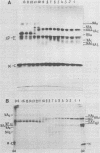
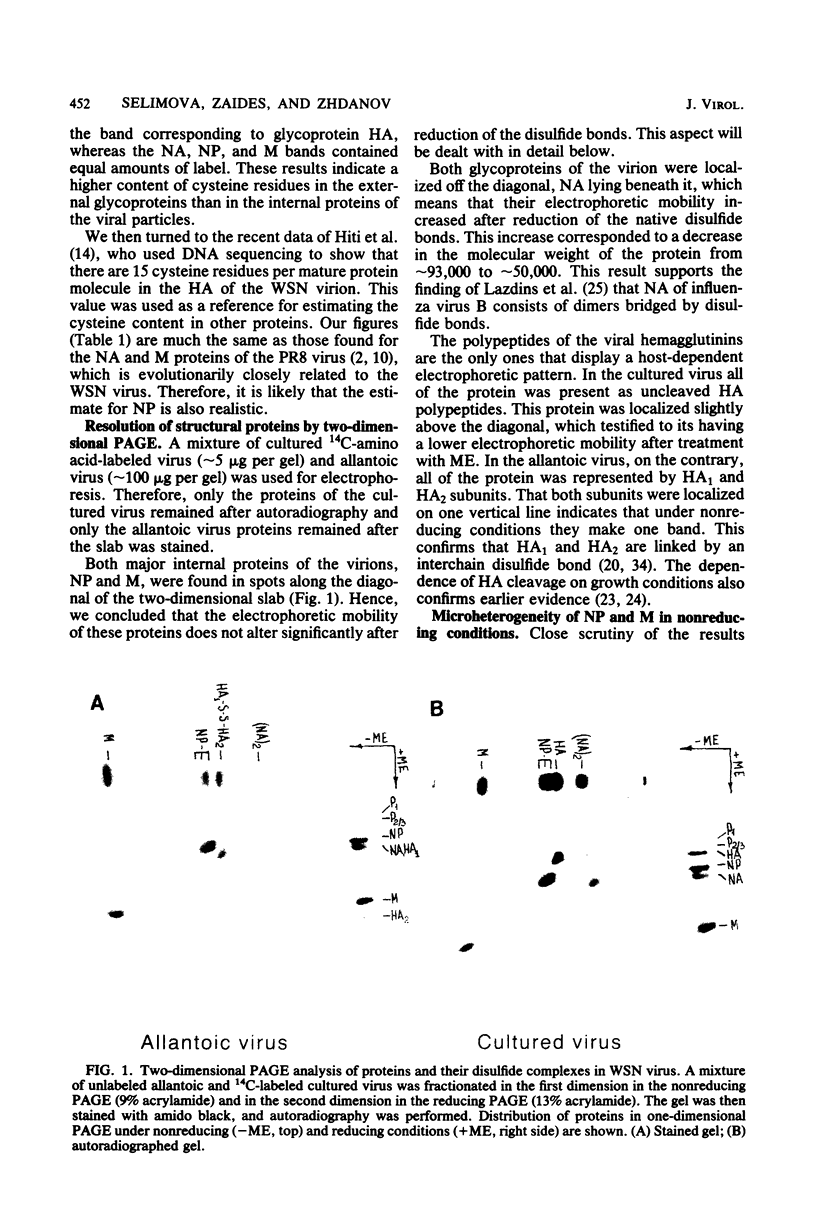
Abstract
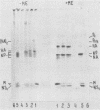
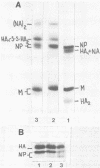
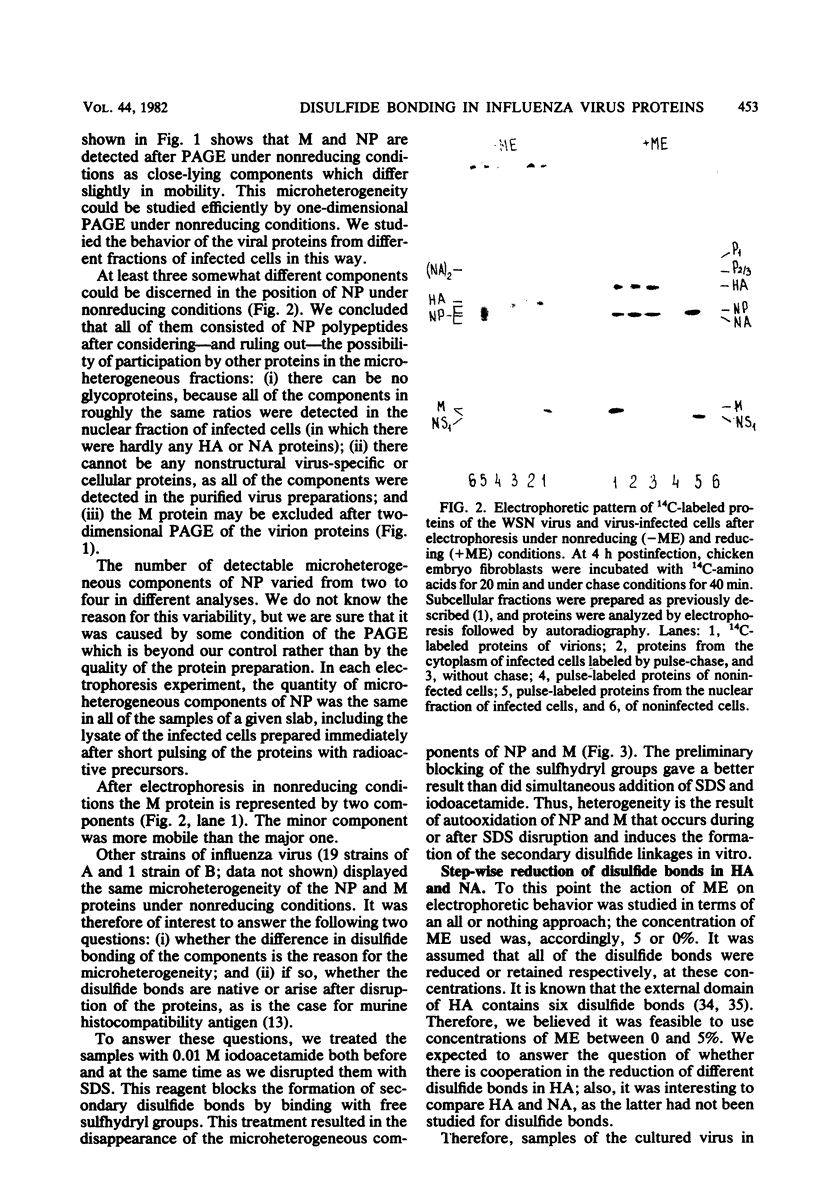
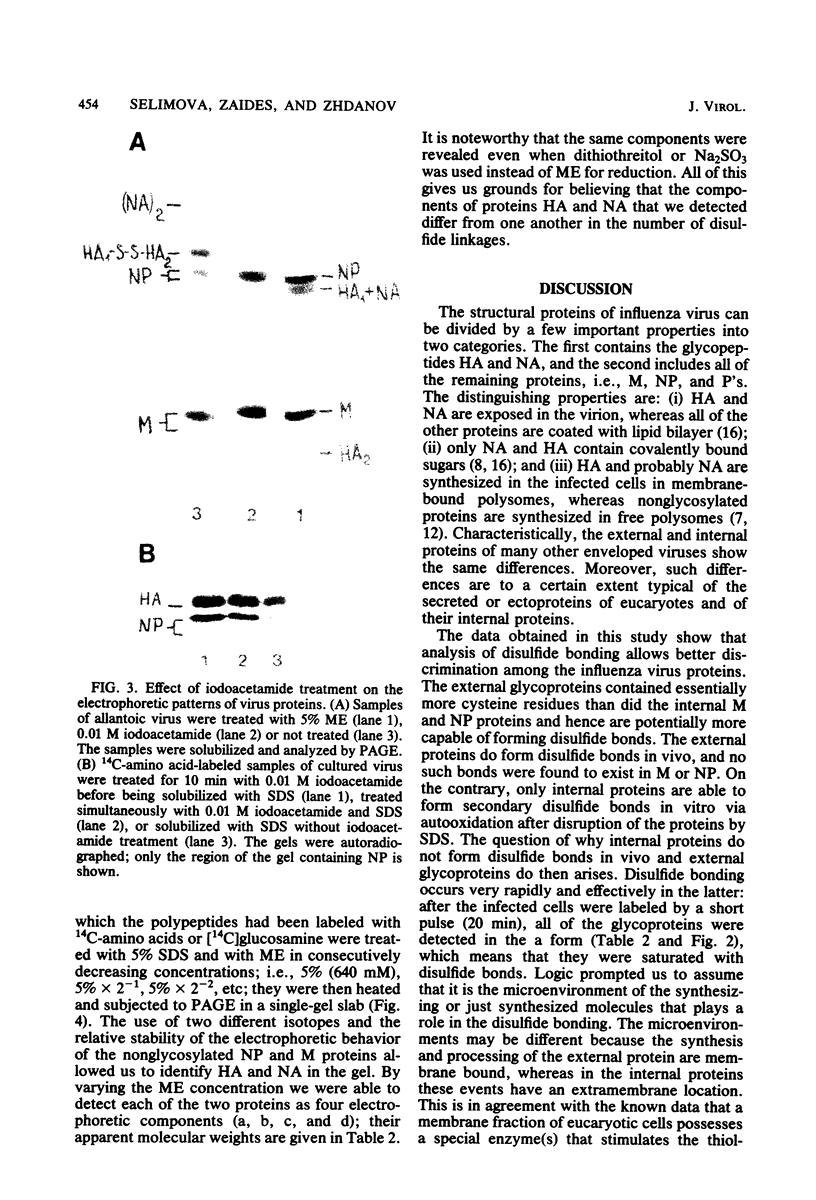
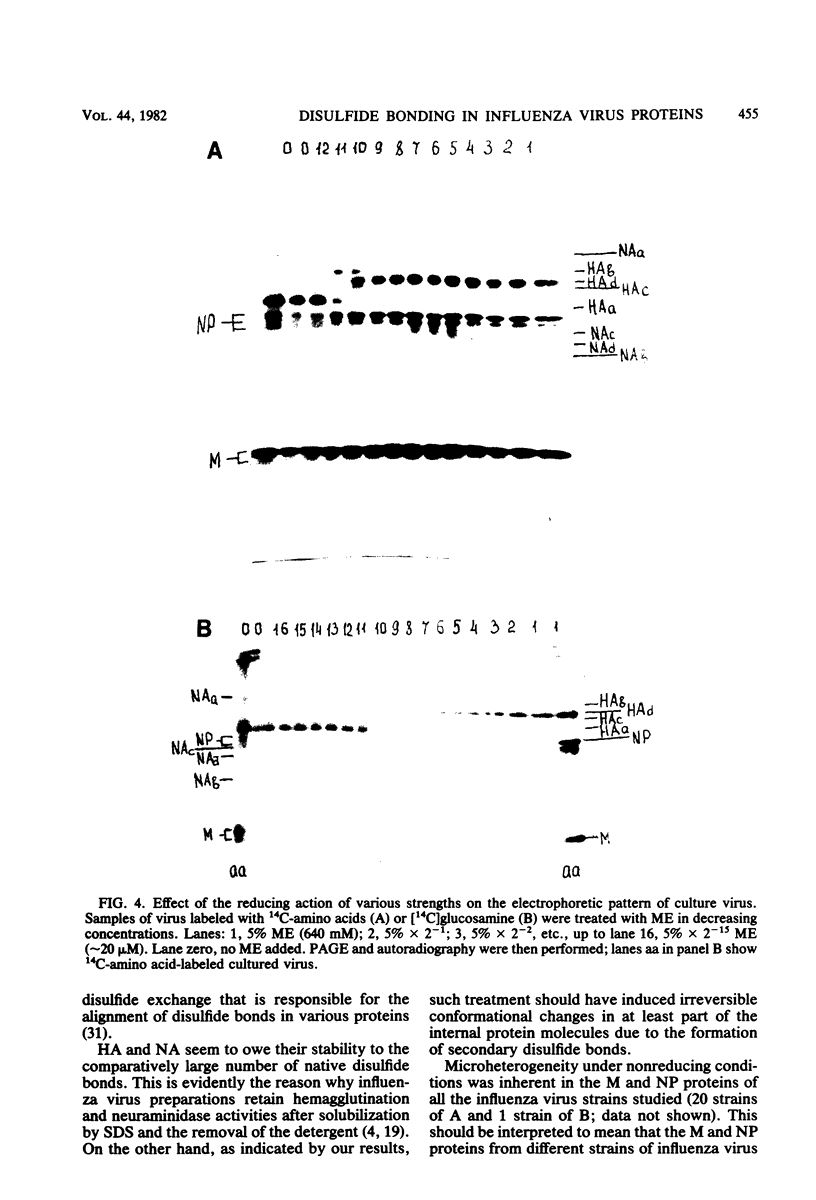
Disulfide bonding in the major proteins of influenza virus A, WSN strain, was studied by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels under reducing and nonreducing conditions. The electrophoretic behavior of the proteins correlated with their localization in the virions and their chemical composition. The internal proteins of the viral particles, i.e. matrix and nucleoproteins, were shown to contain a relatively small number of cysteine residues. Electrophoresis under nonreducing conditions yielded multiple forms of the proteins which could be discriminated by small but readily observable, reproducible differences in their migration rates in the gel. the multiplicity of the protein forms was caused by the formation of intramolecular disulfide bonds in matrix and nucleoproteins that arose during or after solubilization in sodium dodecyl sulfate. On the other hand, we failed to detect native inter- and intramolecular linkages in matrix and nucleoproteins. External glycoproteins of the virions (HA and NA) had, in contrast to the internal ones, a higher number of cysteine residues and native disulfide bonds. At least three disulfide linkages were revealed in HA and NA in our experiments. In uncleaved HA all of the linkages were intramolecular. In NA at least one disulfide bond linked two identical polypeptides into a dimer. It was established that the reduction of the different disulfide linkages in HA and NA required different concentrations of the reducing agent.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akishpaeva O. T., Selimova L. M., Zaides V. M., Bukrinskaia A. G. Protsessing belkov, indutsiruemykh virusom grippa v zarazhennykh kletkakh. Vopr Virusol. 1980 Mar-Apr;(2):202–208. [PubMed] [Google Scholar]
- Allen H., McCauley J., Waterfield M., Gething M. J. Influenza virus RNA segment 7 has the coding capacity for two polypeptides. Virology. 1980 Dec;107(2):548–551. doi: 10.1016/0042-6822(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Bucher D. J., Kilbourne E. D. A 2 (N2) neuraminidase of the X-7 influenza virus recombinant: determination of molecular size and subunit composition of the active unit. J Virol. 1972 Jul;10(1):60–66. doi: 10.1128/jvi.10.1.60-66.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D. J. Purification of neuraminidase from influenza viruses by affinity chromatography. Biochim Biophys Acta. 1977 Jun 10;482(2):393–399. doi: 10.1016/0005-2744(77)90253-4. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Distinct subunits of the ribonucleoprotein of influenza virus. J Mol Biol. 1969 Jun 28;42(3):485–499. doi: 10.1016/0022-2836(69)90237-x. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Henning R., Milner R. J., Reske K., Cunningham B. A., Edelman G. M. Subunit structure, cell surface orientation, and partial amino-acid sequences of murine histocompatibility antigens. Proc Natl Acad Sci U S A. 1976 Jan;73(1):118–122. doi: 10.1073/pnas.73.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiti A. L., Davis A. R., Nayak D. P. Complete sequence analysis shows that the hemagglutinins of the H0 and H2 subtypes of human influenza virus are closely related. Virology. 1981 May;111(1):113–124. doi: 10.1016/0042-6822(81)90658-9. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Jou W. M., Verhoeyen M., Devos R., Saman E., Fang R., Huylebroeck D., Fiers W., Threlfall G., Barber C., Carey N. Complete structure of the hemagglutinin gene from the human influenza A/Victoria/3/75 (H3N2) strain as determined from cloned DNA. Cell. 1980 Mar;19(3):683–696. doi: 10.1016/s0092-8674(80)80045-6. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Becht H. On the structure of the influenza virus envelope. Virology. 1972 Mar;47(3):579–591. doi: 10.1016/0042-6822(72)90547-8. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R., Orlich M., Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975 Dec;68(2):426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Separation of two polypeptide chains from the hemagglutinin subunit of influenza virus. Virology. 1971 Jul;45(1):275–288. doi: 10.1016/0042-6822(71)90134-6. [DOI] [PubMed] [Google Scholar]
- Law S. W., Dugaiczyk A. Homology between the primary structure of alpha-fetoprotein, deduced from a complete cDNA sequence, and serum albumin. Nature. 1981 May 21;291(5812):201–205. doi: 10.1038/291201a0. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Proteolytic cleavage of the hemagglutinin polypeptide of influenza virus. Function of the uncleaved polypeptide HA. Virology. 1973 Mar;52(1):199–212. doi: 10.1016/0042-6822(73)90409-1. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Haslam E. A., White D. O. The polypeptides of influenza virus. VI. Composition of the neuraminidase. Virology. 1972 Sep;49(3):758–765. doi: 10.1016/0042-6822(72)90532-6. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Schild G. C. The polypeptide composition of influenza A viruses. Virology. 1971 May;44(2):396–408. doi: 10.1016/0042-6822(71)90270-4. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Hightower L. E. Identification of the P proteins and other disulfide-linked and phosphorylated proteins of Newcastle disease virus. J Virol. 1981 Jan;37(1):256–267. doi: 10.1128/jvi.37.1.256-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. W., Dopheide T. A. Completion of the amino acid sequence of a Hong Kong influenza hemagglutinin heavy chain: sequence of cyanogen bromide fragment CN1. Virology. 1980 May;103(1):37–53. doi: 10.1016/0042-6822(80)90124-5. [DOI] [PubMed] [Google Scholar]
- Waterfield M., Scrace G., Skehel J. Disulphide bonds of haemagglutinin of Asian influenza virus. Nature. 1981 Jan 29;289(5796):422–424. doi: 10.1038/289422a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S., Brownlee G. G. Nucleotide sequence of the haemagglutinin gene of a human influenza virus H1 subtype. Nature. 1981 Jul 2;292(5818):72–75. doi: 10.1038/292072a0. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G., Skehel J. J., Charlwood P. A., Brand C. M. The size and shape of influenza virus neuraminidase. Virology. 1973 Feb;51(2):525–529. doi: 10.1016/0042-6822(73)90457-1. [DOI] [PubMed] [Google Scholar]