Abstract
Aims
Recent evidence indicates that the activity of energy-dissipating ion channels in the mitochondria can influence the susceptibility of the heart to ischaemia-reperfusion injury. In this study, we describe the effects of 4′-chlorodiazepam (4-ClDzp), a well-known ligand of the mitochondrial benzodiazepine receptor, on the physiology of both isolated cardiomyocytes and intact hearts.
Methods and results
We used current- and voltage-clamp methods to determine the effects of 4-ClDzp on excitation-contraction coupling in isolated rabbit heart cells. At the level of the whole heart, we subjected rabbit hearts to ischaemia/reperfusion in order to determine how 4-ClDzp influenced the susceptibility to arrhythmias and contractile dysfunction. In isolated rabbit cardiomyocytes, 4-ClDzp evoked a significant reduction in the cardiac action potential that was associated with a decrease in calcium currents and peak intracellular calcium transients. In intact perfused normoxic rabbit hearts, 4-ClDzp mediated a dose-dependent negative inotropic response, consistent with the observation that 4-ClDzp was reducing calcium influx. Hearts that underwent 30 min of global ischaemia and 30 min of reperfusion were protected against reperfusion arrhythmias and post-ischaemic contractile impairment when 4-ClDzp (24 μM) was administered throughout the protocol or as a single bolus dose given at the onset of reperfusion. In contrast, hearts treated with cyclosporin-A, a classical blocker of the mitochondrial permeability transition pore, were not protected against reperfusion arrhythmias.
Conclusion
The findings indicate that the effects on 4-ClDzp on both mitochondrial and sarcolemmal ion channels contribute to protection against post-ischaemic cardiac dysfunction. Of clinical relevance, the compound is effective when given upon reperfusion, unlike other pre-conditioning agents.
Keywords: Arrhythmia, Mitochondrial ion channels, Ischaemia, Reperfusion
1. Introduction
Mitochondrial ion channels represent an important novel therapeutic target for the mitigation of ischaemic injury in heart. Recently, a cardioprotective role for one specific channel complex, which is thought to include the mitochondrial benzodiazepine receptor (mBzr) (see reference 1 for review) and an inner membrane anion channel (IMAC), has been elucidated.2,3 The mBzr plays an important role in regulating mitochondrial membrane potential (ΔΨm)3,4 and apoptosis5 in mammalian cardiac myocytes.
Ligands to the mBzr have been shown to inhibit at least two classes of ion channel in the mitochondrial inner membrane, the so-called mitochondrial mega channel, which has properties that resemble the macroscopic mitochondrial permeability transition inhibited by cyclosporin A (CsA), and the CsA-insensitive mitochondrial IMAC6 or centumpicosiemen anion channel (Mcts).7 Furthermore, mBzR ligands have been shown to stabilize mitochondrial membrane potential (ΔΨm) in isolated cardiomyocytes subjected to oxidative stress.3 Given that oscillations in ΔΨm were shown to directly influence action potential duration,3 the mBzR complex may be an important target for stabilizing cardiac excitability in the face of oxidative stress. The prototypical mBzR ligand, 4′chlorodiazepam (4-ClDzp), can reduce the lability in action potential duration in cells and prevent post-ischaemic arrhythmias in guinea pig hearts, leading to the proposal that regions of myocardium undergoing mitochondrial depolarization (‘metabolic sinks’) contribute to the electrical inhomogeneity that results in arrhythmias. Although numerous studies provide evidence that 4-ClDzp targets the mitochondrion, less is known about its possible influence on cellular excitation-contraction coupling, which could, theoretically, contribute to its protective effects.
In this study, we examined the effects of the most commonly used ligand to the mBzr, 4-ClDzp, on cellular action potentials, membrane currents, and Ca2+ transients in isolated myocytes. At the level of the whole heart, we then investigated which actions of the compound might contribute to the cardioprotection provided by 4-ClDzp in a large animal global ischaemia model.
2. Methods
2.1 Animal model
Adult male New Zealand white rabbits (n = 36; body weight 2.1-3.3 kg) were housed in an animal facility with a 12:12 light/dark cycle and fed standard rabbit chow (Harlan High Fiber Rabbit Diet 2031). On the day of sacrifice, all rabbits were anesthetized by sequential administration of a ketamine and acepromazine cocktail (50 and 5 mg/kg, respectively; i.m. injection) followed by 28 mg/kg of sodium pentobarbital delivered via a marginal ear vein. This study was conducted with prior approval from the Johns Hopkins University Animal Care and Use Committee and in accordance with guidelines established in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2 Cell isolation
Rabbits used for myocyte experiments (n = 13) were anesthetized and hearts were placed on a modified Langendorff apparatus for the isolation of ventricular myocytes using protocols similar to those previously described.8 Hearts were perfused with calcium-free Tyrode’s solution containing (in mM) 140 NaCl, 1 MgCl2, 10 HEPES, 5 KCl, pH 7.5 (NaOH) for 5 min, followed by 8 min of perfusion with Tyrode’s solution plus the addition of 1 mg/mL collagenase, 0.17 mg/mL protease, and 10 μM CaCl2. After the digestion solution, the left ventricle was cut away and minced in Tyrode’s solution containing 10 μM CaCl2. The myocyte suspension was filtered and cells were gravity-precipitated for 15 min. A series of four steps were used to gradually titrate calcium up to a final concentration of 2 mM and cells were stored in DMEM (plus HEPES). Cardiomyocytes were placed in an incubator at 5% CO2 (balance room air) at 37°C and were used 2-8 h post-dispersion.
2.3 Whole-cell patch clamp
Myocytes were perfused in a heated (37°C) flow-through chamber of an inverted microscope with an external solution of (in mM) 140 NaCl, 0.1 MgCl2, 1 HEPES, 0.5 KCl, 10 glucose, and 2 CaCl2. Borosilicate glass micropipettes (1-3MΩ resistance) were used to establish the whole-cell patch-clamp configuration using an Axopatch 1-D Amplifier (Axon Instruments). Cells were equilibrated with pipette solution containing (in mM) 130 K-glutamate, 9 KCl, 0.5 MgCl2, 10 HEPES, 10 NaCl, 5 MgATP, and 80 μM indo-1 pentapotassium salt (Molecular Probes) and dialysed for 5 min prior to experimentation. Membrane currents were collected in voltage-clamp mode with a depolarizing step from the -80 mV holding potential to a test potential of 10 mV for 200 ms. For action potential waveforms, current-clamp mode was established, and action potentials were evoked by a 5 ms depolarizing step at a rate of either 0.2 or 1 Hz. Between 0.2 and 1 Hz, we detected no statistically significant frequency-related differences in mean current amplitude or action potential duration, so the data were pooled and analysed accordingly. Action potential and membrane current waveforms were transferred to a personal computer and analysed with custom written software. (Ionview, B.O’R.). Indo-1 calcium transients were obtained during the step to 10 mV and were analysed using previously established methods.9,10 All recordings were collected at a sampling rate of 1000 Hz, and action potential waveforms were corrected to a glutamate offset potential of 13 mV.
2.4 Calcium current experiments
A separate group of myocytes were used for calcium current experiments. Myocytes were equilibrated in potassium-free bath solution containing (in mM) 128 NaCl, 5 CsCl, 1 MgCl2, 10 NaHEPES, 2 CaCl2, and 10 glucose (pH 7.4). Cells were patch-clamped as described earlier with pipettes containing (in mM) 110 CsCl, 0.4 MgCl2, 5 MgATP, 10 HEPES, 5 BAPTA, 20 TEA-Cl, and 5 glucose (pH 7.2). After equilibration at a holding potential of -80 mV, cells were depolarized to -40 mV for 10 ms to inactivate Na currents, followed by a 150 ms test pulse ranging from -30 to 50 mV in 10 mV increments (interpulse interval of 2 s), after which cells returned to the holding potential of -80 mV.
2.5 Haemodynamic experiments
A separate group of rabbits (n = 28) were used in ischaemia-reperfusion experiments. Animals were anesthetized as described earlier. Upon the absence of reflexes, hearts were excised via midline thoracotomy and were placed on the cannula of a modified Langendorff apparatus attached to a Powerlab system (AD Instruments). Hearts were perfused with gassed (95/5% O2/CO2) buffer containing (in mM) 118 NaCl, 24 NaHCO3, 1.2 KH2PO4, 4.75 KCl, 1.2 MgSO4, 2.0 CaCl2, and 10 glucose. A buffer-filled latex balloon (Harvard apparatus) was inserted through the mitral valve into the left ventricle and inflated to a pressure of 5 ± 1 mmHg. Hearts were suspended in a buffer-filled heating chamber maintained at 37°C and two electrodes were placed into the bath for volume-conducted electrocardiogram recordings. Hearts were perfused with constant pressure (73 ± 1 mmHg), and online coronary flow rate, heart rate, left ventricular developed pressure (LVDP), maximal rates of contraction and relaxation, and electrocardiograms were monitored throughout the experiment and recorded on a personal computer. Haemodynamic parameters were analysed using Chart software (version 5, AD Instruments).
2.6 Drug administration and ischaemia/reperfusion
After a 20 min equilibration period, hearts were subjected to the following protocols: (i) control: 10 min normoxic perfusion, 30/30 min ischaemia/reperfusion (n = 7); (ii) 4-ClDzp: 10 min perfusion with 4-ClDzp at concentrations of 12 μM (n = 4), 24 μM (n = 6), or 64 μM (n = 1), 30/30 min ischaemia/reperfusion; (iii) 4-ClDzp bolus: 10 min normoxic perfusion, 30/30 min ischaemia/reperfusion with a bolus of 300 μM 4-ClDzp given at the onset of reperfusion (n = 5); (iv) CsA: 10 min normoxic perfusion with 0.2 μmol/L CsA, 30/30 min ischaemia/reperfusion (n = 5). For the delivery of 4-ClDzp, a stock solution of 4-ClDzp was prepared (using dimethyl sulphoxide as the solvent), and 4-ClDzp was added to fresh perfusion solutions at the beginning of each experiment (one heart). Administration of dimethyl sulphoxide stock in the same concentrations alone had no effect on haemodynamic parameters or volume-conducted ECG (data not shown). With the exception of the bolus experiments, the compounds were present in the perfusate for the duration of the protocol (i.e. also during reperfusion). Preliminary results indicated that 24 μM 4-ClDzp was the lowest concentration of drug that sufficiently provided protection against reperfusion arrhythmias, so this concentration was used in all subsequent cardiomyocyte experiments to characterize the cellular effects of the drug. In all intact heart experiments, global ischaemia was established by stopping the perfusion to the heart, and reperfusion began when perfusion was restored.
2.7 Determination of arrhythmias
Arrhythmias were characterized in accordance with the Lambeth Conventions11 and scores were tabulated for the entire 30 min reperfusion period using Score A as described previously by Curtis and Walker.12 Briefly, each heart was given a score based on the following criteria: 0: <50 ventricular premature beats; 1: 50-499 ventricular premature beats; 2: >500 ventricular premature beats and/or one episode of spontaneously reverting ventricular tachycardia or ventricular fibrillation; 3: more than one episode of spontaneously reverting ventricular tachycardia or fibrillation (<1 min total combined duration); 4: 1-2 min of total combined ventricular tachycardia or fibrillation; 5: >2 min of ventricular tachycardia or fibrillation.12
2.8 Determination of haemodynamic parameters
Baseline measurements of LVDP, coronary flow, maximal rates of contraction and relaxation were made by taking the 30 s average during the last minute before the onset of ischaemia.
2.9 Materials
All materials were obtained from Sigma-Aldrich unless otherwise noted.
2.10 Statistical analysis
Data are expressed as mean ± standard error, and statistics were calculated using Microsoft Excel for paired t-tests and SPSS software for ANOVA. Drug-induced changes in action potential duration, calcium current amplitude, as well as baseline effects of the drug on the whole heart were analysed by using a paired t-test. Arrhythmia scores were compared with a one-way ANOVA, and planned between-group contrasts were made using Dunnett’s t-test based on the a priori hypothesis that blocking the mBzr would decrease arrhythmias when compared with control.2 Between-group differences in the development of ischaemic contracture and in the recovery of LVDP during reperfusion were analysed using repeated-measures ANOVA and Dunnett’s post hoc method of multiple comparisons.
3. Results
3.1 Action potential duration
Administration of 24 μM 4-ClDzp reduced the duration of the action potential by 36% (Figure 1 A). The times to 50 and 90% repolarization were decreased to a similar extent, indicative of a change in the net currents flowing during phase 2 of the action potential (Figure 1B, P < 0.05).
Figure 1.
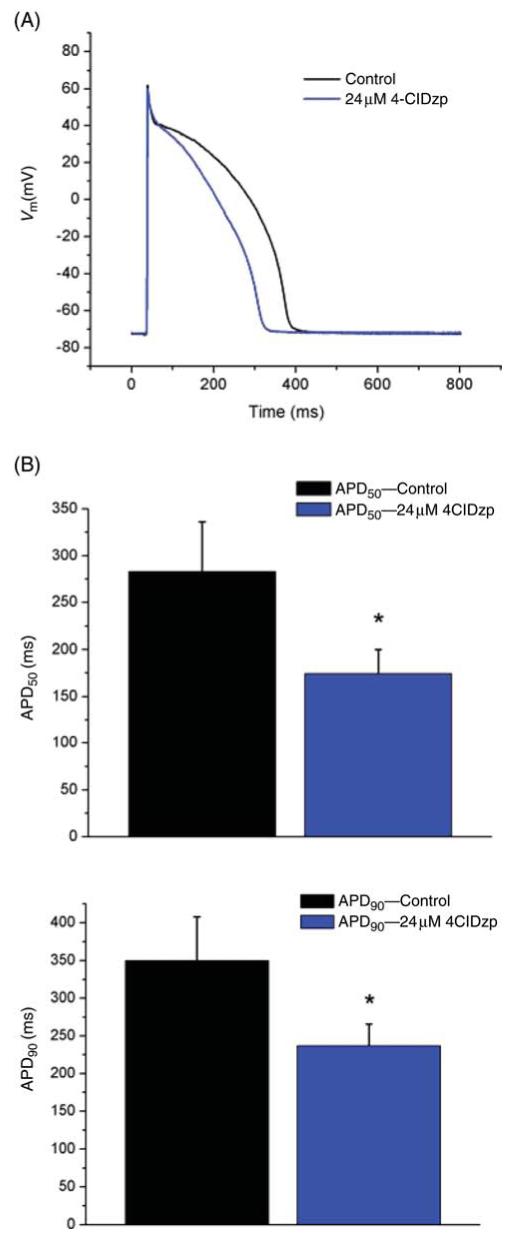
Effects of 4′-chlorodiazepam (4-ClDzp) on rabbit ventricular action potential duration. (A) Representative action potential tracings approximating the mean action potential morphology in the study. (B) Mean time to 50 and 90% repolarization in myocytes used in the study (n = 16 cells for each group); *P < 0.05 vs. control (paired t-test).
3.2 Effects on excitation-contraction coupling
Consistent with the effects of 4-ClDzp to decrease the duration of the action potential plateau, voltage-clamp recordings of the total inward currents revealed a decrease in total inward current at 50 and 150 ms after the test pulse to 10 mV (Figure 2A and B). The late component of this current largely consists of overlapping L-type Ca2+ and K+ currents. When experimental conditions were optimized for measurement of ICa, peak ICa was reduced significantly at test pulses from -10 to +50 mV (Figure 2D and E). A decrease in Ca2+ current would also diminish the trigger for Ca2+ release from the sarcoplasmic reticulum and could explain the negative inotropic effects of the compound. Hence, we also measured intracellular Ca2+ transients. 4-ClDzp (24 μM) treatment led to a significant reduction in the amplitude of the cytosolic calcium transient, as reported using indo-1 (Figure 2E and F). Peak calcium concentration was reduced by 28% in the presence of 24 μM 4-ClDzp (P < 0.05).
Figure 2.
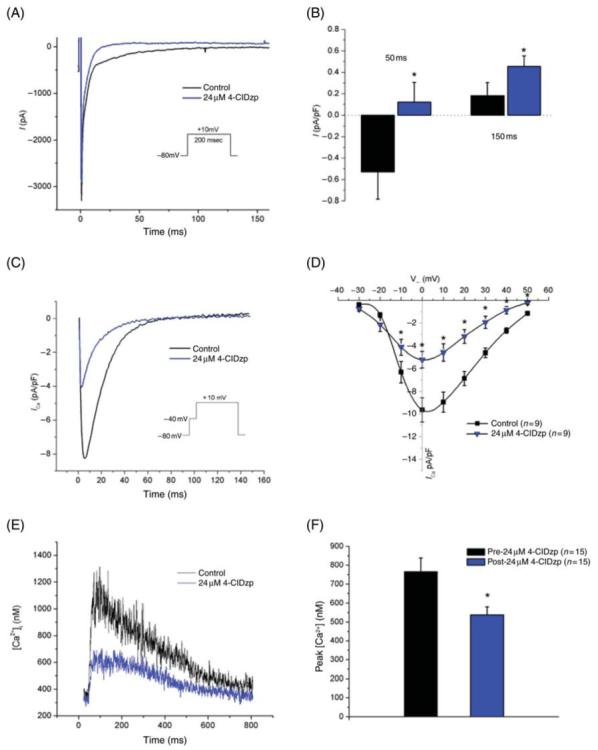
Transmembrane currents and calcium transients recorded from rabbit cardiomyocytes before and after the addition of 24 μM 4′-chlorodiazepam (4-ClDzp). (A) Representative current tracing from the same myocytes before (black line) and after (blue line) exposure to 4′-chlorodiazepam. (B) Current density for rabbit myocytes (n = 12) at 50 and 150 ms after a test pulse to 10 mV without (black bars) and with (blue bars) 4′-chlorodiazepam. (C) Representative calcium transients from the same cell in the absence (black line) and presence (blue line) of 4′-chlorodiazepam. (D) Current-voltage plot for calcium currents in the study. (E) Indo-1-mediated intracellular calcium transient recordings from the same cell under control (black line) and 4′-chlorodiazepam (blue line) conditions. (F) Peak calcium concentrations in rabbit cardiomyocytes with and without the addition of 4′-chlorodiazepam. *P < 0.05 vs. myocytes under control conditions .
3.3 4′-chlorodiazepam effects on contractility
Administration of 4-ClDzp caused a dose-dependent decrease in LVDP, ranging from little effect at 12 μM to a 60% decrease in LVDP in 64 μM 4-ClDzp (Figure 3). The effect on LVDP was entirely due to a decrease in the peak systolic pressure, as end-diastolic pressure was not elevated in any of the groups after receiving 4-ClDzp (data not shown). Baseline effects of 24 μM 4-ClDzp are presented in Table 1. Although previous studies have shown no effect of 4-ClDzp on heart rate at concentrations <10 μM,13,14 our findings indicate that higher concentrations of 4-ClDzp led to a negative chronotropic effect that bordered on statistical significance. As a partial blocker of ICa at a concentration of 24 μM, our results are consistent with previous studies showing that calcium channel blockers act as both negative intropes and negative chronotropes in isolated heart preparations.15
Figure 3.
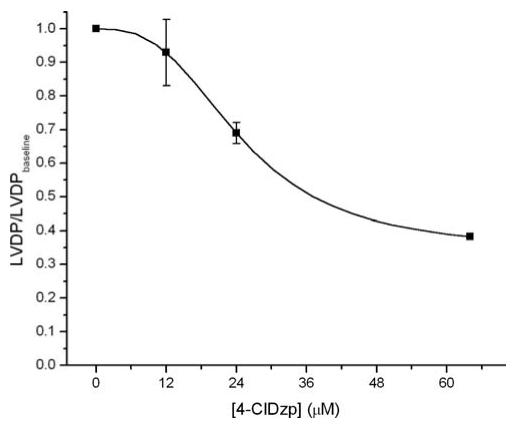
Dose-response curve describing the negative inotropic effect of various concentrations of 4′-chlorodiazepam (4-ClDzp) on left ventricular developed pressure (LVDP) in intact rabbit hearts. Animal numbers were n = 7, 4, 6, and 1 for 0, 12, 24, and 64 μM 4′-chlorodiazepam.
Table 1.
Effects of 24 μM 4′-chlorodiazepam on baseline myocardial haemodynamics
| Baseline (n = 7) | 24 μM 4-ClDzp (n = 6) | P-value | |
|---|---|---|---|
| LVDP (mmHg) | 98 ± 9 | 73 ± 6 | 0.04 |
| +dP/dt | 2144 ± 122 | 1446 ± 127 | 0.002 |
| -dP/dt | -1975 ± 188 | -1315 ± 135 | 0.006 |
| HR (b.p.m.) | 202 ± 8 | 179 ± 6 | 0.06 |
3.4 Left ventricular performance and reperfusion arrhythmias
Representative ECG traces and cumulative arrhythmia scores from whole-heart experiments are presented in Figures 4 and 5. Hearts that were perfused with 4-ClDzp, either when administered before ischaemia or immediately upon reperfusion, had a significantly reduced arrhythmia score, tabulated for the entire 30 min reperfusion period (P < 0.05 for both the 24 μM pre-treatment group and the bolus group when compared with controls). In contrast, treatment with CsA, a classical blocker of the permeability transition pore, was not effective at attenuating post-ischaemic arrhythmias (Figures 4D and 5).
Figure 4.
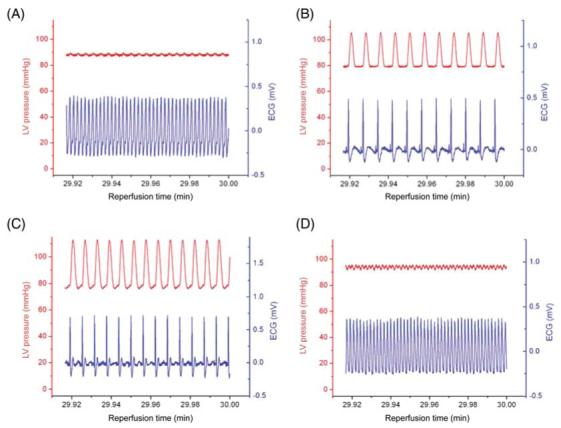
Representative left ventricular pressure tracings and ECG waveforms for the last 5 s of reperfusion in (A) control hearts, (B) hearts receiving 24 μM 4′-chlorodiazepam (4-ClDzp) beginning 10 min prior to ischaemia through the duration of reperfusion, (C) hearts receiving a bolus of 4′-chlorodiazepam at the onset of reperfusion, and (D) hearts receiving 0.2 μM cyclosporin-A 10 min prior to ischaemia through the duration of reperfusion .
Figure 5.
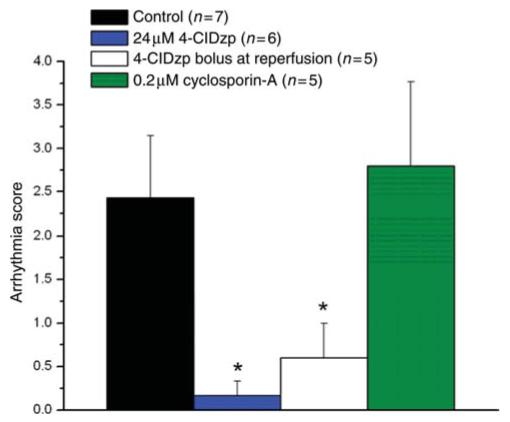
Arrhythmia scores tabulated for the entire 30 min reperfusion period for hearts in the study. *P < 0.05 vs. control hearts (ANOVA with Dunnett’s t-test) .
Development of ischaemic contracture was prominent in hearts, beginning at about 15 min into ischaemia. Increased end-diastolic pressure was observed in the control group, and this contracture was delayed in hearts that received 24 μM 4-ClDzp prior to ischaemia. End-diastolic pressure was significantly lower for the last 4 min of ischaemia in hearts that received 24 μM 4-ClDzp prior to ischaemia compared with control hearts (P < 0.05, RM ANOVA), (Figure 6 A). As expected, there was no difference in the development of ischaemic contracture in the control vs. 4-ClDzp bolus group, indicating that the mechanism of the post-ischaemic protection was distinct from a change in the events occurring during the ischaemic period.
Figure 6.
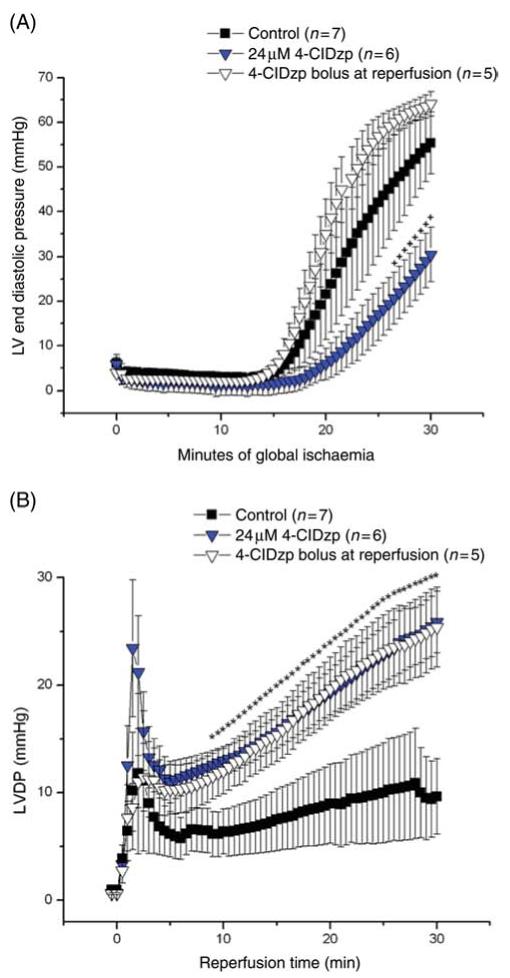
(A) Left ventricular end-diastolic pressure for rabbit hearts during the 30 min ischaemic period. +P < 0.05 for 24 μM 4′-chlorodiazepam (4-ClDzp) when compared with control (repeated measures ANOVA). (B) Left ventricular developed pressure (LVDP) for the 30 min reperfusion period for hearts receiving 4′-chlorodiazepam either prior to ischaemia (24 μM 4′-chlorodiazepam) or as a bolus at the onset of reperfusion. *P < 0.05 for both 4′-chlorodiazepam groups when compared with control (repeated measures ANOVA) .
Recovery of LVDP at reperfusion was significantly enhanced when hearts received 4-ClDzp when compared with control. Administration of 24 μM 4-ClDzp led to greatly improved recovery of LVDP, from 8.5 min of reperfusion throughout the duration of the protocol (P < 0.05, RM ANOVA). At the end of the 30 min protocol, LVDP averaged 10 ± 4 mmHg in the control group, compared with 24 ± 2 mmHg in the 4-ClDzp group (Figure 6B). Recovery was biphasic, consisting of a transient increase and decrease in contractility immediately upon reperfusion, followed by a slow, sustained recovery that was substantially greater in the 4-ClDzp treatment groups. The negative inotropic effect of 4-ClDzp (demonstrated earlier) abolished the initial transient increase in LVDP, however the steady-state recoveries were similar between the 4-ClDzp treatment groups (LVDP was 26 ± 4 mmHg after 30 min of reperfusion in the bolus dose group; Figure 6B).
Hearts that received 4-ClDzp as a bolus at the onset of reperfusion also showed significant protection against reperfusion electrophysiological dysfunction. Arrhythmia scores were similar to those of the continuous 4-ClDzp treatment group (Figure 5). Again, although the protection against ischaemic contracture observed by continuous 4-ClDzp administration could, in theory, contribute to the post-ischaemic protective effect against electrical and contractile dysfunctions, the equivalent protection afforded by the compound given at reperfusion provides evidence that 4-ClDzp is acting primarily to prevent injury during the crucial minutes when flow is restored.
4. Discussion
In this study, we sought to characterize the cellular effects of 4-ClDzp, a ligand to the mBzr, in order to understand the mechanism(s) behind its cardioprotective effect. Previous studies have shown that ligands to the mBzr can protect the heart against infarction4 and arrhythmias2,16 following ischaemia. In the present study, we show that although 4-ClDzp has a partial inhibitory effect on excitation-contraction coupling, it not only reduces the incidence of reperfusion arrhythmias in the rabbit heart but also markedly improves post-ischaemic contractile performance when administered prior to ischaemia or as a bolus at reperfusion.
4.1 Cardioprotection provided by 4′-chlorodiazepam
Previous work by our group has shown that heart cells exposed to oxidative stress undergo periodic oscillations in action potential duration,17 which were later found to be caused by oscillations in mitochondrial membrane potential (ΔΨm) and the consequent activation of ATP-sensitive K+ channels in the sarcolemma.3 The lability in action potential profile elicited by oscillations in ΔΨm served as a substrate for arrhythmia in the intact heart.2 A ligand to the mBzr, 4-ClDzp, stopped the oscillations in ΔΨCm3 and was found to stabilize the action potential and significantly decrease the incidence of arrhythmias at the organ level.2 In earlier studies, both the stability of ΔΨm and the protection from arrhythmias evoked by 4-ClDzp were not mimicked by CsA, the classical blocker of the permeability transition pore,2,3 supporting the view that mitochondrial ion channels open in a hierarchical fashion in response to oxidative stress.18 This study corroborates our earlier work that 4-ClDzp is cardioprotective and re-emphasizes the importance of the mBzr complex as a therapeutic target for preventing arrhythmias that involve a mitochondrial ion channel target ‘upstream’ from the classical permeability transition pore. Previous patch-clamp studies of isolated mitoplasts support the idea that two main mitochondrial ion channel types are inhibited by 4-ClDzp (Ro5-4864), a CsA-sensitive large conductance channel and a CsA-insensitive 100pS IMAC or Mcts.6,7 Our current working hypothesis is that IMAC is the principal target of 4-ClDzp and underlies the phenomenon of mitochondrial ROS-induced ROS release.18
In addition to the 4-ClDzp-mediated protection against arrhythmia, we also provide novel evidence that 4-ClDzp can protect against mechanical dysfunction. Administration of 4-ClDzp delayed the onset of ischaemic contracture and also led to significantly improved recovery of LVDP at the onset of reperfusion. Importantly, application of a bolus of 4-ClDzp at the onset of reperfusion, a strategy with pertinent clinical relevance, also led to significantly greater contractile function. It is noteworthy that despite the negative inotropic effect of the drug, and that the extent of ischaemic contracture was identical to the control group (Figure 6), exposure to the drug at reperfusion only still led to functional recovery during reperfusion.
It is plausible to suggest that as a partial inhibitor of ICa (discussed in what follows), 4-ClDzp was able to delay the onset of ischaemic contracture by reducing the calcium influx during the 30 min ischaemia (as hearts received the drug prior to ischaemia). However, since this compound preserves electrical excitability and the action potential plateau for much longer into the ischaemic period,2 one would actually expect greater total Ca2+ influx during ischaemia. This suggests that the main effect on ischaemic contracture was mediated by more than just inhibition of ICa. It is likely that the stabilizing effect of 4-ClDzp on mitochondrial function helps to maintain the energy supplies required for Ca2+ homeostasis both during and after ischaemia. Moreover, the improved recovery upon reperfusion with a bolus dose of 4-ClDzp argues that delaying the ischaemic contracture is not a major factor determining the outcome after ischaemia (Figure 6). At the onset of reperfusion, 4-ClDzp leads to improved developed pressure by a combination of at least two factors. Most importantly, the anti-arrhythmic properties that decreased the incidence of ventricular fibrillation and tachycardia allow the heart to successfully fulfil its role as a pump. Secondly, the compound may also help to reduce calcium influx (through both direct Ca2+ channel inhibition and preservation of mitochondrial function) in early reperfusion, which could improve diastolic function and allow for better LVDP.
4.2 Effects of 4′-chlorodiazepam on cellular electrophysiology
Although the protective properties of mBzr ligands are clear, very little is known about the effects of these drugs on cellular action potentials or contractility. In order to gain insight into the protective properties of mBzr ligands, we sought to characterize the cellular effects of 4-ClDzp in isolated rabbit cardiomyocytes.
In this study, 4-ClDzp had significant effects on the duration of the cellular action potential, the peak calcium current, and the intracellular calcium transient. The shortening of the action potential, notably in the plateau phase, which we observed in isolated rabbit myocytes, is consistent with earlier experiments using guinea pig myocytes.19-21 Consistent with phase 2 shortening of the action potential, cells that were exposed to 4-ClDzp displayed diminished calcium currents and transients. Notably, previous studies reported that some mBzr ligands decrease the inward calcium current (ICa) in cardiomyocytes,20,22,23 neuroblastoma cells,24 and adrenal glomerulosa cells.25 Our findings demonstrating reduced ICa, shortening of the action potential (beginning in the plateau phase), and a reduction in the amplitude of intracellular calcium transients are consistent with these previous studies that showed a partial inhibition of ICa by 4-ClDzp.
At the level of the whole organ, we found a dose-dependent negative inotropic effect, which also supports the notion that 4-ClDzp may be decreasing the ICa. This observation is in agreement with a previous study in isolated rat hearts,13 as well as in guinea pig papillary muscle preparations, where the 4-ClDzp-mediated decrease in tension was reversed by increasing the extracellular calcium concentration.26
4.3 The protection from 4′-chlorodiazepam cannot be accounted for purely by reducing ICa
Our results indicate that 4-ClDzp, at a concentration that provides protection against arrhythmias, is also reducing ICa. Although a very large number of anti-arrhythmic agents act by reducing calcium influx (see references 27,28 for review), the cardioprotective effects of 4-ClDzp are distinct from traditional class IV anti-arrhythmic agents for several reasons.
First, our previous work indicated that cardiomyocytes exposed to oxidative stress exhibit oscillations in membrane currents that occur independently from intracellular calcium concentrations.17 In the subsequent work using quiescent cardiomyocytes, where L-type calcium channels are not active, oscillations in ΔΨm evoked by oxidative stress were stabilized by the addition of 4-ClDzp.3
Secondly, Akar et al.2 showed that global ischaemia induces gradual shortening of the cellular action potential (by activating IKATP) that was delayed in hearts that received 4-ClDzp. Given that 4-ClDzp shortened the action potential in isolated myocytes in our study, but that 4-ClDzp prevented the shortening of the cellular action potential in the intact organ during ischaemia,2 the properties of the compound that act to maintain ΔΨm and prevent ATP depletion more likely underlie the effects of the compound during ischaemia.
Thirdly, administration of 4-ClDzp as a bolus at the onset of reperfusion successfully reduced the incidences of both arrhythmia and mechanical dysfunction to the same extent as hearts that were pre-treated with the drug. To the contrary, although a number of studies have noted cardioprotection elicited by pre-ischaemic treatment of calcium channel blockers,29-32 the cardioprotective properties of calcium channel blockers are not evident when administered at the onset of reperfusion. For example, post-ischaemic treatment with the ICa blocker verapamil was ineffective at attenuating myocardial infarction31 or stunning.30 Given the important clinical implications of drugs that are effective when administered at reperfusion, agents (such as 4-ClDzp) that act by stabilizing ΔΨm at reperfusion represent clinically relevant strategies for preventing electrical and mechanical dysfunctions.
Finally, it should be noted that pleiotropic effects of 4-ClDzp we describe may actually strengthen the case for its clinical use. Given the propensity for the single compound to have desirable properties of reducing calcium overload and also stabilizing ΔΨm in the face of metabolic stress, its use in mitigating reperfusion injury may be essentially ‘killing two birds with one stone’.
In conclusion, this study provides novel evidence, using a large animal model, that ligands to the mBzr can significantly reduce the incidence of arrhythmia and LV dysfunction, and that the drug may also have beneficial effects in reducing calcium overload in the face of ischaemia-reperfusion challenge.
Acknowledgments
Conflict of interest: none declared.
Funding
This study was funded by National Institutes of Health Grant (R37-HL-54598; B.O’R). D.A.B. is supported by an institutional training grant awarded to The Johns Hopkins School of Medicine (HL0 7227-31; Dr David A. Kass, Program Director).
References
- 1.Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol Ther. 2006;110:503–524. doi: 10.1016/j.pharmthera.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Akar FG, Aon MA, Tomaselli GF, O’Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535. doi: 10.1172/JCI25371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 4.Leducq N, Bono F, Sulpice T, Vin V, Janiak P, Fur GL, et al. Role of peripheral benzodiazepine receptors in mitochondrial, cellular, and cardiac damage induced by oxidative stress and ischemia/reperfusion. J Pharmacol Exp Ther. 2003;306:828–837. doi: 10.1124/jpet.103.052068. [DOI] [PubMed] [Google Scholar]
- 5.Chelli B, Falleni A, Salvetti F, Gremigni V, Lucacchini A, Martini C. Peripheral-type benzodiazepine receptor ligands: mitochondrial permeability transition induction in rat cardiac tissue. Biochem Pharmacol. 2001;61:695–705. doi: 10.1016/s0006-2952(00)00588-8. [DOI] [PubMed] [Google Scholar]
- 6.Garlid KD, Beavis AD. Evidence for the existence of an inner membrane anion channel in mitochondria. Biochim Biophys Acta. 1986;853:187–204. doi: 10.1016/0304-4173(87)90001-2. [DOI] [PubMed] [Google Scholar]
- 7.Kinnally KW, Zorov DB, Antonenko YN, Snyder SH, McEnery MW, Tedeschi H. Mitochondrial benzodiazepine receptor linked to inner membrane ion channels by nanomolar actions of ligands. Proc Natl Acad Sci USA. 1993;90:1374–1378. doi: 10.1073/pnas.90.4.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassani JW, Bassani RA, Bers DM. Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys J. 1995;68:1453–1460. doi: 10.1016/S0006-3495(95)80318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 10.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O’Rourke B. Elevated cytosolic Na+decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- 12.Curtis MJ, Walker MJ. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res. 1988;22:656–665. doi: 10.1093/cvr/22.9.656. [DOI] [PubMed] [Google Scholar]
- 13.Edoute Y, Giris J, Ben-Haim SA, Lochner A, Weizman A, Hayam G, et al. Ro 5-4864 and PK 11195, but not diazepam, depress cardiac function in an isolated working rat heart model. Pharmacology. 1993;46:224–230. doi: 10.1159/000139049. [DOI] [PubMed] [Google Scholar]
- 14.Grupp IL, French JF, Matlib MA. Benzodiazepine Ro 5-4864 increases coronary flow. Eur J Pharmacol. 1987;143:143–147. doi: 10.1016/0014-2999(87)90746-1. [DOI] [PubMed] [Google Scholar]
- 15.Millard RW, Grupp G, Grupp IL, DiSalvo J, DePover A, Schwartz A. Chronotropic, inotropic, and vasodilator actions of diltiazem, nifedipine, and verapamil. A comparative study of physiological responses and membrane receptor activity. Circ Res. 1983;52:I29–I39. [PubMed] [Google Scholar]
- 16.Mestre M, Bouetard G, Uzan A, Gueremy C, Renault C, Dubroeucq MC, et al. PK 11195, an antagonist of peripheral benzodiazepine receptors, reduces ventricular arrhythmias during myocardial ischemia and reperfusion in the dog. Eur J Pharmacol. 1985;112:257–260. doi: 10.1016/0014-2999(85)90505-9. [DOI] [PubMed] [Google Scholar]
- 17.O’Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–966. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 18.Aon MA, Cortassa S, Maack C, O’Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mestre M, Carriot T, Belin C, Uzan A, Renault C, Dubroeucq MC, et al. Electrophysiological and pharmacological characterization of peripheral benzodiazepine receptors in a guinea pig heart preparation. Life Sci. 1984;35:953–962. doi: 10.1016/0024-3205(84)90661-1. [DOI] [PubMed] [Google Scholar]
- 20.Holck M, Osterrieder W. The peripheral, high affinity benzodiazepine binding site is not coupled to the cardiac Ca2+ channel. Eur J Pharmacol. 1985;118:293–301. doi: 10.1016/0014-2999(85)90140-2. [DOI] [PubMed] [Google Scholar]
- 21.Le Fur G, Mestre M, Carriot T, Belin C, Renault C, Dubroeucq MC, et al. Pharmacology of peripheral type benzodiazepine receptors in the heart. Prog Clin Biol Res. 1985;192:175–186. [PubMed] [Google Scholar]
- 22.Bolger GT, Abraham S, Oz N, Weissman BA. Interactions between peripheral-type benzodiazepine receptor ligands and an activator of voltage-operated calcium channels. Can J Physiol Pharmacol. 1990;68:40–45. doi: 10.1139/y90-005. [DOI] [PubMed] [Google Scholar]
- 23.Campiani G, Fiorini I, De Filippis MP, Ciani SM, Garofalo A, Nacci V, et al. Cardiovascular characterization of pyrrolo[2,1-d][1,5]benzothiazepine derivatives binding selectively to the peripheral-type benzodiazepine receptor (PBR): from dual PBR affinity and calcium antagonist activity to novel and selective calcium entry blockers. J Med Chem. 1996;39:2922–2938. doi: 10.1021/jm960162z. [DOI] [PubMed] [Google Scholar]
- 24.Watabe S, Yoshii M, Ogata N, Tsunoo A, Narahashi T. Differential inhibition of transient and long-lasting calcium channel currents by benzodiazepines in neuroblastoma cells. Brain Res. 1993;606:244–250. doi: 10.1016/0006-8993(93)90991-u. [DOI] [PubMed] [Google Scholar]
- 25.Python CP, Rossier MF, Vallotton MB, Capponi AM. Peripheral-type benzodiazepines inhibit calcium channels and aldosterone production in adrenal glomerulosa cells. Endocrinology. 1993;132:1489–1496. doi: 10.1210/endo.132.4.8384990. [DOI] [PubMed] [Google Scholar]
- 26.Mestre M, Carriot T, Belin C, Uzan A, Renault C, Dubroeucq MC, et al. Electrophysiological and pharmacological evidence that peripheral type benzodiazepine receptors are coupled to calcium channels in the heart. Life Sci. 1985;36:391–400. doi: 10.1016/0024-3205(85)90126-2. [DOI] [PubMed] [Google Scholar]
- 27.Ackerman MJ, Clapham DE. Ion channels—basic science and clinical disease. N Engl J Med. 1997;336:1575–1586. doi: 10.1056/NEJM199705293362207. [DOI] [PubMed] [Google Scholar]
- 28.Katz AM. Selectivity and toxicity of antiarrhythmic drugs: molecular interactions with ion channels. Am J Med. 1998;104:179–195. doi: 10.1016/s0002-9343(97)00388-4. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramaniam R, Chawla S, Mackenzie L, Schwiening CJ, Grace AA, Huang CL. Nifedipine and diltiazem suppress ventricular arrhythmogenesis and calcium release in mouse hearts. Pflugers Arch. 2004;449:150–158. doi: 10.1007/s00424-004-1321-2. [DOI] [PubMed] [Google Scholar]
- 30.Adachi T, Miura T, Suzuki K, Iimura O. Effects of verapamil on myocardial stunning in xanthine-oxidase deficient hearts: pre-treatment vs. post-ischemic treatment. Basic Res Cardiol. 1994;89:16–28. doi: 10.1007/BF00788674. [DOI] [PubMed] [Google Scholar]
- 31.Lo HM, Kloner RA, Braunwald E. Effect of intracoronary verapamil on infarct size in the ischemic, reperfused canine heart: critical importance of the timing of treatment. Am J Cardiol. 1985;56:672–677. doi: 10.1016/0002-9149(85)91033-1. [DOI] [PubMed] [Google Scholar]
- 32.Baxter GF, Yellon DM. Attenuation of reperfusion-induced ventricular fibrillation in the rat isolated hypertrophied heart by preischemic diltiazem treatment. Cardiovasc Drugs Ther. 1993;7:225–231. doi: 10.1007/BF00878512. [DOI] [PubMed] [Google Scholar]


