Abstract
Positive modulation of GABAA and antagonism of NMDA receptors mediate the discriminative stimulus effects of ethanol. Endogenous neuroactive steroids produce effects similar to ethanol suggesting that these steroids may modulate ethanol addiction. The 4 isomers of the functional esters at C-3 of the 3-hydroxy metabolites of 4-pregnene-3,20-dione (progesterone) [allopregnanolone (3α,5α-P), pregnanolone (3α,5β-P), epiallopregnanolone (3β,5α-P), epipregnanolone (3β,5β-P)], a synthetic analogue of steroids modified by endogenous sulfation [pregnanolone hemisuccinate (3α,5β-P HS)], and a structurally-similar, adrenally-derived steroid [3α-hydroxy-5-androstan-17-one (3α,5α-A, androsterone)], were assessed for ethanol-like discriminative stimulus effects 30 or 60 min after administration in male (n=9) and female (n=8) cynomolgus monkeys (Macaca fascicularis) trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval. The 3α-hydroxysteroids completely substituted for ethanol (80% of cases) whereas the 3β-hydroxysteroids and 3α,5β-P HS rarely substituted for ethanol (6% of cases). There were no sex differences. Compared to monkeys trained to discriminate 2.0 g/kg ethanol, 3α,5β-P and 3α,5α-A substituted more potently in monkeys trained to discriminate 1.0 g/kg ethanol. Compared to the 5β-reduced isomer (3α,5β-P), the 5α isomer of pregnanolone (3α,5α-P) substituted for ethanol with 3–40-fold greater potency but was least efficacious in female monkeys trained to discriminate 2.0 g/kg ethanol. The data suggest that the discriminative stimulus effects of lower doses (1.0 g/kg) of ethanol are mediated to a greater extent by 3α,5β-P-and 3α,5α-A-sensitive receptors compared to higher doses (2.0 g/kg). Furthermore, the discriminative stimulus effects of ethanol appear to be mediated by activity at binding sites that are particularly sensitive to 3α,5α-P.
Introduction
Alcohol abuse and alcoholism afflicts approximately 18 million people and is the third leading cause of preventable death in the United States (Li et al., 2007). Epidemiological data suggest that individuals are not at equal risk for developing alcoholism (Grant et al., 2004). Individuals differ in the subjective effects of ethanol (i.e., the feelings associated with alcohol intoxication) which may be related to how much alcohol they typically drink (Holdstock and de Wit, 2000) and/or their physiological state (Adinoff et al., 2003). One approach to characterizing individual risk for alcoholism is to identify the pharmacological basis for the subjective effects of ethanol (Krystal et al., 2003, 2006). Drug discrimination procedures have been useful for translating subjective effects associated with drug use into behavioral assays of drug-receptor mechanisms. Once established, the basis of the discriminative stimuli of drugs, including ethanol, can be used to investigate candidate receptors and variation with dose, sex, or genotype. Research from the past 60 years indicates that the stimulus effects of ethanol resemble classic sedative hypnotics (e.g., benzodiazepines) as well as classic and dissociative anesthetics (e.g., barbiturates and ketamine) (see the Drug Discrimination Bibliographic Database: www.dd-database.org). This study addresses the pharmacological characteristics of endogenous neuroactive steroids, particularly pregnenolone derivatives that are neuromodulators at γ-aminobutyric acid (GABA)A and N-methyl-D-aspartate (NMDA) receptors, in substituting for the discriminative stimulus effects of ethanol.
Previous studies have shown that the GABAA receptor-active progesterone derivative, allopregnanolone (3α,5α-P), substitutes in female cynomolgus monkeys trained to discriminate 1.0 g/kg (Grant et al., 1996, 1997) or 2.0 g/kg ethanol (Green et al., 1999). Furthermore, the sensitivity of female monkeys to the stimulus effects of 1.0 g/kg ethanol is greater when circulating progesterone levels peak (i.e., luteal phase, 4–12 ng/ml compared to follicular phase, < 1 ng/ml) (Grant et al., 1997), an effect that was blunted in monkeys trained to discriminate 2.0 g/kg ethanol (Green et al., 1999). Thus, circulating levels of endogenous neuroactive steroids appear additive to exogenously administered ethanol in producing ethanol-like discriminative effects. This effect of ethanol training dose on the substitution of neuroactive steroids is unexplored in male monkeys.
The pharmacological effects of neuroactive steroids vary with stereochemical configuration and ionic charge of C-3 esters (Fig. 1). In vitro, GABAA receptors are positively modulated by the 3α-hydroxy metabolites of progesterone, pregnanolone (3α,5β-P) and allopregnanolone (3α,5α-P), in addition to androsterone (3α,5α-A), a metabolite of dehydroepiandrosterone (DHEA) and testosterone (Majewska et al., 1986; Paul and Purdy, 1992). In contrast, the 3β-isomers epipregnanolone (3β,5β-P) and epiallopregnanolone (3β,5α-P) do not positively modulate GABAA receptors (Park-Chung et al., 1999) but may act as GABAA receptor antagonists (Wang et al., 2002). This study evaluated the role of C-3 steroidal stereochemistry and ionic charge of pregnanolone isomers in substituting for the discriminative stimulus effects of 1.0 or 2.0 g/kg ethanol in male and female cynomolgus monkeys. A negatively-charged sulfate or hemisuccinate (HS) at C-3 abolishes GABAA receptor positive modulation or induces negative modulation of GABAA or NMDA receptors (Park-Chung et al., 1994, 1999). In rats, 3α,5β-P HS has sedative, hypnotic and anticonvulsant effects (Weaver et al., 1997), possibly due to inhibition of NMDA-induced Ca2+ currents at NMDA receptors (Park-Chung et al., 1997). Non-competitive NMDA receptor antagonists partially substitute for ethanol in cynomolgus monkeys (Vivian et al., 2002). Thus, to address neuroactive steroid-sensitive NMDA receptor contributions to the discriminative stimulus effects of ethanol, the current study evaluated the substitution of 3α,5β-P HS for ethanol. The most potent GABAA receptor positive modulator, 3α,5α-P, is generated in greater quantities from human gonads compared to adrenals (Genazzani et al., 1998), whereas DHEA is produced exclusively by the adrenals (Simard et al., 2005). By evaluating androsterone substitution for ethanol, the current study compared the ethanol-like effects of gonadal versus adrenal steroids.
Fig. 1.
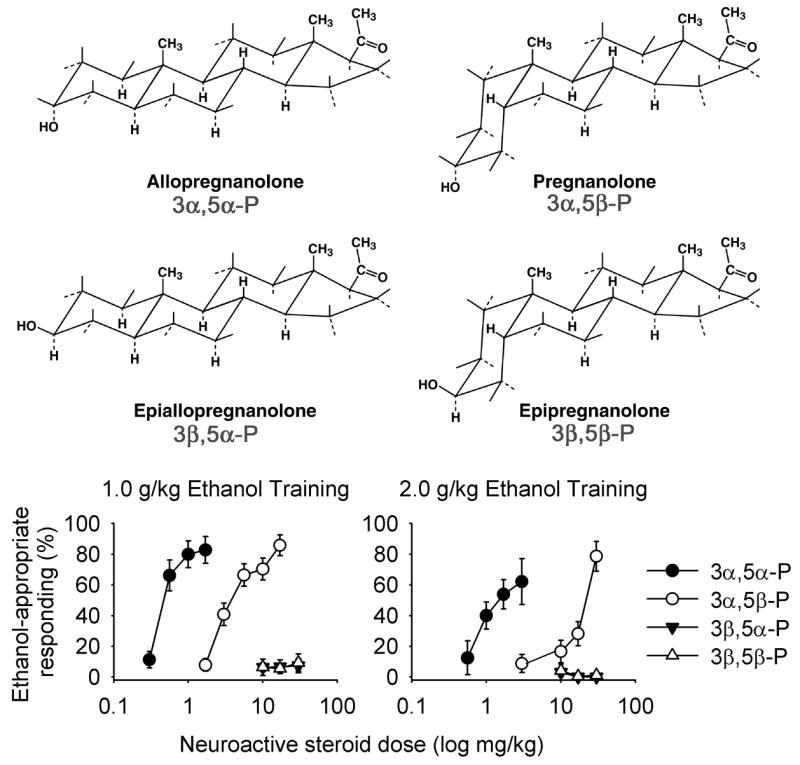
Structures of pregnanolone isomers. The –OH group is axial for the 5β and equatorial for the 5α steroids. Shown below is the mean (± SEM) ethanol-appropriate responding (1.0 and 2.0 g/kg training groups shown separately) produced by the four neuroactive steroid isomers collapsed across the 30- and 60-min pre-treatment intervals. Means include data from both male and female cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) from water. Each point represents data from substitution tests in 4–10 monkeys.
Methods
Subjects
Male (n = 9, 4.9 – 7.1 kg) and female (n = 8, 2.9 – 5.1 kg) adult cynomolgus monkeys (Macaca fascicularis) were housed in stainless steel cages (7.6×6.0×7.0 m) in a temperature- (23±1°C) and humidity- (40–60%) controlled colony room with a 12-h light dark cycle (lights on at 0600 hours). The cages were modified for social housing. The monkeys were housed individually during training and feeding (3–4 h). At all other times, they were housed in groups of two, three, or four. The monkeys were fed a nutritionally complete diet (Purina Mills, St Louis, MO) with daily fruit supplements in addition to food during experimental sessions. Water was always available except during training and testing. The females were trained with small food treats to present for external vaginal swabbing to determine the daily status of menses. Female monkeys were trained to participate in awake venipuncture to collect blood samples (3 ml) for tri-weekly assessment of menstrual cyclicity via progesterone assay (Emory Endocrine Core). The luteal phase was indicated by progesterone levels ≥ 4 ng/ml. The origin and housing conditions of the monkeys have been described (Grant et al., 1996, 2000). The study was conducted in accordance with the Wake Forest University Animal Care and Use Committee and the Guidelines of the Committee on the Care and Use of Laboratory Animal Resources (NRC 1996).
Apparatus
Ventilated and sound-attenuating experimental chambers (1.50 × 0.74 × 0.76 m; Med Associates, Inc., St. Albans, VT) that accommodated a primate chair (1.17 × 0.61× 0.61 m; Plas Labs, Lansing, MI) were used for the experimental sessions. Two retractable levers were within arm’s reach of a monkey sitting in a primate chair, set in a panel (0.48 × 0.69 m) 0.72 m from the bottom of the chamber. The panel had three colored lights (amber, green and red) above each lever and a central white light. Two house lights were set at the rear of the chamber close to the ceiling. A monkey in a primate chair could access 1-g banana-flavored pellets (P.J. Noyes, Lancaster, NH) delivered into a food tray attached to the primate chair. The pellets were delivered from a feeder set outside of the chamber connected to vinyl tubing that led to the food tray. Control of the apparatus and data acquisition were controlled by a PC- or Macintosh-compatible computer connected to an interface (Med Associates or National Instruments) programmed with LabView software (National Instruments, Austin, TX).
Procedure
Experimental design
The experiment included 4 groups of monkeys in a 2 (sex: male or female) ×2 (training dose: 1.0 or 2.0 g/kg ethanol) design. Six male (4864, 4865, 4867, 4892, 4889, 4995) and four female (2830, 2852, 2913, 3123) monkeys were trained to discriminate 1.0 g/kg ethanol (20% w/v, 5 ml/kg) from water. Three male (4890, 4891, 5496) and four female (2835, 3220, 5400, 5999) monkeys were trained to discriminate 2.0 g/kg ethanol (20% w/v, 10 ml/kg) from water. The training conditions were the same as described in Grant et al. (1997, 2000).
Discrimination training
For each session, the monkey was seated in a primate chair and wheeled into the chamber. After learning to respond on the levers for banana pellets, the response requirement was increased from fixed-ratio (FR)-1 to a final schedule of FR-15 to FR-100. A final FR was selected that resulted in each subject receiving the maximum number of pellets in approximately 5 min. Males received 20–25 pellets and females received 10–15 pellets per session. A session ended either after all the pellets were delivered or 30 min elapsed, whichever occurred first. With each response, the center colored light above the lever shut off and re-illuminated (< 1 s). Upon completion of the FR requirement and delivery of a banana pellet, the central white light shut off and re-illuminated (< 1 s).
After response rates on the final FR schedule were > 0.25 responses/s for 3 consecutive sessions, the monkeys were habituated to nasogastric (i.g.) gavage. An infant feeding tube (5 French, 1.7 × 381 mm) was passed down one nostril, through the esophagus and into the stomach. For 5 consecutive sessions, the monkeys received tap water (5 ml/kg, i.g.), were wheeled into the chamber, and 30 min later only the “water-appropriate” lever was extended. For the next 5 sessions, the monkeys were administered a training dose of ethanol (1.0 or 2.0 g/kg, i.g.), wheeled into the chamber, and 30 min later only the “ethanol-appropriate” lever extended into the chamber. After the 30-min pre-treatment interval, the house lights and the central white light were illuminated. Following these 10 sessions, either ethanol or water was administered, and 30 min later both the water- and ethanol-appropriate levers extended into the chamber. A reinforcer was delivered after the monkey completed the number of responses in its final FR on the substance-appropriate lever consecutively, i.e., responding uninterrupted by a switch to the substance-inappropriate lever. Responding on the substance-inappropriate lever reset the response count for the substance-appropriate lever to zero.
A double-alternating pattern (i.e., ethanol, ethanol, water, water) characterized discrimination training sessions. The training session condition was changed if ≥ 70% of responses in the first FR and ≥ 90% of total session responses were to the substance-appropriate lever. The discrimination was acquired when, for 5 consecutive sessions, ≥ 70% of the responses in the first FR, and ≥ 90% of total session responses, were to the substance-appropriate lever.
Substitution testing
During twice-weekly test sessions, a single dose of 3α,5α-P (range of doses tested, 0.17–10 mg/kg, s.c.), 3α,5β-P (1.0, −30 mg/kg, s.c.), 3α,5β-P-HS (3.9–39.5 mg/kg, s.c.), 3β,5β-P (10– 30 mg/kg, s.c.), 3β,5α-P (10–30 mg/kg, s.c.) or 3α,5α-A (3.0–56 mg/kg, s.c.) was administered, and 30 or 60 min later both the water- and ethanol-appropriate levers extended into the chamber. In previous studies, 3α,5α-P substituted for ethanol between 15 and 60 minutes after administration, with no substitution at 90 min (Grant et al., 1996). Tests were conducted after both 30- and 60-min pre-treatment intervals to evaluate the time-course of ethanol-like effects for the steroids tested. Responding on either lever was reinforced according to the monkey’s final FR. Between test sessions, training sessions occurred. If responding did not meet discrimination criteria during a training session, training was continued until the criteria were met for 3 consecutive sessions. In a majority of cases, each test drug dose was administered both after an ethanol training session and after a water training session. The range of doses tested was selected for each monkey to encompass the dose range resulting in substitution in prior studies (e.g., Grant et al., 1996, 1997; Bowen et al., 1999). Monkeys were first tested with intermediate doses, followed by an equal distribution of higher and lower doses. If a monkey did not eat its entire next meal, indicating reduced appetite, or exhibited tremors after administration of a test drug, a higher dose was not tested. Visible signs of tremors included slight shaking of the body and slowed ambulation when returned to the home cage. Tremors were typically evident for approximately 2 hours.
Drugs
Anhydrous ethanol (1.0 or 2.0 g/kg; Warner-Graham Co., Cockeysville, MD) was diluted to 20% w/v with tap water and was administered in a 5–10 ml/kg (i.g.) volume followed by a 5 ml flush of water 30 min before each training session. The neuroactive steroids 3α-hydroxy-5α-pregnan-20-one (3α,5α-P), 3α-hydroxy-5β-pregnan-20-one (3α,5β-P), 3α,5β-P HS, 3β-hydroxy-5β-pregnan-20-one (3β,5β-P), 3β-hydroxy-5α-pregnan-20-one (3β,5α-P) and 3α-hydroxy-5α-androstan-17-one (3α,5α-A) (Sigma, St. Louis, MO) were diluted in concentrations ≤ 10 mg/ml and administered in injection volumes of 0.5–2 ml/kg (s.c.). All drugs were prepared fresh daily. All steroids were purified by R. Purdy for the configurations listed, and 3α,5α-P was synthesized as in Purdy et al. (1990). All steroids were suspended in hydroxypropyl β-cyclodextrin (25% w/v; Cerestar, Hammond, IN). The synthetic analogue 3α,5β-P HS was tested instead of 3α,5β-P sulfate because 3α,5β-P HS is unaffected by endogenous sulfatases, has a higher pKa and therefore more freely crosses the blood-brain barrier, and like 3α,5β-P sulfate, negatively-modulates NMDA receptor function (Weaver et al., 1997).
Data analysis
Percent of total responding on the ethanol-appropriate lever (total responses on the ethanol-appropriate lever/total responses) and response rate (total responses/session time) were calculated for each monkey and session if at least one FR was completed. For a given dose, mean ethanol-appropriate responding was calculated from test sessions occurring after ethanol and water training sessions. Complete and partial substitution were operationally defined, respectively, as ≥ 80% and ≥ 20–79% of responses on the ethanol-appropriate lever, as in Grant et al. (1997, 2000). When a drug completely substituted for ethanol, the ED50 was computed via linear interpolation between the two doses that encompassed the 50% effect (Holtzman et al. 1991). Baseline response rate was calculated by averaging response rates during each ethanol and water training session immediately preceding a test session. Ethanol-appropriate responding and percent of baseline response rate were analyzed with mixed-factor ANOVAs for each test drug with pre-treatment interval as the within-subjects factor, and sex, training dose, and drug dose as between-subjects factors. Including drug dose as a within-subjects factors would have omitted some subjects from the analysis as not all the same doses were tested in each subject. Mean ED50 for each test drug and pre-treatment interval was analyzed with 2 (sex: male, female) × 2 (training dose: 1.0 and 2.0 g/kg) ANOVAs. To compare ED50 values, test drug was treated as a within-subjects factor although this analysis omitted subjects not tested with each drug. The ED50 values were log transformed prior to analysis to normalize skewed distributions. Huynh-Feldt corrections were used when repeated factors were included and adjusted degrees of freedom are cited throughout the manuscript. Interactions were evaluated with Bonferroni-corrected pairwise comparisons. For all tests, α was 0.05.
Results
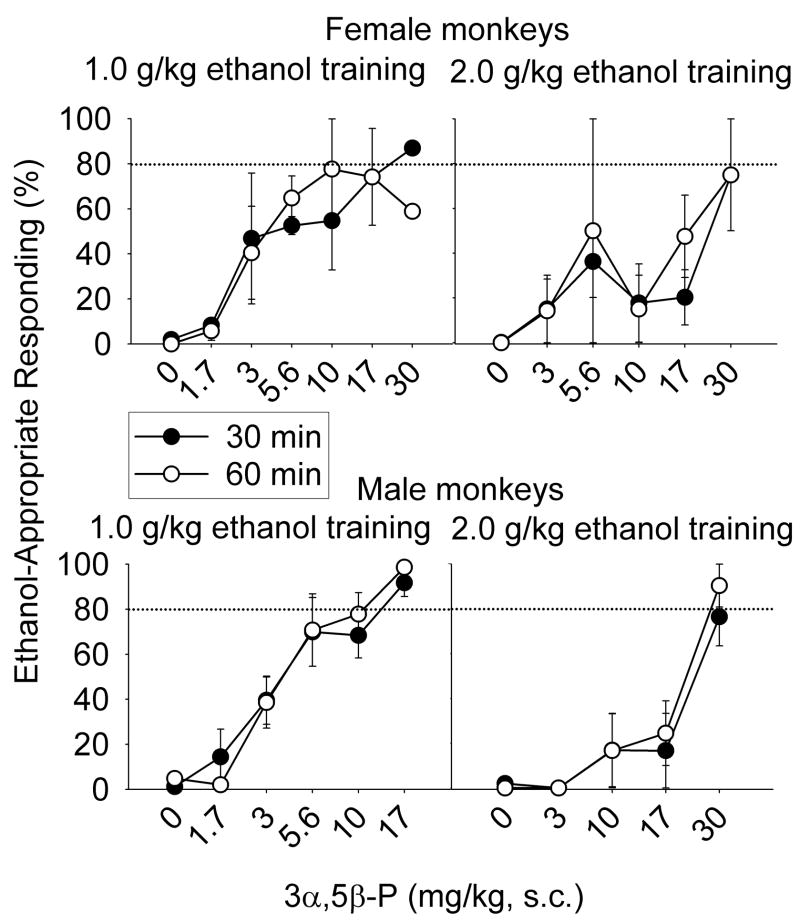
The 3α-hydroxy steroids 3α,5α-P, 3α,5β-P, and 3α,5α-A completely substituted for ethanol in 29/32, 28/34, and 24/36 dose-response determinations, respectively. For all four groups and each pre-treatment interval, percent ethanol-appropriate responding increased as a function of dose for 3α,5α-P [F(8, 48) = 7.42, p < 0.001] (Fig. 2), 3α,5β-P [F(7, 60) = 8.14, p < 0.001] (Fig. 3), and 3α,5α-A [F(6, 59) = 8.94, p < 0.001] (Fig. 4). The potency (ED50) of 3α,5α-P to substitute for ethanol after either pre-treatment interval did not differ between the training doses or between males and females. Despite the absence of a training dose effect, Figure 2 suggests a trend for 3α,5α-P to substitute less potently for the discriminative stimulus effects of 2.0 g/kg ethanol compared to 1.0 g/kg ethanol, in male and female monkeys at both pre-treatment intervals. Figure 2 also suggests that 3α,5α-P was less efficacious in female monkeys trained to discriminate 2.0 g/kg ethanol compared to female monkeys trained to discriminate 1.0 g/kg ethanol. Indeed, the only group showing partial, rather than full, substitution, after 3α,5α-P administration were females trained to discriminate 2.0 g/kg ethanol. However, as shown in Supplemental Figure 1, the difference between full (1.0 g/kg ethanol females) and partial (2.0 g/kg ethanol females) substitution was due to 1 less monkey showing substitution in the 2.0 g/kg ethanol trained group. In every male tested, 3α,5α-P completely substituted for ethanol.
Fig. 2.
Mean (± SEM) percentage of total session responding on the ethanol-appropriate lever 30- (closed circles) or 60-min (open circles) after administration of 3α,5α-pregnanolone (P) in cynomolgus monkeys trained to discriminate 1.0 (female, n=2–4; male, n=1–5) or 2.0 g/kg (female, n=2–4; male, n=1–3) ethanol from water with a 30-min pre-treatment interval. Additional doses were tested, but are not shown, as indicated by the dose-response curves for individual monkeys shown in Supplemental Figure 1.
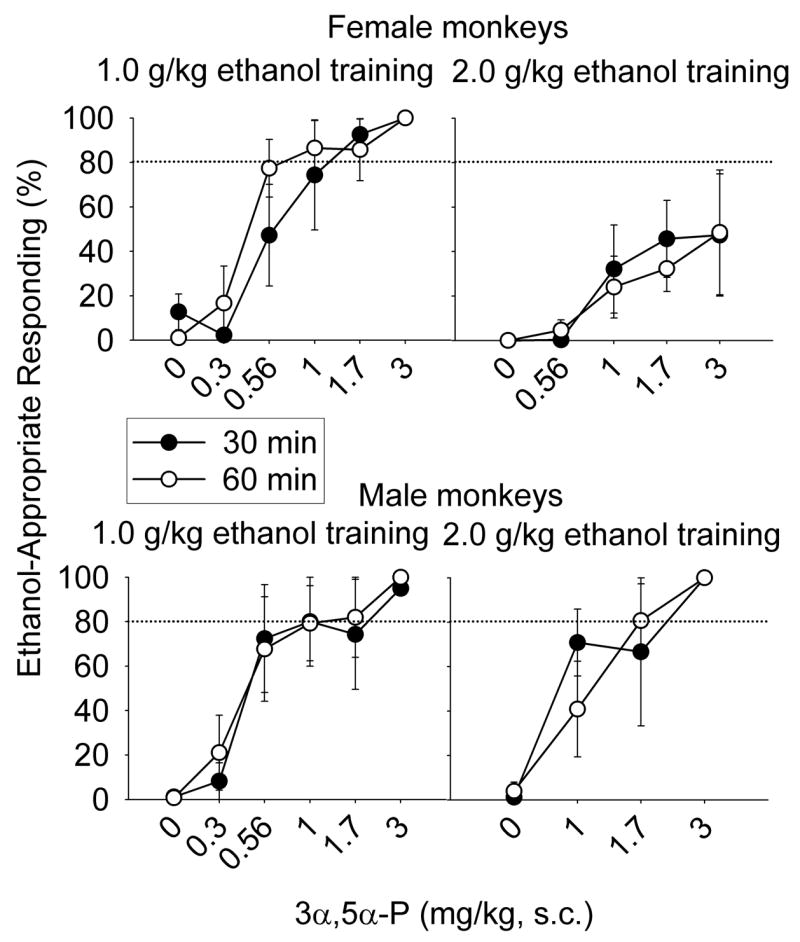
Fig. 3.
Mean (± SEM) percentage of total session responding on the ethanol-appropriate lever 30- (closed circles) or 60-min (open circles) after administration of 3α,5β-pregnanolone (P) in cynomolgus monkeys trained to discriminate 1.0 (female, n=1–4; male, n=4–6) or 2.0 (female, n=2–4; male, n=2–3) g/kg ethanol from water with a 30-min pre-treatment interval. Additional doses were tested, but are not shown, as indicated by the dose-response curves for individual monkeys shown in Supplemental Figure 2.
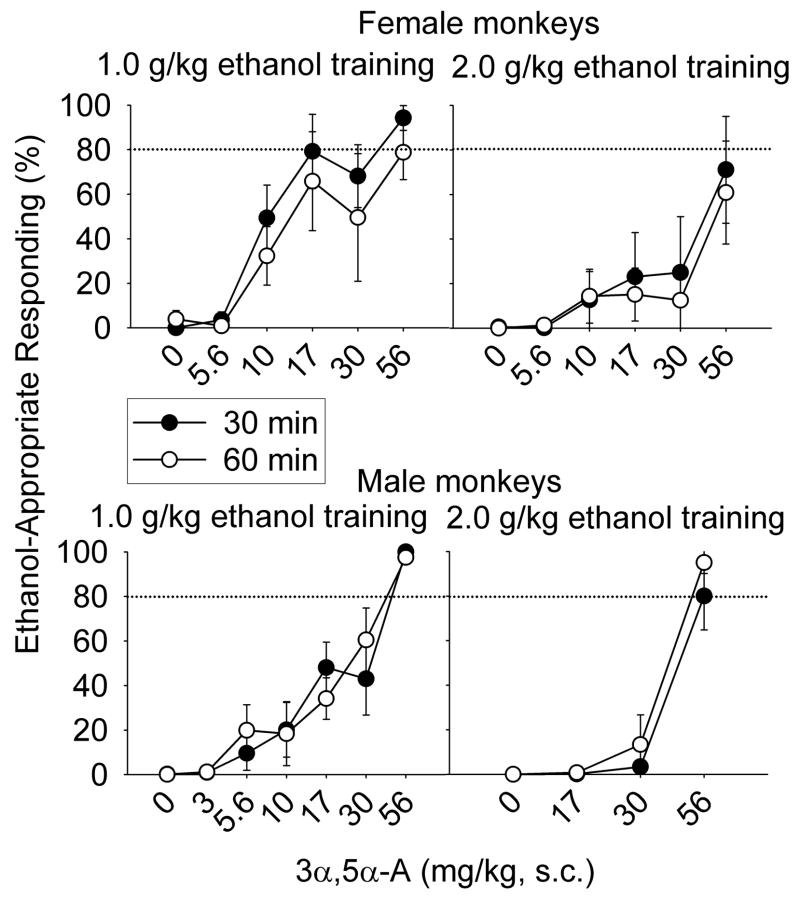
Fig. 4.
Mean (± SEM) percentage of total session responding on the ethanol-appropriate lever 30- (closed circles) or 60-min (open circles) after administration of 3α,5α-androsterone (A) in female and male cynomolgus monkeys trained to discriminate 1.0 (female, n=3–4; male, n=2–6) or 2.0 (female, n=3–4; male, n=2–3) g/kg ethanol from water with a 30-min pre-treatment interval. Dose-response curves for individual monkeys are shown in Supplemental Figure 3.
The potency (ED50) of 3α,5β-P to substitute for ethanol did not vary with sex or training dose after a 30-min pretreatment interval. With a 60-min pre-treatment interval, however, 3α,5β-P more potently substituted for ethanol in male and female monkeys trained to discriminate 1.0 g/kg compared to monkeys trained to discriminate 2.0 g/kg ethanol [main effect of training dose, F(1, 11) = 16.1, p = 0.002] (Fig. 3). In terms of efficacy, mean ethanol-appropriate responding met the criterion for complete substitution for 1.0 g/kg ethanol at both pre-treatment intervals in males and at the 30-min pre-treatment interval in females. In females trained to discriminate 2.0 g/kg ethanol, mean ethanol-appropriate responding 30 and 60 min after 3α,5β-P administration met the criterion for partial substitution. However, 3/4 and 4/4 female monkeys, respectively, showed complete substitution for 2.0 g/kg ethanol 30 and 60 min after administration, although only with the highest dose tested in 6/7 cases (Supplemental Figure 2). In males trained to discriminate 2.0 g/kg ethanol, 3α,5β-P partially substituted for ethanol 30 min after administration, and completely substituted after a 60-min pretreatment. All cases of complete substitution of 3α,5β-P in males trained to discriminate 2.0 g/kg ethanol were observed with the highest dose tested (Supplemental Figure 2). In general, however, ethanol-appropriate responding in cases of partial substitution was > 70%, suggesting minor variation in the efficacy of 3α,5β-P to substitute for ethanol across sex, training dose, and pre-treatment interval. Supplemental Figure 2 shows partial substitution of 3α,5β-P for ethanol in 2 female (1 trained to 1.0 g/kg ethanol and 1 trained to 2.0 g/kg ethanol) and 3 male (1 trained to discriminate 1.0 g/kg and 2 trained to discriminate 2.0 g/kg ethanol) monkeys.
Main effects of training dose indicated that 3α,5α-A substituted more potently for 1.0 g/kg compared to 2.0 g/kg ethanol with both the 30-min [F(1, 7) = 9.0, p = 0.02] and the 60-min [F(1, 11) = 7.9, p = 0.03] pre-treatment interval for both sexes (Fig. 4). The potency of 3α,5α-A to substitute for ethanol did not differ between the sexes. In males trained to discriminate 1.0 or 2.0 g/kg ethanol, 3α,5α-A completely substituted after each pre-treatment interval. In females trained to discriminate 1.0 g/kg ethanol, 3α,5α-A showed complete substitution after a 30-min pre-treatment and partial substitution after a 60-min pretreatment. In contrast, group means showed that 3α,5α-A only partially substituted in females trained to discriminate 2.0 g/kg ethanol, indicating reduced efficacy. Across both pre-treatment intervals, 3α,5α-A completely substituted for 2.0 g/kg ethanol in females in 5 cases, with 4 of these cases occurring with the highest dose tested. All cases of complete substitution in males trained to discriminate 2.0 g/kg ethanol occurred with the highest dose of 3α,5α-A tested (Supplemental Figure 3). Supplemental Figure 3 shows that partial substitution was observed in 4 male (2 trained to 1.0 g/kg ethanol and 2 trained to 2.0 g/kg ethanol) and 3 female (1 trained to 1.0 g/kg ethanol and 2 trained to 2.0 g/kg ethanol) monkeys.
A main effect of test drug in a 3 (test drug: 3α,5α-P, 3α,5β-P, 3α,5α-A) × 2 (sex) × 2 (training dose) × 2 (pre-treatment interval) mixed factor ANOVA, F(2, 24) = 155.75, p < 0.001, indicated that potency to substitute for the discriminative stimulus effects of ethanol differed among the 3α-hydroxy steroids, with rank order potency corresponding to 3α,5α-P > 3α,5β-P > 3α,5α-A (Fig. 1, Table 1).
Table 1.
Average (± SEM) potency (ED50, mg/kg, s.c.) of test drugs to substitute for ethanol (i.g.). The ratio of monkeys showing ≥ 80% ethanol-appropriate responding with at least one test drug dose to the number of monkeys tested is listed in parentheses
| Test Drug | Training pretreatment (min) | ED50 (mg/kg)
|
|||
|---|---|---|---|---|---|
| 1.0 g/kg Ethanol training | 2.0 g/kg Ethanol training | ||||
| Females | Males | Females | Males | ||
| 3α,5α-P | 30 | 0.40±0.05 (3/4) | 0.89±0.38 (5/5) | 1.43±0.30 (3/4) | 0.70±0.20 (3/3) |
| 60 | 0.75±0.21 (4/4) | 0.83±0.37 (5/5) | 1.78±0.27 (3/4) | 1.07±0.15 (3/3) | |
| 3α,5β-P | 30 | 11.74±5.15 (4/4) | 4.28±1.47 (5/6) | 16.24±4.43 (3/4) | 17.0 (1/3) |
| 60** | 3.83±1.03 (3/4) | 4.70±1.15 (6/6) | 11.65±3.32 (4/4) | 19.25±2.25 (2/3) | |
| 3α,5α-A | 30* | 9.02±1.75 (3/4) | 13.69±2.33 (4/6) | 32.78±11.44 (3/4) | 44.48 (3/4) |
| 60* | 10.90±1.17 (3/4) | 16.69±3.90 (4/6) | 28.71±15.21 (2/4) | 43.93±1.32 (2/4) | |
| 3β,5α-P | 30 | No suba (0/4) | No sub (0/4) | No sub (0/4) | No sub (0/3) |
| 60 | (1/4)b | No sub (0/4) | No sub (0/4) | No sub (0/3) | |
| 3β,5β-P | 30 | No sub (0/4) | No sub (0/6) | No sub (0/4) | No sub (0/2) |
| 60 | No sub (0/4) | 25.0 (1/6) | No sub (0/4) | No sub (0/2) | |
| 3α-5β-P HS | 30 | (1/4)b | No sub (0/2) | No sub (0/3) | No sub (0/2) |
| 60 | 42.41 (1/4) | 30.95 (1/2) | No sub (0/3) | No sub (0/2) | |
sub, substitution
ED50 not calculable
p < 0.05,
p < 0.01; main effect of training dose
As predicted, 3α,5β-P HS, 3β,5α-P, and 3β,5β-P rarely (5/84 dose-response determinations) substituted for ethanol. For the 30- and 60-min pretreatment intervals, respectively, complete substitution for ethanol was observed in 1/11 and 2/11 monkeys tested with 3α,5β-P HS, in 0/15 and 1/15 monkeys tested with 3β,5α-P, and in 0/16 and 1/16 monkeys tested with 3β,5β-P (Table 1; Supplemental Figure 4, Supplemental Figure 5, Supplemental Figure 6).
During baseline sessions, female monkeys trained to discriminate 1.0 and 2.0 g/kg ethanol averaged, respectively, 2.98 ± 0.24 and 1.73 ± 0.15 responses/s; male monkeys trained to discriminate 1.0 and 2.0 g/kg ethanol averaged, respectively, 1.84 ± 0.12 and 1.20 ± 0.10 responses/s. Baseline response rate was significantly greater for females compared to males [F(1, 15) = 6.12, p = 0.03] and for monkeys trained to discriminate 1.0 g/kg ethanol compared to monkeys trained to discriminate 2.0 g/kg ethanol [F(1, 15) = 4.65, p = 0.048]. Response rates during substitution tests were therefore expressed as percent of baseline (Supplemental Figure 7). Percent of baseline response rate was lower after the 60-min compared to the 30-min pre-treatment intervals. This trend was statistically significant only for 3α,5β-P [F(1, 79) = 4.54, p = 0.04], 3α,5β-P HS [F(1, 22) = 26.78, p < 0.001], and 3β,5β-P [F(1, 49) = 10.01, p = 0.003] according to main effects of pre-treatment interval. The 3α-hydroxysteroids generally increased response rate above baseline in monkeys trained to discriminate 1.0 g/kg [mean ± SEM: 3α,5α-P, 105.2 ± 5.5; 3α,5β-P, 110.6 ± 4.9; 3α,5α-A, 111.8 ± 4.0], but decreased response rates below baseline in monkeys trained to discriminate 2.0 g/kg ethanol [mean ± SEM: 3α,5α-P, 79.2 ± 7.3; 3α,5β-P, 87.4 ± 6.9; 3α,5α-A, 86.2 ± 5.6]. This training dose difference was not shared with the 3β-hydroxysteroids and 3α,5β-P HS, which decreased response rates below baseline in monkeys trained to discriminate 1.0 g/kg ethanol [3α,5β-P HS, 88.3 ± 11.3; 3β,5β-P, 83.5 ± 4.8; 3β,5α-P, 86.2 ± 6.8] and 2.0 g/kg ethanol [3α,5β-P HS, 81.0 ± 10.3; 3β,5β-P, 74.6 ± 6.6; 3β,5α-P, 70.4 ± 7.2]. Males showed greater decreases in response rate compared to females for all drugs tested, but this effect was significant only for 3α,5β-P [F(1, 79) = 5.08, p = 0.03; 3α,5α-P, F(1, 69) = 0.57; 3α,5α-A, F(1, 80) = 3.53, p = 0.06; 3α,5β-P HS, F(1, 22) = 0.55; 3β,5β-P, F(1, 49) = 0.51; 3β,5α-P, F(1, 46) = 0.76].
Discussion
Humans produce four neuroactive isomers of pregnanolone (Havlíková et al., 2006) withdistinct pharmacological properties in vitro (Park-Chung et al., 1997, 1999). The data show distinct differences in efficacy of 3α versus 3β isomers of pregnanolone to produce ethanol-like discriminative stimulus effects in non-human primates. In contrast to the 3β isomers, the 3α isomers of pregnanolone and androsterone showed ethanol-like effects in a majority of tests, across sex, training dose and pretreatment times. Furthermore, 3α,5β-P but not 3α,5β-P HS substituted for ethanol. Thus, addition of a negatively-charged group at C-3 prevents the ethanol-like discriminative stimulus effects of 3α,5β-P. A main implication from the data is that individual differences in bioconversion of the precursors progesterone or DHEA/testosterone to 3α- or 3β-neuroactive steroids or sulfate derivatives could underlie individual differences in subjective effects following ethanol consumption. An individual predisposed to synthesize relatively more 3β compared to 3α-pregnanolone, or sulfated compared to non-sulfated pregnanolone, may be less sensitive to the effects of consumed ethanol, perhaps a mechanism for innate or acquired tolerance. This, in turn, could affect the choice to continue drinking ethanol on a given occasion resulting in binge-levels of intake. For example, 3α,5β-P HS reduces ethanol self-administration in rats (O’Dell et al., 2005), but this has not been explored in non-human primates. How endogenous levels of 3β- or sulfated hydroxysteroids influences ethanol self-administration, or subjective effects, remains to be explored.
Individual differences in the metabolism of progesterone and its derivatives could account for the rare cases in which 3β-hydroxysteroids substituted for ethanol in the monkeys. For example, the 3β isomer 3β,5α-P is increased in women with chronic fatigue syndrome (Murphy et al., 2004), and pharmacokinetic analyses showed dramatically reduced clearance of 3α,5β-P in 1/9 females tested (Dale et al., 1999). The steroidal milieu resulting from enzymatic activity could affect GABAA receptor pharmacology, ethanol sensitivity, and subsequent neuroactive steroid substitution for ethanol. For example, in vitro experiments show that 3α,5α-P treatment increased the expression of GABAA receptor α4 subunits (Zhou and Smith, 2007), which are potently modulated by ethanol (Wallner et al., 2006) and neuroactive steroids due to co-expression with δsubunits (Belelli and Lambert, 2005). Very little data addresses species differences in the role of endogenous enzymes in producing behaviorally-relevant concentrations of neuroactive steroids. Drug discrimination in macaque monkeys appears to provide a pharmacologically accurate animal model with which to study neuroactive steroid mechanisms mediating the subjective effects of ethanol in humans.
These data have implications for species differences in ethanol-like discriminative stimulus effects from neuroactive steroids that positively modulate GABAA receptors. Specifically, the progesterone-derivatives 3α,5α-P, 3α,5β-P and 3β,5β-P substitute for 1.0 and 2.0 g/kg ethanol in male Long-Evans rats (Bowen et al., 1999). Substitution of the latter contrasts with the current findings in monkeys in which the isomers that do not positively modulate GABAA receptors, 3β,5β-P, 3β,5α-P, in addition to 3α,5β-P HS, rarely substituted (only 6% of dose-response determinations) for ethanol. Since the monkey data match in vitro pharmacological data of activity at the GABAA receptor, the substitution of 3β,5β-P for ethanol in rats may be due to species differences in bioconversion of administered neuroactive steroids. Rodents appear to have minimal 5β-reductase activity (RH Purdy, unpublished data) and may not produce the four pregnanolone isomers present in primates. The role of endogenous isomers in GABAA receptor mediation of ethanol-like discriminative stimulus effects in rodents compared to primates is unclear.
Individual differences in endogenous progesterone related to menstrual cycles influences sensitivity to behavioral effects including the discriminative stimulus effects of ethanol. For example, 12% of females with epilepsy suffer from a greater incidence of seizures during the low-progesterone perimenstrual phase (Duncan et al., 1993), which appears to be related to reduced GABAA receptor positive modulation (Reddy et al., 2001; Herzog and Frye, 2003). Female monkeys show reduced potency and efficacy in 3α,5α-P substitution for ethanol in the follicular (perimenstrual) phase of the menstrual cycle (Grant et al., 1997). In contrast to the current design, menstrual cycle effects were previously investigated by collecting a cumulative dose-response curve in a single session. The present experiment found no sex differences in neuroactive steroid substitution for ethanol, although the female monkeys had normal menstrual cycles. The monkeys were trained to discriminate ethanol across all menstrual cycle phases (2–3 times per week), perhaps training them to discriminate a range of ethanol stimulus effects due to differential circulating progesterone derivatives. For each test drug reported here, as indicated by mense records and serum progesterone, 26.4–52.6% and 36.9–69.8% of tests occurred in the luteal and follicular phases, respectively. The substitution data suggest that the potency of 3α,5α-P to substitute for ethanol in female monkeys reflects composite menstrual cycle phase influences.
In addition to the 3α and 3β isomers, the 5α and 5β isomers differed in potency to produce ethanol-like discriminative stimulus effects. The potency of 3α,5α-P was 3–40-fold greater compared to 3α,5β-P. This is consistent with the stereospecificity of effects mediated by 5α and 5β neuroactive steroids in a variety of species and assays (e.g., Simmonds, 1991). Neuroactive steroids act as agonists at GABAA receptors at binding sites that are distinct from the sites at which neuroactive steroids act as positive modulators. As an agonist, 3α,5α-P is more efficacious than 3α,5β-P (Hosie et al., 2006). Since 3α,5α-P substituted for ethanol with greater potency than 3α,5β-P, the agonist site could be an important target for alcoholism pharmacotherapy. Although central nervous system GABAA receptor agonism appears to be insufficient to produce ethanol-like discriminative stimulus effects, as indicated by the lack of substitution of peripherally-administered muscimol (Grant et al., 2000; but see Hodge and Cox, 1998), whether agonism via a neuroactive steroid binding site is sufficient to produce ethanol-like discriminative stimulus effects remains to be determined. Synthetic steroids are currently being developed that could be used to evaluate this hypothesis (e.g., Mennerick et al., 2004).
The training dose of ethanol influenced the potency or efficacy of 3α-hydroxysteroids to substitute for ethanol (Figures 2–4). In cases for which this effect was not statistically significant, the analysis may have been underpowered as only 3 males were trained to discriminate 2.0 g/kg ethanol. In general, 3α,5α-P, 3α,5β-P and 3α,5α-A substituted for ethanol with greater potency in male and female monkeys trained to discriminate 1.0 compared to 2.0 g/kg ethanol. Efficacy was comparatively low in the females trained to discriminate 2.0 g/kg ethanol, where ethanol-appropriate responding after administration of 3α,5α-P, 3α,5β-P, and 3α,5α-A met the criterion for partial substitution. For each of these test drugs, however, partial rather than complete substitution for 2.0 g/kg ethanol was primarily due to substitution patterns in a single female monkey, 5999 (Supplemental Figure 1, Supplemental Figure 2, Supplemental Figure 3). Investigations of the role of serotonin and glutamate receptor systems indicate that multiple receptors mediate the discriminative stimulus effects of ethanol (Grant, 1999). A greater proportion of the ethanol stimulus may involve receptors sensitive to 3α-hydroxysteroids in monkeys trained to discriminate lower, compared to higher, doses of ethanol. In support of this hypothesis, NMDA receptors appear to mediate the discriminative stimulus effects of 2.0 g/kg ethanol in female monkeys (Vivian et al. 2002), the group showing the lowest efficacy of GABAA receptor-sensitive neuroactive steroids in this study. However, the NMDA receptor antagonist 3α,5β-P HS (Park-Chung et al., 1997) did not substitute for ethanol in any group, including females trained to discriminate 2.0 g/kg ethanol. Antagonism of NMDA receptors by 3α,5β-P HS is likely to be similar to its endogenous analog, 3α,5β-P sulfate, which noncompetitively antagonizes NMDA receptors (Park-Chung et al., 1994). The lack of substitution of 3α,5β-P HS in the current study could be due to negation of NMDA effects via inhibition of GABAA receptors (Park-Chung et al., 1999).
The effects of 3α- and 3β-neuroactive steroids and 3α,5β-P HS on rates of responding were distinct from their ethanol-like discriminative stimulus effects. For example, the effect of neuroactive steroids on response rate, but not ethanol-appropriate responding, depended on the pre-treatment interval. This difference could reflect an interaction between regional pharmacokinetics, steroid binding (Sapp et al., 1992), and the brain regions mediating the sedative versus discriminative stimulus effects of ethanol. Indeed, that 3α,5α-A substituted less potently for ethanol compared to 3α,5α-P, and in many cases, 3α,5β-P, could indicate low brain levels of 3α,5α-A in primates. For example, the first of three enzymes involved in the conversion of DHEA to 3α,5α-A, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD) (Simard et al., 2005) is absent from the cerebral cortex, cerebellum, and cerebrum of rhesus monkeys (Martel et al., 1994). To evaluate this hypothesis, additional studies must be conducted to identify regional differences in neuroactive steroid levels following ethanol administration.
Overall, the receptors mediating the effects of neuroactive steroids represent an important avenue for future research regarding alcoholism pharmacotherapy. These data provide key information regarding structure-activity requirements, the effects of ethanol dose, and sex as a first-step toward identifying specific pharmacotherapies targeting neuroactive steroid-sensitive receptors mediating the subjective effects of ethanol. Future studies could address the relationship between endogenous steroid levels, steroidogenic enzyme activity, and sensitivity to the ethanol-like discriminative stimulus effects of neuroactive steroids.
Supplementary Material
Supplemental Figure 1. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5α-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 2. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5β-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 3. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5α-androsterone (A) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 4. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3β,5α-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 5. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3β,5β-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 6. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5β-pregnanolone (P) hemisuccinate (HS) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 7. Mean (± SEM) percent of baseline response rate averaged from the post-ethanol and post-water test sessions when possible for each neuroactive steroid tested for ethanol substitution in female and male cynomolgus monkeys trained to discriminate 1.0 g/kg (circles) or 2.0 g/kg (triangles) ethanol (i.g.). Substitution testing occurred 30 (open symbols) or 60 (closed symbols) min after neuroactive steroid administration.
Acknowledgments
The authors thank Courtney A. Waters for excellent technical expertise.
This research was funded by NIH/NIAAA AA10009, AA 13860, and RR 000163. This work was presented at the 30th Annual Meeting of the Research Society on Alcoholism July 2007 (Helms et al., Alcohol Clin Exp Res S31: 80A).
List of nonstandard abbreviations
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-D-aspartate
- DHEA
dehydroepiandrosterone
- 3α5α-P,
allopregnanolone
- 3α5β-P
pregnanolone
- 3β5α-P
epiallopregnanolone
- 3β5β-P
epipregnanolone
- 3α
5β-P HS, pregnanolone hemisuccinate
- 3α
5α-A, androsterone
References
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nature Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. Ethanol-like discriminative stimulus effects of endogenous neuroactive steroids: effects of ethanol training dose and dosing procedure. J Pharmacol Exp Ther. 1999;289:405–411. [PubMed] [Google Scholar]
- Dale O, Hynne H, Parivar K, Johansson E, Widman M. Pharmacokinetics of eltanolone in male and female patients following intravenous bolus injection. Acta Anaesthesiol Scan. 1999;43:415–20. doi: 10.1034/j.1399-6576.1999.430409.x. [DOI] [PubMed] [Google Scholar]
- Duncan S, Read CL, Brodie MJ. How common is catamenial epilepsy? Epilepsia. 1993;34:827–831. doi: 10.1111/j.1528-1157.1993.tb02097.x. [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Shively CA, Purdy RH. Discriminative stimulus effects of ethanol and 3α-hydroxy-5α-pregnan-20-one in relation to menstrual cycle phase in cynomolgus monkeys (Macaca fascicularis) Psychopharmacology. 1997;130:59–68. doi: 10.1007/s002130050211. [DOI] [PubMed] [Google Scholar]
- Grant KA, Azarov A, Bowen CA, Mirkis S, Purdy RH. Ethanol-like discriminative stimulus effects of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in female Macaca fascicularis monkeys. Psychopharmacology. 1996;124:340–346. doi: 10.1007/BF02247439. [DOI] [PubMed] [Google Scholar]
- Grant KA, Waters CA, Green-Jordan K, Azarov A, Szeliga KT. Characterization of the discriminative stimulus effects of GABAA receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology. 2000;152:181–188. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- Green KL, Azarov AV, Szeliga KT, Purdy RH, Grant KA. The influence of menstrual cycle phase on sensitivity to ethanol-like discriminative stimulus effects of GABAA-positive modulators. Pharmacol Biochem Behav. 1999;64:379–383. doi: 10.1016/s0091-3057(99)00057-x. [DOI] [PubMed] [Google Scholar]
- Havlíková H, Hill M, Kancheva L, Vrbíková J, Pouzar V, }erný I, Kancheva R, Stárka L. Serum profiles of free and conjugated neuroactive pregnanolone epimers in nonpregnant women of fertile age. J Clin Endocrinol Metab. 2006;91:3092–3099. doi: 10.1210/jc.2005-2785. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABAA receptors in specific limbic brain regions. Psychopharmacology. 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Holtzman SG, Cook L, Steinfels FG. Discriminative stimulus effects of spiradoline, a kappa-opioid agonist. Psychopharmacology. 1991;105:447–452. doi: 10.1007/BF02244362. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Krupitsky E, Schütz C, Trevisan L, D’Souza DC. NMDA receptor antagonism and the ethanol intoxication signal. Proc Natl Acad Sci. 2003;1003:176–184. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Staley J, Mason G, Petrakis IL, Kaufman J, Harris RA, Gelemter J, Lappalainen J. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Arch Gen Psychiatry. 2006;63:957–968. doi: 10.1001/archpsyc.63.9.957. [DOI] [PubMed] [Google Scholar]
- Li T-K, Hewitt BG, Grant BF. Is there a future for quantifying drinking in the diagnosis, treatment, and prevention of alcohol use disorders? Alcohol Alcohol. 2007;41:57–63. doi: 10.1093/alcalc/agl125. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Martel C, Melner MH, Gagné D, Simard J, Labrie F. Widespread tissue distribution of steroid sulfatase, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 epimerase (3β-HSD), 17β-HSD 5α-reductase and aromatase activities in the rhesus monkey. Mol Cell Endocrinol. 1994;104:103–111. doi: 10.1016/0303-7207(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF. Selective antagonism of 5α-reduced neurosteroid effects at GABAA receptors. Mol Pharmacol. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Murphy BEP, Abbott FV, Allison CM, Watts C, Ghadirian A-M. Elevated levels of some neuroactive progesterone metabolites, particularly isopregnanolone, in women with chronic fatigue syndrome. Psychoneuroendocrinol. 2004;29:245–268. doi: 10.1016/s0306-4530(03)00026-x. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Purdy RH, Covey DF, Richardson HN, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacol Biochem Behav. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu F-S, Farb DH. 3α-hydroxy-5β-pregnan-20-one sulfate: a negative modulator of the NMDA-induced current in cultured neurons. Mol Pharmacol. 1994;46:146–160. [PubMed] [Google Scholar]
- Park-Chung M, Wu F-S, Purdy RH, Malayev AA, Gibbs TT, Farb DH. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol. 1997;52:1113–1123. doi: 10.1124/mol.52.6.1113. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Research. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kim H-Y, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Sapp DW, Witte U, Turner DM, Longoni B, Kokka N, Olsen RW. Regional variation in steroid anesthetic modulation of [35S]TBPS binding to γ-aminobutyric acidA receptors in rat brain. J Pharmacol Exp Ther. 1992;262:801–808. [PubMed] [Google Scholar]
- Simard J, Ricketts M-L, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 epimerase gene family. Endocr Rev. 2005;26:525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- Simmonds MA. Modulation of the GABAA receptor by steroids. Sem Neurosci. 1991;3:231–239. [Google Scholar]
- Vivian JA, Waters CA, Szeliga KT, Jordan K, Grant KA. Characterization of the discriminative stimulus effects of N-methyl-D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology. 2002;162:273–281. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABAA receptor subtypes. Pharmacol & Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng C-M, Matthews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S. 3β-hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CE, Jr, Marek P, Park-Chung M, Tam SW, Farb DH. Neuroprotective activity of a new class of steroidal inhibitors of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci. 1997;94:14050–10454. doi: 10.1073/pnas.94.19.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Smith SS. Steroid requirements for regulation of the α4 subunit of the GABAA receptor in an in vitro model. Neurosci Lett. 2007;411:61–66. doi: 10.1016/j.neulet.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5α-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 2. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5β-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 3. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5α-androsterone (A) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 4. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3β,5α-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 5. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3β,5β-pregnanolone (P) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 6. Ethanol-appropriate responding 30 (left column) or 60 (right column) min after 3α,5β-pregnanolone (P) hemisuccinate (HS) administration in individual female and male cynomolgus monkeys trained to discriminate 1.0 or 2.0 g/kg ethanol (i.g.) with a 30-min pre-treatment interval.
Supplemental Figure 7. Mean (± SEM) percent of baseline response rate averaged from the post-ethanol and post-water test sessions when possible for each neuroactive steroid tested for ethanol substitution in female and male cynomolgus monkeys trained to discriminate 1.0 g/kg (circles) or 2.0 g/kg (triangles) ethanol (i.g.). Substitution testing occurred 30 (open symbols) or 60 (closed symbols) min after neuroactive steroid administration.