Abstract
Over the last decade there has been an enormous expansion of research focused on defining the role of inflammation in aging, age-related diseases, disability, and frailty. The availability of methods to measure cytokines and other inflammatory mediators or markers with high sensitivity and specificity is critically important. Enzyme-Linked Immuno-Sorbant Assay (ELISA), the most widely used and best validated method is limited by its ability to measure only a single protein in each sample. Recent developments in serum cytokine quantification technology include multiplex arrays. Multiplex Arrays offer the potential of better evaluating the complexity and dynamic nature of inflammatory responses and offer substantial cost and sample savings over traditional ELISA measurements. Despite potential advantages of this new technology, experience with these techniques is limited and it has not emerged to date as the gold standard in inflammatory mediator measurement. This article reviews ELISA and the emerging multiplex technologies, compares the cost and effectiveness of recently developed multiplex arrays with traditional ELISA technology, and provides specific recommendations for investigators interested in measuring serum inflammatory mediators in older adults.
Keywords: ELISA, Multiplex arrays, cytokines, inflammation, aging
INTRODUCTION
The last decade has seen an enormous surge in research focused on the relationship between inflammation and aging, age-related diseases, disability, and frailty(1;2). A large body of literature demonstrates that inflammation, defined as an elevation of serum levels of inflammatory factors in studies of older adults, is strongly associated with atherosclerosis, sarcopenia, osteoporosis, Alzheimer's disease, anemia, and many other age-related pathophysiologic processes and diseases(1-3). Other studies have shown that elevated inflammatory markers are associated with age-related functional decline and frailty(4-6). The activation and propagation of inflammation in older adults involves multiple soluble mediators, that include cytokines, chemokines, C-reactive protein (CRP), and other inflammatory factors(7;8). The ability to reliably measure serum based inflammatory mediators, along with the advancement of cellular and molecular immune-based measurement technologies, has tremendously enhanced the state of research in this field.
Most previous aging cohort studies have reported cross-sectional cytokine levels that were measured at one point in time using the traditional ELISA method. Inflammatory responses to specific insults involve a cascade of well-defined and distinct cellular and molecular events. Moreover, inflammation is a highly dynamic and interactive process(7;8). Therefore, such cross sectional single cytokine measurements likely do not reflect the true complexity of relevant inflammatory processes in vivo. In addition, ELISA frequently requires high volumes of serum and cost can be prohibitive in a research setting. These facts, along with limited availability of stored biological samples from most aging cohort studies has restricted investigators' ability to systematically evaluate the full spectrum of inflammatory mediators and their contribution to aging, age-related diseases, disability, and frailty. Recently, bead-based and electrochemiluminescence-based multiplex assays have been developed and promoted for requiring far less subject serum per measurement, and for being far more cost effective than traditional ELISA measurements. This review will first review both the ELISA and Multiplex measurement technology, and will then compare the technical and cost advantages and disadvantages of each approach. Given the absence of broadly accepted standards for normal age-adjusted cytokine values and the resulting difficulties in comparing inflammatory cytokine measurements between populations, our review will also address methodological issues which may increase the variability of reported cytokine levels. Finally, accepted laboratory practices which help ensure consistency as regards cytokine measurements will be reviewed, particularly when an assay is adapted by another laboratory or when a new assay platform (e.g. multiplex) is introduced. Thus, the goal of this review is to provide information which will permit gerontologists to make informed choices and to obtain optimal data in their inflammation and aging studies.
ELISA
Radioimmunoassay (RIA) was first described by Yalow and Berson in 1959, a discovery for which they won the 1997 Nobel Prize in Physiology or Medicine(9). In search for alternative labels to replace radioactive isotopes, Enzyme-Linked ImmunoSorbent Assay (ELISA) was introduced in the 1970s(10;11). In the typical double antibody sandwich ELISA (Fig. 1A), antibody attached to the bottom of a well provides both antigen capture and immune specificity, while another antibody linked to an enzyme provides detection and an amplification factor. This approach enables accurate and sensitive detection of the antigen, the cytokine of interest. Because of these desirable features, ELISA has been considered the standard cytokine measurement method and is widely utilized in clinical laboratories and biomedical research. ELISA kits for commonly measured cytokines are commercially available, often from multiple vendors. Additional advantages of ELISA include the fact that results are highly quantitative and generally reproducible.
Figure 1.
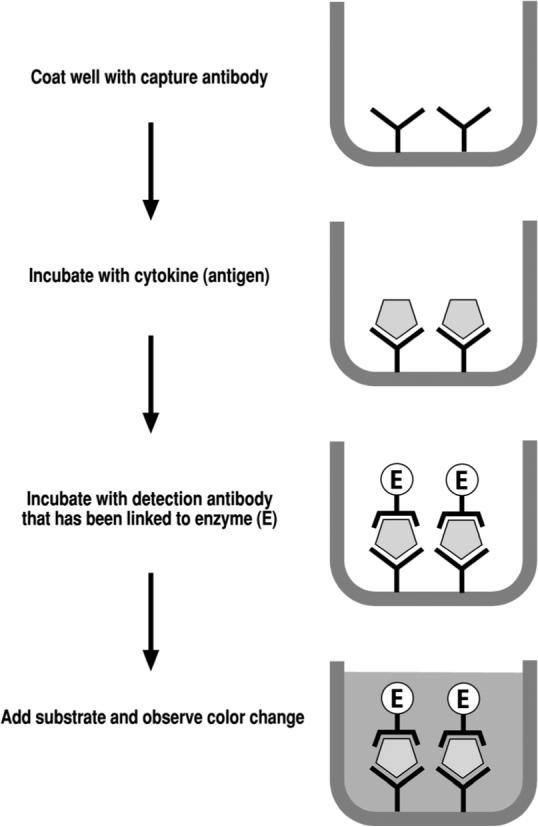

Schematic illustration of the experimental principle for ELISA and bead-based multiplex assays. Both involve capture antibody and detection antibody specific to the cytokine(s) of interest, offering immunological specificity. Enzyme or fluorochrome linked to the detection antibody is the method of detection with signal amplification, offering sensitivity. Panel A illustrates the basic protocol for traditional double antibody sandwich ELISA. Proprietary bead sets provide additional differential detection power in bead-based multiplex arrays (Panel B).
At the same time, several weaknesses have been recognized for this method. ELISA performance is largely dependent on antibody quality, kit manufacturer, as well as operator skills and experience(12). In addition, ELISA permits the measurement of only one cytokine at a time in a given sample aliquot. As discussed earlier, this shortcoming limits the ability of ELISA to meet investigators' needs to include ever-growing numbers of inflammatory molecules in their studies. This concern becomes especially acute when such studies require access to limited amounts of biological material obtained from older adults in the course of longitudinal studies. Difficulties also exist in comparing two cytokine levels measured by two different ELISA assays, each under somewhat different conditions.
Another limitation of ELISA-based assays is that the dynamic range (range over which there is a linear relationship between the cytokine concentration and the absorbance reading) is narrow relative to the range for other technologies such as multiplex assays. Thus, samples with cytokine concentrations above the dynamic range have to be diluted for the assay. Dilution not only reduces the concentration of the cytokine being measured, but may also diminish the concentration of any circulating inhibitors or binding proteins. This is particularly relevant to the use of serum samples, exaggerating differences between samples that have cytokine levels within the dynamic range (do not require dilution in the assay) and samples above the dynamic range (do require dilution).
MULTIPLEX ARRAYS: Technology Review and Advantages
Multiplex arrays have been recently developed from traditional ELISA assays with the purpose of measuring multiple cytokines in the same sample at the same time. They are available in several different formats based on the utilization of flow cytometry, chemiluminescence, or electrochemiluminescence technology. Flow cytometric multiplex arrays, also known as bead-based multiplex assays, represent probably the most commonly used format at the present time. As shown in Fig. 1B, the cytometric bead array (CBA) system from BD Biosciences (www.bdbiosciences.com) and the Luminex multi-analyte profiling (xMAP) technology from Luminex (www.luminexcorp.com) both employ proprietary bead sets which are distinguishable under flow cytometry. Each bead set is coated with a specific capture antibody, and fluorescence or streptavidin-labeled detection antibodies bind to the specific cytokine-capture antibody complex on the bead set. Multiple cytokines in a biological liquid sample can thus be recognized and measured by the differences in both bead sets, with chromogenic or fluorogenic emissions detected using flow cytometric analysis. Commercially available bead-conjugated antibodies permit the measurement of up to 25 different cytokines in the same sample. However, this number can be greatly expanded if the investigator is willing to custom-conjugate antibodies of interest to one of nearly 100 different available beads. Multiplex ELISA from Quansys Biosciences (www.quansysbio.com) coats multiple specific capture antibodies at multiple spots (one antibody at one spot) in the same well on a 96-well microplate. Chemiluminescence technology, which is more sensitive than chromogenic detection in traditional ELISA, is then employed to detect multiple cytokines at the corresponding spots on the plate. Multiplex kits from Meso Scale Discovery (www.mesoscale.com) employ electrochemiluminescence technology with multiple specific capture antibodies coated at corresponding spots on an electric wired microplate. The detection antibody is conjugated to a proprietary tag which is excited with emission beams in the electric field. The proprietary co-reactant in the “read buffer” then further amplifies the signal. Without utilizing the enzymatic or fluorescent detection system, electrochemiluminescence-based multiplex arrays avoid time-dependent signal decay issues. With the later two multiplex formats, up to 9 cytokines can be measured in one sample.
Compared with traditional ELISA, multiplex arrays have a number of advantages including: a) high throughput multiplex analysis; b) less sample volume needed, c) efficiency in terms of time and cost,; d) ability to evaluate the levels of one given inflammatory molecule in the context of multiple others; e) ability to perform repeated measures of the same cytokine panels in the same subjects under the same experimental assay condition; f) ability to reliably detect different proteins across a broad dynamic range of concentrations.
MULTIPLEX ASSAY TECHNOLOGICAL CONSIDERATIONS
In spite these advantages, caution is necessary when considering the application of multiplex arrays in inflammation and aging research. This section details some of the critical issues that have to date prevented this technology from emerging as the gold standard in inflammatory cytokine measurement.
Experience with multiplex arrays remains limited, particularly in the context of human aging studies. Although good correlations between ELISA and multiplex have been reported(13;14), careful side-by-side comparisons are rare. In addition, while concordance between ELISA and multiplex is generally good when using tissue culture supernatant samples, it is much less robust when using serum or plasma samples(15).
Multiplex assays, by their very nature, involve potential interactions between multiple different antibodies and cytokines (antigens) in the sample/assay solution. One cannot assume that a reliable uniplex assay can just be simply added to a functioning multiplex assay. Non-reactivity to all other antibodies must first be established and the lowest amount possible must be used to minimize such cross-reactions. In the authors' experience (unpublished data), some commercially available multiplex arrays may not generate desirable standard curve. Problems can also arise from presence of a broad and varying dynamic range in terms of concentrations of the different proteins being assayed together.
Certain proteins in biological samples, particularly abundant circulating proteins in serum or plasma samples, may affect multiplex results. In the bead-based multiplex arrays, it is important to note that all reactions take place among molecules and antigens which are freely mobile in solution, while ELISA involves the immobilization of the capture antibody and thus of the resultant antigen-antibody-enzyme complexes to the bottom of plastic wells. With these considerations in mind, it is not surprising that these multiplex arrays appear to be much more sensitive than are ELISAs to altered levels of circulating proteins and inhibitors (unpublished data). As many such abundant circulating proteins may change their levels during aging, inflammation or diseases, this can obviously further complicate the picture.
In the above context, it is also important to consider the preparation of biological sample from peripheral blood since serum and plasma are not equivalent(16). Plasma preparation is associated with the removal of fibrinogen, von Willebrand factor and many other proteins, including circulating proteins such as cytokines which often bind to these blood components. In fact, studies indicate that results obtained for even routine biochemistries may differ in serum as opposed to plasma (16-18). Moreover, while cytokine levels obtained via ELISA from serum and plasma may be similar, great attention must be paid to the release of inflammatory mediators from cellular elements during the process of coagulation since release of substances such as VEGF from platelets may strikingly affect cytokine levels (16). However, in the authors' experience (unpublished data), even with careful sample preparation, significant discordance between cytokine levels obtained from serum and plasma is more likely to occur when using Multiplex assays as opposed to ELISA(15). Broader consideration must also be given to the fact that many inflammatory markers which are of great interest to geriatrics (e.g. IL-6(19), TNF-α(20) and others(21) have been shown to bind to circulating carrier proteins such as α2-macroglobulin(19-21). Levels of circulating proteins such as α2-macroglobulin can change with inflammation, aging, disease or frailty, as well as specific assay conditions. Thus, all of these factors could greatly influence the ability of multiplex solution-based assays to detect these specific cytokines by potentially altering the amount of free cytokine available for detection, independently of changes in total cytokine levels. Our own recent experience (unpublished data) has confirmed some of these concerns, demonstrating the ability of denaturing agents which are known to decrease noncovalent binding between proteins, to significantly increase the amount of cytokine detected using Multiplex assays.
Similarly to the multiple comparison issues when conducting microarray data analysis, multiplex data interpretation can be challenging, requiring careful knowledge of the molecular pathways that lead to cytokine regulation, and careful attention to both study design and data analysis.
FINANCIAL CONSIDERATIONS
The use of multiplex technology requires an investment in both equipment and disposable supplies. Nevertheless, once 4 or more cytokines are measured, overall multiplex assay costs are lower than if one chooses to obtain the same information using separate ELISA assays. In addition to requiring smaller sample volumes, multiplex technology also offers savings in terms of time required to complete the assay and decreased technician time. For example, for an investigator wishing to measure four inflammatory biomarkers (IL-1β, IL-6, TNF-α, and IFN-γ), the cost for measuring these four biomarkers in duplicate using individual ELISAs is $61.53 or more per sample and would require a total of 1,100 μl of serum or plasma (Table I). Moreover, each of the four separate assays requires 6 hours, including aliquoting, antibody incubations, washing, absorbance reading and data reduction – so a total of 24 hours of technician time would be required for the four assays. In contrast, Multiplex assays enable multiple determinations to be made simultaneously in the same sample. For example, a typical ‘4-plex’ Multiplex assay system which can determine the same four inflammatory biomarkers entails a cost of $19.20 per sample and requires only 25 μl of serum or plasma (Table I). Thus, an investigator would save $42.33 per sample in terms reagent cost. Additional financial savings in terms of technician time stem from the fact that the multiplex assay can be completed in less than 1/4th of the time required for the completion of 4 separate ELISAs. Finally, sample savings are also substantial, with more than 1 ml (1,050 μl) of serum saved in this example.
Table 1.
ELISA and Multiplex Cost Comparison
| ELISA | Multiplex | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Assay | $/sample* | Company | Sample size | time | Assay | $/sample* | Company | Sample size | time |
| IL-1b HS | $16.53 | R&D Systems | 150 μl | 6h | IL-1b HS | ||||
| IL-6 HS | $16.53 | R&D Systems | 100 μl | 6h | IL-6 HS | $19.20 | MSD | 25 μl | 4.5 h |
| TNF-α | $16.53 | R&D Systems | 200 μl | 6h | TNF-α | ||||
| IFN-γ | $11.94 | R&D Systems | 100 μl | 6h | IFN-γ | ||||
| Totals | $61.53 | per sample | 1100μl | 24h | Totals | $19.20 | per sample | 50 μl | 6h |
Cost per sample is determined by taking the commercial cost of the immunoassay kit and dividing by the number of samples that can be analyzed in duplicate (between 36 and 40, for most immunoassays depending on number of standards and controls run in each assay).
Despite these substantial savings in assay costs, initial investment in multiplex assay equipment costs can be substantial. For example, Luminex technology-based systems require a dedicated analyzer which begins in the $60,000 range, while self-contained multiplexed ELISA systems (Randox Laboratories and MesoScale Discovery) range in price from $90,000 to $140,000. As a result, at many institutions, such equipment is viewed as a shared core resource and is accessible to multiple investigators. In contrast, singleplex ELISA plate readers can cost from between $6,000 to $20,000 depending upon their capabilities (the number of filters for measuring absorbance, ability to measure chemiluminescent or fluorescent signals, ability to stack multiple plates and batch analyze, etc.). Similarly, a singleplex commercial 96-well plate ELISA can cost $350−$700, while a 96-well plate multiplex assay range $600−$1,200, depending on the number of analytes being measured. Thus, multiplexing involves higher costs for hardware.
GOOD LABORATORY PRACTICES
Irrespective of the technique used or the setting in which such measurements are performed, a set of principles defined by the FDA as Good Laboratory Practices (GLP) must be followed in the planning, performing, monitoring, recording, reporting and archiving of all laboratory studies. Quality assurance (QA) represents those activities which are designed to prevent quality problems, optimizing assay precision and accuracy(11). Quality control (QC) describes tests that are applied to individual assays to check the validity of the results. As regards cytokine measurements, standardization is the process of ensuring that all methods for determining the concentration of a particular analyte give the same result, while calibration is the process of assigning values to unknown samples using a standard(11).
Good QA and QC require that controls and standard curves be run with each individual assay so that the inter-assay coefficient of variance can be monitored. Similarly, controls need to be run multiple times in a single assay so that the intra-assay coefficient of variance can be monitored. Monitoring must be ongoing since a drift in the %CV suggesting either increasing inter- or intra-assay variability flags an assay as being questionable. Since there are no WHO (World Health Organization)-accepted standards for normal age-adjusted cytokine levels, comparing results obtained using single ELISA kits from different manufacturers or comparing single-plex to multiplex results can be problematic. An approach designed to get around this issue which has been used by growing numbers of investigators and some manufacturers has been to utilize internal standards, as well as reagents including capture and detection antibodies licensed from R & D Systems, Inc. This approach permits the “standardization” of data to that obtained using the most commonly-cited system (e.g. R&D). Such controls are samples ideally in the same matrix (serum, plasma or saliva) and have a ‘known’ concentration of a particular analyte. Increasingly, these controls are included with commercial kits, but they can also be purchased separately from companies such as Biorad. Such internal and cross-assay comparisons become particularly important when switching from one assay platform to another (e.g. Single ELISA assay to Multiplex).
The design and interpretation of standard curves, especially at concentration extremes represents an additional challenge. In addition to an evaluation of the most current standard curve it is important to generate a standard curve “graveyard” where it is overlaid on top of the historical pattern of all previous curves for a give assay Flexible curve fitting software programs permit both the calculation of ED20 (“estimated dose at 20%”), 50 and 80 for each assay, as well as the back-calculation for all of the standards, providing data which can be used for QC purposes as a means of tracking assay performance. Nevertheless, great caution must be used when interpreting standard curve data results obtained by singleplex ELISA/plate reader, Multiplex reader or RIA software. In all cases, values can be calculated based on a poor or suboptimal standard curve. Log-log transformations of the immunoassay standards are notorious for yielding data skewed at the low or at the high end. Ideally a weighted four parameter logistic regression is used for fitting the standards and calculating control and unknown values. Moreover, it is important to recheck the regression analysis performed in some assays to ensure accuracy.
Interpretation and reporting of cytokine values at extremely low or high concentrations can be especially problematic. One the advantages of the multiplex systems involving solution hybridization is that the dynamic range of such assays appears to be much broader than is the case for traditional ELISAs involving immobilized antibodies. Nevertheless, many sensitivity issues in the very low range of concentration remain unresolved. Manufacturers of both Luminex technology based multiplexing and multiplexed ELISAs claim sensitivities comparable to those of standard ELISAs. Most recently, high sensitivity ELISAs for IL-1β, -6 and -10 kits (from R&D Systems, Inc.) have been reported as having sensitivity of under 1pg/ml (manufacturer reported sensitivities of 0.1, 0.04 and 0.5 pg/ml, respectively). However, the lowest standard in the high sensitivity IL-6 kit is 0.156 pg/ml. Thus, while a theoretical sensitivity of 0.04 pg/ml is impressive, a laboratory would be disingenuous to report calculated values below the lowest standard. Software can certainly be forced to calculate such values, yet any GLP-compliant laboratory would not report values of such questionable precision, instead referring to such results as being “less then the minimum standard”.
RECOMMENDATIONS
As in the case of all research endeavors, the selection of specific inflammatory mediators for study and the choice of suitable measurement tool(s) should be performed in a manner which addresses clinically compelling questions in a physiologically relevant context. Knowledge of the biology that underlies inflammatory pathway activation is also critical to the selection of specific inflammatory mediators for any particular aging study.
ELISA remains the time-tested and best validated method for measuring individual cytokines.
Multiplex arrays offer opportunities to examine physiologically relevant panel(s) of cytokines or entire “classes” of cytokines in a time and cost-efficient fashion. While the use of such technologies in aging research is still at a very early stage, recent reports highlight their usefulness in the setting of cohort(22;23), cross-sectional(24;25) and longitudinal(25) studies.
Substantial critical measurement issues with multiplex assays remain, and marked caution with data interpretation must continue until multiplex assay results are systematically compared with results from ELISAs. In the meantime, significant multiplex data findings should be confirmed by ELISA whenever possible.
One solution to the multiple comparison issue is to analyze multiplex data via classes of cytokines identified by their properties with a priori hypothesis proposed before the study, as reported in this issue by Rudolph et al. (25);
Acknowledgements
Financial Disclosure: There is no conflict of financial interest, relationships or affiliations other than those listed on the title page.
Grant Support: This work was supported in part by the National Institutes of Health, Paul Beeson Career Development Award in Aging Research K23 AG028963 (SXL), R01 AG027236 (JW), P30 AG021334 (JW, SL), R01 AI068265 (JM, DX, GAK) and R01 AG028657 (GAK, JM, DX).
Footnotes
Role of the Sponsors: None
Reference List
- 1.Ferrucci L, Ble A, Bandinelli S, et al. A flame burning within. Aging Clin Exp Res. 2004 Jun;16(3):240–243. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Guralnik JM. Inflammation, hormones, and body composition at a crossroad. Am J Med. 2003 Oct 15;115(6):501–502. doi: 10.1016/j.amjmed.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007 Jan;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993 Feb;41(2):176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 5.Maggio M, Guralnik JM, Longo DL, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006 Jun;61(6):575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leng SX, Xue QL, Tian J, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007 Jun;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 7.Nathan C. Points of control in inflammation. Nature. 2002 Dec 19;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 8.Mak TW, Saunders ME. Cytokines and Cytokine Receptors. The Immune Response. Basic and Clinical Principals. Elsevier Academic Press; San Diego: 2006. pp. 464–516. [Google Scholar]
- 9.Yalow RS, Berson SA. Assay of plasma insulin in human subjects by immunological methods. Nature. 1959 Nov 21;184(Suppl 21):1648–1649. doi: 10.1038/1841648b0. [DOI] [PubMed] [Google Scholar]
- 10.Crowther JA. The ELISA Guidebook. Humana Press; Totowa, NJ: 2001. [Google Scholar]
- 11.The Immunoassay Handbook. 3rd ed. Elsevier; New York: 2005. [Google Scholar]
- 12.Aziz N, Nishanian P, Mitsuyasu R, et al. Variables that affect assays for plasma cytokines and soluble activation markers. Clin Diagn Lab Immunol. 1999 Jan;6(1):89–95. doi: 10.1128/cdli.6.1.89-95.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006 Apr;38(4):317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan SS, Smith MS, Reda D, et al. Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004 Sep;61(1):35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 15.Prabhakar U, Eirikis E, Reddy M, et al. Validation and comparative analysis of a multiplexed assay for the simultaneous quantitative measurement of Th1/Th2 cytokines in human serum and human peripheral blood mononuclear cell culture supernatants. J Immunol Methods. 2004 Aug;291(1−2):27–38. doi: 10.1016/j.jim.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Lundblad RL. Considerations for the Use of Blood Plasma and Serum for Proteomic Analysis. International Journal of Gastroenterology. 2005;1(2):1–12. [Google Scholar]
- 17.Lum G, Gambino SR. A comparison of serum versus heparinized plasma for routine chemistry tests. Am J Clin Pathol. 1974 Jan;61(1):108–113. doi: 10.1093/ajcp/61.1.108. [DOI] [PubMed] [Google Scholar]
- 18.Ladenson JH, Tsai LM, Michael JM, et al. Serum versus heparinized plasma for eighteen common chemistry tests: is serum the appropriate specimen? Am J Clin Pathol. 1974 Oct;62(4):545–552. doi: 10.1093/ajcp/62.4.545. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda T, Hirano T, Nagasawa S, et al. Identification of alpha 2-macroglobulin as a carrier protein for IL-6. J Immunol. 1989 Jan 1;142(1):148–152. [PubMed] [Google Scholar]
- 20.Crookston KP, Webb DJ, Wolf BB, et al. Classification of alpha 2-macroglobulincytokine interactions based on affinity of noncovalent association in solution under apparent equilibrium conditions. J Biol Chem. 1994 Jan 14;269(2):1533–1540. [PubMed] [Google Scholar]
- 21.Garber TR, Gonias SL, Webb DJ. Interleukin-4 and IL-10 bind covalently to activated human alpha2-macroglobulin by a mechanism that requires Cys949. J Interferon Cytokine Res. 2000 Feb;20(2):125–131. doi: 10.1089/107999000312522. [DOI] [PubMed] [Google Scholar]
- 22.Bozza FA, Salluh JI, Japiassu AM, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007 Apr 21;11(2):R49. doi: 10.1186/cc5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariani E, Cattini L, Neri S, et al. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: relationship with zinc status. Biogerontology. 2006 Oct;7(5−6):449–459. doi: 10.1007/s10522-006-9060-8. [DOI] [PubMed] [Google Scholar]
- 24.Njemini R, Bautmans I, Lambert M, et al. Heat shock proteins and chemokine/cytokine secretion profile in ageing and inflammation. Mech Ageing Dev. 2007 Jul;128(7−8):450–454. doi: 10.1016/j.mad.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Rudolph JL, Ramlawi B, Kuchel GA, et al. Chemokines are associated with delirium after cardiac surgery. Journal of Gerontology, Series A: Biological Sciences and Medical Sciences. 2007 doi: 10.1093/gerona/63.2.184. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]


