Abstract
Smads are intermediate effector proteins that transduce the TGF-β signal from the plasma membrane to the nucleus, where they participate in transactivation of downstream target genes. We have shown previously that coactivators p300/CREB-binding protein are involved in TGF-β–mediated transactivation of two Cdk inhibitor genes, p21 and p15. Here we examined the possibility that Smads function to regulate transcription by directly interacting with p300/CREB-binding protein. We show that Smad3 can interact with a C-terminal fragment of p300 in a temporal and phosphorylation-dependent manner. TGF-β–mediated phosphorylation of Smad3 potentiates the association between Smad3 and p300, likely because of an induced conformational change that removes the autoinhibitory interaction between the N- and C-terminal domains of Smad3. Consistent with a role for p300 in the transcription regulation of multiple genes, overexpression of a Smad3 C-terminal fragment causes a general squelching effect on multiple TGF-β–responsive reporter constructs. The adenoviral oncoprotein E1A can partially block Smad-dependent transcriptional activation by directly competing for binding to p300. Taken together, these findings define a new role for phosphorylation of Smad3: in addition to facilitating complex formation with Smad4 and promoting nuclear translocation, the phosphorylation-induced conformational change of Smad3 modulates its interaction with coactivators, leading to transcriptional regulation.
INTRODUCTION
TGF-β is a growth factor that regulates various cellular functions in many cell types (Lyons and Moses, 1990; Massague, 1990; Roberts and Sporn, 1993). Central to this is its ability to inhibit cellular proliferation by causing an arrest in the G1 phase of the cell cycle. In addition, TGF-β regulates the expression of many cellular genes involved in extracellular matrix production and turnover. Clues to the molecular mechanisms through which TGF-β exerts these cellular effects have come from the discovery of the Smad family of proteins.
Smads are intermediate effector molecules of the signaling pathways of the TGF-β superfamily of ligands. To date, at least nine Smads have been cloned (Heldin et al., 1997; Hu et al., 1998; Massague, 1998). Among them, the highly related Smad2 and Smad3 are specific effectors for TGF-β signaling (Macias-Silva et al., 1996; Zhang et al., 1996), and Smad 4 is a common partner for TGF-β superfamily signaling (Hahn et al., 1996; Lagna et al., 1996). Smad 2 and most likely Smad3 are phosphorylated at their extreme C terminus (SSVS) by type I receptor during TGF-β treatment (Macias-Silva et al., 1996; Zhang et al., 1996). This phosphorylation overcomes the autoinhibitory state of Smad2 between its N and C terminus, promoting its interaction with Smad4 and subsequent translocation to the nucleus (Hata et al., 1997). In addition, overexpression of Smad3 and Smad4, which presumably leads to higher absolute levels of basally phosphorylated forms of these proteins, can cause ligand-independent transcriptional activation of certain TGF-β–inducible genes such as plasminogen activator inhibitor 1 (PAI-1) (Zhang et al., 1996); however, the mechanism leading to transcriptional activation is still largely unknown. Smads have been shown to bind DNA directly (Kim et al., 1997; Yingling et al., 1997), and this ability to bind to DNA may correlate, at least in part, with transcriptional activity inherent to Smad molecules and/or in conjunction with coactivator partners (Liu et al., 1997; Dennler et al., 1998; Zawel et al., 1998).
Clues to the biological functions of Smads have also come from the discovery that certain Smads are tumor suppressors mutated in human cancers. Smad4 was originally identified as a tumor suppressor on chromosome 18q, termed DPC4, which is mutated in 50% of human pancreatic cancers (Hahn et al., 1996). Smad4 mutations and deletions have been discovered in other types of cancers, including breast, ovary, head, and neck, and esophageal cancers (Barrett et al., 1996; Kim et al., 1996; Nagatake et al., 1996; Schutte et al., 1996). Smad2 is also defined as a tumor suppressor gene because its mutations have been found in colon and head and neck cancers (Eppert et al., 1996). These findings suggest a role for Smads in cell growth regulation and have lead to the hypothesis that the Smads may be central regulators of TGF-β–mediated growth inhibition (Massague, 1998).
Regulation of the cell cycle in the G1 phase is dependent on the activity of cyclin-dependent kinase (Cdk) complexes, primarily the cyclin D-Cdk4/Cdk6 and cyclin E-Cdk2 complexes. TGF-β has been shown to cause cell cycle arrest by inhibiting the Cdk activities in certain cell types by inducing the expression of the two Cdk inhibitors p15 and p21 (Hannon and Beach, 1994; Datto et al., 1995a; Reynisdottir et al., 1995). To probe the signaling mechanism by which TGF-β regulates cell cycle progression, we previously mapped the TGF-β–responsive elements of the p15 and p21 promoters to Sp1 binding sites in HaCaT cells (Datto et al., 1995a,b; Li et al., 1995). Subsequently, we found that canonical Sp1 binding sites can function as a distinct TGF-β–responsive element for TGF-β–mediated promoter expression, and Sp1 protein, but not family member Sp3, can mediate this response (Li et al., 1998a).
In a separate study, we demonstrated that the coactivator p300 is required for the TGF-β–mediated induction of p15 and p21 (Datto et al., 1997). p300 is a phosphoprotein that was first discovered in anti-E1A cellular immunoprecipitates (Eckner et al., 1994), and it has a functional homologue, CREB-binding protein (CBP), that also binds to E1A (Chrivia et al., 1993). In HaCaT cells, the ability of E1A to abolish TGF-β–mediated growth inhibition, in addition to its binding and inactivation of the retinoblastoma protein Rb, appears to stem from its binding to p300/CBP, which prevents TGF-β–mediated induction of p15 and p21 and relieves cyclin–Cdk repression (Missero et al., 1995; Datto et al., 1997). Although p300/CBP was shown to be required for p15 and p21 induction, the mechanism by which its activity is modulated by the TGF-β signal remains unresolved.
Because p300/CBP appears to be essential in TGF-β–mediated growth inhibitory signaling and because the Smads, by their nature as tumor suppressors, have also been implicated in growth control, we chose to explore the possibility of a functional or physical interaction between these proteins. In this report, we show that Smad3 interacts with p300 in a temporal and TGF-β–regulated phosphorylation-dependent manner. Thus, Smad3 may play a role as a mediator of the TGF-β growth inhibitory signaling pathway. This notion is supported by the recent finding that overexpression of Smad3 and Smad4 could lead to a dramatic ligand-independent transactivation of the p21 promoter in a hepatic cell line (Moustakas and Kardassis, 1998). Furthermore, we provide evidence that the interaction between Smad3 and p300 may be essential for the transcriptional responses of multiple target genes to TGF-β. Specifically, the Smad-dependent induction of the PAI-1 gene by TGF-β is blocked by E1A but not by an E1A mutant deficient in p300 binding, implicating the interaction between Smad3 and p300 as an important requirement for TGF-β signaling.
MATERIALS AND METHODS
Antibodies and Reagents
Human TGF-β1 was a generous gift from Amgen. Anti-HA was from Boehringer Mannheim (Indianapolis, IN). Anti-Smad3 antibody was generated against a specific peptide (DAGSPNLSPNPMSPAHNNLD) in the linker region of Smad3 and purified in this laboratory; anti-Smad4 (sc-7966) and anti-p300 (sc-584 AC) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). TNT SP6-coupled reticulocyte lysate system was from Promega (Madison, WI). Calf intestine alkaline phosphatase (CIAP) and potato acid phosphatase (PAP) were from Boehringer Mannheim.
Cell Culture
Human HaCaT cells were a generous gift from Drs. P. Baukamp and N. Fusenig (Institute of Biochemistry and Molecular Biology, Heidelberg, Germany). They were grown in MEM supplemented with 10% FBS and 2 mM l-glutamine (Life Technologies, Gaithersburg, MD). COS cells were maintained in DMEM with 10% FBS.
Plasmids
HA-tagged Smad3 has been described previously (Yingling et al., 1996). pCMV5-Smad3 C-HA (aa 199–424), Smad4C-HA (aa 266–552), Smad3-Flag, Smad3NL-Flag, Smad3CΔC-Flag, and Smad3ΔC-Flag were generous gifts from Dr. Rik Derynck (Zhang et al., 1997). GST-p300M (aa 744-1571) and GST-p300C (aa 1572–2414) were generous gifts from Dr. Yang Shi (Lee et al., 1995). PAI-1-Luc (Zhang et al., 1996), 3TP-Lux (Wrana et al., 1992), p15P113-Luc (Li et al., 1995), p21P-Luc (Datto et al., 1995b), and Gl1xkB (Li et al., 1998b) have been described previously.
GST Pull-down Assays
The bacterial strain TOPP1 containing GST-p300M and GST-p300C was grown in 5 ml of Luria broth media overnight at 37°C. The next day, the cultures were transferred to flasks containing 50 ml of Luria broth and shaken vigorously for 1 h (optical density, ∼0.6) at 37°C. Isopropylthio-β-d-galactaside (0.5 mM) was then added to the culture and shaken vigorously for another 3 h at 37°C. Cells were sonicated four times on ice in 30 s intervals. Lysates were clarified by centrifugation at 7000 rpm before addition of 200 μl of a 50% slurry of lysis buffer-equilibrated glutathione beads. After a 4 h incubation at 4°C, the beads were pelleted by centrifugation at 1000 rpm and washed three times in lysis buffer before resuspension in 1 ml of lysis buffer. In GST pull-down assays, equal amounts of cell lysates or in vitro translated product were incubated with immobilized GST beads in lysis buffer (50 mM Tris-HCL, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 50 mM NaF, 1 mM sodium orthovanadate, 1 mM DTT, 1 mM phenylmethyl-sulfonyl fluoride and protease inhibitors) at 4°C for 2 h. After the beads were washed four times with lysis buffer, the bound proteins were eluted by boiling in 1× Laemmli sample buffer and subjected to immunoblot analysis. For phosphatase treatment, cell lysates were made with or without phosphatase inhibitor 1 mM sodium orthovanadate, 50 mM NaF, 20 mM β-glycerophosphate, and 0.1 mM sodium molybdate, and then treated with 2 U CIAP and 2 μg PAP at 37°C for 15 min. Thirty micrograms of treated lysates were used as control in immunoblotting, and the rest of the lysates were used for GST pull-down assays.
Luciferase Assays
Transfections were performed by using a standard DEAE–dextran transfection protocol (Li et al., 1995). Briefly, 150,000 cells were plated onto each well of a six-well plate and grown overnight. The cells were then washed once with PBS and incubated in serum-free MEM containing 100 μM chloroquine. The DEAE–dextran mixture containing DNA was then added to the cells and incubated for 3 h. The cells were then glycerol-shocked for 2 min and incubated in medium containing 10% FBS. Twelve hours after transfection, 100 pM TGF-β1 was added, and TGF-β–induced luciferase activity was assayed after 24 h. Luciferase assays were performed as described previously (Li et al., 1995).
Immunoprecipitation and Western Blot Analysis
Cells after treatment were harvested in lysis buffer described above. Agarose-conjugated p300 antibodies (5 μl) were added into ∼300 μg of lysate and incubated at 4°C for at least 3 h. The beads were washed three times with 0.5 ml of lysis buffer. Then loading buffer containing N-ethylmaleimide instead of DTT was added and incubated at room temperature for 20 min to shift the heavy chain of antibodies to a higher position before loading on the gel for Western blot analysis.
Proteins from HaCaT lysates or transfected COS lysates were resolved by SDS-PAGE and transferred to Immobilon-P (Millipore, Bedford, MA). The membranes were then blocked in 5% nonfat milk in 1× PBS and 0.1% Tween 20. The blots were incubated with primary antibody in block solution for 1 h at room temperature and subsequently washed three times in PBS/Tween. The appropriate secondary antibody was added for 1 h at room temperature. After three washes with PBS/Tween, the immunoreactive proteins were visualized by ECL (Amersham, Buckinghamshire, UK) and autoradiography.
RESULTS
Smad3 Binds the p300 C-Terminal Fragment
To examine whether p300 and Smad3 can interact with each other, we performed pull-down experiments using two p300 fragments, GST-p300M (aa 744-1571) and GST-p300C (1572–2414) (Lee et al., 1995), with COS cell lysates containing overexpressed HA-tagged Smad3 and the N-terminal–truncated Smad3 (aa 199–424), termed Smad3C. As shown in Figure 1A, Smad3C can interact strongly with GST-p300C but not GST-p300M. Intriguingly, the full-length Smad3 interacts only weakly with both GST-p300M and GST-p300C.
Figure 1.
p300 C-terminal region interacts with Smad3C and Smad4C. (A) p300C interacts with Smad3C. HA-tagged full-length Smad3 and Smad3 C-terminal fragment (aa 199–424) constructs were transfected into COS cells as indicated. Cells were harvested 48 h after transfection, and GST pull-downs were performed using GST-p300M (aa 744-1571) and GST-p300C (aa 1572–2414). The bound proteins were analyzed by immunoblotting with antibodies against HA. The lysates in lanes 1 and 2 represent ∼12% of the amount used for the pull-down. (B) A region between aa 199 and 381 of Smad3 interacts with p300. Smad3 and truncated forms were in vitro-translated using rabbit reticulocyte lysates. Ten microliters of the 35S-labeled proteins were incubated with GST-p300C beads for 2 h at 4°C, and the bound proteins were analyzed by SDS-PAGE followed by fluorography. (C) Smad4C can interact with p300C. HA-Smad4 and Smad4C (aa 266–552) were transfected into COS cells, and the lysates were pulled down with GST-p300C as in A.
To further define the region of interaction on Smad3, as well as to determine whether this association is direct, 35S-labeled in vitro-translated full-length Smad3 and the indicated fragments were used to perform additional pull-down experiments with GST-p300C (Figure 1B). Consistent with the pull-down experiment with COS lysates, full-length Smad3 and the Smad3 N-terminal fragment were found to interact weakly with p300C, in comparison with the strong interactions between p300C and Smad3ΔC or p300 and Smad3CΔC. This suggests that the region of interaction with p300 is between aa 199 and 381 of Smad3. Most importantly, these results indicate that deletion of either the N or distal C terminus of Smad3 can strongly enhance its interaction with p300, suggesting that the unmodulated conformation of full-length Smad3 may be inaccessible to p300 interaction.
To determine whether p300 could also interact with Smad4, the binding partner of Smad3, we repeated the GST-p300C pull-down experiments with COS lysates containing overexpressed Smad4 (aa 1–552) and Smad4C (aa 266–552). As shown in Figure 1C, p300 was found to associate with Smad4C but not with full-length Smad4. This result suggests that Smad4C, when overexpressed, also has the ability to interact with p300.
TGF-β Induces the Association between Smad3 and p300
Because either the N- or the C-terminal truncated Smad3 protein fragment interacts with p300 more strongly than full-length protein, we reasoned that the conformation of unstimulated Smad3 is likely to be autoinhibitory in a manner similar to that previously demonstrated for Smad2 (Hata et al., 1997). Hence, TGF-β type I receptor-mediated phosphorylation of the SSVS motif in the C-terminal region of Smad3 and subsequent relief of the autoinhibited conformation of this protein are necessary for the interaction with p300 to occur. To test this hypothesis, we treated HaCaT cells with TGF-β for increasing lengths of time and used GST-p300C to pull down endogenous Smad3. The total amount of Smad3 protein did not change with up to 4 h of TGF-β treatment of these cells (Figure 2A). At time 0, we found that GST-p300C did not interact with endogenous Smad3; however, in lysates from cells treated with TGF-β for 10 min up to 2 h, GST-p300 was readily able to interact with endogenous Smad3. Complex formation between Smad3 and p300 peaks at 30 min and completely diminishes by the 4 h time point (Figure 2A). This time course of observed interaction parallels that for Smad phosphorylation after TGF-β treatment (Yingling et al., 1996). This result strongly suggests that the interaction between Smad3 and the coactivator p300 is a TGF-β–regulated event that correlates directly with the phosphorylation of Smad3.
Figure 2.
TGF-β regulates the interaction of Smad3 and p300 in a temporal and phosphorylation-dependent manner. (A) The time course of interaction between Smad3 and p300 after TGF-β treatment. HaCaT cells were treated with TGF-β for 0–4 h. Total cell lysates were used for the GST-p300C pull-down assay as described in MATERIALS AND METHODS. Bound proteins were separated by SDS-PAGE and immunoblotted with antibodies against Smad3. (B) The association of Smad3 and p300 is phosphorylation dependent. HaCaT cells were treated with TGF-β for 0 and 30 min, and then lysates were treated with phosphatases CIAP and PAP, as described in MATERIALS AND METHODS, and used for the GST-p300C pull-down assay as in A. Bound proteins were separated by SDS-PAGE and immunoblotted with antibodies against Smad3. (C) Endogenous Smad3, but not Smad4, interacts with GST-p300C after TGF-β treatment. HaCaT lysates treated with TGF-β for 0 and 30 min were precipitated by GST-p300C and then immunoblotted with antibodies against Smad3 and Smad4. (D) Smad3 interacts with p300 in vivo. HaCaT cells were incubated with TGF-β for 30 min. Lysate (300 μg) was used for immunoprecipitation using agarose-conjugated antibodies against p300 and then immunoblotted with antibodies against Smad3. Thirty micrograms of lysates were loaded on the gel to shown the correct size of Smad3.
To further probe the mechanism underlying the TGF-β–induced temporal association between Smad3 and p300, we treated HaCaT lysates with phosphatases (CIAP and PAP) to determine whether phosphorylation was the underlying event required for this association. In the phosphatase-treated lysates of cells incubated with TGF-β for 30 min, the interaction of Smad3 with GST-p300C was almost completely abolished (Figure 2B). It is also of note that without exogenous phosphatase treatment, this interaction is greatly reduced in the absence of phosphatase inhibitors in the lysis buffer (Figure 2B, lane 7) and suggests that this reduced association is a result of Smad3 dephosphorylation by endogenous phosphatases. As a control, it is demonstrated that the total amount of Smad3 protein is not affected by the indicated phosphatase incubation conditions. These results demonstrate that the TGF-β–induced conformational change of Smad3, most likely through the phosphorylation of Smad3 at its C-terminal region, is required for its interaction with p300. This notion is further supported by the results shown in Figure 1B, in which it is demonstrated that Smad3ΔC and Smad3CΔC, both of which lack the SSVS site of phosphorylation, have a much stronger affinity for p300C, and suggests that these sites of phosphorylation are not required for the interaction of Smad3 with p300 but rather that the phosphorylation-induced conformational change of Smad3 is the essential event.
We also determined whether Smad4, when expressed at endogenous levels, can bind to p300 in a TGF-β–regulated manner in the same system. In HaCaT lysates either untreated or incubated with TGF-β for 30 min, Smad4 was not able to associate with GST-p300C, whereas Smad3 was TGF-β–inducibly associated with p300 in the same experiment (Figure 2C). Thus, although both Smad3 and Smad4 have the potential to interact with p300 as demonstrated by the COS overexpression experiment (Figure 1, A and C), only endogenous Smad3 but not Smad4 can interact with p300 during TGF-β treatment. This is probably because only Smad3 can undergo phosphorylation during TGF-β treatment, which will lead to a conformational change favorable for the interaction with p300.
To demonstrate an in vivo interaction between Smad3 and p300, we performed immunoprecipitation and Western blot analysis using HaCaT cell lysates. Cells untreated or treated with TGF-β for 30 min were harvested, immunoprecipitated with agarose-conjugated p300 antibodies, and blotted with the anti-Smad3 antibody. As shown in Figure 2D, the association between Smad3 and p300 is observed only after TGF-β treatment, a result fully consistent with that of the GST pull-down assay. Taken together, these results indicate that Smad3 interacts with p300 in a temporal and ligand-induced phosphorylation-dependent manner.
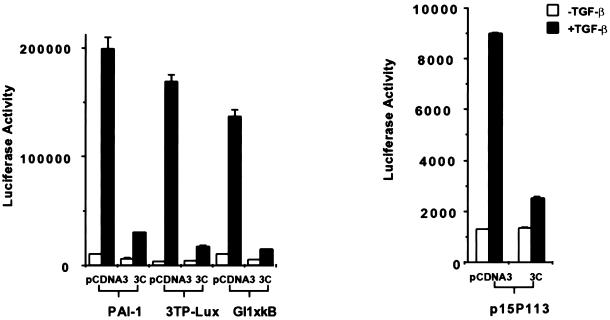
Overexpression of Smad3C Has a Squelching Effect on Multiple TGF-β–regulated Promoters
To further explore the functional significance of the interaction between Smad3 and p300, we tested whether the overexpression of Smad3C (aa 199–424), a Smad3 fragment that can constitutively bind to p300, could affect TGF-β–mediated transactivation of multiple target genes. As shown in Figure 3, cotransfection of Smad3C with PAI-1-Luc, 3TP-Lux, Gl1XκB, a minimal responsive reporter construct containing NFκB sites (Li et al., 1998a,b), and the minimal promoter for the p15 gene, p15P113-Luc, caused a dramatic decrease in TGF-β–induced transcriptional activity. This broad spectrum of transcriptional inhibition by the overexpressed Smad3C may be the result of a sequestration of a common factor, likely the coactivator p300/CBP, although we cannot rule out the possibility that titration of endogenous Smad4 or other factors also plays a role in this process. These results nevertheless suggest that p300, and possibly the interaction between Smad3 and p300, may be required for the mediation of TGF-β–signaling pathways leading to the activation of multiple genes involving different families of transcription factors.
Figure 3.
Overexpression of Smad3C has a squelching effect on multiple TGF-β–responsive reporter genes. Three micrograms of HA-tagged Smad3C were cotransfected with 3 μg of different reporter constructs into HaCaT cells as indicated. The total DNA amount was kept constant by adding pCDNA3.
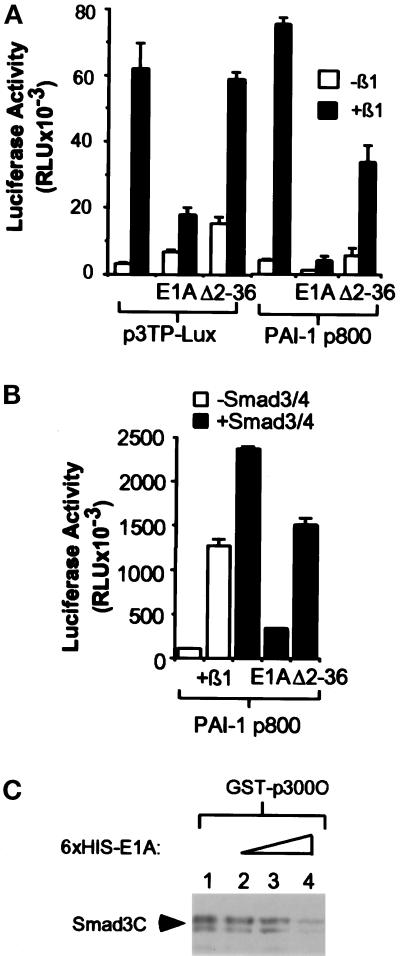
E1A Competes with Smad3 for Binding to p300
We and others have shown previously that the adenoviral oncoprotein E1A is able to antagonize TGF-β–mediated transcription and growth inhibition (Pietenpol et al., 1990; Missero et al., 1991; Abraham et al., 1992; Datto et al., 1997). This activity of E1A is dependent on its ability to bind to two main target proteins, p300/CBP and pRB. The demonstration here that Smad3 interacts with p300 in a temporal and phosphorylation-dependent manner indicates that this interaction may be important for the transactivation ability of Smads. To test whether E1A can act to block Smad-mediated transcription activation in a p300-dependent manner, we examined the effect of E1A on the TGF-β–induced expression of PAI-1-Luc and 3TP-Lux, two reporters that have been shown to require Smads for transcriptional activation. As shown in Figure 4A, E1A can dramatically inhibit the TGF-β–mediated transactivation of these two promoters in HaCaT cells cotransfected with either of these two reporter constructs. Furthermore, the inhibitory effect of E1A on the TGF-β induction of the two promoters was significantly reduced when HaCaT cells were cotransfected with an E1A mutant, Δ2–36, that is severely attenuated in p300 binding (Kraus et al., 1992; Wang et al., 1993). Both promoters have been previously shown to be transcriptionally activated in a ligand-independent manner during cotransfection of Smad3 and Smad4. Consistent with the notion that Smad3 and Smad4 play a role as effectors for TGF-β in this transactivation event by binding to p300, E1A greatly reduced the 20-fold ligand-independent transactivation of the PAI-1 reporter resulting from cotransfected Smad3 and Smad4, whereas cotransfection of the mutant E1A, Δ2–36, only partially affected the Smad3/Smad4 ligand-independent effect (Figure 4B).
Figure 4.
E1A inhibits Smad-dependent transcriptional activation. (A) E1A, but not the p300 binding mutant E1AΔ2–36, blocks transcriptional activation of 3TP-Lux and PAI-1-Luc. HaCaT cells were cotransfected with 3 μg of 3TP-Lux or PAI-1-Luc reporter constructs and 4 μg of the indicated E1A expression constructs. The total amount of DNA was kept constant with the addition of vector control pCDNA3. TGF-β was added 12 h after transfection, and luciferase activity was measured 24 h later. Error bars represent the SD for duplicate transfections in a single experiment. “E1A” stands for wild-type E1A, and “Δ2–36” stands for E1AΔ2–36 mutant. (B) The transcriptional activation induced by Smad3/Smad4 overexpression is inhibited by E1A in a p300-dependent manner. HaCaT cells were cotransfected with 3 μg of PAI-1, 3 μg of Smad3/Smad4, and 4 μg of E1A expression constructs as indicated. The total DNA amount was kept constant with the addition of pCDNA3. After transfection and TGF-β treatment, luciferase activity was measured as above. (C) E1A can compete with Smad3 for interaction with p300. COS-overexpressed HA-tagged Smad3C (aa 199–424) was used to access the ability of Smad3 to interact with p300 in the presence of E1A. Eluted bacterial-produced 6XHis E1A was added in increasing amounts from lanes 2 to 4 to the GST-p300C pull-down reaction. After incubation at 4°C for 2 h, the bound proteins were washed three times with lysis buffer and immunoblotted with antibodies against HA.
Because the E1A binding site of p300 has been previously mapped to the C-terminal region, which is now shown to interact with Smad3, we next tested whether E1A acts to affect TGF-β–induced transcription by competing with Smad3 for p300 binding. Consistent with this model, increasing amounts of bacterially produced 6XHis-tagged E1A decreased the ability of Smad3C to interact with GST-p300C in an in vitro binding assay (Figure 4C). This result implicates a mechanism by which E1A antagonizes TGF-β–mediated transcriptional activation and growth inhibition through its competition with Smad3 for binding to the coactivator p300.
DISCUSSION
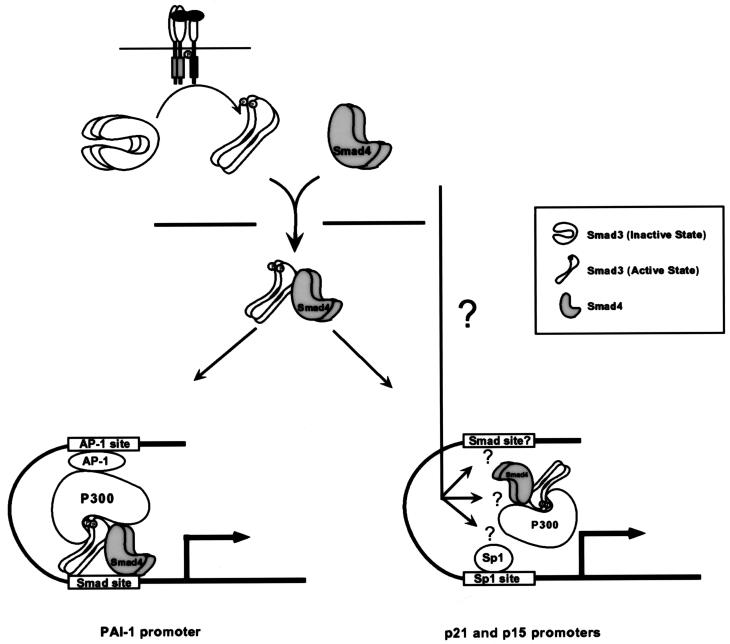
In this report, we present data supporting a model for the mechanism by which Smads function to activate transcription through a TGF-β–regulated interaction with coactivator p300/CBP. In this model (Figure 5), TGF-β treatment initiates a kinase cascade that results in the phosphorylation of Smad3, followed by its heteromerization with Smad4 and subsequent translocation into the nucleus. Once in the nucleus, phosphorylated Smad3 can interact with the coactivator p300/CBP, and likely other transcription factors, to activate transcription from TGF-β target genes. In this sequence of signaling events, the differential association of Smad3 with p300/CBP in a temporal and phosphorylation-dependent manner plays a key role in the regulatory mechanism by which TGF-β activates the transcription of downstream genes. In this context, E1A can prevent the Smad3-dependent activation of target promoters by competing with Smad3 for p300/CBP binding. This model is supported by three recent reports demonstrating the interaction between Smad2 or Smad3 and p300/CBP (Feng et al., 1998; Janknecht et al., 1998; Topper et al., 1998).
Figure 5.
Proposed model for Smad-dependent TGF-β signal transduction pathway. TGF-β treatment initiates a receptor kinase cascade that results in the phosphorylation of Smad3. The phosphorylation of Smad3 weakens the interaction between the N and C terminal regions of Smad3, enabling its interaction with Smad4 and subsequent nuclear translocation of the complex. Once in the nucleus, Smad3 is able to associate with p300 because of the phosphorylation-induced unmasking of the p300 interaction region of Smad3. The Smad3-p300/CBP interaction synergizes with AP1-p300/CBP interaction to activate transcription for PAI-1 promoter. The mechanism underlying the TGF-β–induced transactivation of the p21 and p15 promoters may require both the Smad3-p300/CBP interaction and additional signals that may modulate the interaction between Sp1 and the rest of the transcriptional complex.
Transcriptional activation in general can be regulated at multiple levels: de novo synthesis of a transcription factor, translocation of the transcription factor from cytosol to nucleus, or posttranslational modification. In the case of Smad-mediated signaling, both changes in localization and phosphorylation play a role in their ability to transactivate downstream genes during TGF-β treatment. One model suggests that phosphorylation of the three C-terminal serine residues on Smad2 (SSVS) by the TGF-β type I receptor changes Smad2 conformation to a state in which the Smad2 N-terminal arm, which normally acts to inhibit its biologically active C terminus, dissociates from the C terminus. This, in turn, promotes the association of phosphorylated Smad2 with Smad4 and subsequent translocation of the complex into the nucleus (Hata et al., 1997). Building on this working model, our results suggest that aside from its role in complex formation and nuclear translocation, phosphorylation-induced conformational change is also important for Smad3 nuclear function in terms of promoting interaction with p300/CBP. This may be explained by the possibility that the p300-binding domain of Smad3 is masked when it is in an unphosphorylated autoinhibited conformation. As for Smad4, we were unable to show that endogenous Smad4 interacts with p300/CBP during TGF-β treatment, probably because of the lack of phosphorylation during the treatment; however, because Smad4 is known to interact with Smad3 in a DNA binding complex during TGF-β treatment, it is very likely that Smad4 is contained in a functional complex containing both p300/CBP and Smad3. The detection of Smad4 in such a complex may be difficult in our pull-down experiments because the interaction is through Smad3. Furthermore, an excess amount of GST-p300C could potentially interfere with the association between Smad3 and Smad4.
Once in the nucleus, Smads may cooperate with the coactivator p300/CBP, as well as other transcription factors, to recruit the basal transcriptional machinery to the promoter to initiate transcription. Smads may direct the formation of such higher order complexes to specific promoters through their direct binding to specific DNA sequences (Chen et al., 1997; Yingling et al., 1997; Dennler et al., 1998), as well as potentially to other transcription factors, such as Fos and Jun of the AP-1 complex, a possibility implicated by our previous work suggesting a necessary functional interaction between Smads and AP-1 in the transactivation of the 3TP-Lux reporter (Yingling et al., 1997). Indeed, both the p3TP-Lux and PAI-1 promoters contain Smad-specific binding sequences as well as AP-1 elements that appear to be important in modulating the TGF-β and Smad-dependent responses (Yingling et al., 1997; Dennler et al., 1998). It is also worth noting that both Fos and Jun can directly associate with p300/CBP (Arias et al., 1994) and consequently strengthen the interactions among different components in the preinitiation complex. After it is recruited to specific promoters, p300/CBP may also help to stabilize the preinitiation complex by making additional contacts with TBP and TFIIB (Kwok et al., 1994; Swope et al., 1996; Dallas et al., 1997). Recent studies have suggested an important enzymatic function for p300/CBP as a histone and protein acetyltransferase, paramount to its ability to initiate transcription (Ogryzko et al., 1996; Gu and Roeder, 1997). In this model, binding of p300/CBP to transcription factors, such as Smad3/Smad4 and Jun/Fos, may allow its acetyltransferase activity to acetylate surrounding histones, thereby loosening the chromatin and increasing the accessibility of the preinitiation complex to DNA.
Many other transcription factors also require p300 and CBP for transcriptional activation (Arias et al., 1994; Bhattacharya et al., 1996; Chakravarti et al., 1996; Kamei et al., 1996). Because cellular concentrations of p300 and CBP are limited, one would expect that these transcription factors will compete for p300 and CBP. This has been demonstrated in steroid hormone signaling where overexpression of the nuclear receptor for steroid hormone can inhibit phorbol-ester–activated transcription from AP-1 sites by competing for p300 and CBP (Kamei et al., 1996). Consistent with this, as well as with the finding that a specific region of Smad3 can interact strongly with p300/CBP, transcriptional activation of multiple TGF-β–responsive promoters was dramatically inhibited during Smad3C overexpression (Figure 3), suggesting that p300/CBP may play a critical role in TGF-β signaling. In contrast to the constitutively active Smad2C reported in a previous study (Baker and Harland, 1996), overexpressed Smad3C is inhibitory in our experimental system, possibly because of its sequestration of p300/CBP. This discrepancy could reflect the different molecular characteristics of Smad2 and Smad3 as reported recently: the opposite effect of Smad2 and Smad3 on the transcription of mouse goosecoid gene through binding to FAST2 (Labbe et al., 1998). In addition, different expression levels of these two proteins in the two assaying conditions could also lead to a different outcome in those functional assays. In our transient transfection experiment, for example, an inhibitory effect is observed only when >0.5 μg of Smad3C is transfected; below this amount of transfected DNA, transcription on 3TP-Lux reporter actually increases slightly (our unpublished results).
Functional disruption of Smads and p300/CBP is thought to contribute to the loss of cell cycle control and carcinogenesis (Muraoka et al., 1995; Borrow et al., 1996; Eppert et al., 1996; Hahn et al., 1996). In this regard, the exact role of Smads in the mediation of the growth inhibitory effect of TGF-β, and their connection to transcriptional activation of p15 and p21, two important effectors in TGF-β–mediated growth arrest, are just beginning to be understood. The squelching effect of Smad3C on the transcriptional activation of p15 minimal promoter in response to TGF-β suggests that Smad3 may be required for TGF-β–induced expression of the p15 gene, and consequently TGF-β–mediated cell cycle arrest. To test this possibility, we cotransfected Smad2, Smad3, and Smad4 in various combinations to determine whether overexpression of Smads can activate transcription of the two Cdk inhibitor genes, in comparison to that of the positive control, the PAI-1 promoter. Our results indicate that overexpression of Smad3 and Smad4, or other combinations of different Smads, could not potentiate transcription from the p15 and p21 promoters, whereas the PAI-1 promoter is greatly activated by Smad3 and Smad4 overexpression (our unpublished results). This result is in contrast to the recent report that Smad3 and Smad4 coexpression could potently activate the p21 promoter in a hepatic cancer line, HepG2 (Moustakas and Kardassis, 1998). The discrepancy between the two apparently opposite results is most likely due to the difference in the cell types used in the studies. It is conceivable that a putative, essential signal that acts in conjunction with overexpressed Smads to initiate transcription of the p21 promoter is constitutively active in the HepG2 cells, and in contrast, is only TGF-β–inducible in the HaCaT cells used in this study. This hypothesis is consistent with the observation reported by Moustakas et al. that expression of endogenous p21, as well as activation of the p21 promoter luciferase construct, is constitutively high in HepG2 cells, whereas in HaCaT cells, endogenous p21 levels are barely detectable in untreated culture yet markedly induced by TGF-β. Therefore, although the function of Smads as intermediates of TGF-β signaling may be essential for multiple pathways, the mode of their involvement in transcriptional activation of specific target genes may mechanistically differ in various cell types. In addition, within a distinct cell type such as HaCaT cells, specific TGF-β–responsive genes may require different stimuli for p300-dependent transcription to occur. For example, in HaCaT cells, Smad overexpression alone is sufficient to stimulate transcription of the PAI-1 promoter, yet not that of p21 or p15 genes. For these promoters, Smad overexpression and subsequent nuclear translocation is only one essential component of the complete TGF-β signal. Other distinct, yet to be defined signaling events that are apparently constitutively active in HepG2 cells, yet only TGF-β inducibly so in HaCaT cells, are also required to cooperate with Smads/p300/CBP to fully activate transcription from these promoters.
Combined with other studies, our results suggest a general strategy by which signal-dependent transcriptional activation can occur for a once seemingly disparate group of transcription factors that include Smads, Stats, and NF-κB (Darnell, 1997; Zhong et al., 1998). During stimulation by specific external signals, these transcription factors are phosphorylated and change conformation, form complexes with partner proteins or dissociate from inhibitory sequestration, and translocate from the cytosol into the nucleus. Once in the nucleus, they bind to the coactivator p300 or CBP in a phosphorylation-dependent manner to activate transcription. This general transcriptional activation strategy may be an evolutionarily conserved mechanism that transduces extracellular stimuli into a prompt transcriptional response.
ACKNOWLEDGMENTS
We thank Rik Derynck for his generous gifts of Smad constructs, and Yang Shi for his generous gifts of GST-p300 constructs. TGF-β1 was kindly provided by Amgen, Inc. We thank Yong Yu for technical assistance and members of the Wang Lab for helpful discussion. This work was supported by grant DK-45746 from National Institutes of Health. P.P.H. and N.T.L. were supported by predoctoral fellowships from the National Science Foundation. J.P.F. was supported by a predoctoral fellowship from the Department of Defense. X.-F.W. is a Leukemia Society Scholar.
REFERENCES
- Abraham S E, Carter MC, Moran E. Transforming growth factor beta1 (TGF-beta1) reduces cellular levels of p34cdc2 and this effect is abrogated by adenovirus independently of the E1A-associated pRb binding activity. Mol Biol Cell. 1992;3:655–665. doi: 10.1091/mbc.3.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- Baker J, Harland R. A novel mesoderm inducer, Madr2, functions in the activin signal transduction pathway. Genes Dev. 1996;10:1880–1889. doi: 10.1101/gad.10.15.1880. [DOI] [PubMed] [Google Scholar]
- Barrett MT, Schutte M, Kern SE, Reid BJ. Allelic loss and mutational analysis of the DPC4 gene in esophageal adenocarcinoma. Cancer Res. 1996;56:4351–4353. [PubMed] [Google Scholar]
- Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D’Andrea A, Livingston DM. Cooperation of Stat2 and p300/CBP in signaling by interferon. Nature. 1996;383:344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- Borrow J, et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM. Role of CBP/p300 in nuclear receptor signaling. Nature. 1996;383:99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- Chen X, Weisberg E, Fridmacher V, Watanabe M, Naco G, Whitman M. Smad4 and FAST-1 in the assembly of activin-responsive factor. Nature. 1997;389:85–89. doi: 10.1038/38008. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kowk RPS, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Dallas PB, Yaciuk P, Moran E. Characterization of monoclonal antibodies raised against p300: both p300 and CBP are present in intracellular TBP complexes. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JEJ. STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Datto MB, Hu PP-C, Kowalik TF, Yingling JM, Wang X-F. The viral oncoprotein E1A blocks transforming growth factor β-mediated induction of p21/WAF1/Cip1 and p15/INK4B. Mol Cell Biol. 1997;17:2030–2037. doi: 10.1128/mcb.17.4.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto MB, Li Y, Panus J, Howe DJ, Xiong Y, Wang X-Y. TGF-β mediated growth inhibition is associated with induction of the cyclin-dependent kinase inhibitor, p21. Proc Natl Acad Sci USA. 1995a;92:5545–5549. doi: 10.1073/pnas.92.12.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto MB, Yu Y, Wang X-F. Functional analysis of the transforming growth factor β responsive elements in the WAF1/Cip1/p21 promoter. J Biol Chem. 1995b;270:28623–28628. doi: 10.1074/jbc.270.48.28623. [DOI] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGFb-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R, Ewen ME, Newsome D, Gerdes M, DeCaprio JA, Lawrence JB, Livingston DM. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- Eppert K, et al. MADR2 maps to 18q21 and encodes a TGFβ-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- Feng X-H, Zhang Y, Wu R-Y, Derynck R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for Smad3 in TGF-β-induced transcriptional activation. Genes Dev. 1998;12:2153–2163. doi: 10.1101/gad.12.14.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Hahn SA, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Hata A, Lo RS, Wotton D, Lagna G, Massague J. Mutations increasing autoinhibition inactivate tumor suppressors Smad2 and Smad4. Nature. 1997;388:82–87. doi: 10.1038/40424. [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hu PP-C, Datto MB, Wang X-F. Molecular mechanisms of transforming growth factor-β signaling. Endocr Rev. 1998;19:349–363. doi: 10.1210/edrv.19.3.0333. [DOI] [PubMed] [Google Scholar]
- Janknecht R, Wells NJ, Hunter T. TGF-β-stimulated cooperation of Smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- Kim J, Johnson K, Chen HJ, Carroll S, Laughon A. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature. 1997;388:304–308. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- Kim SK, Fan Y, Papadimitrakopoulou V, Clayman G, Hittelman WN, Hong W, Lotan R, Mao L. DPC4, a candidate tumor suppressor gene, is altered infrequently in head and neck squamous cell carcinoma. Cancer Res. 1996;56:2519–2521. [PubMed] [Google Scholar]
- Kraus VB, Moran E, Nevins JR. Promoter-specific trans-activation by the adenovirus E1A12S product involves separate E1A domains. Mol Cell Biol. 1992;12:4391–4399. doi: 10.1128/mcb.12.10.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok RPS, Lundblasd JR, Chrivia JC, Richards JP, Bachinger HP, Brennan RG, Roberts SGE, Green MR, Goodman RH. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- Labbe E, Silvestri C, Hoodless PA, Wrana JL, Attisano L. Smad2 and Smad3 positively and negatively regulate TGFβ-dependent transcription through the forkhead DNA-binding protein FAST2. Mol Cell. 1998;2:109–120. doi: 10.1016/s1097-2765(00)80119-7. [DOI] [PubMed] [Google Scholar]
- Lagna G, Hata A, Hemmati-Brivanlou A, Massague J. Partnership between DPC4 and SMAD proteins in TGF-beta signaling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- Lee J-S, Galvin KM, See RH, Eckner R, Livingston DM, Moran E, Shi Y. Relief of YY1 transcriptional repression by adenovirus E1A is mediated by E1A-associated protein p300. Genes Dev. 1995;9:1188–1198. doi: 10.1101/gad.9.10.1188. [DOI] [PubMed] [Google Scholar]
- Li JM, Datto MB, Shen X, Hu PP, Yu Y, Wang XF. Sp1, but not Sp3, functions to mediate promoter activation by TGF-β through canonical Sp1 binding sites. Nucleic Acids Res. 1998a;26:2449–2456. doi: 10.1093/nar/26.10.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-M, Nichols MA, Chandrasekharan S, Xiong Y, Wang X-F. Transforming growth factor β activates the promoter of cyclin-dependent kinase inhibitor p15INK4B through an Sp1 consensus site. J Biol Chem. 1995;270:26750–26753. doi: 10.1074/jbc.270.45.26750. [DOI] [PubMed] [Google Scholar]
- Li J-M, Shen X, Hu PP, Wang XF. Transforming growth factor β stimulates the Human Immunodeficiency Virus 1 enhancer and requires NF-κB activity. Mol Cell Biol. 1998b;18:110–121. doi: 10.1128/mcb.18.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RM, Moses HL. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem. 1990;187:467–473. doi: 10.1111/j.1432-1033.1990.tb15327.x. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Abdollah S, Hoodless P, Pirone R, Attisano L, Wrana J. MADR2 is a substrate of the TGF-β receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- Massague J. The transforming growth factor-B family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;17710:28491–19244. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missero C, Filvaroff E, Dotto GP. Induction of transforming growth factor 1 resistance by the E1A oncogene requires binding to a specific set of cellular proteins. Proc Natl Acad Sci USA. 1991;88:3489–3493. doi: 10.1073/pnas.88.8.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Kardassis D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci USA. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong J-M, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1995;12:1565–1569. [PubMed] [Google Scholar]
- Nagatake M, Takagi Y, Osada H, Uchida K, Mitsudomi T, Saji S, Shimokata K, Takahashi T. Somatic in vivo alterations of the DPC4 gene at 18q21 in human lung cancers. Cancer Res. 1996;56:2718–2720. [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional activators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Pietenpol JA, et al. TGF-β1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990;61:777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Schutte M, et al. DPC4 gene in various tumor types. Cancer Res. 1996;56:2527–2530. [PubMed] [Google Scholar]
- Swope DL, Mueller CL, Chrivia JC. CREB-binding protein activates transcription through multiple domains. J Biol Chem. 1996;271:28138–28145. doi: 10.1074/jbc.271.45.28138. [DOI] [PubMed] [Google Scholar]
- Topper JN, DiChiara MR, Brown JD, Williams AJ, Falb D, Collins T, Gimbrone MA., Jr CREB binding protein is a required coactivator for Smad-dependent, transforming growth factor β transcriptional responses in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9506–9511. doi: 10.1073/pnas.95.16.9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Rikitake Y, Carter MC, Yaciuk P, Abraham SE, Zerler B, Moran E. Identification of specific adenovirus E1A N-terminal residues critical to the binding of cellular proteins and to the control of cell growth. J Virol. 1993;67:476–488. doi: 10.1128/jvi.67.1.476-488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang X-F, Massague J. TGF-B signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Yingling JM, Das P, Savage C, Zhang M, Padgett RW, Wang X-F. Mammalian dwarfins are phosphorylated in response to transforming growth factor β and are implicated in control of cell growth. Proc Natl Acad Sci USA. 1996;93:8940–8944. doi: 10.1073/pnas.93.17.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling JM, Datto MB, Wong C, Frederick JP, Liberati NT, Wang X-F. The tumor suppressor, Smad-4, is a TGF-beta inducible, DNA binding protein. Mol Cell Biol. 1997;17:7019–7028. doi: 10.1128/mcb.17.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Feng X-H, Wu R-Y, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Musci T, Derynck R. The tumor suppressor Smad4/DPC4 as a central mediator of Smad function. Curr Biol. 1997;7:270–276. doi: 10.1016/s0960-9822(06)00123-0. [DOI] [PubMed] [Google Scholar]
- Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]