers M, De Repentigny Y, Houle B, Kothary R, et al. Hyperplasia and tumours in lung, breast and other tissues in mice carrying a RAR beta 4-like transgene. EMBO J. 1994;13:5570–5580. doi: 10.1002/j.1460-2075.1994.tb06894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu XC, Lee JJ, Wu TT, Hoque A, Ajani JA, Lippman SM. Increased retinoic acid receptor-beta4 correlates in vivo with reduced retinoic acid receptor-beta2 in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14:826–829. doi: 10.1158/1055-9965.EPI-04-0500. [DOI] [PubMed] [Google Scholar]
- 85.Sommer KM, Chen LI, Treuting PM, Smith LT, Swisshelm K. Elevated retinoic acid receptor beta(4) protein in human breast tumor cells with nuclear and cytoplasmic localization. Proc Natl Acad Sci U S A. 1999;96:8651–8656. doi: 10.1073/pnas.96.15.8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hayashi K, Goodison S, Urquidi V, Tarin D, Lotan R, Tahara E. Differential effects of retinoic acid on the growth of isogenic metastatic and non-metastatic breast cancer cell lines and their association with distinct expression of retinoic acid receptor beta isoforms 2 and 4. Int J Oncol. 2003;22:623–629. [PubMed] [Google Scholar]
- 87.Khuri FR, Lotan R, Kemp BL, Lippman SM, Wu H, Feng L. Retinoic acid receptor-beta as a prognostic indicator in stage I non-small-cell lung cancer. J Clin Oncol. 2000;18:2798–2804. doi: 10.1200/JCO.2000.18.15.2798. [DOI] [PubMed] [Google Scholar]
- 88.Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, Xu XC. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–867. [PubMed] [Google Scholar]
- 89.Soria JC, Xu XC, Liu DD, Lee JJ, Kurie J, Morice RC. Retinoic acid receptor beta and telomerase catalytic subunit expression in bronchial epithelium of heavy smokers. J Natl Cancer Inst. 2003;95:165–168. doi: 10.1093/jnci/95.2.165. [DOI] [PubMed] [Google Scholar]
- 90.Nagpal S, Zelent A, Chambon P. RAR-beta4, a retinoic acid receptor isoform is generated from RAR-beta 2 by alternative splicing and usage of a CUG initiator codon. Proc Natl Acad Sci U S A. 1992;89:2718–2722. doi: 10.1073/pnas.89.7.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zelent A, Mendelsohn C, Kastner P, Krust A, Garnier JM, Ruffenach F. Differentially expressed isoforms of the mouse retinoic acid receptor beta generated by usage of two promoters and alternative splicing. EMBO J. 1991;10:71–81. doi: 10.1002/j.1460-2075.1991.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peng X, Maruo T, Cao Y, Punj V, Mehta R, Das Gupta TK. A novel RARbeta isoform directed by a distinct promoter P3 and mediated by retinoic acid in breast cancer cells. Cancer Res. 2004;64:8911–8918. doi: 10.1158/0008-5472.CAN-04-1810. [DOI] [PubMed] [Google Scholar]
- 93.Petty WJ, Li N, Biddle A, Bounds R, Nitkin C, Ma Y. A novel retinoic acid receptor beta isoform and retinoid resistance in lung carcinogenesis. J Natl Cancer Inst. 2005;97:1645–1651. doi: 10.1093/jnci/dji371. [DOI] [PubMed] [Google Scholar]
- 94.Houle B, Pelletier M, Wu J, Goodyer C, Bradley WE. Fetal isoform of human retinoic acid receptor beta expressed in small cell lung cancer lines. Cancer Res. 1994;54:365–369. [PubMed] [Google Scholar]
- 95.Berard J, Delabre JF, Garneau H, Bellehumeur C, Carrier ME. Lung tumorigenesis in transgenic mice expressing RAR-beta1 and RAR-beta3 antisense mRNA. Lung Cancer. 2003;41(S2):S122. [Google Scholar]
- 96.Chen LI, Sommer KM, Swisshelm K. Downstream codons in the retinoic acid receptor beta-2 and beta-4 mRNAs initiate translation of a protein isoform that disrupts retinoid-activated transcription. J Biol Chem. 2002;277:35411–35421. doi: 10.1074/jbc.M202717200. [DOI] [PubMed] [Google Scholar]
- 97.Soprano DR, Scanlon E, Shukri M, Zhang ZP, Soprano KJ. Murine RARbeta4 displays reduced transactivation activity, lower affinity for retinoic acid, and no anti-AP1 activity. J Cell Biochem. 2000;77:604–614. [PubMed] [Google Scholar]
- 98.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 99.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 100.Houle B, Rochette-Egly C, Bradley WE. Tumor-suppressive effect of the retinoic acid receptor beta in human epidermoid lung cancer cells. Proc Natl Acad Sci U S A. 1993;90:985–989. doi: 10.1073/pnas.90.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berard J, Laboune F, Mukuna M, Masse S, Kothary R, Bradley WE. Lung tumors in mice expressing an antisense RARbeta2 transgene. FASEB J. 1996;10:1091–1097. doi: 10.1096/fasebj.10.9.8801172. [DOI] [PubMed] [Google Scholar]
- 102.Faria TN, Mendelsohn C, Chambon P, Gudas LJ. The targeted disruption of both alleles of RARbeta(2) in F9 cells results in the loss of retinoic acid-associated growth arrest. J Biol Chem. 1999;274:26783–26788. doi: 10.1074/jbc.274.38.26783. [DOI] [PubMed] [Google Scholar]
- 103.Treutin PM, Chen LI, Buetow BS, Zeng W, Birkebak TA, Seewaldt VL, et al. Retinoic acid receptor beta2 inhibition of metastasis in mouse mammary gland xenografts. Breast Cancer Res Treat. 2002;72:79–88. doi: 10.1023/a:1014906529407. [DOI] [PubMed] [Google Scholar]
- 104.Cheung B, Hocker JE, Smith SA, Norris MD, Haber M, Marshall GM. Favorable prognostic significance of high-level retinoic acid receptor beta expression in neuroblastoma mediated by effects on cell cycle regulation. Oncogene. 1998;17:751–759. doi: 10.1038/sj.onc.1201982. [DOI] [PubMed] [Google Scholar]
- 105.McGregor F, Wagner E, Felix D, Soutar D, Parkinson K, Harrison PR. Inappropriate retinoic acid receptor-beta expression in oral dysplasias: correlation with acquisition of the immortal phenotype. Cancer Res. 1997;57:3886–3889. [PubMed] [Google Scholar]
- 106.McGregor F, Muntoni A, Fleming J, Brown J, Felix DH, MacDonald DG, et al. Molecular changes associated with oral dysplasia progression and acquisition of immortality: potential for its reversal by 5-azacytidine. Cancer Res. 2002;62:4757–4766. [PubMed] [Google Scholar]
- 107.Muntoni A, Fleming J, Gordon KE, Hunter K, McGregor F, Parkinson E, et al. Senescing oral dysplasias are not immortalized by ectopic expression of hTERT alone without other molecular changes, such as loss of INK4A and/or retinoic acid receptor-beta: but p53 mutations are not necessarily required. Oncogene. 2003;22:7804–7808. doi: 10.1038/sj.onc.1207085. [DOI] [PubMed] [Google Scholar]
- 108.Lin F, Xiao D, Kolluri SK, Zhang X. Unique anti-activator protein-1 activity of retinoic acid receptor beta. Cancer Res. 2000;60:3271–3280. [PubMed] [Google Scholar]
- 109.Delage B, Bairras C, Buaud B, Pallet V, Cassand P. A high-fat diet generates alterations in nuclear receptor expression: prevention by vitamin A and links with cyclooxygenase-2 and beta-catenin. Int J Cancer. 2005;116:839–846. doi: 10.1002/ijc.21108. [DOI] [PubMed] [Google Scholar]
- 110.Huang J, Wu TT, Liang ZD, Jiang Y, Zhang H, Xu XC. Tumor-suppressive effect of retinoid receptor-induced gene-1 (RRIG1) in esophageal cancer. Cancer Res. doi: 10.1158/0008-5472.CAN-06-2472. accepted. [DOI] [PubMed] [Google Scholar]


